Abstract
Scope
Dietary prebiotics show potential in anti-diabetes. Dietary resistant starch (RS) has a favorable impact on gut hormone profiles, including glucagon-like peptide-1 (GLP-1) consistently released, a potent anti-diabetic incretin. Also RS reduced body fat and improved glucose tolerance in rats and mice. In the current project, we hypothesize that dietary-resistant starch can improve insulin sensitivity and pancreatic β cell mass in a type 2 diabetic rat model. Altered gut fermentation and microbiota are the initial mechanisms, and enhancement in serum GLP-1 is the secondary mechanism.
Methods and results
In this study, GK rats were fed an RS diet with 30% RS and an energy control diet. After 10 wk, these rats were mated and went through pregnancy and lactation. At the end of the study, pancreatic β cell mass, insulin sensitivity, pancreatic insulin content, total GLP-1 levels, cecal short-chain fatty acid concentrations and butyrate producing bacteria in cecal contents were greatly improved by RS feeding. The offspring of RS-fed dams showed improved fasting glucose levels and normal growth curves.
Conclusion
Dietary RS is potentially of great therapeutic importance in the treatment of diabetes and improvement in outcomes of pregnancy complicated by diabetes.
Keywords: Fermentation, Glucagon-like peptide-1, Glycemic control, Resistant starch, Short- chain fatty acid
1 Introduction
With the explosion of diabetes reaching epidemic proportion, maternal hyperglycemia is becoming a health threat to pregnant women worldwide. Maternal hyperglycemia patients consist of diabetic pregnant women and those diagnosed with gestational diabetes mellitus (GDM). Maternal hyperglycemia increases risks in labor for mothers. Studies also showed by contributing to the abnormal fetal environment, maternal hyperglycemia might predispose offspring to develop metabolic disorders [1, 2]. Approaches for treatment of maternal hyperglycemia are limited due to safety concerns for the fetus.
Resistant starches (RS) are dietary carbohydrates that resist digestion in the small intestine and reach the large intestine where they are fermented by bacteria to produce short-chain fatty acids. We have shown that feeding RS decreases body fat accumulation in rodents, increases gut glucagon-like peptide-1 (GLP-1) gene expression and plasma level, and improves glucose tolerance in Streptozotocin-induced diabetic mice [3–5]. GLP-1 is a potent incretin by the L enteroendocrine cells of the distal intestine [6]. GLP-1 has been shown to possess multiple effects on glucose metabolism such as promoting pancreatic β-cell mass [7, 8], stimulating glucose-dependent insulin secretion [9] and inhibiting glucagon secretion [10].
However, the effects of dietary RS on maternal hyper-glycemia remain unknown. In this study, therefore, we tested the impact of RS feeding on improving glycemic control in pregnant type 2 diabetes rats – Goto–Kakizaki (GK) rats. It is indicated that fermentation plays an important role in the effects of dietary-resistant starch [11]. Therefore, we investigated several markers involved in the fermentation process as well as uncoupling protein-1 (UCP-1) to explore the underlying mechanism. UCP-1 (Thermogenin) is highly present in brown adipose tissue (BAT). It helps accumulated protons in the inter-membrane space in mitochondria to return to the matrix resulting in the production of heat instead of ATP like a short circuit [12]. Our previous research demonstrated that RS feeding increased fatty acid oxidation [11].
Owing to its ability to resist being broken down completely by digestive enzymes, it was reported that RS in the diet was accompanied by gastrointestinal (GI) effects including flatus, abdominal discomfort and diarrhea, which could exert a negative impact on gestation and fetal growth [13]. Therefore, pup size, their growth curve and fat pad weights were observed to elucidate these possibilities.
2 Methods and materials
2.1 Animals and diet
Twenty female GK rats aged 5 wk and weighing 80–100 g along with 10 age-matched female Wistar rats at the beginning of the study were obtained from Charles River (Wilmington, MA, USA). They were housed individually in hanging wire-mesh cages in a temperature-controlled room (22±1°C) on a 12 h/12 h light/dark cycle with the light on at 7 am. Rats were acclimated for 1 wk to a powdered diet and to the cages. Water and assigned diet were available ad libitum during the experiment except as noted. The protocols (#604P) were approved by the Pennington Biomedical Research Institutional Animal Care and Use Committee.
The composition of the two experimental diets used in this study is listed in Table 1. The RS diet contained 30% (weight/weight) type 2 resistant starch (Hi-Maize® corn-starch containing 60% amylose; National Starch Food Innovation, Bridgewater, NJ, USA). The metabolizable energy value for the Hi-Maize®-260 cornstarch was determined as 2.8 kcal/g [14]. The equal energy density control (EC) diet had 100% amylopectin cornstarch (Amioca®; National Starch Food Innovation) as the carbohydrate source and equal energy density as the RS diet (3.3 kcal/g) by using purified non-fermentable cellulose (Dyets, Bethlehem, PA, USA) to dilute the energy density (Table 1).
Table 1.
Experimental diet composition
| Ingredients | Control
|
RS
|
||
|---|---|---|---|---|
| Grams | kcal | Grams | kcal | |
| 100% amylopectin High amylose starch | 424.5 | 1485.8 | 0 | 0 |
| 60% amylose/40% amylopectin | 0 | 530.7 | 1486 | |
| Sucrose | 100 | 400 | 100 | 400 |
| Casein | 200 | 716 | 200 | 716 |
| Soybean oil | 70 | 591.5 | 70 | 591.5 |
| Cellulose | 156.2 | 0 | 50 | 0 |
| Mineral mix | 35 | 30.8 | 35 | 30.8 |
| Vitamin mix | 10 | 38.7 | 10 | 38.7 |
| Choline chloride | 1.3 | 0 | 1.3 | 0 |
| L-cystine | 3.0 | 12 | 3.0 | 12 |
| 1000g/kg | 3.3kcal/g | 1000g/kg | 3.3kcal/g | |
2.2 Experimental design
After 1 wk of acclimation, GK rats were randomly grouped and divided into two diet treatment groups, RS and EC, and stratified by their weight. Wistar rats served as a genetic control and were fed the EC diet. The three groups of rats were fed their assigned diets throughout the experiment. Food intake and body weight were measured three times per week. After they were on the diets for 70 days, animals in all three groups were mated with male Wistar rats by caging two opposite gender rats together. Pregnancy was confirmed by the presence of a vaginal plug. After rats in these three groups delivered, their pups were weighed and litter size reduced to six pups. Ten male pups from each group were randomly chosen and raised to 8 wk old on standard chow diet (#5001, Dietlab, USA). Food intake and body weight were measured three times a week.
The dams were sacrificed via decapitation when pups were weaned. Different fat pads (ovarian fat, perirenal fat and abdominal fat) were removed and weighed. Total abdominal body fat used for body fat calculation was the sum of ovarian fat, perirenal fat, and abdominal fat. The GI tract was removed and weighed after removal of mesenteric fat. Disemboweled weight was calculated by subtracting GI weight from body weight. The weight of the cecal contents was determined by subtraction of empty cecum weight from full cecum weight.
2.3 Plasma assays
Blood was collected and centrifuged at 4000 × g for 20 min to extract serum. Serum GLP-1 was measured by radio-immunoassay with RIA kits from Millipore Research. (Billerica, MA, USA). Serum insulin was measured using rat ELISA kits from Crystal Chem (Downers Grove, IL, USA).
2.4 Immunohistochemical staining and morphometry
Pancreases were removed from decapitated rats, weighed and fixed in 10% buffered neutral formalin for at least 48 h and embedded in paraffin. Each pancreatic block was sectioned serially at 5μm throughout the length to avoid any bias. Twelve pancreases were examined as total for three groups. Adjacent sections were obtained one in every 20 sections through the specimen and immunostained for insulin by an immunofluorescent method [15]. The primary anti-insulin serum was purchased from Invitrogen (CA, USA). It was a guinea pig anti-porcine insulin serum with a 1:200 dilution in PBS. The second antibody was Alexa Fluor 488 goat anti-guinea pig immunoglobulin at a concentration of 5 μg/mL diluted in PBS, also from Invitrogen. The sections were visualized using fluorescence microscopy (Zeiss Axioplan 2 upright microscope, Semrock Brightline filter set – FITC-3540B cube with EX 482/35 nm, EM 536/40 nm and D506). Counterstaining with haematoxylin was performed on each section to facilitate nuclear identification. Quantitative evaluation was performed using nanozoomer digital pathology software (Hamamatsu, Japan). The areas occupied by insulin positive cells as well as the area of the total pancreatic cells were analyzed in each section. The average percent of β cells to the total pancreatic area of each section was calculated as the relative β cell density.
2.5 Cecal butyrate-producing bacterial DNA expression
DNA was extracted from rats’ cecal contents using a QIAamp DNA Stool Mini kit (QIAGEN, Valencia, CA, USA). The gene transcription for butyrate producing bacteria was determined using the SYBR® Green method of quantitative real–time PCR (qRT-PCR) assay, and results were expressed as a relative fold change of GK EC control group. The sequences of the primers for targeted bacterial groups are listed in Table 2. Real-time RT-PCR reaction mixture was 10 μL of total volume, including 3 μL of DNA sample, 5 μL of 2 × SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), 0.5 μL of reverse/forward primers at 10 μM, 0.5 μ: of bovine serum albumin (BSA) (final concentration 2.5 mg/mL), and 0.5μL of nuclease free water. The reaction conditions were 50°C for 2 min, 95°C for 10 min for one cycle, then 40 cycles of 95°C for 15 s, 60°C for 1 min, then 78°C for 30 s. The dissociation step was included to verify specificity through analyzing the melting curve of the amplified product.
Table 2.
The sequences of primers for real-time RT-PCR. F: forward primer, R: reverse primer, P: probe
| Gene | Sequence |
|---|---|
| 16S universal primers | F-TGSTGCAYGGYYGTCGTCA |
| R-ACGTCRTCCMCNCCTTCCTC | |
| Bifidobacterium spp. | F-GGGTGGTAATGCCGGATG |
| R-TAAGCCATGGACTTTCACACC | |
| Bacteroidetes | F-GAA GGT CCC CCA CAT TG |
| R-CAA TCG GAG TTC TTC GTG | |
| Lactobacillus spp. | F-TGG ATG CCT TGG CAC TAG GA |
| R-AAA TCT CCG GAT CAA AGC TTA CTTAT | |
| Clostridial cluster IV | F-TTA CTG GGT GTA AAG GG |
| R-TAG AGT GCT CTT GCG TA | |
| Clostridium cluster XIV | F-AAA TGA CGG TAC CTG ACT AA |
| R-CTT TGA GTT TCA TTC TTG CGA | |
| Cyclophilin | F-CCCACCGTGTTCTTCGACAT |
| R-TG CAAACAG CTCG AAG CAG A | |
| P-CAAGGGCTCGCCATCAGCCG |
2.6 Measurement of UCP-1 mRNA expression
Total RNA was extracted from BAT using Trizol from Sigma (St. Louis, MO, USA). The gene transcription for UCP-1 was determined using real-time reverse transcriptase polymerase chain reaction, and the results were expressed as a ratio to the expression of the constitutive gene cyclophilin. The sequence of the primers and probe for rat cyclophilin is listed in Table 3. The probe and primers for UCP-1 (assay identification no. Rn00562126_m1) were purchased from Applied Biosystems. The real-time RT-PCR reaction mixture was 10 μL of total volume, including 9 ng of sample RNA, 1 μL of 10 × Taqman buffer, 5.5 mM MgCl2, dATP, dCTP, dUTP and dGTP each 0.3 mM, 500 nM forward primers, 500 nM reverse primers, 200 nM Taqman probes, 7.5U RNase inhibitor, 5 U MuLV reverse transcriptase, 0.3 U AmpliTaq Gold DNA polymerase and RNase-free H2O. Each sample was tested in duplicate. The one-step real-time RT-PCR reaction conditions were 48°C for 30 min, 95°C for 10 min for one cycle, 95°C for 15 s and 60°C for 1 min for 40 cycles.
Table 3.
Food intakes, disemboweled body weight and percentages of body fat/disemboweled body weight in GK rats fed with resistant starch or energy control diet
| Group | Cumulative food intake (g)
|
Disemboweled body weight (g)
|
% Body fat/disemboweled body weight
|
|||
|---|---|---|---|---|---|---|
| Total | Abdominal | Ovarian | Perirenal | |||
| GK-EC | 1442.0±108.0 | 274±15.3 | 6.12 ± 1.3 | 1.85±0.37 | 3.31 ± 0.74 | 0.96±0.24 |
| GK-RS | 1565.6±163.9 | 262 ± 20.3 | 5±0.68* | 1.52 ± 0.3* | 2.7 ± 0.46* | 0.77±0.12* |
GK-EC: GK rats fed control diet, GK-RS: GK rats fed resistant starch diet, Data are mean±SD for group of 10 rats.
p<0.05 versus EC-treated GK rats.
2.7 Oral glucose tolerance test
The oral glucose tolerance test was performed in female rats on the 16th day of gestation. After an overnight fast, a blood sample was collected for insulin measurement. Blood glucose was measured prior and 30, 60, and 120 min after the administration of glucose by gavage (2.0 g/kg body weight) with a glucometer (Abbott Laboratories, North Chicago, IL, USA). For the offspring blood glucose was measured prior and 120 min after the administration of glucose when they were 56 days old. HOMA-IR was calculated to indicate insulin sensitivity.
2.8 Pancreatic insulin content
Approximately, 150 mg of pancreas was weighed and placed into 2 mL of an acid–ethanol solution (75% ethanol, 1.5% HCL 12 N, 23.5% distilled water). After an overnight incubation at −20°C, the tissue was homogenized at 4°C and then underwent another overnight incubation at −20°C. The diluted supernatant was used to determine insulin content by ELISA (Crystal Chem, Downers Grove, IL, USA) [16].
2.9 Cecal contents pH and short-chain fatty acid analysis
Cecal contents (0.5 grams) were weighed and put into a plastic centrifuge tube with 4.5 mL of distilled water. The sample was mixed thoroughly by vortex and pH was measured using a pH meter. Then 1 mL of acid (250 g/L of metaphosphoric acid containing 2 g/L of ethyl butyric acid) containing an internal standard was added to the mixture. The sample was centrifuged at 4°C and filtered through a Millipore syringe filter. The supernatant was transferred to a GC autosampler vial and stored at 0°C until short fatty acids were quantitatively determined by gas chromatography (GLC). Concentrations of individual SCFA were measured by GLC using a Shimadzu GC2010 equipped with a 15-m EC-1000 column that had an internal diameter of 0.53 mm and a film thickness of 1.2 μm (Alltech Associates, Deerfield, IL, USA). The reagent preparation procedure and temperature gradient for SCFA analysis were adapted from Grigsby et al. [17] and Bateman et al. [18], respectively.
2.10 Statistical analysis
Data are presented as mean±SD. Statistical analyses were performed using the Statistical Analysis System (SAS 9.1). Kruskal–Wallis test was used to examine the influence of treatment on all measurements and Mann–Whitney U-test was used for pairwise comparison among multiple groups, and P<0.05 was regarded as significant.
3 Results
3.1 Dam data
3.1.1 Fat pad weights
Compared with GK rats fed the control diet, dietary resistant starch significantly decreased fat/disemboweled weight (p<0.05) (Table 3).
3.1.2 Insulin sensitivity and fasting glucose levels
Dietary-resistant starch improved insulin sensitivity in pregnant GK rats as indicated by HOMA-IR. Also resistant starch-fed pregnant GK rats had lower fasting glucose and fasting serum insulin concentrations compared with EC-fed GK rats (p<0.05) (Fig. 1). However there was no difference found in ∆AUC between these two groups.
Figure 1.
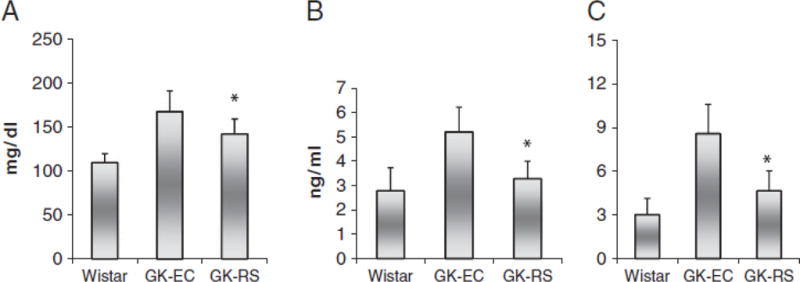
Fasting glucose concentrations (A), fasting serum insulin levels (B) and HOMA-IR (C) measured on the 16th day of gestation in GK rats fed with RS or EC diet and Wistar rats fed the EC diet. Data are mean±SD for group of 10 rats. *p<0.05 versus EC-GK.
3.1.3 Immunohistochemistry and pancreatic insulin content
There were fewer islets in the GK groups than in normal Wistar rats; the GK-EC rats had the least. Pancreatic insulin content was not significantly different between RS-GK rats and EC-fed GK rats (p>0.05). The β cell relative densities were 0.758±0.24% in Wistar rats, 0.301±0.09% in GK-EC rats and 0.56±0.13% in GK-RS rat, respectively (p<0.05). In the GK-EC rats, the large islets displayed disrupted configuration, irregular capsules and cells unevenly stained with the anti-insulin sera. In contrast, islets in Wistar rats were round in shape, clearly boundary defined, and homogeneously stained β cells (Fig. 2).
Figure 2.
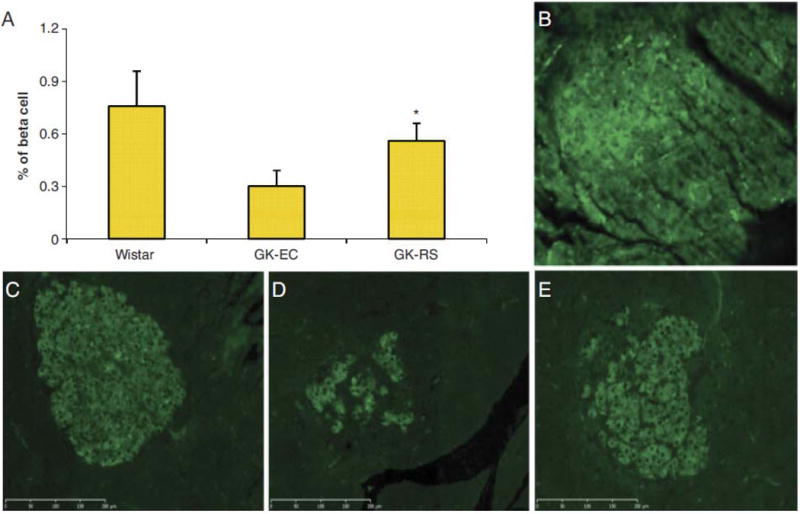
β cell densities (A) in GK rats fed RS or EC diet and Wistar rats fed the EC diet. Data are mean ±SD. *p<0.05 versus EC-GK. Immunohistochemical staining in pancreatic islets of non-primary antibody control (B), Wistar rats (C), GK—EC rats (D), and GK-RS rats (E).
3.1.4 Cecal content pH and short-chain fatty acids
The pH values for both cecal contents and feces were lower in RS-fed pregnant GK rats, whereas the weights of full GI and cecal contents were higher in RS-fed pregnant GK rats than EC-fed GK rats (p<0.05) (Table 4). Short-chain fatty acid concentrations including acetate, butyrate and propionate, were elevated in cecal contents of pregnant GK rats fed RS (p<0.05) (Table 4).
Table 4.
Fermentation parameters among three groups
| Group | Cecal content pH
|
Feces pH
|
Full GI weight (g)
|
Cecal content weight (g)
|
Cecal short-chain fatty acid
|
Serum total GLP-1 (PM)
|
||
|---|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate (mmol/L) | ||||||
| Wistar-EC | 8.6±0.42 | 8.14±0.34 | 19.2±2.97 | 3.04±0.68 | 5.03 ± 0.99 | 0.93±0.15 | 0.77 ±0.25 | 11.0±6.3 |
| GK-EC | 8.7 ± 0.22 | 8.14±0.37 | 14.8±0.81 | 1.84±0.37 | 4.56±0.84 | 0.82±0.12 | 0.70±0.18 | 7.99±2.1 |
| GK-RS | 6.9 ±0.37* | 5.6 ± 0.46* | 27.6±5.2* | 6.27±2.2* | 6.2±1.39* | 1.8±0.55* | 1.38±0.58* | 43.5±23.7* |
Wistar-EC: Wistar rats fed control diet, GK-EC: GK rats fed control diet, GK-RS: GK rats fed resistant starch diet, Cecal content pH, feces pH values, full GI weight, cecal content weight, cecal short-chain fatty acid concentrations, and serum total GLP-1 concentrations in GK rats fed with RS or EC diet and Wistar rats on EC diet. Data are mean ± SD for group of 10 rats.
p<0.05 versus EC-treated GK rats.
3.1.5 Microflora expressions in cecum
The microflora engaged in converting resistant starch to butyrate are Bacteroides spp., Bifidobacterium spp., Lactobacillus spp., Clostridial cluster IV and Clostridial cluster XIV [19–22]. The population of bacteria involved in butyrate production in the cecum was increased in RS-fed pregnant GK rats. Bacteroides, Bifidobacterium, Lactobacillus and Clostridial cluster IV populations were increased compared with those in EC-fed GK rats (p<0.05) (Fig. 3). No significant difference was observed in Clostridial cluster XIV.
Figure 3.
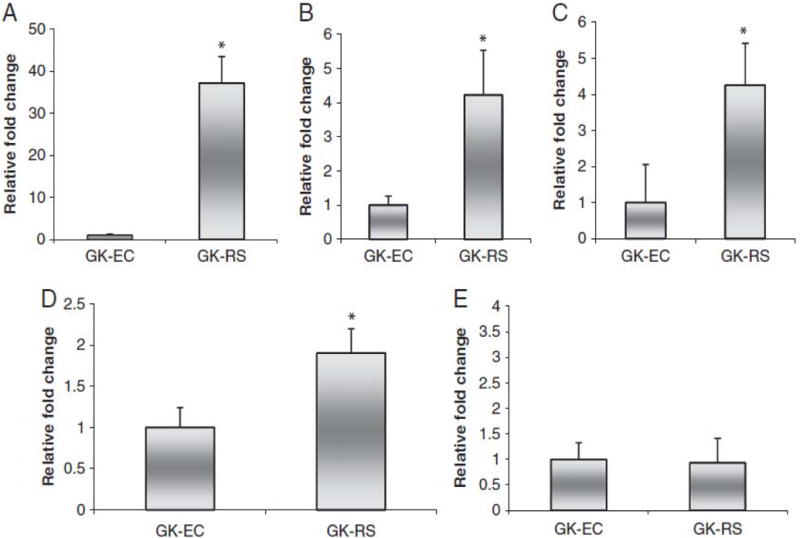
Fold changes of Bacteroides spp. (A), Bifidobacterium spp. (B), Lactobacillus spp. (C), Clostridial cluster IV(D), and Clostridial cluster XIV (E) measured with RT-PCR in GK rats fed RS or EC diet. Data are mean ± SD for group of 10 rats. *p<0.05 versus EC-GK.
3.1.6 Serum GLP-1 concentration and UCP-1 gene expression in BAT
The total serum GLP-1 concentration was increased in GK rats fed with RS, compared with GK rats fed with EC (p<0.05) (Table 4). There was a trend of increased mRNA expression of UCP-1 in BAT of rats fed RS (p=0.08).
3.1.7 Food intake and disemboweled weight
There were no statistical differences in food intake between control and RS-fed rats. This demonstrated no or minimal discomfort with the consumption of resistant starch at the levels fed. Because RS-fed rats had significantly heavier GI contents, the disemboweled body weight was used to exclude GI contents from body weight. There was no significant difference for disemboweled body weight between control and RS-fed rats (Table 3).
3.1.8 Offspring data
There were no statistical differences in litter size between pups born to control and RS-fed GK rats. No significant differences for body weight, percentage of body fat and food intake were found between offspring from control and RS-fed GK rats (not shown). No significant difference was detected in growth rate between offspring of GK rats fed resistant starch and EC diet. It demonstrated no or minimal side effects on pregnancy with the consumption of resistant starch at the levels in their diet.
Pups born to GK rats fed resistant starch had lower fasting glucose compared with offspring from GK rats fed the EC diet (p<0.05) (Fig. 4). However, there was no difference found in fasting serum insulin concentration, 2-h glucose level and insulin sensitivity (HOMA-IR) between these two groups. Pancreatic insulin content was increased in pups born to GK rats fed resistant starch compared with those born to GK rats fed the EC (Fig. 4). No significant difference was found in β cell density between offspring from the two groups.
Figure 4.
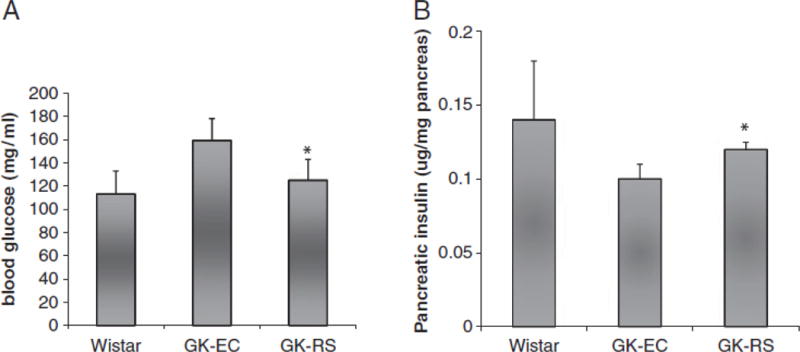
Fasting glucose (A) and pancreatic insulin contents (B) were measured in 8-wk-old pups born to GK-RS, GK-EC and Wistar-EC dams. Data are mean7SD. *po0.05 versus EC-GK.
The pH value for cecal contents was not significantly different between pups born to GK rats on the resistant starch or the EC diet (7.38±0.75 versus 7.91±0.65, p>0.05). As indicated by cecal pH value, there was no fermentation difference in the cecum between the two groups, so short-chain fatty acids concentrations and microfloral populations were not measured.
4 Discussion
In this study, we investigated the effects of dietary-resistant starch on improving maternal hyperglycemia. Our results demonstrated that dietary-resistant starch increases insulin sensitivity and pancreatic β cell mass in pregnant GK rats, a non-obesity type 2 diabetes model that demonstrates reduced β cell mass and defective insulin response to glucose [15, 23–25]. Specifically, we measured fat pad changes, fasting insulin and glucose concentrations, pancreatic insulin content and β cell density in the pregnant GK rats in the context of resistant starch feeding. To our knowledge, our findings provide the first direct evidence that dietary-resistant starch alters pancreatic β cell density in GK rats.
Feeding resistant starch to pregnant GK rats significantly increased insulin sensitivity. The result is consistent with the observation that RS-fed rats had reduced fat pads. The decreased body fat in RS-fed rats is most likely the result of increased energy expenditure. Human studies have shown fatty acid oxidation is significantly increased after consumption of resistant starch [26]. Our previous data also suggested dietary-resistant starch enhanced energy expenditure in mice [5]. Dietary RS was also associated with increased expression of pro-opiomelanocortin (POMC) in the arcuate nucleus of the hypothalamus in rats [27], which is critical in promoting energy expenditure [28], and increased protein expression of adiponectin in white adipose tissue (unpublished data). We found the butyrate levels were elevated in the cecal contents of GK rats with the feeding of resistant starch. Gao and his coworkers reported that the addition of sodium butyrate in the diet led to improved insulin sensitivity, facilitated fatty oxidation and increased energy expenditure in mice; it protected mice from diet-induced obesity [29]. Different from the butyrate in the diet, butyrate from fermentation has a local effect in the lower gut where butyrate is avidly absorbed by the colonocytes and L-endocrine cells to stimulate GLP-1 expression [4]. Some butyrate may reach the liver to inhibit hepatic lipolysis (unpublished data). However, whether the butyrate from fermentation enters systemic blood is still uncertain. Therefore, the effect of the butyrate from fermentation may be indirect through the production of gut hormones. GLP-1 is reported to increase energy expenditure. Higher fasting plasma GLP-1 levels are associated with higher rates of energy expenditure and fat oxidation in human subjects [30]. It has also been reported that GLP-1 plays a role in post-prandial energy expenditure and that GLP-1 stimulates pro-opiomelanocortin neurons in the arcuate nucleus via GLP-1 receptors [31]. We found a trend of increased UCP-1 expression in resistant starch-fed GK rats, compared with EC-fed GK rats, which is in accordance with what Aziz et al reported. They observed that a high amylose starch diet led to a higher insulin sensitivity index (QUICKI), and elevated mRNA expression of UCP-1 in diet-induced obese rats [32]. UCP-1 diverts energy from ATP synthesis to thermogenesis, which increases energy expenditure [33].
Another major finding is that feeding resistant starch significantly increased β cell density in GK rats. This result raises an interesting question: what causes such changes in resistant starch-fed GK rats? Resistant starch potentially has three major effects as a part of the diet: metabolizable energy dilution, a bulking effect, and fermentation to produce short-chain fatty acids and increase GLP-1 [3, 5]. In our study, control and resistant starch diets have the same energy density, so the energy dilution effect can be excluded [14]. The bulking effect is the prevention of food intake caused by GI distension [34]. But our results indicated there was no difference in food intake between resistant starch-fed GK rats and EC-fed rats. Thus, the hypothesis most likely for the mechanism of β cell density was narrowed down to the fermentation of resistant starch and the subsequent increases of GLP-1. Elevated plasma GLP-1 was previously observed before in both normal Sprague–Dawley (SD) and diet-induced obese rats fed resistant starch [27, 32]. The GLP-1 increase is consistent over a 24-h period in SD rats [5]. Gut gene expression data from our group also verified that dietary RS dramatically up-regulated the expression of the proglucagon (GLP-1) gene in a sample of cells from the cecum in rats compared with rats consuming an energy control diet [4].
In order to clarify the process from dietary resistant starch fermentation associated with increased production of GLP-1 in resistant starch-fed pregnant GK rats, we investigated several important steps in fermentation. First, we measured cecal and fecal pH values, cecal content weight and found the existence of fermentation demonstrated by the decrease of pH values as well as increased cecal content weight in resistant starch-fed GK rats. Second, augmented butyrate-producing bacterial population was confirmed except for Clostridial cluster XIV which might be due to its absolute reduction in type 2 diabetes [35]. Third, short-chain fatty acids including acetate, butyrate and propionate were elevated in resistant starch-fed GK rats. Finally, an increase in plasma GLP-1 was observed. Our previous study has indicated that butyrate promoted GLP-1 expression in vitro [4].
The action of GLP-1, as a potent incretin, includes stimulating proinsulin gene expression [36] and inhibiting glucagon secretion [10]. It also mediates glucose-dependent insulin secretion via GLP-1 receptors expressed on β cells [9], inhibits gastric acid secretion and delays gastric emptying [37], as well as promotes an increase in pancreatic β-cell mass through enhancing b cell proliferation and inhibiting apoptosis [7, 8].
GLP-1 was shown to be able to delay the onset of type 2 diabetes and improve pancreatic insulin content and total β cell mass in GK rats when applied postnatally for 5 days [38]. This hormone was also reported to reduce apoptosis in human islets [39]. A GLP-1 receptor agonist demonstrated similar effects. Exendin-4 not only improved glucose tolerance in diabetic rats via expansion of β cell volume [40], but also prevented the development of diabetes in rats exposed to intrauterine growth retardation [7]. Further studies are needed for a conclusive determination for the role of increased GLP-1 in dietary-resistant starch-induced improvement in glycemic control in pregnant GK rats.
During the last few years, it has been suggested that the experience of hyperglycemia may contribute to an endocrine pancreas defect in the offspring [41]. Although the development of diabetes in GK rats results from both genetic and environmental determinants, Gauguier and coworkers [42] reported that offspring of GK females crossed with Wistar males had a more marked hyperglycemia than those of Wistar females crossed with GK males, suggesting a role of the intrauterine environment. However, not all studies agreed with this conclusion [43]. Gill-Randall and co-workers developed a rat embryo transfer technique to examine the weight of genetic factors and intrauterine hyperglycemia [44]. They showed that Wistar embryos implanted into the uterus of GK mothers were more hyperglycemic in adulthood than those that were reared in Wistar mothers [44], this clearly illustrating the notion that an intrauterine hyperglycemic environment is a risk factor for developing hyperglycemia in offspring in adult-hood. Impaired gene expression and disturbed organogenesis attributed to increased oxidative stress [45, 46] and reduced angiogenesis induced by hyperglycemia [47] are potential mechanisms associated with this anomaly. Tight glycemic control before conception and intensive glucose control maintained during pregnancy is suggested by ADA in order to reduce the prevalence of type 2 diabetes in offspring [48].
In conclusion, dietary-resistant starch improved insulin sensitivity, pancreatic insulin content and β cell density in pregnant GK rats. The increased production of GLP-1 resulting from fermentation of resistant starch was linked to the mechanism of improved glycemic control in pregnant GK rats. In this study, we also showed that feeding resistant starch to GK dams resulted in lower fasting glucose and enhanced pancreatic insulin content. These favorable effects may result from the improvement of maternal hyperglycemia that in turn changes the intrauterine environment. Our findings provide evidence to apply resistant starch as an intervention in prevention and treatment of diabetes associated with pregnancy.
Acknowledgments
This paper was approved for publication by the Director of Louisiana Agricultural Experiment Station as publication number 2010-239-5307. This research was supported by LSU Agcenter. National Starch Food innovation Company provided the researchers with Hi-Maize and Amioca as a gift.
Abbreviations
- BAT
brown adipose tissue
- GI
gastrointestinal
- GLP-1
glucagon-like peptide-1
- GK
Goto–Kakizaki
- RS
resistant starch
- UCP-1
uncoupling protein 1
Footnotes
Conflict of interest statement: M. J. K. has received research funding from the National Starch Food Innovation Company.
References
- 1.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 2.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 3.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring) 2006;14:1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, et al. Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity. 2006;14:683–689. doi: 10.1038/oby.2006.77. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Martin RJ, Tulley RT, Raggio AM, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 7.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52:734–740. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45:1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA, Heimesaat MM, Behle K, Holst JJ, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Martin RJ, Tulley RT, Raggio AM, et al. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J Agric Food Chem. 2009;57:8844–8851. doi: 10.1021/jf901548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak LP, Britton JH, Kozak UC, Wells JM. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1998;263:12274–12277. [PubMed] [Google Scholar]
- 13.Grabitske HA, Slavin JL. Gastrointestinal effects of low-digestible carbohydrates. Crit Rev Food Sci Nutr. 2009;49:327–360. doi: 10.1080/10408390802067126. [DOI] [PubMed] [Google Scholar]
- 14.Tulley RT, Appel MJ, Enos TG, Hegsted M, et al. Comparative methodologies for measuring metabolizable energy of various types of resistant high amylose corn starch. J Agric Food Chem. 2009;57:8474–8479. doi: 10.1021/jf900971c. [DOI] [PubMed] [Google Scholar]
- 15.Movassat J, Saulnier C, Serradas P, Portha B. Impaired development of pancreatic b-cell mass is a primary event during the progression to diabetes in the GK rat. Diabetologia. 1997;40:916–925. doi: 10.1007/s001250050768. [DOI] [PubMed] [Google Scholar]
- 16.Zeng CJ, Zhang L, Yang HX. Effects of severe hyperglycaemia in pregnancy and early overfeeding on islet development and insulin resistance. Zhonghua Fu Chan Ke Za Zhi. 2010;45:658–663. [PubMed] [Google Scholar]
- 17.Grigsby KN, Kerley MS, Paterson JA, Weigel JC. Site and extent of nutrient digestion by steers fed a low-quality bromegrass hay diet with incremental levels of soybean hull substitution. J Anim Sci. 1992;70:1941–1949. doi: 10.2527/1992.7061941x. [DOI] [PubMed] [Google Scholar]
- 18.Bateman HG, Williams CC, Chung YH. Effects of supplemental zinc in high quality diets on ruminal fermentation degradation of urea in vitro and in vivo. Prof Anim Sci. 2002;18:363–367. [Google Scholar]
- 19.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbio. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 20.Bird AR, Brown IL, Topping DL. Starches, resistant starches, the gut microflora. Curr Issues Intest Microbiol. 2000;1:25–37. [PubMed] [Google Scholar]
- 21.Sato T, Matsumoto K, Okumura T, Yokoi W, et al. Isolation of lactate-utilizing butyrate-producing bacteria from human feces and in vivo administration of Anaerostipes caccae strain L2 and galacto-oligosaccharides in a rat model. FEMS Microbiol Ecol. 2008;66:528–536. doi: 10.1111/j.1574-6941.2008.00528.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Bjursell MK, Himrod J, Deng S, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 23.Movassat J, Saulnier C, Portha B. Beta-cell mass depletion precedes the onset of hyperglycaemia in the GK rat, a genetic model of non-insulin-dependent diabetes mellitus. Diabetes Metab. 1995;21:365–370. [PubMed] [Google Scholar]
- 24.Movassat J, Calderari S, Fernández E, Martín MA, et al. Type 2 diabetes – a matter of failing beta-cell neogenesis? Clues from the GK rat model. Diabetes Obes Metab. 2007;9:187–195. doi: 10.1111/j.1463-1326.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 25.Portha B. Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab Res Rev. 2005;21:495–504. doi: 10.1002/dmrr.566. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int. 2004;87:761–768. [PubMed] [Google Scholar]
- 27.Shen L, Keenan MJ, Martin RJ, Tulley RT, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17:40–45. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu AW, Barsh GS. MC4R neurons weigh in differently. Nat Neurosci. 2006;9:15–16. doi: 10.1038/nn0106-15. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, Yin J, Zhang Z, Ward RE, et al. butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26:1623–1631. doi: 10.1016/j.peptides.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci. 2007;27:7125–7129. doi: 10.1523/JNEUROSCI.1025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Aziz AA, Kenney LS, Goulet B, Abdel-Aal el-S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J Nutr. 2009;139:1881–1889. doi: 10.3945/jn.109.110650. [DOI] [PubMed] [Google Scholar]
- 33.Puigserver P, Wu Z, Park CW, Graves R, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 34.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 35.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 38.Tourrel C, Bailbe D, Lacorne M, Meile MJ, et al. Persistent improvement of type 2 diabetes in the Goto–Ka-kizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 39.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 40.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 41.Simmons R. Developmental origins of adult metabolic disease. Endocrinol Metab Clin North Am. 2006;35:193–204. doi: 10.1016/j.ecl.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Gauguier D, Nelson I, Bernard C, Parent V, et al. Higher maternal than paternal inheritance of diabetes in GK rats. Diabetes. 1994;43:220–224. doi: 10.2337/diab.43.2.220. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Halim SM, Guenifi A, Luthman H, Grill V, et al. Impact of diabetic inheritance on glucose tolerance and insulin secretion in spontaneously diabetic GK–Wistar rats. Diabetes. 1994;43:281–288. doi: 10.2337/diab.43.2.281. [DOI] [PubMed] [Google Scholar]
- 44.Gill-Randall R, Adams D, Ollerton RL, Lewis M, Alcolado JC. Type 2 diabetes mellitus – genes or intrauterine environment? An embryo transfer paradigm in rats. Diabetologia. 2004;47:1354–1359. doi: 10.1007/s00125-004-1464-x. [DOI] [PubMed] [Google Scholar]
- 45.Loeken MR. Advances in understanding the molecular causes of diabetes-induced birth defects. J Soc Gynecol Investig. 2006;13:2–10. doi: 10.1016/j.jsgi.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z, Reece EA. Experimental mechanisms of diabetic embryopathy and strategies for developing therapeutic interventions. J Soc Gynecol Investig. 2005;12:549–557. doi: 10.1016/j.jsgi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Larger E, Marre M, Corvol P, Gasc JM. Hyperglycemia-induced defects in angiogenesis in the chicken chorioallantoic membrane model. Diabetes. 2004;53:752–761. doi: 10.2337/diabetes.53.3.752. [DOI] [PubMed] [Google Scholar]
- 48.American Diabetes Association. Preconception care of women with diabetes. Diabetes Care. 2004;27:S76–S78. doi: 10.2337/diacare.27.2007.s76. [DOI] [PubMed] [Google Scholar]


