Abstract
There are several interrelated mechanisms involving iron, dopamine, and neuromelanin in neurons. Neuromelanin accumulates during aging and is the catecholamine-derived pigment of the dopamine neurons of the substantia nigra and norepinephrine neurons of the locus coeruleus, the two neuronal populations most targeted in Parkinson’s disease. Many cellular redox reactions rely on iron, however an altered distribution of reactive iron is cytotoxic. In fact, increased levels of iron in the brain of Parkinson’s disease patients are present. Dopamine accumulation can induce neuronal death; however, excess dopamine can be removed by converting it into a stable compound like neuromelanin, and this process rescues the cell. Interestingly, the main iron compound in dopamine and norepinephrine neurons is the neuromelanin-iron complex, since neuromelanin is an effective metal chelator. Neuromelanin serves to trap iron and provide neuronal protection from oxidative stress. This equilibrium between iron, dopamine, and neuromelanin is crucial for cell homeostasis and in some cellular circumstances can be disrupted. Indeed, when neuromelanin-containing organelles accumulate high load of toxins and iron during aging a neurodegenerative process can be triggered. In addition, neuromelanin released by degenerating neurons activates microglia and the latter cause neurons death with further release of neuromelanin, then starting a self-propelling mechanism of neuroinflammation and neurodegeneration. Considering the above issues, age-related accumulation of neuromelanin in dopamine neurons shows an interesting link between aging and neurodegeneration.
Keywords: Iron, dopamine, melanin, human neuromelanin, Parkinson's disease
1. Introduction
Roles for neuromelanin (NM), iron and dopamine (DA) in brain aging and Parkinson's disease (PD) have been reported in multiple studies. In particular, iron deposition and accumulation in reactive forms has been associated with brain aging and neurodegenerative diseases including PD. The dual protective and toxic roles that NM can play in DA neurons of the substantia nigra (SN) have also been detailed, as have toxic roles of DA itself in the degeneration of neurons in PD.
These three factors interact with each other in multiple ways and influence neuronal survival in human SN. Indeed, NM binds iron and can play a protective or damaging role depending on the level of bound iron. DA can be oxidized by iron to form DA-o-quinone, which can enter into toxic pathways forming adducts with amino acid residues (mainly cysteine residues) of different proteins. Alternatively cytosolic DA-o-quinone can react with cysteine to form cysteinyl-DA compounds, precursors of NM synthesis. NM accumulates in DA neurons of the SN during aging as a physiological process, and in some circumstances can play a neurodegenerative role through the activation of microglia. Another potential toxic process involving NM is based on the high expression of major histocompatibility complex class I (MHC-I) in NM-containing organelles which suggests the presentation of antigenic peptides by MHC-I on neuronal membrane of catecholaminergic neurons containing NM.
It thus appears that iron, DA and NM interact by multiple pathways. In this review we describe these pathways and the conditions that maintain them in health or drive them toward neurodegeneration.
2. Iron cellular role: a critical balance between physiological and harmful effects
2.1. Iron biochemistry
Iron is a transition metal with an ubiquitous distribution in the biosphere, and, due to its ability to participate in electron-transfer reactions, is essential for normal cellular function. Reversible redox cycling between the oxidation states of iron, ferrous and ferric iron, is utilized in a variety of reactions essential to life (Aisen et al., 2001; Dlouhy and Outten, 2013). In contrast, excess iron can be harmful generating toxic reactive oxygen species (ROS): thus, organisms, organs and cells which are strictly dependent on iron chemistry need to manage a delicate balance between the physiologically beneficial effects of iron and serious consequences deriving from iron deficiency or iron overload. Iron in biological systems is mostly present in a complex that consists of ferrous and ferric iron in equilibrium, which are associated with chelating ligands, including small (i.e., citrate, ATP, etc.) or large biomolecules (i.e., carrier proteins, storage proteins and complexes, enzymes, etc.). Biological complexes of iron display a great variety of stability constants, depending on the oxidation state and ligand to which iron species is bound (Crichton 2009).
Major roles of iron in cell biology include oxygen transport/storage/delivery as a heme cofactor in hemoglobin and myoglobin, as well as heme cofactor in many oxidase and oxygenase enzymes, and in electron transfer proteins (the cytochromes) for mitochondrial respiration. The iron also forms Fe-S clusters, the prosthetic groups of many important proteins of the mitochondrial respiratory chain (Crichton 2009; Dlouhy and Outten, 2013): since the brain has a high energy demand, iron is essential for generating ATP by electron transport in brain mitochondria. These Fe-S clusters are also enclosed in many enzymes, with functions ranging from general cell metabolism to DNA repair (Rouault 2012). Iron is also involved in DNA synthesis, since ribonucleotide reductase is iron-dependent (Kolberg et al., 2004; Tomter et al., 2013), and under anaerobic condition a particular class of this enzyme contains a Fe-S cluster (Tamarit et al., 2000).
Notably, in the brain iron is essential in the synthesis and metabolism of neurotransmitters including DA, norepinephrine, epinephrine and serotonin. Each of these monoamine neurotransmitters are synthesized by iron-dependent enzymes: phenylalanine hydroxylase, tyrosine hydroxylase, tryptophan hydroxylase (Beard 2003; Flydal et al., 2013; Windahl et al., 2008). Iron also affects other steps of neurotransmitters metabolism, such as uptake, extracellular concentration, interaction with receptors (Beard et al., 1994; Bianco et al., 2008; Burhans et al., 2005; Youdim et al., 1989), and catabolism. Last but not least, myelination requires adequate levels of iron (Todorich et al., 2009), and the oligodendrocytes that produce myelin maintain the highest iron concentrations of the brain (Connor et al., 1990; Connor and Menzies, 1995). Iron is a required co-factor for the synthesis of lipid components of myelin, and is further required for oligodendrocyte development (Badaracco et al., 2010; Todorich et al., 2009).
In contrast to these roles in healthy brain function, when iron levels exceed the cellular iron sequestration capacity of storage proteins or other molecules, iron in the labile iron pool (defined as a pool of chelatable and redox-active iron in complexes of low stability) may increase, becoming harmful and leading to oxidative damage and cell death (Kakhlon and Cabantchik, 2002; Kruszewski 2003; Zecca et al., 2008a). Iron can induce oxidative stress due to its central role in generating hydroxyl radical, the most harmful cellular ROS (Crichton and Ward, 2014; Dlouhy and Outten, 2013). ROS can directly damage DNA and mitochondrial DNA, causing a variety of DNA lesions (Melis et al., 2013), and affect DNA expression by epigenetic events (Kwok 2010). In addition, ROS may cause several direct oxidative modifications of amino acid residues, leading to the formation of protein carbonyl derivatives, an important ROS-induced post translational modification of proteins often used as a general marker of oxidative protein damage (Dalle-Donne et al., 2003a, b). Protein carbonylation has been associated with functional alterations in a variety of structural and enzymatic proteins, leading to their elimination or accumulation with final cell injury (Dalle-Donne et al., 2003a). Furthermore, ROS can promote the peroxidation of polyunsaturated fatty acids in membrane lipids, leading to alterations and functional loss of membranes (Català 2009). Secondary by-products of lipid peroxidation are highly reactive aldehydes such as 4-hydroxy-2-nonenal, malondialdehyde, and acrolein (Esterbauer et al., 1991), which can cause irreversible modification of different biomolecules like phospholipids, proteins, and DNA, resulting in impaired function: notably, proteins are particularly susceptible to reactions with 4-hydroxy-2-nonenal by carbonylation (Dalle-Donne et al., 2003a; Perluigi et al., 2012). Introduction of carbonyl groups into proteins can also occur by secondary reactions of amino acid residues with other reactive carbonyl compounds derived from oxidation of carbohydrates (glycoxidation products) (Dalle-Donne et al., 2003a, b). Finally, excessive ROS can also cause the release of reactive iron from Fe-S cluster proteins of the mitochondrial respiratory chain, as well as from other iron storage proteins in other cell compartments, to further feed ROS production via the Fenton reaction.
The hypothesis of metal-based neurodegeneration is strongly suggested by multiple in vitro studies showing that the aggregation of some proteins linked to neurodegenerative disorders, including α-synuclein and hyper-phosphorylated tau, can be triggered by elevated iron levels as a consequence of disrupted iron homeostasis (Hashimoto et al., 1999; Li et. al., 2010; Yamamoto et al., 2002). Iron can directly promote amyloid-β aggregation (House et al., 2004; Schubert and Chevion, 1995) and iron levels can modulate amyloid precursor protein pathways (Exley et al., 2012; Silvestri and Camaschella, 2008). Iron excess can also facilitate neurotoxic processes through mechanisms apart from Fenton chemistry. In particular, catecholamines such as DA may be oxidized to highly reactive/toxic quinones via oxidation by ferric iron (Paris et al., 2005a; Sulzer and Zecca, 2000).
Neurodegenerative mechanisms originated from iron toxicity can eventually lead to classical apoptosis signaled by oxidative stress (Ott et al., 2007) or to the more recently identified ferroptosis, which is a non-apoptotic, iron dependent, and oxidative cell death (Dixon et al., 2012).
2.2. A brief overview on iron metabolism
The total amount of iron in human adult body is about 3.5–4.0 g (approximately 50 mg/Kg), most of which is found in hemoglobin of erythrocytes and their precursors. The iron pool is derived also from damaged red blood cells after their destruction by macrophages for iron recycling. Additional iron may be obtained through mobilization of cellular iron stores in proteins, particularly from hepatocytes or other cell types (Muñoz et al., 2009; Theil and Le Brun, 2013). In addition to hemoglobin, iron is distributed among myoglobin, iron containing proteins (enzymes that catalyze a wide array of reactions, cytochromes, etc.), and a variety of binding, transport and storage proteins (i.e., transferrin, lactoferrin, ferritin, etc.) in multiple cell types (Andrews 2005; Muñoz et al., 2009).
Systemic iron homeostasis is a semi-closed system, since there appears to be no controlled mechanism for iron excretion. Thus, the maintenance of body iron balance mainly depends on intestinal iron uptake, which is strictly controlled. Iron management in the body begins with absorption through enterocytes but also involves its utilization in erythroid cells, storage/mobilization in hepatocytes, recycling from macrophages, and redistribution among multiple cell types. All of these mechanism are critically regulated by factors balancing between iron deficiency and iron overload (Andrews 2008; Crichton 2009). After the liver, brain contains one of the highest iron concentrations among the organs in the body (Bush et al., 1995; Hallgren and Sourander, 1958; Zecca et al., 2004a).
Normal cellular iron homeostasis provides for optimal cell function, allowing cells to benefit from iron use while avoiding toxicity derived from its altered concentration and distribution, by maintaining an equilibrium of available iron concentrations between cellular compartments and buffering molecules (Hentze et al., 2010). The regulation of iron homeostasis at the cellular level is to a large degree controlled at the level of translation of mRNA of proteins directly involved in cellular iron absorption, transport, storage, and mobilization (Muckenthaler et al., 2008).
The brain represents a “privileged” compartment which under normal conditions does not strictly respond to alterations of peripheral iron (Ward et al., 2014). This is probably because the brain is protected by barriers (Abbott et al., 2010), most prominently the blood-brain barrier (BBB), composed of endothelial cells clamped together by tight junctions, a basal lamina, pericytes, and astrocytic perivascular end-feet (Abbott et al., 2006). The BBB is an efficient physical barrier that regulates entry of nutrients, e.g., iron in different molecular forms essential for brain development and functions. Another barrier separating the brain from systemic circulation is the blood-cerebrospinal fluid-barrier, formed by the epithelial cells of the choroid plexus facing the cerebrospinal fluid (Abbott et al., 2010).
Mechanisms by which iron is transported into the brain from blood and how iron is then redistributed/stored from the interstitial fluid and cerebrospinal fluid between neurons, oligodendrocytes, astrocytes and microglia are only partially understood. Recent findings on brain iron metabolism have been summarized and critically discussed (Crichton et al., 2011; Ke and Qian, 2007; Mills et al., 2010; Moos et al., 2007; Rouault 2013; Rouault and Cooperman, 2006; Ward et al., 2014; Zecca et al., 2004a; Zucca et al., 2011).
Iron may cross the endothelial cells of the BBB either as a low molecular weight complex or by endocytosis of the iron-transferrin complex via its specific receptor (Moos et al., 2007). While most iron transport across the BBB is mediated by transferrin and its receptor, non-transferrin-bound iron (in complexes with low molecular weight molecules, such as citrate, ascorbate and ATP, or chelated by other proteins such lactoferrin, melanotransferrin, etc.) can independently cross the BBB (Ke and Qian, 2007). Additionally, iron may enter the brain via the epithelial cells of the choroid plexus using proteins involved in iron transport/homeostasis that have been identified in other polarized cells (Rouault et al., 2009). Even if iron levels are efficiently maintained in the brain, some iron must be exported in physiological condition, although little is known about this mechanism. It is possible that multiple iron molecular forms exit the brain via cerebrospinal fluid circulation, which drains interstitial fluid, and its successive reabsorption into the venous drainage system through the epithelium of the arachnoid villi, which allows cerebrospinal fluid movement from the brain to blood (Bradbury 1997).
A common crossroads in multiple mechanisms of neurodegeneration is an impairment of iron homeostasis, a condition affected by several factors including aging, mitochondrial dysfunction, oxidative stress, protein aggregation, etc. The increased accumulation of iron in specific brain regions observed in normal aging is enhanced in many neurodegenerative diseases (Rouault 2013; Ward et al., 2014), and is often associated with oxidative stress and cellular damage. It remains unclear whether the iron accumulation observed in some neurodegenerative disorders is a primary event or a secondary effect, in particular for diseases specifically affecting iron-rich areas, such as the SN in PD. The age-related accumulation of iron in some brain regions may thus provide an important contributing factor to neurodegenerative processes, for which aging constitutes the major risk factor.
3. Iron in DA oxidation
DA oxidation to o-quinones has been proposed to play a role in the degeneration of dopaminergic neurons containing NM, since these o-quinones can participate in neurotoxic reactions (Segura-Aguilar et al., 2014). It is important to remember that DA oxidation to o-quinones is an essential event required for NM synthesis, and that the SN dopaminergic neurons of the nigrostriatal system that are lost during PD contain NM. DA oxidation to o-quinones proceeds in a sequential manner, since DA is first oxidized to DA-o-quinone, which is stable only at pH below 2.0 (Segura-Aguilar and Lind, 1989). DA-o-quinone at physiological pH undergoes cyclization to leukoaminochrome, which is subsequently oxidized to aminochrome reducing oxygen to superoxide radical (Segura-Aguilar et al., 2014), with a rate constant for the intramolecular cyclization of DA-o-quinone to aminochrome of 0.15 s−1 (Tse et al., 1976). While it is more stable than DA-o-quinone at physiological pH, aminochrome is unstable as well, and rearranges to 5,6-dihydroxyindole, with a rate constant of 0.06 min−1, and can further auto-oxidize to 5,6-indolequinone reducing oxygen to superoxide radical (Bisaglia et al., 2007; Napolitano et al., 2011). Nuclear magnetic resonance studies performed during DA oxidation with tyrosinase revealed that aminochrome is the only o-quinone detected until 40 min, when 5,6-indolequinone can also be detected (Bisaglia et al., 2007). The 5,6-indolequinone is also unstable since it can polymerize, and after further steps can generate the final NM pigment (Fig. 1, 2), that accumulates with aging in the cell bodies of human SN DA neurons (Zecca et al., 2002).
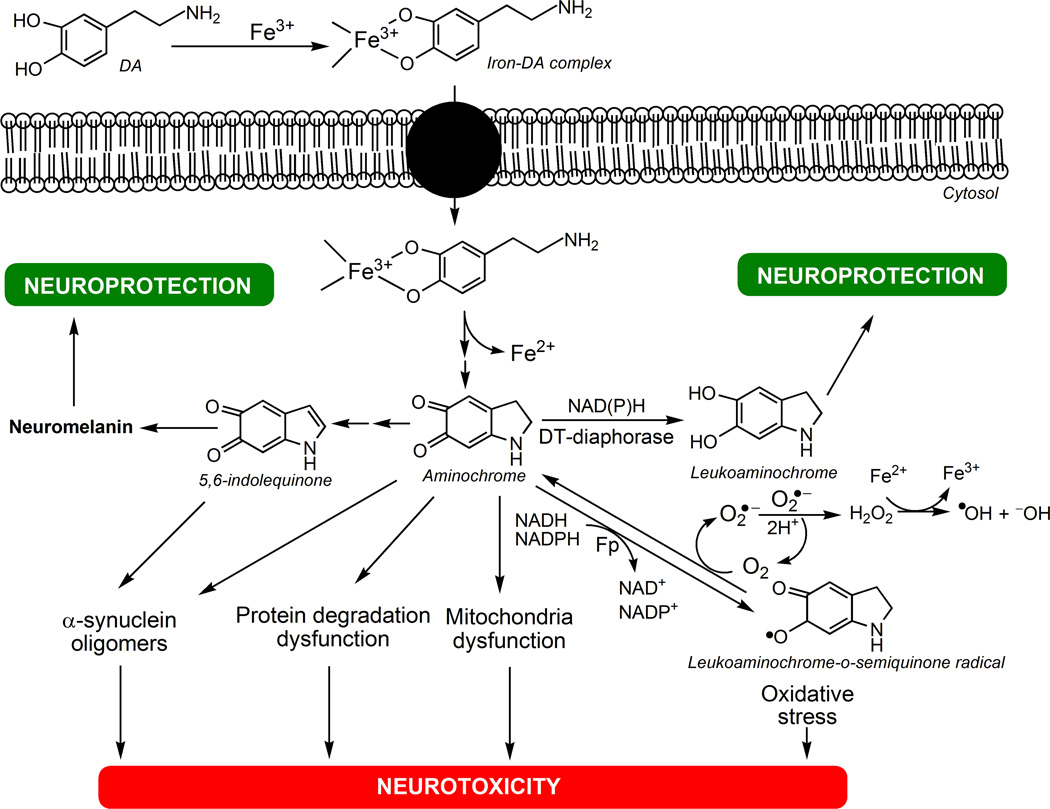
Fig. 1. The neurotoxicity of iron-DA complex.
DA forms complex with ferric iron (Fe3+) that is taken up into the cell by DA transporters. Iron-DA complex undergoes a redox reaction where ferric iron is reduced to ferrous iron (Fe2+) and DA oxidizes to aminochrome. Then aminochrome can polymerize to NM via 5,6-indolequinone affording neuroprotection, or can be two-electron reduced by DT-diaphorase to leukoaminochrome, preventing aminochrome-induced neurotoxic reactions such as: i) α-synuclein aggregation to neurotoxic oligomers; ii) protein degradation dysfunction; iii) mitochondria dysfunction; iv) oxidative stress caused by one-electron reduction of aminochrome to leukoaminochrome-o-semiquinone radical catalyzed by flavoenzymes (Fp). Leukoaminochrome-o-semiquinone radical can auto-oxidize to aminochrome by reducing dioxygen (O2) to superoxide radical anion (O2•−): then the dismutation of two superoxide radicals regenerates O2 and yields hydrogen peroxide (H2O2), which in presence of ferrous iron forms the hydroxyl radical (•OH) by Fenton's reaction, leading to high oxidative stress.
Fig. 2. DA oxidation to o-quinones.
DA oxidizes to DA-o-quinone that can form adducts with parkin, resulting in the inactivation of the proteasomal system. DA-o-quinone can form adducts also with mitochondrial complex I, III and V inducing mitochondrial dysfunction. DA-o-quinone can undergo intramolecular cyclization to aminochrome, which can: i) inactivate the proteasome; ii) inactivate mitochondria complex I inducing mitochondrial dysfunction; iii) be one-electron reduced by flavoenzymes to leukoaminochrome-o-semiquinone radical, which auto-oxidizes immediately to aminochrome generating oxidative stress; iv) inactivate the vacuolar ATPase proton pump of lysosomes inducing lysosomal dysfunction; v) induce aggregation of α- and β-tubulin preventing the formation of microtubules required for the fusion of autophagic vacuoles with lysosomes, thus generating autophagic dysfunction; vi) induce α-synuclein aggregation to neurotoxic oligomers. The neurotoxic reactions of aminochrome can be prevented by aminochrome polymerization to NM (via rearrangement to 5,6-dihydroxindole and then to 5,6-indolequinone) or two-electron reduction, catalyzed by DT-diaphorase, of aminochrome to leukoaminochrome that probably can undergo rearrangement to 5,6-dihydroxyindole, the precursor of 5,6-indolequinone, which can be involved as well in NM synthesis.
Additionally, DA-o-quinone, aminochrome and 5,6-indolequinone can participate in neurotoxic reactions. DA-o-quinone forms adducts with several rat mitochondrial proteins including those of complexes I, III and V of the electron transport chain and oxidative phosphorylation, inducing mitochondria dysfunction (Van Laar et al., 2009). In dopaminergic cell lines and in human SN tissues, DA-o-quinone is able to form adducts with parkin, which is an E3 ubiquitin-protein ligase, probably causing an impairment of the proteasome system (LaVoie et al., 2005). DA-o-quinone forms adducts also with other proteins involved in different cellular pathways (ranging from mitochondrial metabolism, protein degradation and processing, neurotransmitter synthesis, etc.), e.g., protein deglycase DJ-1 and ubiquitin carboxyl-terminal hydrolase isozyme L1 in rat and SH-SY5Y cells, tyrosine hydroxylase from rat cells, rat mitochondrial glutathione peroxidase 4, human DA transporter, etc. (Hauser et al., 2013; Van Laar et al., 2009; Whitehead et al., 2001; Xu et al., 1998).
DA-o-quinone has been proposed to be the most reactive o-quinone species responsible for the oxidative stress induced by DA oxidation (Bisaglia et al., 2010). The depletion of glutathione in dopaminergic neurons would be the consequence of the rapid nucleophilic addition of glutathione to DA-o-quinone with a rate constant of 200 s−1, generating 5-S-glutathionyl-DA (Tse et al., 1976). The latter compound undergoes enzymatic degradation to yield 5-S-cysteinyl-DA (Shen et al., 1996). However, the question is whether DA-o-quinone-dependent depletion of glutathione leading to oxidative stress is a neurotoxic reaction or contributes to generate NM, which appears to be neuroprotective in certain conditions (see section 9.1.). Reductive hydrolysis of human NM pigment showed that cysteinyl-DA is one of the main components of this pigment (Wakamatsu et al., 2003).
Aminochrome interacts with human α-synuclein, inducing and stabilizing the formation of neurotoxic oligomers (Muñoz et al., 2015; Norris et al., 2005). Recently, by using a rat cell line with stable over-expression of wild type α-synuclein and a siRNA against the DT-diaphorase enzyme, it has been demonstrated that the neurotoxicity of α-synuclein oligomers depends on the silencing of DT-diaphorase enzyme (Muñoz et al., 2015). This enzyme is a flavoenzyme that catalyzes the two-electron reduction of aminochrome to leukoaminochrome, thus preventing the formation of neurotoxic α-synuclein oligomers induced by aminochrome. Interestingly, DT-diaphorase prevents α-synuclein fibrillation, probably because leukoaminochrome can stabilize α-synuclein in the monomer state (Muñoz et al., 2015).
In addition, aminochrome forms adducts with complex I of electron transport chain in SH-SY5Y cells differentiated into a DA phenotype, inducing mitochondria dysfunction and inhibiting ATP production (Aguirre et al., 2012). Aminochrome also induces protein degradation dysfunction by: i) impairing the proteasomal system in different in vitro models (Xiong et al., 2014; Zafar et al., 2006; Zhou and Lim, 2009); ii) inhibiting autophagy by preventing the adequate formation of microtubules required for the fusion of autophagic vacuoles and lysosomes in rat and human cell lines (Huenchuguala et al., 2014; Muñoz et al., 2012a; Paris et al., 2010); iii) inducing lysosomal dysfunction in human cell lines by affecting lysosomal acidification required for protein degradation inside these organelles (Huenchuguala et al., 2014). Aminochrome can induce oxidative stress when it is one-electron reduced by flavoenzymes to leukoaminochrome-o-semiquinone radical, which is extremely reactive with oxygen and auto-oxidizes immediately to aminochrome generating a redox cycling between aminochrome and leukoaminochrome-o-semiquinone radical, depleting both NADH or NADPH and oxygen, the latter being reduced to superoxide radical with consequent formation of hydroxyl radical, as demonstrated in rat cell lines experiments (Arriagada et al., 2004). The 5,6-indolequinone, which is derived from aminochrome rearrangement, interacts with proteins such as α-synuclein, inducing the formation of neurotoxic oligomers in vitro (Bisaglia et al., 2007).
Several enzymes have been reported to be able to catalyze DA oxidation to o-quinone in vitro, such as prostaglandin H synthase (Hastings 1995; Mattamal et al., 1995), cytochrome P450 (Segura-Aguilar 1996; Segura-Aguilar et al., 1998; Thompson et al., 2000), tyrosinase (Jimenez et al., 1984; Segura-Aguilar et al., 1998), xanthine oxidase (Foppoli et al., 1997), lactoperoxidase (Galzigna et al., 1999; Segura-Aguilar et al., 1998), DA β-monooxygenase (Terland et al., 1997), and brain peroxidases (Galzigna et al., 2000). Oxygen also catalyzes DA oxidation to o-quinone in the absence of metal ions at pH 7.4 (Linert et al., 1996). Inorganic reagents are also able to oxidize DA to o-quinone, for instance sodium periodate (Jimenez et al., 1984) or manganese(III), both under aerobic and anaerobic conditions (Segura-Aguilar and Lind, 1989). Interestingly, high exposure to manganese both during the welding and in the mining industry can induce parkinsonian syndromes (Park 2013). Copper sulfate catalyzes DA oxidation to aminochrome by forming the complex of copper-DA that is neurotoxic only in rat SN cell lines expressing DA transporters (Paris et al., 2001). The high selectivity of copper-DA complex for DA transporters may explain the existence of parkinsonian syndromes in young workers highly exposed to copper (Caviedes and Segura-Aguilar, 2001), and the high prevalence of parkinsonian symptoms in Wilson´s disease patients (Taly et al., 2007). Aerobic DA oxidation to o-quinone can also be catalyzed by iron, as demonstrated in vitro by both free iron ions (trivalent or bivalent) and iron complexes with cyanide ions, such as potassium ferrous cyanide and potassium ferric cyanide (Zhou et al., 2010).
The formation of iron-DA complex, in the form of iron(III)-DA, has also been proposed as a selective mechanism of neurotoxicity in dopaminergic neurons or cells expressing monoaminergic transporters such as DA, norepinephrine, and serotonin transporters (Paris et al., 2005a, b). The selectivity of iron-DA complex neurotoxicity depends on the ability of cells to take up such neurotoxic complex. It was reported that PC-12 cells exposed to iron showed an increase of intracellular iron that was blocked when the cells were treated with an inhibitor of DA synthesis. It was also shown that iron was accumulated in dopaminergic vesicles, thus supporting the idea that iron-DA complex is required for iron uptake (Ortega et al., 2007).
In vitro experiments confirmed the formation of iron-DA complex and its stability (Arreguin et al., 2009). Thus, the neurotoxic effects of iron-DA complex in rat cell lines depend on: i) uptake into the cells via monoaminergic transporters; ii) oxidation of DA to aminochrome and reduction of ferric to ferrous iron; iii) DT-diaphorase inhibition (Paris et al., 2005a, b). Iron(III) is also able to form similar complexes with norepinephrine and serotonin (Siraki et al., 2000). Interestingly, the iron-norepinephrine complex prevents iron-DA complex neurotoxic effects in cells derived from rat SN, probably because of a competition between norepinephrine and DA in complex formation with ferric iron (Paris et al., 2005b). The oxidation of DA to aminochrome with concomitant reduction of ferric iron to ferrous iron finally results in the release of aminochrome and ferrous iron from the iron-DA complex (Fig. 1). This idea is also supported by the formation of a dark pigment in the test tube after 3–4 h incubation as a consequence of aminochrome polymerization. As discussed above, free aminochrome can be one-electron reduced by flavoenzymes that use NADH or NADPH, generating leukoaminochrome-o-semiquinone radical that immediately auto-oxidizes to aminochrome by reducing dioxygen to superoxide radical. The dismutation of two superoxide radicals generates hydrogen peroxide, which in the presence of ferrous iron forms the hydroxyl radical producing high oxidative stress (Fig. 1, 2). Additionally, aminochrome can perturb general cellular iron homeostasis: in SH-SY5Y cells differentiated into a dopaminergic phenotype, aminochrome decreased expression of the iron export transporter ferroportin 1, and increased expression of the iron import transporter divalent metal transporter 1, thus leading to iron accumulation (Aguirre et al., 2012).
4. Role of DA oxidation in neurodegenerative processes of PD
The degenerative process in PD initiates long before the appearance of motor symptoms. Pre-motor symptoms include sleep fragmentation, olfactory disturbances, and depression (Wolters and Braak, 2006). Different stages of PD have been proposed in the development of the disease (Braak et al., 2003, 2004), based on the appearance of α-synuclein aggregates in pathology, in which the disease initiates in the enteric plexus, olfactory bulb and motor component of cranial nerve X (stage 1) and continues to the locus coeruleus (LC), caudal raphe nuclei, and magnocellular reticular formation (stage 2). Five years before the onset of motor symptoms, the disease progresses to the SN, amygdala central subnucleus, pedunculopontine tegmental nucleus, and Meynert's nucleus (stage 3). At stage 4 lesions principally extend to temporal mesocortex. After 10 years of the motor symptoms the disease progresses to tertiary sensory association areas and prefrontal cortex (stage 5). In stage 6, the secondary and then the primary motor and sensory areas are affected (Braak et al., 2003, 2004; Hawkes et al., 2010).
The appearance of α-synuclein aggregates, while clearly indicating a problem in the handling of a protein by particular cells, is not necessarily identical to the pattern of neuronal death: it is possible that some of the aggregation provide a successful protective response. As recently reviewed (Sulzer and Surmeier, 2013), the neuronal populations that most clearly show loss in pathological studies are the following. i) In the peripheral nervous system, the most clearly documented neuronal loss is of noradrenergic neurons innervating the heart and skin. Loss of catecholaminergic neurons of the enteric nervous system is widely suspected but not \ clearly confirmed. ii) There is a loss of neurons of the dorsal motor nucleus of the vagus in PD. This region has many pigmented neurons, but there are differences in the literature regarding if and when these are lost. A potentially important factor is that cholinergic dorsal motor nucleus of the vagus neurons also express tyrosine hydroxylase and aromatic acid decarboxylase, and thus might synthesize catecholamines. iii) The death of the NM-containing dopaminergic neurons of the SN pars compacta, is by far the most noted and confirmed instance of neuronal loss, as reported for nearly a century. The loss of SN NM is seen in all PD patients. Some Lewy pathology has been reported in the neighboring ventral tegmental area and retrorubral field neurons as well, but neuronal loss in those two regions is variable. iv) Virtually all PD patients exhibit substantial loss of NM-containing noradrenergic LC neurons. v) Additional regions of the central nervous system show less clear loss. The serotonergic raphe nuclei neurons are lost in PD, particularly in the median raphe less so in the raphe obscurus. The pedunculopontine nucleus, which includes a mixture of cholinergic, glutamatergic and gamma-aminobutyric acid-ergic neurons, has been reported to show neuron loss in PD, although this may not be specific to PD and is seen in progressive supranuclear palsy and Alzheimer's disease. The existence and targeting of neuronal loss in the olfactory system is controversial. Several other regions have been reported to have neuronal loss in PD, many in single studies. These include for example the hypothalamus, the intralaminar nuclei of the thalamus and possibly the nucleus basalis of Meynert, and DA neurons of the retina (Surmeier and Sulzer, 2013).
Nearly five decades after the introduction of L-3,4-dihydroxyphenylalanine (L-DOPA) in the treatment of PD, the molecular mechanism involved in the loss of NM-containing dopaminergic neurons remains unknown. The discovery of several alterations in proteins linked to the genetic form of PD resulted in an enormous input in basic research: i.e., leucine-rich repeat serine/threonine-kinase 2, α-synuclein, E3 ubiquitin-protein ligase parkin, probable cation-transporting ATPase 13A2, protein deglycase DJ-1, serine/threonine-protein kinase PINK1 mitochondrial, etc. (Abbas et al., 1999; Bonifati et al., 2003; Hattori et al., 1998; Kachergus et al., 2005; Polymeropoulos et al., 1997; Ramirez et al., 2006; Trinh and Farrer, 2013; Valente et al., 2004).
In general, the present scientific consensus is that multiple factors are involved in the degenerative process resulting in the loss of NM-containing dopaminergic neurons during PD, including neuroinflammation, oxidative stress, protein degradation dysfunction, aggregation of α-synuclein to neurotoxic oligomers, endoplasmic reticulum stress, and mitochondrial dysfunction (Ebrahimi-Fakhari et al., 2012a, b; Exner et al., 2012; Hauser and Hastings, 2013; Kalia et al., 2013; Martinez-Vicente and Vila, 2013; Mercado et al., 2013; Mullin and Schapira, 2013; Rohn 2012; Taylor et al., 2013).
A central issue is to identify the neurotoxin (if any) responsible for the degeneration of dopaminergic neurons in the nigrostriatal system during PD. The fact that the exogenous neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces in humans a severe parkinsonism in a very short period together with a rapid and severe depletion of dopaminergic neurons (Ballard et al., 1985; Williams 1984), strongly suggests that such a neurotoxin must be of endogenous origin, since the neurodegenerative process in PD initiates years before the motor symptoms and the progression of the disease is also very slow. Interestingly, the products of DA oxidation (DA-o-quinone, aminochrome and 5,6-indolequinone) are directly involved in mitochondria dysfunction, protein degradation alteration, α-synuclein aggregation to neurotoxic oligomers and oxidative stress in dopaminergic neurons (Fig. 1, 2), suggesting that these o-quinones can play an important role in the degenerative process, resulting in the loss of SN dopaminergic neurons containing NM (Aguirre et al., 2012; Arriagada et al., 2004; Bisaglia et al., 2007; Dibenedetto et al., 2013; Hauser and Hastings, 2013; Huenchuguala et al., 2014; LaVoie et al., 2005; Martinez-Vicente et al., 2008; Muñoz et al., 2012a, 2015; Norris et al., 2005; Paris et al., 2010; Van Laar et al., 2009; Whitehead et al., 2001; Xiong et al., 2014; Xu et al., 1998; Zafar et al., 2006; Zhou and Lim, 2009).
It is important to remark that the formation of o-quinones by DA oxidation occurs inside the neurons that are lost in the disease, and probably the cell death induced by these o-quinones during DA oxidation is a focal event that explains why the neurodegeneration is very slow in PD. Aminochrome is the most stable and studied of these o-quinones and can participate in both neurotoxic and neuroprotective reactions (Fig. 1, 2). The neurotoxic reactions in which aminochrome is involved are: i) α-synuclein aggregation to neurotoxic oligomers both in vitro and in cell line experiments (Dibenedetto et al., 2013; Muñoz et al., 2015; Norris et al., 2005); ii) protein degradation dysfunction that includes proteasome system impairment in different in vitro models (Xiong et al., 2014; Zafar et al., 2006; Zhou and Lim, 2009), inhibition of the fusion of autophagic vacuoles with lysosomes (Huenchuguala et al., 2014; Muñoz et al., 2012a; Paris et al., 2010), and lysosomal dysfunction (Huenchuguala et al., 2014) in rat and human cell lines; iii) oxidative stress with the formation of hydroxyl radicals during one-electron reduction of aminochrome to leukoaminochrome-o-semiquinone radical, which is extremely unstable in the presence of oxygen in a rat cell line (Arriagada et al., 2004); iv) mitochondrial dysfunction by inhibiting complex I of electron transport chain with a significant decrease in ATP in SH-SY5Y cells (Aguirre et al., 2012).
The o-quinones formed during DA oxidation seem to play an important role in the loss of dopaminergic neurons containing NM of the nigrostriatal system during PD. However, DA oxidation to o-quinones finally resulting in the formation of NM seems to be a normal event since this pigment is present in SN of healthy individuals. A possible reason why DA oxidation forms NM pigment and does not induce neurotoxicity is the presence of enzymes in SN that prevent aminochrome neurotoxicity, such as DT-diaphorase in dopaminergic neurons of SN (Lozano et al., 2010; Schultzberg et al., 1988) and glutathione S-transferase M2-2 in astrocytes (Huenchuguala et al., 2014) in both rat and human cells.
As discussed above, DT-diaphorase, also called NQO1 or NAD(P)H:quinone oxidoreductase 1, catalyzes the two-electron reduction of aminochrome to leukoaminochrome (Segura-Aguilar and Lind, 1989). This enzyme is the unique flavoenzyme that reduces quinones with two electrons to hydroquinones. It is reasonable to suppose that leukoaminochrome can then undergo rearrangement to 5,6-dihydroxyindole, the precursor of 5,6-indolequinone: the latter compound can polymerize leading to the formation of NM pigment, resulting in neuroprotection, or can interact with proteins like α-synuclein, inducing neurotoxicity (Fig. 1, 2). Two-electron reduction of aminochrome catalyzed by DT-diaphorase prevents the formation of α-synuclein neurotoxic oligomers and fibrils: this enzyme can stabilize the monomer form of α-synuclein in rat cell lines (Muñoz et al., 2015), inhibiting the neurotoxic effects of the α-synuclein oligomers generated when aminochrome (Muñoz et al., 2015) and 5,6-indolequinone (Bisaglia et al., 2007) interact with α-synuclein. DT-diaphorase prevents protein degradation dysfunction by inhibiting aminochrome-induced proteasome impairment in rat and rabbit cells (Xiong et al., 2014; Zafar et al., 2006) and aminochrome-induced autophagy impairment in a rat cell line (Muñoz et al., 2012b). Moreover, DT-diaphorase can block aminochrome-induced mitochondria dysfunction, decrease of mitochondrial membrane potential, and decrease of ATP production in rat cell cultures (Arriagada et al., 2004; Fuentes et al., 2007; Muñoz et al., 2012b; Paris et al., 2011). DT-diaphorase could act as a neuroprotective enzyme preventing one-electron reduction of aminochrome to leukoaminochrome-o-semiquinone radical, with formation of hydroxyl radicals and oxidative stress (Arriagada et al., 2004). Finally, as demonstrated in rat cell lines, DT-diaphorase can also prevent aminochrome-induced cell death (Arriagada et al., 2004; Fuentes et al., 2007; Lozano et al., 2010; Muñoz et al., 2012b; Paris et al., 2010, 2011).
Glutathione S-transferase M2-2 catalyzes glutathione conjugation of DA-o-quinone leading to 5-S-glutathionyl-DA (Dagnino-Subiabre et al., 2000), which is then degraded to 5-S-cysteinyl-DA, and the latter compound has been detected in SN and other human brain regions rich in DA (Rosengren et al., 1985), in NM pigment from healthy human SN (Carstam et al., 1991; Wakamatsu et al., 2003), and interestingly in the cerebrospinal fluid of PD patients (Cheng et al., 1996; Rosengren et al., 1985). Glutathione S-transferase M2-2 also conjugates aminochrome with glutathione to form 4-S-glutathionyl-5,6-dihydroxyindoline, which is stable in the presence of superoxide radicals, oxygen, and hydrogen peroxide (Baez et al., 1997; Segura-Aguilar et al., 1997). Therefore, glutathione S-transferase M2-2 prevents aminochrome-induced toxicity in astrocytes by conjugating aminochrome with glutathione. Indeed, the silencing of glutathione S-transferase M2-2 expression with a siRNA in a human cell line model of astrocytes induced autophagic and lysosomal dysfunction (Huenchuguala et al., 2014). Interestingly, although glutathione S-transferase M2-2 is only expressed in astrocytes, the enzyme may also protect dopaminergic neurons against aminochrome toxicity. This may be because dopaminergic neurons internalize glutathione S-transferase M2-2 secreted by astrocytes, thus acquiring this protective enzyme against aminochrome neurotoxicity, as suggested in human-derived cell cultures experiments (Cuevas et al., 2015).
The recently emerged role of 3,4-dihydroxyphenylacetaldehyde (DOPAL), the primary DA metabolite, in neuronal degeneration must be included among the reactive species containing carbonyl groups (Goldstein et al., 2014). DOPAL results from DA oxidation by monoamine oxidase, particularly isoform A in the catecholaminergic neurons (Westlund et al., 1985; Youdim et al., 2006), and under normal conditions DOPAL is rapidly converted to the corresponding acid by the action of aldehyde dehydrogenase (Eisenhofer et al., 2004). As the activity of aldehyde dehydrogenase is apparently reduced in patients with sporadic PD (Goldstein et al., 2013), the possibility of build-up of reactive DOPAL is therefore to be taken into account. DOPAL contains two reactive functional groups, the aldehyde and the catechol, which can be involved in protein modification or contribute to the complex DA oxidative oligomerization process (Goldstein et al., 2014). DOPAL could also auto-oxidize to semi-quinone radical and o-quinone in vitro, with possible formation of toxic ROS (Anderson et al., 2011), as reported for aminochrome or DA. However, the most intriguing effect appears to be related to the capacity of DOPAL to induce oligomerization of α-synuclein in a cell-free system, in SH-SY5Y dopaminergic cell cultures and in vivo (Burke et al., 2008). DOPAL is far more active in this oligomerization process than DA itself, and this may explain its connection with DA cytotoxicity, since proto-fibrillar α-synuclein permeabilizes vesicles in vitro forming pores in the membrane, thereby enhancing leakage of endogenous DA in the cytosol (Volles and Lansbury, 2002).
5. Definition, classification and basic physicochemical properties of natural melanins
According to a recent review on melanins and melanogenesis by an international group of experts, melanins are ubiquitous pigments of diverse structure and origin derived by the oxidation and polymerization of tyrosine, or related compounds, in animals or phenolic compounds in lower organisms (d’Ischia et al., 2013; Wakamatsu and Ito, 2002). From a biological and chemical point of view, at least four distinct types of melanin pigments can be distinguished: eumelanins, pheomelanins, NMs and pyomelanins. The first three types of melanins are present, to a varying degree, in humans. The most studied eumelanins are black-to-brown insoluble pigments derived at least in part from the oxidative polymerization of L-DOPA via 5,6-dihydroxyindole intermediates (Prota 2000; Wakamatsu and Ito, 2002). Pheomelanins are yellow-to-reddish brown, alkali-soluble, sulfur-containing pigments derived from the oxidation of cysteinyldopa precursors via benzothiazine and benzothiazole intermediates (Ito and Wakamatsu, 2006). NM pigments are extensively discussed in the following chapters of this review and will not be addressed in this chapter. The least studied melanins, the pyomelanins, are dark pigments produced mainly by microorganisms from homogentisate (d’Ischia et al., 2013).
In pigmented human and animal tissues, melanins are typically synthesized by specialized cells, known as melanocytes, in the form of discernible membrane bound pigment granules named melanosomes (Boissy and Hornyak, 2006; Quevedo and Holstein, 2006). A number of structural proteins and enzymes are involved in the biogenesis of melanin (Brilliant 2006; Hearing 2006; Solano and Garcia-Borron, 2006; Takeda and Shibahara, 2006).
The mature pigment granules vary in size and shape depending on the type of pigment cells responsible for biogenesis of the melanosomes. Thus melanosomes from the ink sac of Sepia officinalis are spherical in shape and about 150 nm in diameter (Kollias et al., 1991; Liu and Simon, 2003), while melanosomes in the human retinal pigment epithelium (RPE) are typically elongated and much larger, having dimension of 2–3 µm long and 1 µm wide (Boulton 1998; Sarna 1992; Simon et al., 2008). In normal human skin, the ultrastructure of melanosomes is related to the type of melanin they produce (Sarna and Swartz, 2006). Typical eumelanosomes have ellipsoidal lamellar structure with melanin deposited in a uniform pattern. Pheomelanosomes in contrast are round and granular with an uneven deposition of pigment within the melanosomes (Liu et al., 2005).
Although melanocytes are also found in hair follicles and occasionally in the dermis, heritable, i.e., constitutive, human skin color is predominantly determined by the amount and type of melanin in the epidermis (Quevedo and Holstein, 2006). Pigmentation of the human skin is regulated by environmental factors, among which ultraviolet radiation plays a key role, and by a variety of endocrine, paracrine and autocrine factors (Abdel-Malek and Kadekaro, 2006). Distinct changes in the location and morphology of melanin granules occur during their turnover in the human epidermis. Thus, mature melanosomes formed in the basal melanocyte are transferred via its dendrites to adjacent keratinocytes (Scott 2006). On incorporation into keratinocytes, melanosomes fuse with lysosomes forming secondary lysosomes (Byers 2006). Lysosomal hydrolase degrades melanosomes as the keratinocytes move to the epidermal surface. Larger melanosomes, characteristic for heavily pigmented human skin, appear to be less susceptible to lysosomal degradation than smaller melanosomes formed in lightly pigmented skin (Jimbow et al., 1999; Quevedo and Holstein, 2006). Thus, in lightly pigmented human skin the degraded melanosomes only persists as so-called “melanin dust” in the suprabasal layer of the epidermis (Brenner and Hearing, 2008). It must be stressed that such an enzymatic degradation of melanosomes only reduces the size of the granules to finer particles without actually degrading their melanin component. In fact, there are no known enzymes that can actually decompose melanin. The physicochemical properties of melanosomes degraded in secondary lysosomes of the keratinocytes have not been systematically studied, and it is questionable if such “melanin dust” is indeed more effective in shielding the epidermis from penetration and damage of ultraviolet light than non-degraded melanosomes (Jimbow et al., 1999). Equally important is the issue of whether melanin in the human skin undergoes significant chemical modification during the relatively short lifetime of the pigment granules (Halprin 1972).
Interestingly, such changes were recently determined in melanin from human RPE. Employing electron paramagnetic resonance (EPR) spectroscopy for specific detection of melanin in isolated RPE cells, the content of melanin in a number of samples, obtained postmortem from donors of different age, has been determined (Sarna et al., 2003). It has been demonstrated that aging is accompanied by gradual loss of RPE melanin (Sarna et al., 2003) and its oxidative modifications (Ito et al., 2013). The authors postulated that these phenomena result from chronic photochemical reactions leading to irreversible oxidative cleavage and cross-linking of the melanin. It should be emphasized that human RPE, consisting of postmitotic cells, is a unique pigmentary system, in which melanin is synthesized early during fetal development and shows little or no metabolic turnover afterward. Although biological consequences of such an in vivo photoaging of RPE melanosomes are not yet fully understood, it could be argued that the reduction in the content of melanin and its oxidative modification lower the ability of melanin to protect human RPE cells, as well as other cells of the outer retina, against oxidative stress (Ito et al., 2013; Sarna et al., 2003). Human RPE, regardless skin and eye colors, contains mainly eumelanin (Ito et al., 2013), while human uveal melanocytes contain both eumelanin and pheomelanin and their relative content depends on skin and iris colors (Prota et al., 1998; Wakamatsu et al., 2008; Wielgus and Sarna, 2005).
Another distinct extracutaneous location of melanin in humans is the inner ear, where biological functions of the pigment remain speculative (Boissy and Hornyak, 2006). Extensively dendritic, heavily pigmented melanocytes residing beneath the epithelial layer of each respective area are found in stria vascularis and modiolus of the cochlea (Meyer zum Gottesberge 1988). Both eumelanin and pheomelanin are synthesized by the otic melanocytes, and the otic pigments appear to be similar to those from eyes and hair (Inoue et al., 1984).
In spite of significant research, the precise molecular structure of melanin pigments has not been determined. To a large extent, this is due to intractable character of the pigments, which are mostly insoluble in the majority of solvents and naturally present as complex pigment granules containing not only melanin but also protein matrix and lipid membrane bilayer. Results of numerous studies, carried out on synthetic and natural eumelanins, in which atomic force microscopy, EPR spectroscopy, nuclear magnetic resonance spectroscopy, X-ray diffraction, mass spectrometry and advanced quantum chemical calculations were used, seem to support a stacked-aggregate model of the eumelanin structure (d’Ischia et al., 2013; Meredith and Sarna, 2006; Zajac et al., 1994). In eumelanin from sepia ink, at least three distinct levels of structural organization are identified. Relatively small planar oligomers stacked along the z-axis with the stacking spacing of about 3.4 Å are responsible for the first level of aggregation, forming the fundamental nanoaggregates (protomolecules). The second level of aggregation, involving protomolecules, leads to formation of filaments which are small aggregated substructures. The third level of aggregation results in formation of large particles of the size 100–200 nm (Clancy et al., 2000; Zajac et al., 1994). It has been postulated that the high molecular heterogeneity of eumelanin, determined by heterogeneous character of its monomeric units and oligomers, is responsible for unusual physicochemical properties including absorption of light (Meredith and Sarna, 2006).
Eumelanins are commonly viewed as efficient antioxidants and photoprotective agents (Brenner and Hearing, 2008; Sarna 1992; Sarna and Swartz, 2006; Zareba et al., 2014). Indeed, synthetic eumelanins have been shown to efficiently scavenge reactive free radicals (Rozanowska et al., 1999) and quench excited electronic states of certain molecules (Sarna et al., 1985; Ye et al., 2003). The ability of eumelanin to sequester redox active metal ions has been demonstrated in vitro, in case of synthetic L-DOPA-melanin (Pilas et al., 1988), bovine eye melanin (Sarna et al., 1976), porcine RPE melanosomes, both extracellularly and intracellularly (Kaczara et al., 2012), and sepia melanin (Liu et al., 2004; Liu and Simon, 2005). Synthetic and human NM, as well as bovine RPE melanosomes prevented the peroxidation of unsaturated lipids in model systems consisting of liposomes made of unsaturated lipids (Korytowski et al., 1995; Zadlo et al., 2007, 2009).
Another intriguing property of melanin with unknown biological implications is its electrical conductivity, seemingly typical for amorphous semiconductors (McGinness 1972; McGinness et al., 1974). However, a recent study employing muon spin relaxation, EPR and electrical conductivity measurements, demonstrated that synthetic eumelanin is actually a hybrid semiconductor, in which both electrons and ions participated in electrical conductivity (Mostert et al., 2012). The study underscored the role of comproportionation reaction, which drives the production of extrinsic free radicals and hydronium ions in synthetic and natural eumelanins from different sources (Felix et al., 1978; Sarna and Swartz, 2006).
6. NM synthesis and structure
NM is a pigment present in different neurons of the human central nervous system and is particularly abundant in DA neurons of the SN and in the noradrenergic neurons of LC (Zecca et al., 2004b, 2008b). NM pigment appears as a black and insoluble molecule composed of different components: melanin, proteins, lipids and metal ions. NM pigment is located within cytoplasmic organelles, with variable sizes ranging from 0.5 to 3.0 µm, surrounded by a double membrane, together with lipid bodies and proteins (Sulzer et al., 2008; Zecca et al., 2008b). NM pigment concentration increases with aging, beginning its formation and accumulation early in the life (Zecca et al., 2002, 2004b).
Although the origin of NM-containing organelles is not completely clear, it has been proposed that NM synthesis is driven by an excess of cytosolic catecholamines not accumulated into synaptic vesicles, since NM synthesis was inhibited in rat neuronal cultures by the over-expression of synaptic vesicular monoamine transporter 2 (Sulzer et al., 2000), the transporter which sequesters monoamines from the cytosol into synaptic vesicles for subsequent neurotransmission (Liu and Edwards, 1997). The regulatory role of this transporter on NM biosynthesis in human SN was also supported by immunohistochemical studies on post-mortem human brains which have correlated the presence of high levels of vesicular monoamine transporter 2 with relatively low levels of NM pigment, and vice-versa, indicating an inverse relationship between vesicular monoamine transporter 2 expression and NM content (Liang et al., 2004). In rats exposed to bright light for up to 90 days an accumulation of NM pigment in neurons was found with a simultaneous decrease of tyrosine hydroxylase-positive neurons in the SN, thus probably showing a neurodegenerative effect of NM (Romeo et al., 2013).
The biosynthetic pathway leading to NM formation is complex and only partly understood (Zucca et al., 2014). It appears to differ from that leading to peripheral melanins, explaining the notable differences in the structural organization of the two pigments. The synthesis of natural melanins present in skin, hair, and eye is controlled enzymatically by tyrosinase, which is essential in the initial energy demanding step of the process, in which tyrosine is oxidized to dopaquinone (Solomon et al., 2014). This reactive intermediate undergoes a series of redox and condensation reactions, collectively known as the Raper-Mason scheme, leading to eumelanin and pheomelanin (Agrup et al., 1982; Simon and Peles, 2010; Wakamatsu and Ito, 2002). The biosynthesis of eumelanin precursors requires the participation of two additional enzymes, known as the tyrosinase-related proteins, namely 5,6-dihydroxyindole-2-carboxylic acid oxidase and L-dopachrome tautomerase (Garcìa-Borron and Solano, 2002; Wakamatsu and Ito, 2002), whereas the precursors of pheomelanin are generated starting from the addition of cysteine to dopaquinone (Di Donato and Napolitano, 2003; Napolitano et al., 2008). To explain the structural organization of the two components in the final melanin pigment, it is important to note that the kinetics of the initial steps of eumelanin and pheomelanin formation are different, and in particular that the reaction of cysteine with dopaquinone is faster than the steps involved in eumelanin synthesis (Ito and Wakamatsu, 2008). Thus, pheomelanin is formed earlier, until most cysteine and cysteine-containing peptides are consumed, and only after that eumelanin accumulates from residual dopaquinone (Simon and Peles, 2010). This is the basis for the formulation of the “casing model” of organization of the pigment granules within the melanosome (Greco et al., 2011; Ito 2006; Simon et al., 2008), with an inner pheomelanic core surrounded by the photoprotective, antioxidant eumelanic coat (Liu et al., 2005; Peles et al., 2009). It is currently unknown whether the two components are covalently linked or simply associated through non-covalent interactions.
In the case of NM, there is no evidence for enzymatic control of the oxidative process leading to the formation of the pigment, since tyrosinase was not found in human SN where NM is most abundant (Ikemoto et al., 1998; Tribl et al., 2007). Other enzymes than tyrosinase might be involved in NM biosynthesis (as discussed in chapter 3), but to date a specific enzymatic synthesis pathway for NM has not been unequivocally proven; additionally, the existence of an auto-oxidation process of catecholamines must be also taken into account for NM biosynthesis (Zucca et al., 2014). The substrate principally involved in the oxidative polymerization is either DA, in the SN and most other brain areas, or norepinephrine in the LC, although different catecholamines seem to be involved in the synthesis of human NM pigment (Wakamatsu et al., 2014; Zecca et al., 2008b). In this NM pigment, the melanic groups are covalently bound to aliphatic chains and peptides moieties (Engelen et al., 2012); dolichols and dolichoic acid were identified as the major components of NM lipids (Fedorow et al., 2005; Ward et al., 2007; Zecca et al., 2000, 2008b). The initial events of the NM biosynthesis are the most difficult to trace, but the emerging view is that three actors appear to be involved: the cytosolic catecholamines, iron(III) ions, and "seeds" of aggregated oligomers of peptides/proteins (Fig. 3). The presence of iron(III) strongly promotes the auto-oxidation of catecholamine into a reactive quinone (Sulzer and Zecca, 2000). This, in turn, is trapped by the peptide/protein seeds present in the cytoplasm, particularly those containing cysteine residues, initiating a conjugation process that is formally similar to that occurring in the formation of pheomelanin and eumelanin pigment, but with two important differences. The first one is that the process occurs on a preformed core of peptide/protein, and the second one is that iron remains trapped into the melanic component. As the resulting material accumulates in the cytosol, it is transferred to autophagic vacuoles that fuse with lysosomes and other autophagic vacuoles, forming the NM-containing organelles (Zucca et al., 2014). Lipids bodies are likely formed by the fusion with other organelles and by transportation of dolichols inside the NM-containing organelles (Fig. 3).
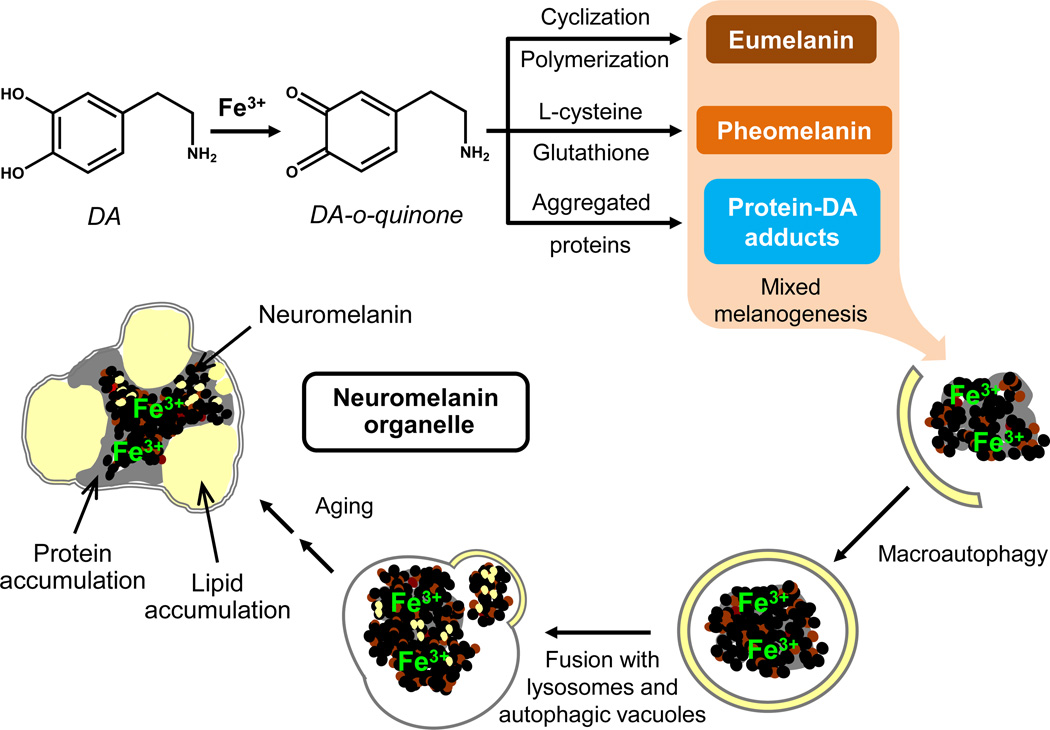
Fig. 3. Possible mechanisms for the synthesis of NM pigment and for the formation of NM-containing organelles.
Excess of DA present in the cytosol can be oxidized to DA-o-quinone by ferric iron in a catalytic reaction. In the formation of NM pigment, DA-o-quinone can undergo three different pathways: i) cyclization, further oxidation and polymerization to give eumelanin; ii) reaction with L-cysteine or glutathione to give cysteinyl-DA compounds then oxidized to pheomelanin; and iii) conjugation with protein residues to give DA-protein adducts. The latter two reactions seem to be faster and lead to the formation of a protein-pheomelanin core, which is then coated by eumelanin, according to the mixed melanogenesis model. Iron(III) is incorporated into the melanic portion of the forming NM pigment. The resulting undegradable and insoluble pigment is taken into autophagic vacuoles that fuse with lysosomes and other autophagic vacuoles containing lipids, proteins, etc., leading to the formation of NM-containing organelles. These double membrane bounded organelles contain NM pigment along with its components, abundant lipid bodies, and protein matrix. This process continues during the entire neuron life and results in the accumulation of NM-containing organelles with aging. This scheme is modified from Zucca and colleagues (2014) by permission of Springer Publishing.
Determination of the relative amounts of the three components in human NM pigment is a difficult task, in view of the limited amount of biological material available. Previous estimates based on chemical degradative, mass spectrometry and spectrophotometric methods (Dzierzega-Lecznar et al., 2006; Wakamatsu and Ito, 2002; Wakamatsu et al., 2003, 2012; Zecca et al., 1992) have been recently updated by using elemental analysis, nuclear magnetic resonance spectroscopy, infrared spectroscopy and amino acid analyses, and show some significant variability depending also on the brain area from which NM pigment is extracted (Engelen et al., 2012). Melanin, with an approximate ratio of 1:3 between pheomelanic and eumelanic components, is most abundant in NM pigment isolated from SN (~56 %), with lower amounts of lipid (~18 %) and protein (~12 %). By contrast, NM pigments isolated from most other human brain areas contain lipids as the major component (>40 %), with protein at similar level (~12 %), and variable amounts of melanin, but always lower than in SN (Engelen et al., 2012).
NM pigments of different brain areas consist of granular aggregates that are 200–400 nm in size, or varying shapes, composed of smaller spherical substructures of about 30 nm in diameter (Bush et al., 2006, 2009; Zecca et al., 2008b). The structure proposed for the melanic portion of human NM pigments resembles that of other natural melanins: the NM pigment architecture is that of a pheomelanin core surrounded by eumelanin surface, forming spherical electron-dense aggregates according to the "casing model" of mixed melanogenesis (Ito 2006).
The difference in the biosynthetic processes leading to pigment melanins and NM is reflected in the overall structure and morphology of the resulting particles. The size of these roughly spherical particles of NM pigment, with a diameter of about 30 nm, is similar to that observed for melanins (Simon et al., 2008), but the inner structural organization is different. In the case of melanins, the only ordered structural element appears to be the stacked arrangement of planar indole oligomeric units (or "protomolecules") of eumelanin, which are separated by about 3.4 Å, as deduced on the basis of early X-ray scattering and scanning tunnelling microscopy (Cheng et al.,1994a, b; Gallas et al., 1999; Meredith and Sarna, 2006; Zajac et al., 1994). The updated model of stacked organization of eumelanin protomolecules derives from a wealth of recent studies on poly-DA synthesis and properties (d'Ischia et al., 2014). In contrast, the melanic component of NM pigment does not grow as an ordered phase as in natural melanins, but rather as a "tree", the branches of which initiate at the peptide/protein seed attachment points. Analysis of NM pigment isolated from several human brain areas by X-ray powder diffraction shows a complete lack of the typical pattern of π-stacking arrangement between extended aromatic molecules, but a different motif characterized by ordered systems separated by about 4.7 Å (Zecca et al., 2008b). This separation is far too large to be associated with stable, noncovalent π-stacking interactions between aromatic rings, particularly for polycyclic systems, which typically arrange in parallel displaced disposition at distances of 3.4–3.8 Å (Bissantz et al., 2010; Salonen et al., 2011; Tiekink and Zukerman-Schpector, 2012), and must be attributed to a different type of ordered structure. Indeed, the 4.7 Å metric parameter corresponds to the separation between hydrogen-bonded backbones of peptide chains arranged in β-sheets, and is strikingly similar to the X-ray diffraction pattern observed for amyloid cross-β structures (Greenwald and Riek, 2010; Makin and Serpell, 2005). This supports the view that NM biosynthesis starts from a seed of oligomeric peptide or protein material accumulated in the cytosol to which oxidized DA, norepinephrine, or other catecholamines bind initiating the melanization process (Fig. 3). Recent work on synthetic melanin-protein conjugates complies with this model and supports the proposed biosynthetic pathway (Ferrari et al., 2013).
7. Synthetic models of NM
Studying the structure and function of human NM is a difficult task because of the small amounts that can be extracted from human brain tissues. Therefore, the preparation of synthetic melanins as models of NM provides a useful means to elucidate its synthesis, structure, and reactivity. Good models of NM are appealing as they could be used to induce and study neurodegenerative and neuroinflammatory processes in cell culture lines and in vivo models. Synthetic melanins able to induce chronic neuroinflammation, through microglia activation, and neuronal degeneration could reproduce what really occurs in a PD brain, replacing the exogenous toxins that have been used so far that cause an acute insult disrupting dopaminergic neurons, i.e. rotenone, paraquat, 6-hydroxydopamine or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Hattori and Sato, 2007).
An intriguing application of synthetic melanins has been recently presented by Viceconte and coworkers (2015): in vivo and in vitro experiments showed that synthetic melanins are able to induce microglia activation, as previously reported using human NM (Wilms et al., 2003; Zhang et al., 2011), through a caspase-dependent mechanism (Viceconte et al., 2015). In addition to significant up-regulation of a variety of pro-inflammatory mediators upon microglia activation, significant increase of caspase-8 and caspase-3 expression was observed in response to melanin treatment, in absence of cell death (Viceconte et al., 2015). Although this study uses a synthetic melanin that represents only the melanic portion of human NM, it provides a new and interesting approach to the study of molecular mechanisms of inflammation during PD.
The synthesis of suitable models of NM is not straightforward due to the many components present in the pigment: melanin, peptides, lipids, and metal ions. To date, the synthetic counterparts of human NM have been mainly represented by melanins produced by oxidation of phenolic precursors (Aime et al., 1997; Bridelli et al., 1999; Crippa et al., 2010; Double et al., 2000; Wakamatsu et al., 2003), in some cases with addition of iron ions. These models are insoluble in water and in most organic solvents. In general, synthetic models of NM can be divided in two types corresponding to the components of natural melanins: eumelanins and pheomelanins. Pheomelanins differ from eumelanins by the presence of a sulfur source which is provided by L-cysteine or glutathione. A variety of phenolic precursors can be used for the preparation of pheomelanins and eumelanins: L-tyrosine, L-DOPA, DA or norepinephrine (d’Ischia et al., 2013; Manini et al., 2007). To resemble NM from human SN, the precursor of choice is DA, since chemical degradative analyses demonstrated that NM from human SN mainly consists of DA-derived and cysteinyl-DA-derived units (Wakamatsu et al., 2003), although other catecholamines may participate in its synthesis (Wakamatsu et al., 2014). Notably, in vitro experiments confirmed that DA (derived from L-DOPA) is likely the most important precursor in NM biosynthesis (Sulzer et al., 2000).
Oxidation of DA to form the melanin can be carried out with two methods: a fast enzymatic oxidation by tyrosinase or the slower auto-oxidation in air. In some cases, also hydrogen peroxide has been used, but an excess of peroxide could degrade the forming melanin (d’Ischia et al., 2013). Although, as discussed above, it seems that the melanic portion of NM is not synthesized enzymatically, both this method and simple auto-oxidation process can be used to prepare NM models, though it is still unclear which preparation leads to better models of human NM (Bridelli et al., 1999; Crippa et al., 2010).
In addition to the melanic portion, the protein portion can be also incorporated in advanced NM models. To date, only bovine serum albumin has been employed in synthetic studies, although it is neither a human nor a neuronal protein. However, the advantage of using this protein is its availability and extensive knowledge of its structure and binding properties (Anand and Mukherjee, 2013). A first model was prepared by tyrosinase-mediated oxidation of DA in the presence of albumin, but the resulting adduct was not well characterized (Aime et al., 2000). A series of better characterized albumin-melanins were more recently prepared by auto-oxidation of DA in the presence of albumin and iron ions (Ferrari et al., 2013). Here, using suitable ratios of protein to DA, it was possible to obtain water soluble albumin-melanin conjugates. These soluble models have the advantage of allowing more detailed analysis by various techniques including nuclear magnetic resonance spectroscopy, UV spectroscopy, and circular dichroism; notably, the concentration of soluble NM models can be straightforwardly controlled in cell culture and in vivo studies.
Multiple studies have compared synthetic melanins with natural NM extracted from human brain tissues, in particular from SN. Since NM has a typical EPR spectrum, with a signal at g = 4.3 assigned to iron(III) and a signal at g = 2.0 due to the organic radical of melanin (Zecca and Swartz, 1993), this technique is useful for determining if the synthetic melanins can be related to the natural pigment. Furthermore, EPR spectra give useful information on the interaction between melanin and iron(III) ions. Interestingly, iron-containing synthetic melanins show very similar EPR spectra (Aime et al., 1997; Ferrari et al., 2013; Shima et al., 1997), regardless of the methods used for their preparation, and comparable to those of natural NM pigment. However, only a careful quantitative analysis enables to show that two different iron sites are present in the synthetic melanins (Ferrari et al., 2013), as well as in natural NM pigment: small iron oxy-hydroxy aggregates similar to those present in ferritin iron core, which are not EPR-detectable but detectable by Mössbauer spectroscopy (Zecca et al., 2001a), and mononuclear iron(III) ions (EPR-detectable), as observed in natural NM (Fig. 4). A difference in the amount of iron in the two sites was observed between eumelanins and pheomelanins, since pheomelanic moieties seem to favor the formation of mononuclear iron centers (Ferrari et al., 2013). The presence of two different sites has been also confirmed by magnetic susceptibility experiments, in which it was observed that the amount of mononuclear iron is higher in synthetic models in comparison with human NM (Bolzoni et al., 2002). The preparation of NM models has been very useful also to identify infrared peaks of the pigment due to the melanic component when using DA-melanins alone (Bridelli et al., 1999; Double et al., 2000), and the peaks due to peptides using albumin-melanins (Engelen et al., 2012). The comparison of these spectra with those of human NM led to the identification of several peaks assigned to lipids bound to NM.
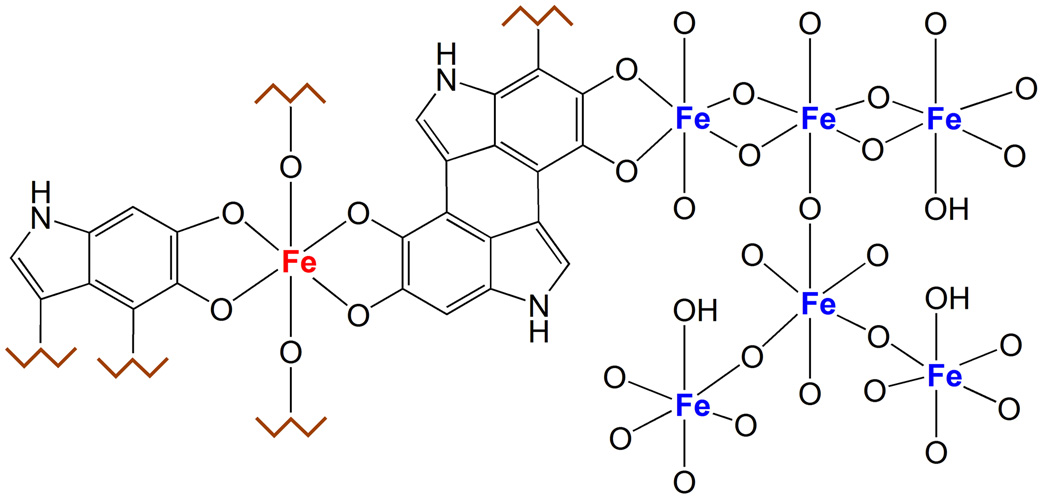
Fig. 4. Iron centers in NM pigment.
The figure shows the two types of iron centers present in the NM pigment. The multinuclear iron cluster, similarly to ferritin, contains iron(III) ions (blue colored in the figure) coupled by oxy-hydroxy bridges and surrounded by catechol groups of NM. In this site, iron is probably stored with high affinity and maintained in a redox inactive state, and is principally detected by Mössbauer spectroscopy. The other iron site consists of mononuclear centers, where iron (red colored) is coordinated by oxygen atoms of catechols moieties, and possibly by hydroxo groups. This could be a low affinity binding site occupied only in case of iron overload, when the high affinity centers are saturated; in iron overload condition as occurring in PD, the mononuclear iron could be still redox reactive and catalyze the production of toxic species, for example via the Fenton’s reaction. Iron in this site is principally detected by EPR spectroscopy.
Another structural comparison between human NM and its models was performed by 13C-nuclear magnetic resonance spectroscopy (Aime et al., 2000). In this work an albumin-melanin was compared with NM pigments isolated from SN of healthy subjects and PD patients. The spectrum of the albumin-melanin was more similar to that of NM pigment from the PD patients, suggesting the presence of a more extended protein portion in the pigment in the disease state.
In addition to the employment for investigating the NM structure and its physical properties, the synthetic analogues can help elucidate its reactivity and role. In particular, NM models provide insight into the reactivity of the iron ions bound to NM pigment. For instance, synthetic melanins, like human NM, lower the production of hydroxyl radicals by iron(III) (Zecca et al., 2008a). Furthermore, iron bound to synthetic melanins is not able to oxidize DA, suggesting that melanin can protect from oxidative stress by chelating metal ions present in solution (Zecca et al., 2008a). This occurs when iron is present in low amounts, but when the synthetic melanins are saturated with ferric ions, they promote the formation of hydroxyl radicals (Zareba et al., 1995). This is probably due to the presence of two iron sites with different redox activity or different accessibility to substrates, as discussed in the following chapter.
Additional studies are needed to obtain better NM models. Proteins other than albumin should be employed in the synthesis of NM models, possibly using neuronal proteins that could be present into natural NM. The availability of realistic models of NM with known composition and structure will provide the ability to conduct structure/activity experiments that establish which components of NM structure interact with receptors of microglia, and with neurons and cellular organelles to identify processes involved in brain aging and neurodegeneration.
8. Iron-NM complex as major iron compound in DA neurons
Human NM pigment has the ability to chelate various transition metal ions, in particular iron, copper and zinc (Bohic et al., 2008; Zecca and Swartz, 1993; Zecca et al., 1994, 1996, 2001b, c, 2008b). Iron is the most abundant of these metal ions, and of particular interest since NM is the main iron storage molecule in DA neurons of human SN (Fig. 5) (Zecca et al., 2001a, b, 2004b). Iron binding to the pigment prevents Fenton's reaction and ascorbate oxidation, as observed in in vitro experiments by using both human and synthetic NM (Zecca et al., 2008a). In the pigment isolated from SN of healthy subjects, the concentration of iron is about 10 µg/mg of NM pigment (Zecca et al., 2004b, 2008b; Zucca et al., 2006), although NM can bind even higher quantities of iron (Shima et al., 1997; Zecca et al., 1996, 2004b).
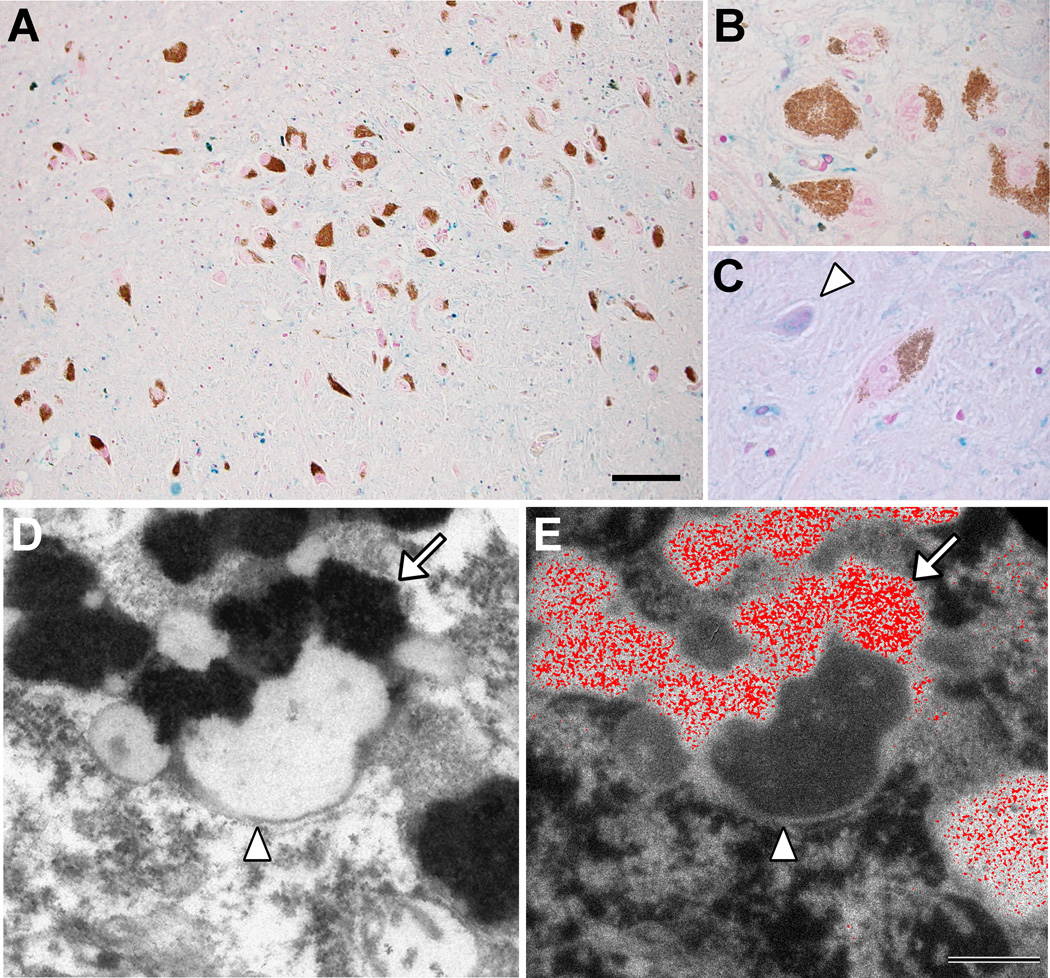
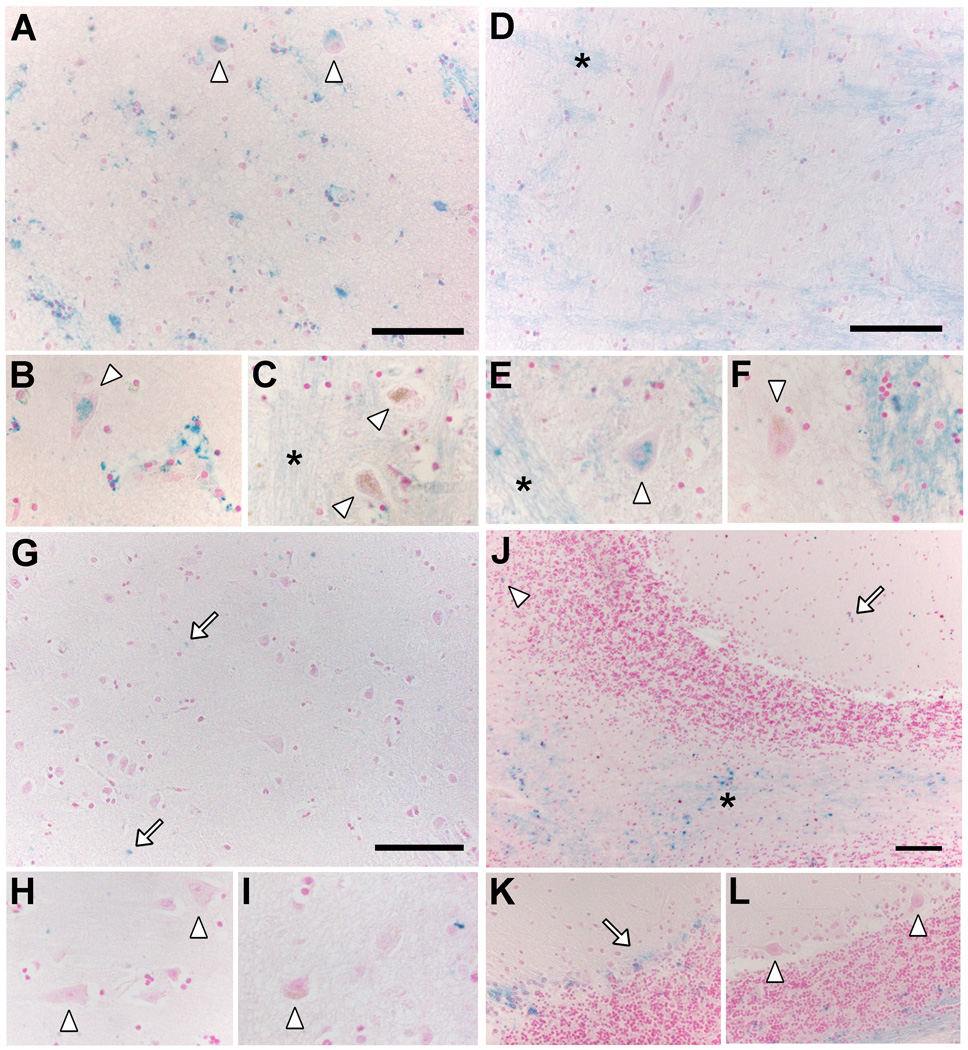
Fig. 5. Iron deposits in healthy aged SN.
(A, B, C) Histochemistry with modified Perls’ staining for detection of reactive ferric iron deposits (for details see Zecca et al., 2004b) in normal human SN tissue sections (88 years old). Many iron deposits are present in glial cells and are stained in blue: the overview (A, scale bar = 100 µm) shows that blue iron deposits are abundant in the whole SN parenchyma, while are absent in NM-containing neurons (brown-colored for the presence of NM pigment in the cytoplasm), as confirmed at higher magnification showing a group of NM-containing neurons (B). However NM-free neurons of the SN contain large amounts of iron deposits (arrowhead in C). Panels A, B, C are modified from Zucca and colleagues (2011) by permission of Royal Society of Chemistry Publishing. (D, E) NM-containing organelles of normal human SN (89 years old) observed using electron imaging. In the left panel (D), transmission electron microscopy shows the classical structure of NM-containing organelles present in SN neurons: these organelles contain large amount of dark NM pigment (arrow) closely associated with lipid bodies (arrowhead) as previously reported (Sulzer et al., 2008; Zecca et al., 2008b). The elemental iron distribution map obtained by electron spectroscopic imaging (E, scale bar = 500 nm) clearly reveals that large amounts of iron deposits are localized inside the NM pigment of the organelle (iron element is shown in red color). This unpublished finding strongly confirms the ability of NM pigment to scavenge iron forming stable NM-iron complexes. Electron spectroscopic imaging were performed by using a LEO 912AB electron microscope as described by Pezzati and colleagues (1997). For tissue treatments and ethics policies refer to: Zecca et al., 2008b; Engelen et al., 2012.
Two distinct iron-binding sites are present in the human NM pigment with different affinity for the metal; these have been classified as high and low affinity sites (Double et al., 2003; Fasano et al., 2006; Zecca et al., 2008a). The presence of two sites in the pigment from human SN was confirmed by means of EPR (Aime et al., 1997; Lopiano et al., 2000; Zecca and Swartz, 1993), magnetic susceptibility (Bolzoni et al., 2002), and Mössbauer spectroscopy (Double et al., 2003; Zecca et al., 2001a). Both sites bind iron(III), to a large extent as chelates with the oxygen donor atoms of the catechol groups present in the melanic portion of NM pigment (Enochs et al., 1993; Kropf et al., 1998). The identification and characterization of the high affinity and low affinity iron binding sites was also performed using synthetic models of NM pigment (Ben-Shachar et al., 1991; Bolzoni et al., 2002; Double et al., 2003; Ferrari et al., 2013). Nevertheless, the structural differences between high and low affinity sites are still unclear.
Data collected by Mössbauer spectroscopy on the pigment isolated from human SN indicate that one of the sites is similar to that of ferritin core (Galazka-Friedman et al., 1996; Gerlach et al., 1995, Zecca et al., 2001a), and the presence of this structure was confirmed by magnetic susceptibility measurements (Bolzoni et al., 2002). In this site, iron ions are coupled by oxy-hydroxy bridges in multinuclear complexes similar to that observed with ferritin. However, in the NM pigment iron(III) ions are bound to the catechols of eumelanin rather than amino acid residues, thus providing a different environment than ferritin. In addition, the iron domain inside NM seems to be significantly smaller than that of ferritin core (Aime et al., 1997; Bolzoni et al., 2002). Moreover, it seems that most of the NM-bound iron is in a ferritin-like cluster (Aime et al., 1997; Gerlach et al., 1995), although the techniques used cannot give accurate quantitative data. Thus, a reliable quantification is needed.
The other iron binding site was characterized by means of EPR. The EPR spectra of NM from human SN typically show a signal at g = 4.3 assigned to high spin iron(III), which is coordinated by oxygen atoms in mononuclear, rhombic, six-coordinated centers (Aime et al., 1997; Lopiano et al., 2000; Shima et al., 1997; Zecca and Swartz, 1993). A signal at g = 2.0 is also detected, indicating the presence of an organic radical delocalized on melanin (Aime et al., 1997; Enochs et al., 1993; Zecca and Swartz, 1993; Zecca et al., 1996, 2008b). Temperature-dependent measurements demonstrated an interaction between these two signals, indicating that, also in this case, iron is bound to the melanic portion of NM pigment. Interestingly, EPR spectra of human NM pigments treated with iron chelators showed a reduction in the intensity of the signal at g = 4.3 and/or an increase in the signal at g = 2.0 (Aime et al., 1997; Lopiano et al., 2000; Shima et al., 1997; Zecca et al., 1996). These results suggest that the iron bound in mononuclear centers can be extracted from NM pigment. The presence of an exchangeable iron inside NM pigment was also confirmed by infrared spectroscopy (Bridelli et al., 1999). Indeed, an increase of the amplitude in the absorption band of phenolic groups was detected after iron depletion by chelating agent ethylenediaminetetraacetic acid (Bridelli et al., 1999), indicating that the iron was bound to the oxygen atoms of the catechols of melanin. Nevertheless, this technique does not indicate whether the exchangeable iron was in the ferritin-like core or in the mononuclear iron cluster.
From EPR analyses on NM pigment after iron removal by a chelating agent, it appears that the iron stored in ferritin-like centers is likely bound with high affinity to the NM pigment, where it is not redox reactive (Aime et al., 1997; Lopiano et al., 2000; Shima et al., 1997; Zecca et al., 1996). This domain could therefore provide a safe storage system for iron within the neurons. Nevertheless, it may be that when the high affinity sites are saturated, as in case of iron overload observed in PD (as described in sections 9.1. and 10.2.), the iron ions also bind to the low affinity sites of the pigment by catechol residues in mononuclear centers, from where it can be easily released, causing iron-mediated toxicity.
In summary, iron is bound by the NM pigment in two types of sites: the first site is an oxy-hydroxy bridged iron(III) cluster surrounded by catechols groups of the melanic portion of NM pigment, while the second site is a mononuclear iron, directly coordinated by oxygen atoms of catechols moieties (Fig. 4). However, it is not completely clear which one of these sites coordinates iron with high or low affinity. In addition to the chemical structure of the iron centers, the accessibility of iron ions should also be considered. Future experiments aiming to characterize the NM iron-binding sites should take into account not only the structure of these centers but also their accessibility and reactivity towards small binding ligands and biological substrates.
9. NM and iron: neuroprotective and neurodegenerative effects
9.1. Neuroprotective roles of human NM
The synthesis of NM is itself a protective process, which is sustained by excess DA in the cytosol not accumulated by synaptic vesicles, as demonstrated in cell cultures experiments (Sulzer et al., 2000). DA in the cytosol can be oxidized to form DA-o-quinone via iron mediated catalysis and then can follow two different pathways: eumelanin or pheomelanin (Wakamatsu et al., 2003, 2012). Both these pathways concur to NM formation (Fig. 3), as discussed above in chapters 3 and 6. Without NM synthesis, the accumulation of cytosolic DA would induce the neurotoxic effects described in chapter 4. Analysis by photoelectron emission microscopy shows that the oxidation potential of human NM pigment corresponds to that of eumelanin, thus demonstrating that NM structure is composed of a pheomelanic core surrounded by eumelanin surface (Bush et al., 2006). This oxidation potential is low and not sufficiently reductive to generate a high level of oxidative processes and consequent neurodegenerative effects.
Human NM pigment can bind different metals forming stable complexes, blocking neurotoxic effects that could take place through redox reactions or other processes (Zecca and Swartz, 1993; Zecca et al., 1994, 1996, 2001b, c, 2008b). This functional property of NM pigment is due to the presence of dihydroxyindole groups within its structure, as discussed above. Iron is the most abundant metal contained in NM isolated from human SN and other brain areas, and this is of special interest due to its contribution to oxidative stress within neurons. It is interesting that NM in SN tissue is not completely saturated with iron, and is able to chelate higher quantities of this metal (Shima et al., 1997; Zecca et al., 1996, 2004b). Indeed, NM from SN can accumulate high amounts of iron, up to 55-fold higher than tissue concentrations (Zecca et al., 2008b).
As discussed above, NM appears to possess two types of iron binding sites. In conditions of iron overload, as in PD (Dexter et al., 1987, 1989; Earle 1968; Sofic et al., 1988), the high affinity sites of NM would be saturated, and iron could bind to the low affinity sites as well. In the latter sites, iron is bound into a low stability complex and could be easily released, playing a toxic role by participating to redox processes (Double et al., 2003) and oxidizing ascorbate, a major antioxidant system in brain cells (Rice 2000). The overload of iron on NM can also catalyze DA oxidation with formation of further DA-o-quinone, inducing oxidative modification of proteins with a consequent cascade of neurotoxic processes (Segura-Aguilar et al., 2014; Zecca et al., 2008a).
Under conditions of iron overload onto NM, the rate of NM degradation by hydrogen peroxide could increase as well (Zecca et al., 2008a). This process is likely to occur in microglia when NM released by dying neurons is phagocytosed and degraded, so that reactive iron (Zecca et al., 2008a; Zhang et al., 2011) and other toxins previously accumulated by NM (D'Amato et al., 1986; Karlsson and Lindquist, 2013; Lindquist et al., 1988; Oestergren et al., 2004; Salazar et al., 1978) are suddenly released and can cause cell death. An increased level of redox-active iron has been described in NM of SN from PD patients (Faucheux et al., 2003; Good et al., 1992; Jellinger et al., 1992). Thus, a protective role of NM may be hypothesized when iron is bound under physiological conditions, but a toxic effect of the NM-iron complex occurs when iron overload is present in the brain.
9.2. Neurodegenerative role of human NM
NM has long been suggested as a critical factor underlying neuronal vulnerability in PD (Hirsch et al., 1988; Marsden 1983). In the SN, the DA neurons containing NM appear to have different vulnerability in PD depending on their NM content. Some studies suggested that neurons with the highest NM content are more susceptible to death, while other studies reported that lightly pigmented neurons are more vulnerable in PD (Gibb 1992; Hirsch et al., 1988; Kastner et al., 1992; Mann and Yates, 1983; Pakkenberg et al., 1991). In any case, PD patients have a severe decrease of NM concentration in SN of about 60 % compared to the age matched controls (Zecca et al., 2002).
The intraneuronal role of NM in increasing neuronal vulnerability may be also related to the high content of MHC-I in NM-containing organelles of neurons in human SN and LC; these neurons that specifically degenerate in PD express MHC-I, while other neurons not targeted by PD have low or absent MHC-I expression. In SN and LC of PD subjects, CD8+ cells were sometimes observed in close proximity to neurons containing NM and expressing MHC-I, suggesting that they may trigger neuronal death. In cultured DA murine neurons, the expression of MHC-I can be induced by factors released from microglia activated by NM or by α-synuclein (both found extracellularly in PD brains), or alternatively induced by high cytosolic DA and its increase of oxidative stress. Then, MHC-I can bind antigenic peptides derived by cleavage of foreign or native proteins and present them on neuronal membrane, so that cytotoxic CD8+ lymphocytes target the neuron inducing degeneration (Cebrián et al., 2014). The presence of high number of infiltrating cells, in brains of both PD patients and mouse models of the disease, is likely to be due in part to increased BBB leakage, a conditions that occurs in aging and PD (Brochard et al., 2009; Farrall and Wardlaw, 2009; Kortekaas et al., 2005). Since NM also accumulates during aging, such a neuroinflammatory mechanism could provide a link between aging, NM, and PD. An in vitro study has reported that murine dendritic cells can develop a mature phenotype after the exposure to human NM (Oberländer et al., 2011). Dendritic cells phagocytized NM pigment and secreted interleukine-6 and tumor necrosis factor α, while triggering T cell proliferation. However, the density of dendritic cells in the SN is much lower than microglia, and their response to NM activation is slower than microglia: therefore, the role of dendritic cells in neuroinflammatory and neurodegenerative mechanisms of PD may be scarce compared to microglia.
NM likely contributes to the progression of neurodegenerative mechanisms by fueling a neuroinflammatory process that involves the activation of microglia (Block et al., 2007). Indeed, a typical feature in the parkinsonian SN is the occurrence of NM released from dying neurons in the extracellular space, together with intense microglial activation (Banati et al., 1998; Langston et al., 1999; McGeer et al., 1988). In vitro and in vivo experiments showed that activated microglia can induce neuronal death by releasing various proinflammatory molecules, ROS and reactive nitrogen species and by directly attacking neuronal bodies and processes (Zecca et al., 2008c; Zhang et al., 2011). Due to its insolubility, the released NM granules could remain for long periods in the extracellular space of the human SN, and provide a source of chronic inflammation: this extraneuronal NM moreover slowly releases to the extracellular milieu the toxic compounds adsorbed into the NM structure. In addition, the extracellular NM particles can be phagocytosed and degraded by activated microglia cells, with the consequence that the toxic compounds and redox active metals previously immobilized into NM can be released, which could further exacerbate microglial activation and neuronal damage, as demonstrated by several in vitro and in vivo models of PD (Zecca et al., 2008c; Zhang et al., 2011, 2013). Consistent with studies reporting an age-related loss of SN pigmented neurons, extraneuronal granules of NM have been reported in the SN of non-diseased elderly subjects in association with microglial activation (Beach et al., 2007).
In cell culture studies, the treatment of microglia cultures with human NM pigment produces chemotaxis: microglia cells move toward NM granules, actively phagocytose NM granules, and release neurotoxic molecules including tumor-necrosis factor α, interleukin 6, nitric oxide (Wilms et al., 2003) and reactive molecules such as superoxide and hydrogen peroxide (Zhang et al., 2011, 2013). Interestingly, the injection of NM into rat SN causes intense microglia activation and degeneration of DA neurons with a moderate astrocytosis (Zecca et al. 2008c; Zhang et al., 2011, 2013).
Mice-derived cell cultures studies clearly demonstrated that the neurodegeneration induced by NM-activated microglia likely involves macrophage antigen complex-1 and phagocytic oxidase of microglia (Zhang et al., 2011, 2013), although other factors could play a key role. The inflammatory molecules released from microglia activated by NM can diffuse through brain tissue, and induce vascular reaction with the consequent efflux of blood leukocytes including macrophages into the brain parenchyma, where these inflammatory molecules and leukocytes further extend the brain inflammation.
Then, it seems that human NM has a dual role: protective and/or toxic, depending mostly on the cellular context and conditions (Zecca et al., 2003; Zucca et al., 2014).
10. Iron in brain aging and PD
10.1. Iron changes in brain aging
The concentration of total iron increases during aging in human SN, putamen, globus pallidus, caudate nucleus, and cortices. However, it is not known why iron increases only in these regions (Hallgren and Sourander, 1958; Hebbrecht et al., 1999; Ramos et al., 2014; Zecca et al., 2004b). The distribution of total iron in normal adult human brain is inhomogeneous and the highest content is found in putamen, globus pallidus, and caudate nucleus, with lower concentrations in the cortical grey matter, white matter, midbrain (i.e., SN), and cerebellum, and the lowest iron concentrations are found in the pons, LC, and medulla (House et al., 2012; Ramos et al., 2014; Zecca et al., 1996, 2001b, c, 2004b). In aging, changes are observed in iron’s various molecular forms (ferritin, NM, transferrin, hemosiderin, and others) and in the distribution of iron molecules between neurons and glial cells (Connor et al., 1990; Zecca et al., 2001a, 2004b).
Several factors contribute to the increase of total iron concentration with aging, including BBB permeability, inflammatory state, redistribution of iron within the brain, and changes in iron homoeostasis (Conde and Streit, 2006; Farrall and Wardlaw, 2009). The accumulation of iron in neurons can directly induce cellular damage with the mechanisms reported in section 2.1. Glial iron accumulation in aging can damage neurons by inducing an inflammatory state through increased release of pro-inflammatory cytokines (Williams et al., 2012) and establishing neuroinflammation and neurodegeneration (Xu et al., 2012).
The changes in iron, NM and ferritins concentration in human SN and LC during aging have been carefully investigated (Zecca et al., 2001a, 2004b). These studies provide important reference values and mechanisms to understand the role of iron in PD neurodegeneration, which mainly targets catecholamine neurons of SN and LC. In healthy subjects, a linear increase in the total concentration of iron in the SN is found throughout life, and this is higher than that measured in the LC, where total iron concentration remains stable throughout life (Zecca et al., 2004b; Zucca et al., 2006). In the SN, the concentrations of ferritin heavy chain and ferritin light chain (the two isoforms of the iron-storage protein ferritin) increase in aging, while these are lower and remain constant in the LC; thus, iron may contribute to neurodegeneration more in the SN than the LC (Zecca et al., 2001a, 2004b; Zucca et al., 2006).
NM pigment is present in all types of neurons as NM-iron complexes, and the amount of iron bound to NM varies depending on the neurons in each human brain area (Zecca et al., 1996, 2001c, 2004b, 2008b). The NM-iron complex is the main iron compound in catecholaminergic neurons (as discussed in chapter 8) and its concentration, measured by accurate method for NM quantitation, increases during aging in the SN and LC, although the iron content in NM pigment from LC remains much lower than that found in NM from SN, consistently with the hypothesis that LC neurons are less vulnerable than SN neurons to iron-mediated damage (Zecca et al., 2004b; Zucca et al., 2006).
In healthy aged SN, histochemical investigations showed many deposits of reactive ferric iron by Perls’ staining in glial cells and in NM-free neurons, indicating that ferritin is not sufficiently expressed to chelate these amounts of iron (Zecca et al., 2004a, b; Zucca et al., 2006). In contrast, in NM-containing neurons, reactive ferric iron deposits were not detectable by Perls’ staining (Fig. 5), because iron is efficiently sequestered by NM into a stable complex, as shown by EPR and Mössbauer spectroscopy. Moreover, these spectroscopic methods have shown that NM in catecholamine neurons is not saturated with iron, even in elderly subjects where the highest accumulation of iron occurs in glial cells. This indicates that NM is an effective buffer of intraneuronal iron (Zecca et al., 1996, 2001a, 2004b). The content of NM-iron complexes increases with age also in neurons of the premotor cortex, putamen, and cerebellum; this is likely a consequence of increased iron content and mobilization taking place in these neurons during normal aging (Zecca et al., 2008b).
In the aged human brain, as discussed above, the putamen and globus pallidus have a high iron content and Perls’ staining shows many deposits of iron in fibers, glial cells and neurons without visible NM pigment, while neurons containing NM do not have detectable iron deposits. In cerebellum and premotor cortex, histochemically detected iron levels are lower than in putamen and globus pallidus. Both neurons and glia of cerebellum and premotor cortex are relatively free of iron deposits. In the cerebellum, numerous deposits of iron can be found by Perls’ staining in the white matter, in contrast with the relative sparsity observed in granular and molecular layers of the cerebellar cortex (Fig. 6). Thus, regions with high iron content have more histochemically detectable iron deposits in neurons and glia. This is consistent with previous reports of low total iron concentrations in LC tissue, where Perls' staining did not show any evident iron deposit in neurons and glial cells (Zecca et al., 2004a, b; Zucca et al., 2006, 2011).
Fig. 6. Iron deposits in different regions of healthy aged brain.
(A, B, C) Putamen of 71 years old subject. (A, scale bar = 100 µm). Reactive ferric iron deposits visualized by Perls’ histochemistry (for details see Zecca et al., 2004b) are clearly abundant in the whole parenchyma and principally localized in glial cells (diffuse blue staining) and fibers (asterisk in C). Iron deposits are clearly present in the cytoplasm of two neurons not containing NM (arrowhead in A), as confirmed at higher magnification (arrowhead in B). NM-containing neurons of putamen (brown-colored for the presence of NM pigment) do not show blue staining in their cytoplasm (arrowheads in C). (D, E, F) Globus pallidus of 71 years old subject. (D, scale bar = 100 µm). The iron distribution in the globus pallidus is similar to that observed in the putamen. Iron deposits are abundant and mainly present in glial cells (diffuse blue staining) and in fibers (asterisks in D and E). Cytoplasm of neurons not containing NM (arrowhead in E) is clearly positive for iron staining, while NM-containing neuron (slightly brown-colored for the presence of NM pigment) apparently does not contain iron deposits (arrowhead in F). (G, H, I) Premotor cortex of 72 years old subject. Iron deposits in the premotor cortex are fewer if compared to other previous brain areas (G, scale bar = 100 µm). Scarce iron deposits are mainly present in glial cells (arrows indicate some deposits in G) and are completely absent in both neurons without visible NM (arrowheads in H) and in NM-containing neurons (arrowhead in I, indicating a neuron with weak brown-colored pigmentation due to the presence of NM). (J, K, L) Cerebellum of 80 years old subject. (J, scale bar = 100 µm). Iron deposits are scattered in the different layers of the cerebellar architecture (J): the arrow indicates iron in the molecular layer, arrowhead in the granular layer and asterisk in the white matter. However the most intense staining is localized in the white matter of the cerebellum. Panels K and L show at higher magnification the Purkinje cells layer: iron staining is sometimes observed in the middle layer between molecular and granular layers (arrow in K), while Purkinje cells apparently do not contain iron deposits in their cytoplasm (arrowhead in L). The presence of NM pigments has been recently established by means of different techniques in neurons of putamen, globus pallidus, premotor cortex, cerebellum, and other human brain areas (Engelen et al., 2012; Zecca et al., 2008b). However, the concentration of NM pigment in these areas is lower than that observed in SN and LC (Zecca et al., 2004b, 2008b). For tissue treatments and ethics policies refer to: Zecca et al., 2008b; Engelen et al., 2012.
An increased pro-inflammatory situation is present in the brains of the elderly (Conde and Streit, 2006). The number of glial cells increase during normal aging of the human brain, and there is an elevated immunoreactivity of astrocytic and microglial markers (Conde and Streit, 2006). Infiltration of lymphocytes could be also present at the same time, representing an important risk factor for neurodegeneration (Brochard et al., 2009). There is also an augmented permeability of the BBB that increases with aging, and all these aging-related changes can lead to region-specific increases in brain iron (Block et al., 2007; Conde and Streit, 2006; Farrall and Wardlaw, 2009).
Iron deposits and ferritin content generally increase with age in microglia and astrocytes of the human cortex, cerebellum, hippocampus, basal ganglia, and amygdala. The largest amounts of iron and iron-binding proteins, such as ferritin and transferrin, are contained in oligodendrocytes (Connor et al., 1990). A group of ferritin-positive microglia cells with aberrant dystrophic-type morphology is found in aged human brain. The iron phagocytosed by the subpopulations of ferritin-positive microglia cells could be a source of toxic species inducing cellular degeneration. Dystrophic and ferritin-positive microglia might therefore contribute to the pathogenesis of neurodegenerative disorders due to altered microglial function (Lopes et al., 2008).
A range of aging processes can impair iron homeostasis mechanisms, so that significant amounts of iron are not sufficiently chelated by storage proteins or other molecules. A general phenomenon observed in brain aging is the chemical conversion of iron from the physiological molecular iron molecules, which are mainly soluble, into insoluble molecular forms of iron such as NM and hemosiderin.
10.2. Iron and PD
As discussed above, the basal ganglia are iron-enriched regions of the aged human brain and represent a privileged site of excessive iron accumulation in several neurodegenerative disorders, particularly PD (Rouault 2013; Ward et al., 2014). Interestingly, one of the first studies dealing with the occurrence of abnormal iron deposits in the brain of PD patients was performed at the beginning of the last century (Lhermitte et al., 1924). After that pioneering study, the literature on the connection between iron and PD has become immense, but with still open questions.
PD specifically affects the SN, and several studies have strongly demonstrated an increase of total iron concentration in the SN of PD patients, with peak iron levels apparently correlating with disease severity (Dexter et al., 1987, 1989, 1991; Earle 1968; Riederer et al., 1989; Sofic et al., 1988). The majority of studies have observed a consistent increase in total iron in SN of PD patients, using a variety of semi-quantitative and quantitative analytical techniques (Sian-Hülsmann et al., 2011). However some studies, especially those mainly using Mössbauer spectroscopy, did not report a significant increase in total iron levels in PD patients compared to healthy subjects (Galazka-Friedman 1996; Wypijewska et al., 2010): this discrepancy could be related to low accuracy of Mössbauer spectroscopy, or to inadequate procedures in sample preparation (Hare et al., 2012; Zecca et al., 1996). Similar discrepancies in iron contents have been reported in brain areas involved in disease progression other than SN, such as putamen and globus pallidus (Sian-Hülsmann et al., 2011).
Reasons for the high accumulation of iron in the SN of PD patients and increasing vulnerability of DA neurons have been debated for a long time, and various factors occurring in the disease have been suggested: i) increased permeability or dysfunction of BBB during the disease (Faucheux et al., 1999; Kortekaas et al., 2005); ii) increased pro-inflammatory state (Block et al., 2007; Conde and Streit, 2006; Gao and Hong, 2008), since neuroinflammation can perturb iron homeostasis on different brain cells as demonstrated in in vitro studies that used both mouse and rat cell cultures (Rathore et al., 2012; Urrutia et al., 2013); iii) increased expression of lactoferrin receptors (involved in iron uptake through lactoferrin) in neurons and microvessels of the mesencephalon, with higher lactoferrin receptor immunoreactivity in SN of PD patients with higher dopaminergic nigral loss (Faucheux et al., 1995); iv) increased expression of a specific isoform of the divalent metal ion transporter 1 in the SN neurons of PD patients (Salazar et al., 2008); v) decreased ferroxidase activity of ceruloplasmin (Ayton et al., 2013, Olivieri et al., 2011), an iron-export ferroxidase essential for brain iron metabolism, in both the SN and cerebrospinal fluid form PD patients; vi) dysregulation of transferrin/transferrin receptor type 2 mediated iron transport in dopaminergic SN neurons of PD patients, particularly in their mitochondria (Mastroberardino et al., 2009); vii) mutations in genes coding for proteins involved in iron transport, binding, and metabolism (Borie et al., 2002; Guerreiro et al., 2006; Hochstrasser et al., 2004). Some of these alterations occurring in PD patients have been also observed in animal models of PD (Mastroberardino et al., 2009; Salazar et al., 2008).
Redox-active iron was demonstrated to accumulate in human SN neurons Lewy bodies (Castellani et al., 2000), the pathological inclusions typical of PD which are mainly composed of α-synuclein (Goedert 2001). Moreover, in vitro studies showed that iron induces a conformational change of α-synuclein to the β-sheet conformation, forming fibrils that are present in Lewy bodies (el-Agnaf et al., 2002; Uversky et al., 2001). The accumulation of both α-synuclein and iron in the SN could in principle establish local conditions required for α-synuclein-mediated reactive species formation, as demonstrated in vitro by Turnbull and colleagues (2001): the formation of hydrogen peroxide from α-synuclein followed by its conversion to hydroxyl radicals via Fenton's reaction can establish the conditions for α-synuclein aggregation (Hashimoto et al., 1999).
The cellular distribution of iron has been also deeply investigated. It should be noted that iron content in cells may change when an iron-rich area, like SN, begins to degenerate. After neuronal degeneration, activated microglia and perivascular macrophages phagocytose debris and other cellular components (e.g., NM, iron, iron-containing proteins, etc.) released by degenerating cells (Andersen et al., 2014; Block et al., 2007; Zhang et al., 2011). As a consequence, these scavenger cells become iron rich and additionally in activated microglia iron homeostasis is perturbed as well as ferritin expression, as demonstrated in cell cultures (Cheepsunthorn et al., 2001; Rathore et al., 2012; Urrutia et al., 2013). Furthermore, dying microglia and macrophages can in turn release their iron thus enhancing iron-mediated oxidative stress of adjacent cells (Andersen et al., 2014). Semiquantitative histochemical methods showed that iron deposits were particularly increased in astrocytes, macrophages, reactive microglia cells associated with extracellular NM, and non-pigmented neurons of the SN of PD patients (Morris and Edwardson, 1994; Jellinger et al., 1990), together with an increase of ferritin reactivity in proliferating microglia (Jellinger et al., 1990).
The reported changes in ferritin immunoreactivity in the SN of PD patients are not consistent, likely due to differences in specificity of antibodies used. Up-regulation of ferritin expression was reported by a group of studies (Jellinger et al., 1990; Riederer et al., 1989), and this event could be a normal physiological response in case of high iron levels (Muckenthaler et al., 2008) like those occurring in PD. In contrast, several studies reported unchanged levels (Mann et al., 1994) or a decrease of SN ferritin levels in PD patients (Connor et al., 1995; Dexter et al., 1990, 1991). Increases of iron and decreases of ferritin levels are indicative of higher iron loading in SN ferritins of PD patients (Griffiths et al., 1999). Moreover, the decreased ferritin synthesis in PD may be due to the sustained activity of iron regulatory protein 1 observed in post-mortem PD brain (Faucheux et al., 2002).
The high levels of iron detected in SN of PD patients might exceed iron buffering capacity of complexes, such as NM and ferritins, thus leading to neurotoxicity. Interestingly, studies by means of different methodologies have reported that more iron accumulates within NM granules in SN pigmented neurons of patients with PD than in controls (Good et al., 1992; Jellinger et al., 1992). This NM-associated iron is redox-active, and was most increased in patients with the most severe neuronal loss (Faucheux et al., 2003). These findings suggest that an overload of redox-active iron in NM occurs during PD, thus enhancing oxidative stress and significantly contributing to the selective degeneration of NM-containing neurons of the SN.
11. Conclusions
A multitude of studies have attempted to determine the pathogenic steps involved in PD. However, an important series of neuroprotective pathways that serve to effectively block PD pathogenesis are frequently ignored. Over the past three decades, there has been increasing evidence that the dysregulation of cytosolic DA and reactive metals, most prominently iron, plays an important role in pathogenesis. If so, NM synthesis may provide such a protective pathway. Moreover, NM blocks reactive iron forming stable complex then preventing iron toxicity. Under conditions in which these steps are no longer sufficient or are disturbed, iron and NM may initiate a series of toxic and neuroinflammatory steps that build up a “vicious cycle” which sustains the progression of disease. Of these steps, the activation of neuroinflammation by iron and NM could be of particular importance, and is under investigation in many studies. An optimistic scenario suggested by the complex interplay of these multiple steps is that there are multiple possible points at which disease progression may be blocked, and so elucidating these pathways promises a range of future clinical directions to halt or modify the disease.
Highlights.
In this review the multiways interactions/modulations between iron, dopamine oxidation, neuromelanin and their role in brain are discussed.
Each of these components is discussed in details before describing the interactions among them.
The relationship between iron and dopamine oxidation with consequences on cells is presented.
An overview of synthesis and properties of peripheral melanins is given.
Neuromelanin synthesis, structure and interaction with iron are reviewed, considering their protective/toxic pathways in neurons.
The role of iron and neuromelanin and their possible detrimental effects in brain aging and Parkinson’s disease are appraised.
Acknowledgements
FAZ, EF, and LZ were supported by Italian Ministry of Education, University, and Research (MIUR) - National Research Programme (PNR) - CNR Flagship “InterOmics” Project (PB.P05), by PNR - CNR Aging program 2012–2014, and by Lombardy Region - CNR 2013–2015 Framework Agreement, MbMM Project (18089/RCC). LZ and LC also acknowledge the MIUR-Research Projects of National Interest (PRIN) 2010–2011 prot. 2010M2JARJ. LZ also thanks Grigioni Foundation for Parkinson’s disease, Milano, Italy. JSA, PM and IP were supported by FONDECYT 1100165. DS was supported by the Parkinson’s Disease and JPB Foundations, and NIH grants DA07418 and DA10154.
FAZ, EF and LZ also thank Dr. Maria Carla Panzeri (Advanced Light and Electron Microscopy BioImaging Center - San Raffaele Scientific Institute) for expert assistance in electron spectroscopic imaging, and Mrs. Chiara Bellei for her skillful technical assistance. Authors also thank the Section of Legal Medicine and Insurances, Department of Biomedical Sciences for Health at University of Milano for providing brain tissue samples.
Abbreviations
- BBB
blood-brain barrier
- DA
dopamine
- DOPAL
3,4-dihydroxyphenylacetaldehyde
- EPR
electron paramagnetic resonance
- L-DOPA
L-3,4-dihydroxyphenylalanine
- LC
locus coeruleus
- MHC-I
major histocompatibility complex class I
- NM
neuromelanin
- PD
Parkinson's disease
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- SN
substantia nigra
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas N, Lücking CB, Ricard S, Dürr A, Bonifati V, De Michele G, Bouley S, Vaughan JR, Gasser T, Marconi R, Broussolle E, Brefel-Courbon C, Harhangi BS, Oostra BA, Fabrizio E, Böhme GA, Pradier L, Wood NW, Filla A, Meco G, Denefle P, Agid Y, Brice A. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum. Mol. Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, Kadekaro AL. Human Pigmentation: Its regulation by Ultraviolet Light and by Endocrine, Paracrine and Autocrine Factors. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 410–420. [Google Scholar]
- Agrup G, Hansson C, Rorsman H, Rosengren E. The effect of cysteine on oxidation of tyrosine, dopa, and cysteinyldopas. Arch. Dermatol. Res. 1982;272:103–115. doi: 10.1007/BF00510400. [DOI] [PubMed] [Google Scholar]
- Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura-Aguilar J, Núñez MT. The dopamine metabolite aminochrome inhibits mitochondrial complex I and modifies the expression of iron transporters DMT1 and FPN1. Biometals. 2012;25:795–803. doi: 10.1007/s10534-012-9525-y. [DOI] [PubMed] [Google Scholar]
- Aime S, Bergamasco B, Biglino D, Digilio G, Fasano M, Giamello E, Lopiano L. EPR investigations of the iron domain in neuromelanin. Biochim. Biophys. Acta. 1997;1361:49–58. doi: 10.1016/s0925-4439(97)00014-8. [DOI] [PubMed] [Google Scholar]
- Aime S, Bergamasco B, Casu M, Digilio G, Fasano M, Giraudo S, Lopiano L. Isolation and 13C-NMR Characterization of an Insoluble Proteinaceous Fraction From Substantia Nigra of Patients With Parkinson’s Disease. Mov. Disord. 2000;15:977–981. doi: 10.1002/1531-8257(200009)15:5<977::aid-mds1032>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- Anand U, Mukherjee S. Binding, unfolding and refolding dynamics of serum albumins. Biochim. Biophys. Acta. 2013;1830:5394–5404. doi: 10.1016/j.bbagen.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Andersen HH, Johnsen KB, Moos T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol. Life Sci. 2014;71:1607–1622. doi: 10.1007/s00018-013-1509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NC. Molecular control of iron metabolism. Best Pract. Res. Clin. Haematol. 2005;18:159–169. doi: 10.1016/j.beha.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreguin S, Nelson P, Padway S, Shirazi M, Pierpont C. Dopamine complexes of iron in the etiology and pathogenesis of Parkinson's disease. J. Inorg. Biochem. 2009;103:87–93. doi: 10.1016/j.jinorgbio.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Arriagada C, Paris I, Sanchez de las Matas MJ, Martinez-Alvarado P, Cardenas S, Castañeda P, Graumann R, Perez-Pastene C, Olea-Azar C, Couve E, Herrero MT, Caviedes P, Segura-Aguilar J. On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol. Dis. 2004;16:468–477. doi: 10.1016/j.nbd.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Ayton S, Lei P, Duce JA, Wong BX, Sedjahtera A, Adlard PA, Bush AI, Finkelstein DI. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013;73:554–559. doi: 10.1002/ana.23817. [DOI] [PubMed] [Google Scholar]
- Badaracco ME, Siri MV, Pasquini JM. Oligodendrogenesis: the role of iron. Biofactors. 2010;36:98–102. doi: 10.1002/biof.90. [DOI] [PubMed] [Google Scholar]
- Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35:949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov. Disord. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Lue LF, Connor DJ, Caviness JN, Sabbagh MN, Adler CH. Marked microglial reaction in normal aging human substantia nigra: correlation with extraneuronal neuromelanin pigment deposits. Acta Neuropathol. 2007;114:419–424. doi: 10.1007/s00401-007-0250-5. [DOI] [PubMed] [Google Scholar]
- Beard J. Iron deficiency alters brain development and functioning. J. Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol. Biochem. Behav. 1994;48:621–624. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Riederer P, Youdim MB. Iron-melanin interaction and lipid peroxidation: implications for Parkinson's disease. J. Neurochem. 1991;57:1609–1614. doi: 10.1111/j.1471-4159.1991.tb06358.x. [DOI] [PubMed] [Google Scholar]
- Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J. Neurochem. 2008;106:205–215. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J. Biol. Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- Bisaglia M, Soriano ME, Arduini I, Mammi S, Bubacco L. Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: implications for mitochondrial dysfunction in Parkinson disease. Biochim. Biophys. Acta. 2010;1802:699–706. doi: 10.1016/j.bbadis.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Bissantz C, Kuhn B, Stahl M. A medicinal chemist's guide to molecular interactions. J. Med. Chem. 2010;53:5061–5084. doi: 10.1021/jm100112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bohic S, Murphy K, Paulus W, Cloetens P, Salomé M, Susini J, Double K. Intracellular chemical imaging of the developmental phases of human neuromelanin using synchrotron X-ray microspectroscopy. Anal. Chem. 2008;80:9557–9566. doi: 10.1021/ac801817k. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Hornyak TJ. Extracutaneous Melanocytes. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 91–107. [Google Scholar]
- Bolzoni F, Giraudo S, Lopiano L, Bergamasco B, Fasano M, Crippa PR. Magnetic investigations of human mesencephalic neuromelanin. Biochim. Biophys. Acta. 2002;1586:210–218. doi: 10.1016/s0925-4439(01)00099-0. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Borie C, Gasparini F, Verpillat P, Bonnet AM, Agid Y, Hetet G, Brice A, Dürr A, Grandchamp B French Parkinson's disease genetic study group. Association study between iron-related genes polymorphisms and Parkinson's disease. J. Neurol. 2002;249:801–804. doi: 10.1007/s00415-002-0704-6. [DOI] [PubMed] [Google Scholar]
- Boulton ME. Melanin and the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. pp. 68–85. [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bradbury MW. Transport of iron in the blood-brain-cerebrospinal fluid system. J. Neurochem. 1997;69:443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84:539–849. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridelli MG, Tampellini D, Zecca L. The structure of neuromelanin and its iron binding site studied by infrared spectroscopy. FEBS Lett. 1999;457:18–22. doi: 10.1016/s0014-5793(99)01001-7. [DOI] [PubMed] [Google Scholar]
- Brilliant MH. Molecular Regulation of Melanin Formation: Melanosome Transport Proteins. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 230–241. [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr. Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Bush VJ, Moyer TP, Batts KP, Parisi JE. Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin. Chem. 1995;41:284–294. [PubMed] [Google Scholar]
- Bush WD, Garguilo J, Zucca FA, Albertini A, Zecca L, Edwards GS, Nemanich RJ, Simon JD. The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4785–4789. doi: 10.1073/pnas.0604010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush WD, Garguilo J, Zucca FA, Bellei C, Nemanich RJ, Edwards GS, Zecca L, Simon JD. Neuromelanins isolated from different regions of the human brain exhibit a common surface photoionization threshold. Photochem. Photobiol. 2009;85:387–890. doi: 10.1111/j.1751-1097.2008.00476.x. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Byers HR. Melanosome Processing in Keratinocytes. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 181–190. [Google Scholar]
- Carstam R, Brinck C, Hindemith-Augustsson A, Rorsman H, Rosengren E. The neuromelanin of the human substantia nigra. Biochim. Biophys. Acta. 1991;1097:152–160. doi: 10.1016/0925-4439(91)90100-n. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Siedlak SL, Perry G, Smith MA. Sequestration of iron by Lewy bodies in Parkinson's disease. Acta Neuropathol. 2000;100:111–114. doi: 10.1007/s004010050001. [DOI] [PubMed] [Google Scholar]
- Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Caviedes P, Segura-Aguilar J. The price of development in Chile: overcoming environmental hazards produced by heavy industrial exploitation. Neuroreport. 2001;12:A25. [Google Scholar]
- Cebrián C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD, Sulzer D. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat. Commun. 2014;5:3633. doi: 10.1038/ncomms4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Cheng FC, Kuo JS, Chia LG, Dryhurst G. Elevated 5-S cysteinyldopamine/homovanillic acid ratio and reduced homovanillic acid in cerebrospinal fluid: possible markers for and potential insights into the pathoetiology of Parkinson's disease. J. Neural Transm. 1996;103:433–446. doi: 10.1007/BF01276419. [DOI] [PubMed] [Google Scholar]
- Cheng J, Moss SC, Eisner M, Zschack P. X-ray characterization of melanins--I. Pigment Cell Res. 1994a;7:255–262. doi: 10.1111/j.1600-0749.1994.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Moss SC, Eisner M. X-ray characterization of melanins--II. Pigment Cell Res. 1994b;7:263–273. doi: 10.1111/j.1600-0749.1994.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Clancy CM, Nofsinger JB, Hanks RK, Simon JD. A hierarchical self-assembly of eumelanin. J. Phys. Chem. B. 2000;104:7871–7873. [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J. Neuropathol. Exp. Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL. Cellular management of iron in the brain. J. Neurol. Sci. 1995;134(Suppl.):33–44. doi: 10.1016/0022-510x(95)00206-h. [DOI] [PubMed] [Google Scholar]
- Connor JR, Menzies SL, St Martin SM, Mufson EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J. Neurosci. Res. 1990;27:595–611. doi: 10.1002/jnr.490270421. [DOI] [PubMed] [Google Scholar]
- Crichton RR. Inorganic biochemistry of iron metabolism from molecular mechanisms to clinical consequences. Chichester: John Wiley & Sons; 2009. p. 461. 461. [Google Scholar]
- Crichton RR, Ward RJ. Metal based neurodegeneration: from molecular mechanisms to therapeutic strategies. 2nd edn. Chichester: John Wiley & Sons; 2014. [Google Scholar]
- Crichton RR, Dexter DT, Ward RJ. Brain iron metabolism and its perturbation in neurological diseases. J. Neural Transm. 2011;118:301–314. doi: 10.1007/s00702-010-0470-z. [DOI] [PubMed] [Google Scholar]
- Crippa PR, Eisner M, Morante S, Stellato F, Vicentin FC, Zecca L. An XAS study of the sulfur environment in human neuromelanin and its synthetic analogs. Eur. Biophys. J. 2010;39:959–970. doi: 10.1007/s00249-009-0462-9. [DOI] [PubMed] [Google Scholar]
- Cuevas C, Huenchuguala S, Muñoz P, Villa M, Paris I, Mannervik B, Segura-Aguilar J. Glutathione Transferase-M2-2 Secreted from Glioblastoma Cell Protects SH-SY5Y Cells from Aminochrome Neurotoxicity. Neurotox. Res. 2015;27:217–228. doi: 10.1007/s12640-014-9500-1. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A, Cassels BK, Baez S, Johansson AS, Mannervik B, Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem. Biophys. Res. Commun. 2000;274:32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003a;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003b;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- D'Amato RJ, Lipman ZP, Snyder SH. Selectivity of the parkinsonian neurotoxin MPTP: toxic metabolite MPP+ binds to neuromelanin. Science. 1986;231:987–989. doi: 10.1126/science.3080808. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2:1219–1220. doi: 10.1016/s0140-6736(87)91361-4. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J. Neurochem. 1989;52:1830–1830. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Vidailhet M, Ruberg M, Agid F, Agid Y, Lees AJ, Wells FR, Jenner P, Marsden CD. Decreased ferritin levels in brain in Parkinson's disease. J. Neurochem. 1990;55:16–20. doi: 10.1111/j.1471-4159.1990.tb08814.x. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Dibenedetto D, Rossetti G, Caliandro R, Carloni P. A molecular dynamics simulation-based interpretation of nuclear magnetic resonance multidimensional heteronuclear spectra of α synuclein•dopamine adducts. Biochemistry. 2013;52:6672–6683. doi: 10.1021/bi400367r. [DOI] [PubMed] [Google Scholar]
- Di Donato P, Napolitano A. 1,4-benzothiazines as key intermediates in the biosynthesis of red hair pigment pheomelanins. Pigment Cell Res. 2003;16:532–539. doi: 10.1034/j.1600-0749.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- d'Ischia M, Wakamatsu K, Napolitano A, Briganti S, Garcia-Borron JC, Kovacs D, Meredith P, Pezzella A, Picardo M, Sarna T, Simon JD, Ito S. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 2013;26:616–633. doi: 10.1111/pcmr.12121. [DOI] [PubMed] [Google Scholar]
- d'Ischia M, Napolitano A, Ball V, Chen CT, Buehler MJ. Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014;47:3541–3550. doi: 10.1021/ar500273y. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy AC, Outten CE. The iron metallome in eukaryotic organisms. Met. Ions Life Sci. 2013;12:241–278. doi: 10.1007/978-94-007-5561-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Zecca L, Costi P, Mauer M, Griesinger C, Ito S, Ben-Shachar D, Bringmann G, Fariello RG, Riederer P, Gerlach M. Structural characteristics of human substantia nigra neuromelanin and synthetic dopamine melanins. J. Neurochem. 2000;75:2583–2589. doi: 10.1046/j.1471-4159.2000.0752583.x. [DOI] [PubMed] [Google Scholar]
- Double KL, Gerlach M, Schünemann V, Trautwein AX, Zecca L, Gallorini M, Youdim MB, Riederer P, Ben-Shachar D. Iron-binding characteristics of neuromelanin of the human substantia nigra. Biochem. Pharmacol. 2003;66:489–494. doi: 10.1016/s0006-2952(03)00293-4. [DOI] [PubMed] [Google Scholar]
- Dzierzega-Lecznar A, Kurkiewicz S, Stepien K, Chodurek E, Riederer P, Gerlach M. Structural investigations of neuromelanin by pyrolysis-gas chromatography/mass spectrometry. J. Neural Transm. 2006;113:729–734. doi: 10.1007/s00702-005-0446-6. [DOI] [PubMed] [Google Scholar]
- Earle KM. Studies on Parkinson's disease including x-ray fluorescent spectroscopy of formalin fixed brain tissue. J. Neuropathol. Exp. Neurol. 1968;27:1–14. doi: 10.1097/00005072-196801000-00001. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Wahlster L, McLean PJ. Protein degradation pathways in Parkinson's disease: curse or blessing. Acta. Neuropathol. 2012a;124:153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, McLean PJ, Unni VK. Alpha-synuclein's degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy. 2012b;8:281–283. doi: 10.4161/auto.8.2.18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- el-Agnaf OM, Irvine GB. Aggregation and neurotoxicity of alpha-synuclein and related peptides. Biochem. Soc. Trans. 2002;30:559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- Engelen M, Vanna R, Bellei C, Zucca FA, Wakamatsu K, Monzani E, Ito S, Casella L, Zecca L. Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS One. 2012;7:e48490. doi: 10.1371/journal.pone.0048490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enochs WS, Nilges MJ, Swartz HM. Purified human neuromelanin, synthetic dopamine melanin as a potential model pigment, and the normal human substantia nigra: characterization by electron paramagnetic resonance spectroscopy. J. Neurochem. 1993;61:68–79. doi: 10.1111/j.1471-4159.1993.tb03538.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Exley C, House E, Polwart A, Esiri MM. Brain burdens of aluminum, iron, and copper and their relationships with amyloid-β pathology in 60 human brains. J. Alzheimers Dis. 2012;31:725–730. doi: 10.3233/JAD-2012-120766. [DOI] [PubMed] [Google Scholar]
- Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol. Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Fasano M, Bergamasco B, Lopiano L. Modifications of the iron-neuromelanin system in Parkinson's disease. J. Neurochem. 2006;96:909–916. doi: 10.1111/j.1471-4159.2005.03638.x. [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Nillesse N, Damier P, Spik G, Mouatt-Prigent A, Pierce A, Leveugle B, Kubis N, Hauw JJ, Agid Y, et al. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9603–9607. doi: 10.1073/pnas.92.21.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson's disease. Lancet. 1999;353:981–982. doi: 10.1016/S0140-6736(99)00641-8. [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Martin ME, Beaumont C, Hunot S, Hauw JJ, Agid Y, Hirsch EC. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson's disease. J. Neurochem. 2002;83:320–330. doi: 10.1046/j.1471-4159.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, Hirsch EC. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson's disease. J. Neurochem. 2003;86:1142–1148. doi: 10.1046/j.1471-4159.2003.01923.x. [DOI] [PubMed] [Google Scholar]
- Fedorow H, Pickford R, Hook JM, Double KL, Halliday GM, Gerlach M, Riederer P, Garner B. Dolichol is the major lipid component of human substantia nigra neuromelanin. J. Neurochem. 2005;92:990–995. doi: 10.1111/j.1471-4159.2004.02975.x. [DOI] [PubMed] [Google Scholar]
- Felix CC, Hyde JS, Sarna T, Sealy RC. Interactions of melanin with metal ions. Electron spin resonance evidence for chelate complexes of metal ions with free radicals. J. Am. Chem. Soc. 1978;100:3922–3926. [Google Scholar]
- Ferrari E, Engelen M, Monzani E, Sturini M, Girotto S, Bubacco L, Zecca L, Casella L. Synthesis and structural characterization of soluble neuromelanin analogs provides important clues to its biosynthesis. J. Biol. Inorg. Chem. 2013;18:81–93. doi: 10.1007/s00775-012-0951-7. [DOI] [PubMed] [Google Scholar]
- Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life. 2013;65:341–349. doi: 10.1002/iub.1150. [DOI] [PubMed] [Google Scholar]
- Foppoli C, Coccia R, Cini C, Rosei MA. Catecholamines oxidation by xanthine oxidase. Biochim. Biophys. Acta. 1997;1334:200–206. doi: 10.1016/s0304-4165(96)00093-1. [DOI] [PubMed] [Google Scholar]
- Fuentes P, Paris I, Nassif M, Caviedes P, Segura-Aguilar J. Inhibition of VMAT-2 and DT-diaphorase induce cell death in a substantia nigra-derived cell line--an experimental cell model for dopamine toxicity studies. Chem. Res. Toxicol. 2007;20:776–783. doi: 10.1021/tx600325u. [DOI] [PubMed] [Google Scholar]
- Gałazka-Friedman J, Bauminger ER, Friedman A, Barcikowska M, Hechel D, Nowik I. Iron in parkinsonian and control substantia nigra--a Mössbauer spectroscopy study. Mov. Disord. 1996;11:8–16. doi: 10.1002/mds.870110104. [DOI] [PubMed] [Google Scholar]
- Gallas JM, Littrell KC, Seifert S, Zajac GW, Thiyagarajan P. Solution structure of copper ion-induced molecular aggregates of tyrosine melanin. Biophys. J. 1999;77:1135–1142. doi: 10.1016/S0006-3495(99)76964-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzigna L, Schiappelli MP, Rigo A, Scarpa M. A rat brain fraction and different purified peroxidases catalyzing the formation of dopaminochrome from dopamine. Biochim Biophys Acta. 1999;1427:329–336. doi: 10.1016/s0304-4165(99)00037-9. [DOI] [PubMed] [Google Scholar]
- Galzigna L, De Iuliis A, Zanatta L. Enzymatic dopamine peroxidation in substantia nigra of human brain. Clin. Chim. Acta. 2000;300:131–138. doi: 10.1016/s0009-8981(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Borrón JC, Solano F. Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002;15:162–173. doi: 10.1034/j.1600-0749.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Trautwein AX, Zecca L, Youdim MB, Riederer P. Mössbauer spectroscopic studies of purified human neuromelanin isolated from the substantia nigra. J. Neurochem. 1995;65:923–926. doi: 10.1046/j.1471-4159.1995.65020923.x. [DOI] [PubMed] [Google Scholar]
- Gibb WR. Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson's disease. Brain Res. 1992;581:283–291. doi: 10.1016/0006-8993(92)90719-p. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson's disease. J. Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014;144:268–282. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good PF, Olanow CW, Perl DP. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson's disease: a LAMMA study. Brain Res. 1992;593:343–346. doi: 10.1016/0006-8993(92)91334-b. [DOI] [PubMed] [Google Scholar]
- Greco G, Panzella L, Gentile G, Errico ME, Carfagna C, Napolitano A, d'Ischia M. A melanin-inspired pro-oxidant system for dopa(mine) polymerization: mimicking the natural casing process. Chem. Commun. (Camb) 2011;47:10308–10310. doi: 10.1039/c1cc13731j. [DOI] [PubMed] [Google Scholar]
- Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Dobson BR, Jones GR, Clarke DT. Iron in the basal ganglia in Parkinson's disease. An in vitro study using extended X-ray absorption fine structure and cryo-electron microscopy. Brain. 1999;122:667–673. doi: 10.1093/brain/122.4.667. [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Bras JM, Santana I, Januario C, Santiago B, Morgadinho AS, Ribeiro MH, Hardy J, Singleton A, Oliveira C. Association of HFE common mutations with Parkinson's disease, Alzheimer's disease and mild cognitive impairment in a Portuguese cohort. BMC Neurol. 2006;6:24. doi: 10.1186/1471-2377-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Halprin KM. Epidermal "turnover time"--a re-examination. Br. J. Dermatol. 1972;86:14–19. doi: 10.1111/j.1365-2133.1972.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Hare DJ, Gerlach M, Riederer P. Considerations for measuring iron in post-mortem tissue of Parkinson's disease patients. J. Neural Transm. 2012;119:1515–1521. doi: 10.1007/s00702-012-0898-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J. Neurochem. 1995;64:919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Hattori N, Sato S. Animal models of Parkinson's disease: similarities and differences between the disease and models. Neuropathology. 2007;27:479–483. doi: 10.1111/j.1440-1789.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- Hattori N, Kitada T, Matsumine H, Asakawa S, Yamamura Y, Yoshino H, Kobayashi T, Yokochi M, Wang M, Yoritaka A, Kondo T, Kuzuhara S, Nakamura S, Shimizu N, Mizuno Y. Molecular genetic analysis of a novel Parkin gene in Japanese families with autosomal recessive juvenile parkinsonism: evidence for variable homozygous deletions in the Parkin gene in affected individuals. Ann. Neurol. 1998;44:935–941. doi: 10.1002/ana.410440612. [DOI] [PubMed] [Google Scholar]
- Hauser DN, Hastings TG. Mitochondrial dysfunction and oxidative stress in Parkinson's disease and monogenic parkinsonism. Neurobiol. Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser DN, Dukes AA, Mortimer AD, Hastings TG. Dopamine quinone modifies and decreases the abundance of the mitochondrial selenoprotein glutathione peroxidase 4. Free Radic. Biol. Med. 2013;65:419–427. doi: 10.1016/j.freeradbiomed.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson's disease. Parkinsonism Relat. Disord. 2010;16:79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. The Regulation of Melanin Formation. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 191–212. [Google Scholar]
- Hebbrecht G, Maenhaut W, De Reuck J. Brain trace elements and aging. Nucl. Instrum. Methods Phys. Res. B. 1999;150:208–213. [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hochstrasser H, Bauer P, Walter U, Behnke S, Spiegel J, Csoti I, Zeiler B, Bornemann A, Pahnke J, Becker G, Riess O, Berg D. Ceruloplasmin gene variations and substantia nigra hyperechogenicity in Parkinson disease. Neurology. 2004;63:1912–1917. doi: 10.1212/01.wnl.0000144276.29988.c3. [DOI] [PubMed] [Google Scholar]
- House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer's disease. J. Alzheimers. Dis. 2004;6:291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- House E, Esiri M, Forster G, Ince PG, Exley C. Aluminium, iron and copper in human brain tissues donated to the Medical Research Council's Cognitive Function and Ageing Study. Metallomics. 2012;4:56–65. doi: 10.1039/c1mt00139f. [DOI] [PubMed] [Google Scholar]
- Huenchuguala S, Muñoz P, Zavala P, Villa M, Cuevas C, Ahumada U, Graumann R, Nore BF, Couve E, Mannervik B, Paris I, Segura-Aguilar J. Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy. 2014;10:618–630. doi: 10.4161/auto.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto K, Nagatsu I, Ito S, King RA, Nishimura A, Nagatsu T. Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 1998;253:198–200. doi: 10.1016/s0304-3940(98)00649-1. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ito S, Kimura E, Nabeshima M, Takayama M, Ishii T. Chemical Analysis of Melanin in Guinea Pig Inner Ear. Ear Res. Japan. 1984;16:68–71. [Google Scholar]
- Ito S. Encapsulation of a reactive core in neuromelanin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14647–14648. doi: 10.1073/pnas.0606879103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K. Chemistry of melanins. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 282–310. [Google Scholar]
- Ito S, Wakamatsu K. Chemistry of mixed melanogenesis--pivotal roles of dopaquinone. Photochem. Photobiol. 2008;84:582–592. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Pilat A, Gerwat W, Skumatz CM, Ito M, Kiyono A, Zadlo A, Nakanishi Y, Kolbe L, Burke JM, Sarna T, Wakamatsu K. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013;26:357–366. doi: 10.1111/pcmr.12078. [DOI] [PubMed] [Google Scholar]
- Jellinger K, Paulus W, Grundke-Iqbal I, Riederer P, Youdim MB. Brain iron and ferritin in Parkinson's and Alzheimer's diseases. J. Neural Transm. Park. Dis. Dement. Sect. 1990;2:327–340. doi: 10.1007/BF02252926. [DOI] [PubMed] [Google Scholar]
- Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben-Shachar D, Youdim MB. Iron-melanin complex in substantia nigra of parkinsonian brains: an x-ray microanalysis. J. Neurochem. 1992;59:1168–1171. doi: 10.1111/j.1471-4159.1992.tb08362.x. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Quevedo WC, Fitzpatrick TB, Szabo G. Biology of Melanocytes. In: Freeberg IA, Eisen AZ, Wolf K, Austen KF, Goldsimth LA, Katz SI, Fitzpatrick TB, editors. Fiotzpatrick’s Dermatology in General Medicine. 5th edition. New York: McGraw Hill; 1999. pp. 192–220. [Google Scholar]
- Jimenez M, Garcia-Carmona F, Garcia-Canovas F, Iborra JL, Lozano JA, Martinez F. Chemical intermediates in dopamine oxidation by tyrosinase, and kinetic studies of the process. Arch. Biochem. Biophys. 1984;235:438–448. doi: 10.1016/0003-9861(84)90217-0. [DOI] [PubMed] [Google Scholar]
- Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J, Gibson JM, Ross OA, Lynch T, Wiley J, Payami H, Nutt J, Maraganore DM, Czyzewski K, Styczynska M, Wszolek ZK, Farrer MJ, Toft M. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczara P, Zaręba M, Herrnreiter A, Skumatz CM, Ządło A, Sarna T, Burke JM. Melanosome-iron interactions within retinal pigment epithelium-derived cells. Pigment Cell Melanoma Res. 2012;25:804–814. doi: 10.1111/pcmr.12008. [DOI] [PubMed] [Google Scholar]
- Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic. Biol. Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann. Neurol. 2013;73:155–169. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O, Lindquist NG. Melanin affinity and its possible role in neurodegeneration. J. Neural Transm. 2013;120:1623–1630. doi: 10.1007/s00702-013-1062-5. [DOI] [PubMed] [Google Scholar]
- Kastner A, Hirsch EC, Lejeune O, Javoy-Agid F, Rascol O, Agid Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson's disease related to their neuromelanin content? J. Neurochem. 1992;59:1080–1089. doi: 10.1111/j.1471-4159.1992.tb08350.x. [DOI] [PubMed] [Google Scholar]
- Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog. Neurobiol. 2007;83:149–173. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kolberg M, Strand KR, Graff P, Andersson KK. Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta. 2004;1699:1–34. doi: 10.1016/j.bbapap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J. Photochem. Photobiol. B. 1991;9:135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Korytowski W, Sarna T, Zareba M. Antioxidant action of neuromelanin: the mechanism of inhibitory effect on lipid peroxidation. Arch. Biochem. Biophys. 1995;319:142–148. doi: 10.1006/abbi.1995.1276. [DOI] [PubMed] [Google Scholar]
- Kropf AJ, Bunker BA, Eisner M, Moss SC, Zecca L, Stroppolo A, Crippa PR. X-ray absorption fine-structure spectroscopy studies of Fe sites in natural human neuromelanin and synthetic analogues. Biophys. J. 1998;75:3135–3142. doi: 10.1016/S0006-3495(98)77755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat. Res. 2003;531:81–92. doi: 10.1016/j.mrfmmm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Kwok JB. Role of epigenetics in Alzheimer's and Parkinson's disease. Epigenomics. 2010;2 doi: 10.2217/epi.10.43. 671-282. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine exposure. Ann. Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Lhermitte J, Kraus WM, McAlpine D. Original Papers: ON THE OCCURRENCE OF ABNORMAL DEPOSITS OF IRON IN THE BRAIN IN PARKINSONISM WITH SPECIAL REFERENCE TO ITS LOCALISATION. J. Neurol. Psychopathol. 1924;5:195–208. doi: 10.1136/jnnp.s1-5.19.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WJ, Jiang H, Song N, Xie JX. Dose- and time-dependent alpha-synuclein aggregation induced by ferric iron in SK-N-SH cells. Neurosci. Bull. 2010;26:205–210. doi: 10.1007/s12264-010-1117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: human midbrain dopamine neurons. J. Comp. Neurol. 2004;473:97–106. doi: 10.1002/cne.20098. [DOI] [PubMed] [Google Scholar]
- Lindquist NG, Larsson BS, Lydén-Sokolowski A. Autoradiography of [14C]paraquat or [14C]diquat in frogs and mice: accumulation in neuromelanin. Neurosci. Lett. 1988;93:1–6. doi: 10.1016/0304-3940(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Linert W, Herlinger E, Jameson RF, Kienzl E, Jellinger K, Youdim MB. Dopamine, 6-hydroxydopamine, iron, and dioxygen--their mutual interactions and possible implication in the development of Parkinson's disease. Biochim. Biophys. Acta. 1996;1316:160–168. doi: 10.1016/0925-4439(96)00020-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu. Rev. Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- Liu Y, Simon JD. The effect of preparation procedures on the morphology of melanin from the ink sac of Sepia officinalis. Pigment Cell Res. 2003;16:72–80. doi: 10.1034/j.1600-0749.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Simon JD. Metal-ion interactions and the structural organization of Sepia eumelanin. Pigment Cell Res. 2005;18:42–48. doi: 10.1111/j.1600-0749.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hong L, Kempf VR, Wakamatsu K, Ito S, Simon JD. Ion-exchange and adsorption of Fe(III) by Sepia melanin. Pigment Cell Res. 2004;17:262–269. doi: 10.1111/j.1600-0749.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hong L, Wakamatsu K, Ito S, Adhyaru B, Cheng CY, Bowers CR, Simon JD. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem. Photobiol. 2005;81:135–144. doi: 10.1562/2004-08-03-RA-259.1. [DOI] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia. 2008;56:1048–1060. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Lopiano L, Chiesa M, Digilio G, Giraudo S, Bergamasco B, Torre E, Fasano M. Q-band EPR investigations of neuromelanin in control and Parkinson's disease patients. Biochim. Biophys. Acta. 2000;1500:306–312. doi: 10.1016/s0925-4439(99)00116-7. [DOI] [PubMed] [Google Scholar]
- Lozano J, Muñoz P, Nore BF, Ledoux S, Segura-Aguilar J. Stable expression of short interfering RNA for DT-diaphorase induces neurotoxicity. Chem. Res. Toxicol. 2010;23:1492–1496. doi: 10.1021/tx100182a. [DOI] [PubMed] [Google Scholar]
- Makin OS, Serpell LC. Structures for amyloid fibrils. FEBS J. 2005;272:5950–5961. doi: 10.1111/j.1742-4658.2005.05025.x. [DOI] [PubMed] [Google Scholar]
- Manini P, Panzella L, Napolitano A, d'Ischia M. Oxidation chemistry of norepinephrine: partitioning of the O-quinone between competing cyclization and chain breakdown pathways and their roles in melanin formation. Chem. Res. Toxicol. 2007;20:1549–1555. doi: 10.1021/tx700254q. [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO. Possible role of neuromelanin in the pathogenesis of Parkinson's disease. Mech. Ageing Dev. 1983;21:193–203. doi: 10.1016/0047-6374(83)90074-x. [DOI] [PubMed] [Google Scholar]
- Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH. Complex I, iron, and ferritin in Parkinson's disease substantia nigra. Ann. Neurol. 1994;36:876–881. doi: 10.1002/ana.410360612. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Neuromelanin and Parkinson's disease. J. Neural. Transm. Suppl. 1983;19:121–141. [PubMed] [Google Scholar]
- Martinez-Vicente M, Vila M. Alpha-synuclein and protein degradation pathways in Parkinson's disease: a pathological feed-back loop. Exp. Neurol. 2013;247:308–313. doi: 10.1016/j.expneurol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, Greenamyre JT. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson's disease. Neurobiol. Dis. 2009;34:417–431. doi: 10.1016/j.nbd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattammal MB, Strong R, Lakshmi VM, Chung HD, Stephenson AH. Prostaglandin H synthetase-mediated metabolism of dopamine: implication for Parkinson's disease. J. Neurochem. 1995;64:1645–1654. doi: 10.1046/j.1471-4159.1995.64041645.x. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGinness JE. Mobility gaps: a mechanism for band gaps in melanins. Science. 1972;177:896–897. doi: 10.1126/science.177.4052.896. [DOI] [PubMed] [Google Scholar]
- McGinness J, Corry P, Proctor P. Amorphous semiconductor switching in melanins. Science. 1974;183:853–855. doi: 10.1126/science.183.4127.853. [DOI] [PubMed] [Google Scholar]
- Melis JP, van Steeg H, Luijten M. Oxidative DNA damage and nucleotide excision repair. Antioxid. Redox Signal. 2013;18:2409–2419. doi: 10.1089/ars.2012.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado G, Valdés P, Hetz C. An ERcentric view of Parkinson's disease. Trends. Mol. Med. 2013;19:165–175. doi: 10.1016/j.molmed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006;19:572–594. doi: 10.1111/j.1600-0749.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM. Physiology and pathophysiology of inner ear melanin. Pigment Cell Res. 1988;1:238–249. doi: 10.1111/j.1600-0749.1988.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Mills E, Dong XP, Wang F, Xu H. Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med. Chem. 2010;2:51–64. doi: 10.4155/fmc.09.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J. Neurochem. 2007;103:1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- Morris CM, Edwardson JA. Iron histochemistry of the substantia nigra in Parkinson's disease. Neurodegeneration. 1994;3:277–282. [PubMed] [Google Scholar]
- Mostert AB, Powell BJ, Pratt FL, Hanson GR, Sarna T, Gentle IR, Meredith P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. U. S. A. 2012;109:8943–8947. doi: 10.1073/pnas.1119948109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- Mullin S, Schapira A. α-Synuclein and mitochondrial dysfunction in Parkinson's disease. Mol. Neurobiol. 2013;47:587–597. doi: 10.1007/s12035-013-8394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J. Gastroenterol. 2009;15:4617–4626. doi: 10.3748/wjg.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinsons Dis. 2012a;2012:920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Paris I, Sanders LH, Greenamyre JT, Segura-Aguilar J. Overexpression of VMAT-2 and DT-diaphorase protects substantia nigra-derived cells against aminochrome neurotoxicity. Biochim. Biophys. Acta. 2012b;1822:1125–1136. doi: 10.1016/j.bbadis.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P, Cardenas S, Huenchuguala S, Briceño A, Couve E, Paris I, Segura-Aguilar J. DT Diaphorase prevents aminochrome-induced alpha-synuclein oligomer formation and neurotoxicity. Toxicol. Sci. 2015;145:37–47. doi: 10.1093/toxsci/kfv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano A, De Lucia M, Panzella L, d'Ischia M. The "benzothiazine" chromophore of pheomelanins: a reassessment. Photochem. Photobiol. 2008;84:593–599. doi: 10.1111/j.1751-1097.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Manini P, d'Ischia M. Oxidation chemistry of catecholamines and neuronal degeneration: an update. Curr. Med. Chem. 2011;18:1832–1845. doi: 10.2174/092986711795496863. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J. Biol. Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- Oberländer U, Pletinckx K, Döhler A, Müller N, Lutz MB, Arzberger T, Riederer P, Gerlach M, Koutsilieri E, Scheller C. Neuromelanin is an immune stimulator for dendritic cells in vitro. BMC Neurosci. 2011;12:116. doi: 10.1186/1471-2202-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri S, Conti A, Iannaccone S, Cannistraci CV, Campanella A, Barbariga M, Codazzi F, Pelizzoni I, Magnani G, Pesca M, Franciotta D, Cappa SF, Alessio M. Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J. Neurosci. 2011;31:18568–18577. doi: 10.1523/JNEUROSCI.3768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega R, Cloetens P, Devès G, Carmona A, Bohic S. Iron storage within dopamine neurovesicles revealed by chemical nano-imaging. PLoS One. 2007;2:e925. doi: 10.1371/journal.pone.0000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergren A, Annas A, Skog K, Lindquist NG, Brittebo EB. Long-term retention of neurotoxic beta-carbolines in brain neuromelanin. J. Neural Transm. 2004;111:141–157. doi: 10.1007/s00702-003-0080-0. [DOI] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J. Neurol. Neurosurg. Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris I, Dagnino-Subiabre A, Marcelain K, Bennett LB, Caviedes P, Caviedes R, Azar CO, Segura-Aguilar J. Copper neurotoxicity is dependent on dopamine-mediated copper uptake and one-electron reduction of aminochrome in a rat substantia nigra neuronal cell line. J. Neurochem. 2001;77:519–529. doi: 10.1046/j.1471-4159.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- Paris I, Martinez-Alvarado P, Cárdenas S, Perez-Pastene C, Graumann R, Fuentes P, Olea-Azar C, Caviedes P, Segura-Aguilar J. Dopamine-dependent iron toxicity in cells derived from rat hypothalamus. Chem. Res. Toxicol. 2005a;18:415–419. doi: 10.1021/tx0497144. [DOI] [PubMed] [Google Scholar]
- Paris I, Martinez-Alvarado P, Perez-Pastene C, Vieira MN, Olea-Azar C, Raisman-Vozari R, Cardenas S, Graumann R, Caviedes P, Segura-Aguilar J. Monoamine transporter inhibitors and norepinephrine reduce dopamine-dependent iron toxicity in cells derived from the substantia nigra. J. Neurochem. 2005b;92:1021–1032. doi: 10.1111/j.1471-4159.2004.02931.x. [DOI] [PubMed] [Google Scholar]
- Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Muñoz P, Couve E, Caviedes P, Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox. Res. 2010;18:82–92. doi: 10.1007/s12640-009-9148-4. [DOI] [PubMed] [Google Scholar]
- Paris I, Muñoz P, Huenchuguala S, Couve E, Sanders LH, Greenamyre JT, Caviedes P, Segura-Aguilar J. Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line. Toxicol. Sci. 2011;121:376–388. doi: 10.1093/toxsci/kfr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RM. Neurobehavioral deficits and parkinsonism in occupations with manganese exposure: a review of methodological issues in the epidemiological literature. Saf. Health Work. 2013;4:123–135. doi: 10.1016/j.shaw.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles DN, Hong L, Hu DN, Ito S, Nemanich RJ, Simon JD. Human iridal stroma melanosomes of varying pheomelanin contents possess a common eumelanic outer surface. J. Phys. Chem. B. 2009;113:11346–11351. doi: 10.1021/jp904138n. [DOI] [PubMed] [Google Scholar]
- Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxid. Redox Signal. 2012;17:1590–1609. doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzati R, Bossi M, Podini P, Meldolesi J, Grohovaz F. High-resolution calcium mapping of the endoplasmic reticulum-Golgi-exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol. Biol. Cell. 1997;8:1501–1512. doi: 10.1091/mbc.8.8.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilas B, Sarna T, Kalyanaraman B, Swartz HM. The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radic. Biol. Med. 1988;4:285–293. doi: 10.1016/0891-5849(88)90049-4. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa, S,, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Prota G. Melanins, melanogenesis and melanocytes: looking at their functional significance from the chemist's viewpoint. Pigment Cell Res. 2000;13:283–293. doi: 10.1034/j.1600-0749.2000.130412.x. [DOI] [PubMed] [Google Scholar]
- Prota G, Hu DN, Vincensi MR, McCormick SA, Napolitano A. Characterization of melanins in human irides and cultured uveal melanocytes from eyes of different colors. Exp. Eye Res. 1998;67:293–299. doi: 10.1006/exer.1998.0518. [DOI] [PubMed] [Google Scholar]
- Quevedo WC, Jr, Holstein TJ. General Biology of Mammalian Pigmentation. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 63–90. [Google Scholar]
- Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Ramos P, Santos A, Pinto NR, Mendes R, Magalhães T, Almeida A. Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J. Trace. Elem. Med. Biol. 2014;28:13–17. doi: 10.1016/j.jtemb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Rathore KI, Redensek A, David S. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia. 2012;60:738–750. doi: 10.1002/glia.22303. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Rohn TT. Targeting alpha-synuclein for the treatment of Parkinson's disease. CNS Neurol. Disord. Drug Targets. 2012;11:174–179. doi: 10.2174/187152712800269678. [DOI] [PubMed] [Google Scholar]
- Romeo S, Viaggi C, Di Camillo D, Willis AW, Lozzi L, Rocchi C, Capannolo M, Aloisi G, Vaglini F, Maccarone R, Caleo M, Missale C, Racette BA, Corsini GU, Maggio R. Bright light exposure reduces TH-positive dopamine neurons: implications of light pollution in Parkinson's disease epidemiology. Sci. Rep. 2013;3:1395. doi: 10.1038/srep01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren E, Linder-Eliasson E, Carlsson A. Detection of 5-S-cysteinyldopamine in human brain. J. Neural. Transm. 1985;63:247–253. doi: 10.1007/BF01252029. [DOI] [PubMed] [Google Scholar]
- Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis. Model Mech. 2012;5:155–164. doi: 10.1242/dmm.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci. 2013;14:551–564. doi: 10.1038/nrn3453. [DOI] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain iron metabolism. Semin. Pediatr. Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Rouault TA, Zhang DL, Jeong SY. Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab. Brain Dis. 2009;24:673–684. doi: 10.1007/s11011-009-9169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rózanowska M, Sarna T, Land EJ, Truscott TG. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic. Biol. Med. 1999;26:518–525. doi: 10.1016/s0891-5849(98)00234-2. [DOI] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nuñez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Sokoloski TD, Patil PN. Binding of dopaminergic drugs by the neuromelanin of the substantia nigra, synthetic melanins and melanin granules. Fed Proc. 1978;37:2403–2407. [PubMed] [Google Scholar]
- Salonen LM, Ellermann M, Diederich F. Aromatic rings in chemical and biological recognition: energetics and structures. Angew. Chem. Int. Ed. Engl. 2011;50:4808–4842. doi: 10.1002/anie.201007560. [DOI] [PubMed] [Google Scholar]
- Sarna T. Properties and function of the ocular melanin--a photobiophysical view. J. Photochem. Photobiol. B. 1992;12:215–258. doi: 10.1016/1011-1344(92)85027-r. [DOI] [PubMed] [Google Scholar]
- Sarna T, Swartz HM. The Physical Properties of Melanins. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 311–341. [Google Scholar]
- Sarna T, Hyde JS, Swartz HM. Ion-exchange in melanin: an electron spin resonance study with lanthanide probes. Science. 1976;192:1132–1134. doi: 10.1126/science.179142. [DOI] [PubMed] [Google Scholar]
- Sarna T, Menon IA, Sealy RC. Photosensitization of melanins: a comparative study. Photochem. Photobiol. 1985;42:529–532. doi: 10.1111/j.1751-1097.1985.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Sarna T, Burke JM, Korytowski W, Rózanowska M, Skumatz CM, Zareba A, Zareba M. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp. Eye. Res. 2003;76:89–98. doi: 10.1016/s0014-4835(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Schubert D, Chevion M. The role of iron in beta amyloid toxicity. Biochem. Biophys. Res. Commun. 1995;216:702–707. doi: 10.1006/bbrc.1995.2678. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Segura-Aguilar J, Lind C. Distribution of DT diaphorase in the rat brain: biochemical and immunohistochemical studies. Neuroscience. 1988;27:763–776. doi: 10.1016/0306-4522(88)90181-9. [DOI] [PubMed] [Google Scholar]
- Scott GA. Melanosome Trafficking and Transfer. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 171–180. [Google Scholar]
- Segura-Aguilar J. Peroxidase activity of liver microsomal vitamin D 25-hydroxylase and cytochrome P450 1A2 catalyzes 25-hydroxylation of vitamin D3 and oxidation of dopamine to aminochrome. Biochem. Mol. Med. 1996;58:122–129. doi: 10.1006/bmme.1996.0039. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Lind C. On the mechanism of the Mn3(+)-induced neurotoxicity of dopamine:prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem. Biol. Interact. 1989;72:309–324. doi: 10.1016/0009-2797(89)90006-9. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione transferases, in particular isoenzyme M2-2, catalyze detoxication of the dopamine metabolite aminochrome. J. Biol. Chem. 1997;272:5727–5731. doi: 10.1074/jbc.272.9.5727. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Metodiewa D, Welch CJ. Metabolic activation of dopamine o-quinones to o-semiquinones by NADPH cytochrome P450 reductase may play an important role in oxidative stress and apoptotic effects. Biochim. Biophys. Acta. 1998;1381:1–6. doi: 10.1016/s0304-4165(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L, Zucca FA. Protective and toxic roles of dopamine in Parkinson's disease. J. Neurochem. 2014;129:898–915. doi: 10.1111/jnc.12686. [DOI] [PubMed] [Google Scholar]
- Shen XM, Xia B, Wrona MZ, Dryhurst G. Synthesis, redox properties, in vivo formation, and neurobehavioral effects of N-acetylcysteinyl conjugates of dopamine: possible metabolites of relevance to Parkinson's disease. Chem. Res. Toxicol. 1996;9:1117–1126. doi: 10.1021/tx960052v. [DOI] [PubMed] [Google Scholar]
- Shima T, Sarna T, Swartz HM, Stroppolo A, Gerbasi R, Zecca L. Binding of iron to neuromelanin of human substantia nigra and synthetic melanin: an electron paramagnetic resonance spectroscopy study. Free Radic. Biol. Med. 1997;23:110–119. doi: 10.1016/s0891-5849(96)00623-5. [DOI] [PubMed] [Google Scholar]
- Sian-Hülsmann J, Mandel S, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J. Neurochem. 2011;118:939–957. doi: 10.1111/j.1471-4159.2010.07132.x. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Camaschella C. A potential pathogenetic role of iron in Alzheimer's disease. J. Cell. Mol. Med. 2008;12:1548–1550. doi: 10.1111/j.1582-4934.2008.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JD, Peles DN. The red and the black. Acc. Chem. Res. 2010;43:1452–1460. doi: 10.1021/ar100079y. [DOI] [PubMed] [Google Scholar]
- Simon JD, Hong L, Peles DN. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J. Phys. Chem. B. 2008;112:13201–13217. doi: 10.1021/jp804248h. [DOI] [PubMed] [Google Scholar]
- Siraki AG, Smythies J, O'Brien PJ. Superoxide radical scavenging and attenuation of hypoxia-reoxygenation injury by neurotransmitter ferric complexes in isolated rat hepatocytes. Neurosci. Lett. 2000;296:37–40. doi: 10.1016/s0304-3940(00)01618-9. [DOI] [PubMed] [Google Scholar]
- Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J. Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- Solano F, Garcia-Borron JC. Enzymology of Melanin Formation. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 230–241. [Google Scholar]
- Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L. Copper Active Sites in Biology. Chem. Rev. 2014;114:3659–3853. doi: 10.1021/cr400327t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox. Res. 2000;1:181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov. Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J. Neurochem. 2008;106:24–36. doi: 10.1111/j.1471-4159.2008.05385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Sulzer D. The pathology roadmap in Parkinson disease. Prion. 2013;7:85–91. doi: 10.4161/pri.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Shibahara S. Transcriptional Regulation of melanocyte Function. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne J-P, editors. The Pigmentary System: Physiology and Pathophysiology. Second edition. Malden, MA, USA: Blackwell Publishing; 2006. pp. 242–260. [Google Scholar]
- Taly AB, Meenakshi-Sundaram S, Sinha S, Swamy HS, Arunodaya GR. Wilson disease: description of 282 patients evaluated over 3 decades. Medicine (Baltimore) 2007;86:112–121. doi: 10.1097/MD.0b013e318045a00e. [DOI] [PubMed] [Google Scholar]
- Tamarit J, Gerez C, Meier C, Mulliez E, Trautwein A, Fontecave M. The activating component of the anaerobic ribonucleotide reductase from Escherichia coli. An iron-sulfur center with only three cysteines. J. Biol. Chem. 2000;275:15669–15675. doi: 10.1074/jbc.275.21.15669. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Main BS, Crack PJ. Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson's disease. Neurochem. Int. 2013;62:803–819. doi: 10.1016/j.neuint.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Terland O, Flatmark T, Tangerås A, Grønberg M. Dopamine oxidation generates an oxidative stress mediated by dopamine semiquinone and unrelated to reactive oxygen species. J. Mol. Cell Cardiol. 1997;29:1731–1738. doi: 10.1006/jmcc.1997.0412. [DOI] [PubMed] [Google Scholar]
- Theil EC, Le Brun NE. Fe Transport and Storage Related to Humans and Pathogens and Oxygen. In: Reedijk J, Poeppelmeier J, Elsevier K, editors. Comprehensive Inorganic Chemistry II. Vol. 3. 2013. pp. 21–33. [Google Scholar]
- Thompson CM, Capdevila JH, Strobel HW. Recombinant cytochrome P450 2D18 metabolism of dopamine and arachidonic acid. J. Pharmacol. Exp. Ther. 2000;294:1120–1130. [PubMed] [Google Scholar]
- Tiekink ERT, Zukerman-Schpector J, editors. The Importance of Pi-Interactions in Crystal Engineering: Frontiers in Crystal Engineering. John Wiley & Sons; 2012. [Google Scholar]
- Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- Tomter AB, Zoppellaro G, Andersen NH, Hersleth HP, Hammerstad M, Røhr AK, Sandvik GK, Strand KR, Nilsson GE, Bell CB, III, Barra AL, Blasco E, Le Pape L, Solomon EI, Andersson KK. Ribonucleotide reductase class I with different radical generating clusters. Coordination Chemistry Reviews. 2013;257:3–26. [Google Scholar]
- Tribl F, Arzberger T, Riederer P, Gerlach M. Tyrosinase is not detected in human catecholaminergic neurons by immunohistochemistry and Western blot analysis. J. Neural Transm. Suppl. 2007;72:51–55. doi: 10.1007/978-3-211-73574-9_8. [DOI] [PubMed] [Google Scholar]
- Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- Tse DC, McCreery RL, Adams RN. Potential oxidative pathways of brain ncatecholamines. J. Med. Chem. 1976;19:37–40. doi: 10.1021/jm00223a008. [DOI] [PubMed] [Google Scholar]
- Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson's disease catalyses the formation of hydrogen peroxide in vitro. Free Radic. Biol. Med. 2001;30:1163–1170. doi: 10.1016/s0891-5849(01)00513-5. [DOI] [PubMed] [Google Scholar]
- Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, González-Billault C, Núñez MT. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 2013;126:541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J. Biol. Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Van Laar VS, Mishizen AJ, Cascio M, Hastings TG. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol. Dis. 2009;34:487–500. doi: 10.1016/j.nbd.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viceconte N, Burguillos MA, Herrera AJ, De Pablos RM, Joseph B, Venero JL. Neuromelanin activates proinflammatory microglia through a caspase-8-dependent mechanism. J Neuroinflammation. 2015;12:5. doi: 10.1186/s12974-014-0228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Ito S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002;15:174–183. doi: 10.1034/j.1600-0749.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Fujikawa K, Zucca FA, Zecca L, Ito S. The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 2003;86:1015–1023. doi: 10.1046/j.1471-4159.2003.01917.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Hu DN, McCormick SA, Ito S. Characterization of melanin in human iridal and choroidal melanocytes from eyes with various colored irides. Pigment Cell Melanoma Res. 2008;21:97–105. doi: 10.1111/j.1755-148X.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Murase T, Zucca FA, Zecca L, Ito S. Biosynthetic pathway to neuromelanin and its aging process. Pigment Cell Melanoma Res. 2012;25:792–803. doi: 10.1111/pcmr.12014. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Tanaka H, Tabuchi K, Ojika M, Zucca FA, Zecca L, Ito S. Reduction of the nitro group to amine by hydroiodic acid to synthesize o-aminophenol derivatives as putative degradative markers of neuromelanin. Molecules. 2014;19:8039–8050. doi: 10.3390/molecules19068039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WC, Guan Z, Zucca FA, Fariello RG, Kordestani R, Zecca L, Raetz CR, Simon JD. Identification and quantification of dolichol and dolichoic acid in neuromelanin from substantia nigra of the human brain. J. Lipid Res. 2007;48:1457–1462. doi: 10.1194/jlr.C700008-JLR200. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230:181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- Whitehead RE, Ferrer JV, Javitch JA, Justice JB. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J. Neurochem. 2001;76:1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- Wielgus AR, Sarna T. Melanin in human irides of different color and age of donors. Pigment Cell Res. 2005;18:454–464. doi: 10.1111/j.1600-0749.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Williams A. MPTP parkinsonism. Br. Med. J (Clin. Res. Ed) 1984;289:1401–1402. doi: 10.1136/bmj.289.6456.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Buchheit CL, Berman NE, LeVine SM. Pathogenic implications of iron accumulation in multiple sclerosis. J. Neurochem. 2012;120:7–25. doi: 10.1111/j.1471-4159.2011.07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. FASEB J. 2003;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- Windahl MS, Petersen CR, Christensen HE, Harris P. Crystal structure of tryptophan hydroxylase with bound amino acid substrate. Biochemistry. 2008;47:12087–12094. doi: 10.1021/bi8015263. [DOI] [PubMed] [Google Scholar]
- Wolters ECh, Braak H. Parkinson's disease: premotor clinico-pathological correlations. J. Neural. Transm. Suppl. 2006;70:309–319. doi: 10.1007/978-3-211-45295-0_47. [DOI] [PubMed] [Google Scholar]
- Wypijewska A, Galazka-Friedman J, Bauminger ER, Wszolek ZK, Schweitzer KJ, Dickson DW, Jaklewicz A, Elbaum D, Friedman A. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat. Disord. 2010;16:329–333. doi: 10.1016/j.parkreldis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Xiong R, Siegel D, Ross D. Quinone-induced protein handling changes: implications for major protein handling systems in quinone-mediated toxicity. Toxicol. Appl. Pharmacol. 2014;280:285–295. doi: 10.1016/j.taap.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Jia Z, Knutson MD, Leeuwenburgh C. Impaired iron status in aging research. Int. J. Mol. Sci. 2012;13:2368–2386. doi: 10.3390/ijms13022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Stokes AH, Roskoski R, Jr, Vrana KE. Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J. Neurosci. Res. 1998;54:691–697. doi: 10.1002/(SICI)1097-4547(19981201)54:5<691::AID-JNR14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer's disease. J. Neurochem. 2002;82:1137–1147. doi: 10.1046/j.1471-4159.2002.t01-1-01061.x. [DOI] [PubMed] [Google Scholar]
- Ye T, Simon JD, Sarna T. Ultrafast energy transfer from bound tetra(4-N,N,N,N-trimethylanilinium)porphyrin to synthetic dopa and cysteinyldopa melanins. Photochem. Photobiol. 2003;77:1–4. [PubMed] [Google Scholar]
- Youdim MB, Ben-Shachar D, Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989;50:607–615. doi: 10.1093/ajcn/50.3.607. discussion 615–617. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- Zadlo A, Rozanowska MB, Burke JM, Sarna TJ. Photobleaching of retinal pigment epithelium melanosomes reduces their ability to inhibit iron-induced peroxidation of lipids. Pigment Cell Res. 2007;20:52–60. doi: 10.1111/j.1600-0749.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- Zadlo A, Burke JM, Sarna T. Effect of untreated and photobleached bovine RPE melanosomes on the photoinduced peroxidation of lipids. Photochem. Photobiol. Sci. 2009;8:830–837. doi: 10.1039/b901820d. [DOI] [PubMed] [Google Scholar]
- Zafar KS, Siegel D, Ross D. A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol. Pharmacol. 2006;70:1079–1086. doi: 10.1124/mol.106.024703. [DOI] [PubMed] [Google Scholar]
- Zajac GW, Gallas JM, Cheng J, Eisner M, Moss SC, Alvarado-Swaisgood AE. The fundamental unit of synthetic melanin: a verification by tunneling microscopy of X-ray scattering results. Biochim. Biophys. Acta. 1994;1199:271–278. doi: 10.1016/0304-4165(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Zareba M, Bober A, Korytowski W, Zecca L, Sarna T. The effect of a synthetic neuromelanin on yield of free hydroxyl radicals generated in model systems. Biochim. Biophys. Acta. 1995;1271:343–348. doi: 10.1016/0925-4439(95)00058-c. [DOI] [PubMed] [Google Scholar]
- Zareba M, Skumatz CM, Sarna TJ, Burke JM. Photic injury to cultured RPE varies among individual cells in proportion to their endogenous lipofuscin content as modulated by their melanosome content. Invest. Ophthalmol. Vis. Sci. 2014;55:4982–4990. doi: 10.1167/iovs.14-14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Swartz HM. Total and paramagnetic metals in human substantia nigra and its neuromelanin. J. Neural Transm. Park. Dis. Dement. Sect. 1993;5:203–213. doi: 10.1007/BF02257675. [DOI] [PubMed] [Google Scholar]
- Zecca L, Mecacci C, Seraglia R, Parati E. The chemical characterization of melanin contained in substantia nigra of human brain. Biochim. Biophys. Acta. 1992;1138:6–10. doi: 10.1016/0925-4439(92)90144-c. [DOI] [PubMed] [Google Scholar]
- Zecca L, Pietra R, Goj C, Mecacci C, Radice D, Sabbioni E. Iron and other metals in neuromelanin, substantia nigra, and putamen of human brain. J. Neurochem. 1994;62:1097–1101. doi: 10.1046/j.1471-4159.1994.62031097.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Shima T, Stroppolo A, Goj C, Battiston GA, Gerbasi R, Sarna T, Swartz HM. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience. 1996;73:407–415. doi: 10.1016/0306-4522(96)00047-4. [DOI] [PubMed] [Google Scholar]
- Zecca L, Costi P, Mecacci C, Ito S, Terreni M, Sonnino S. Interaction of human substantia nigra neuromelanin with lipids and peptides. J. Neurochem. 2000;74:1758–1765. doi: 10.1046/j.1471-4159.2000.0741758.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Gallorini M, Schünemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J. Neurochem. 2001a;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Tampellini D, Costi P, Rizzio E, Giaveri G, Gallorini M. Combined biochemical separation and INAA for the determination of iron and other metals in Neuromelanin of human brain Substantia Nigra. J. Radioanal. Nucl. Chem. 2001b;249:449–454. [Google Scholar]
- Zecca L, Tampellini D, Rizzio E, Giaveri G, Gallorini M. The determination of iron and other metals by INAA in Cortex, Cerebellum and Putamen of human brain and in their neuromelanins. J. Radioanal. Nucl. Chem. 2001c;248:129–131. [Google Scholar]
- Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEBS Lett. 2002;510:216–220. doi: 10.1016/s0014-5793(01)03269-0. [DOI] [PubMed] [Google Scholar]
- Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26:578–580. doi: 10.1016/j.tins.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004a;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG, Karatekin E, Kleinman MH, Turro N, Hornykiewicz O, Zucca FA. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. U. S. A. 2004b;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T. Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson's disease. J. Neurochem. 2008a;106:1866–1875. doi: 10.1111/j.1471-4159.2008.05541.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Bellei C, Costi P, Albertini A, Monzani E, Casella L, Gallorini M, Bergamaschi L, Moscatelli A, Turro NJ, Eisner M, Crippa PR, Ito S, Wakamatsu K, Bush WD, Ward WC, Simon JD, Zucca FA. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl. Acad. Sci. U. S. A. 2008b;105:17567–17572. doi: 10.1073/pnas.0808768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson's disease. Acta Neuropathol. 2008c;116:47–55. doi: 10.1007/s00401-008-0361-7. [DOI] [PubMed] [Google Scholar]
- Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY, Miller DS, Chignell CF, Wilson B, Jackson-Lewis V, Przedborski S, Joset D, Loike J, Hong JS, Sulzer D, Zecca L. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease. Neurotox. Res. 2011;19:63–72. doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zecca L, Wilson B, Ren HW, Wang YJ, Wang XM, Hong JS. Human neuromelanin: an endogenous microglial activator for dopaminergic neuron death. Front. Biosci. (Elite Ed) 2013;5:1–11. doi: 10.2741/e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZD, Lim TM. Dopamine (DA) induced irreversible proteasome inhibition via DA derived quinones. Free Radic. Res. 2009;43:417–430. doi: 10.1080/10715760902801533. [DOI] [PubMed] [Google Scholar]
- Zhou ZD, Lan YH, Tan EK, Lim TM. Iron species-mediated dopamine oxidation, proteasome inhibition, and dopaminergic cell demise: implications for iron-related dopaminergic neuron degeneration. Free Radic. Biol. Med. 2010;49:1856–1871. doi: 10.1016/j.freeradbiomed.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Zucca FA, Bellei C, Giannelli S, Terreni MR, Gallorini M, Rizzio E, Pezzoli G, Albertini A, Zecca L. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: consequences for neuronal vulnerability. J. Neural Transm. 2006;113:757–767. doi: 10.1007/s00702-006-0453-2. [DOI] [PubMed] [Google Scholar]
- Zucca FA, Cupaioli FA, Zecca L. The role of iron in neurodegeneration. In: Milardi D, Rizzarelli E, editors. Neurodegeneration: metallostasis and proteostasis. Cambridge: RSC Publishing; 2011. pp. 174–211. [Google Scholar]
- Zucca FA, Basso E, Cupaioli FA, Ferrari E, Sulzer D, Casella L, Zecca L. Neuromelanin of the human substantia nigra: an update. Neurotox. Res. 2014;25:13–23. doi: 10.1007/s12640-013-9435-y. [DOI] [PubMed] [Google Scholar]