Abstract
Introduction
Oculopharyngeal muscular dystrophy (OPMD) causes ptosis, dysphagia, and limb weakness. Health-related quality of life (HRQoL) and its relationship to physical symptoms was investigated
Methods
The Short Form-36 (SF-36) was completed by 89 participants in the US OPMD Registry. Multiple hierarchical regression was used to determine the relative contributions of dysphagia severity and lower extremity functional impairment to the physical (PCS) and mental (MCS) components of the SF-36.
Results
HRQoL was reduced in OPMD compared with population norms. Lower extremity functional impairment explained a significant proportion of variance in PCS and MCS. Dysphagia symptom severity explained a moderate amount of variance only in MCS. Dysphagia symptom severity had the strongest associations with general health perception and social functioning domains.
Discussion
Lower extremity functional impairment in OPMD deserves attention due to its large influence on HRQoL. Both generic and dysphagia-specific measures are necessary to assess HRQoL in OPMD.
Keywords: Oculopharyngeal muscular dystrophy, quality of life, dysphagia, mobility, rare disease registry
INTRODUCTION
Oculopharyngeal muscular dystrophy (OPMD) is a rare, dominantly inherited muscle disease that causes ptosis, dysphagia, and limb weakness.1 It is caused by short triplet-repeat expansions in the PABPN1 gene.2 Dysphagia may result in malnutrition, aspiration pneumonia, and death.3 It is not known whether overall health-related quality of life (HRQoL) is diminished in OPMD.
The World Health Organization defines quality of life (QOL) as “individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.”4 Assessment of HRQoL is important for several reasons. First, since therapeutic trials should incorporate HRQoL endpoints when evaluating treatment success.5,6 valid measures of HRQoL are needed when designing clinical trials. Second, data on HRQoL may guide allocation of health care resources.7 Third, identification of factors influencing HRQoL may lead to interventions that can improve QOL when cures do not yet exist. HRQoL entails multiple dimensions including physical, psychological, and social function. Factors associated with reduced HRQoL in muscular dystrophies include older age, longer disease duration, greater disease severity, and depressed mood.5,8,9 While several studies have used dysphagia-specific QOL measures in OPMD,10,11 to our knowledge, there have been no prior studies of overall HRQoL and its determinants in a purely OPMD population.
The main goal of this study was to measure HRQoL of a nationally representative sample of individuals with OPMD and to identify factors associated with HRQoL. The SF-36 was used to measure HRQoL, because it is a generic survey that has been used in other muscular dystrophies.5 Because disease severity is a recognized predictor of HRQoL, the independent variables investigated were severity of major physical symptoms, namely dysphagia and lower extremity functional impairment. Secondarily, this study provides preliminary evidence of the validity of several patient-reported outcome measures in OPMD. I hypothesized that 1) HRQoL in OPMD is reduced compared with U.S. general population normative data, and 2) dysphagia symptom severity is the strongest independent predictor of HRQoL in OPMD.
METHODS
Sample
This was a prospective, cross-sectional study of individuals age ≥ 18y with OPMD who enrolled in the US OPMD Registry between December, 2012 and August, 2014. Registry enrollment packets contained a standardized questionnaire eliciting demographic and clinical information and included patient-reported measures of dysphagia and lower extremity function (see below). All surveys were in English and were completed by mail-out/mail-back administration. Details of registry design have been previously reported 12. Participants who enrolled in the registry during this time period also completed the Medical Outcome Study Short Form-36 (version 2) (SF-36) survey. 13 Individuals who did not return completed surveys were contacted once by postal mail to request survey completion.
Inclusion/exclusion criteria
Individuals in the OPMD Registry were classified as definite-genetically diagnosed, definite-clinically-diagnosed, or possible OPMD. Only definite OPMD cases were included in this study. Definite-genetically diagnosed OPMD was defined as either: 1) positive genetic test for OPMD; or 2) late-onset ptosis (or previous corrective surgery for ptosis) and/or dysphagia, and a relative with a positive genetic test for OPMD. Definite-clinically-diagnosed OPMD was defined as: late-onset ptosis (or previous corrective surgery for ptosis) and dysphagia, and positive family history affecting ≥2 generations. Health records for clinically-diagnosed cases were reviewed. Individuals with a negative OPMD gene test and those with clinical data supporting a diagnosis other than OPMD (onset of ptosis or dysphagia before age 30 y, severe external ophthalmoplegia before age 60 y, or clinical or electromyographic myotonia) were excluded. Cases meeting only some of the clinical diagnostic criteria and none of the exclusion criteria were classified as possible OPMD and were not included.
This study was approved by the University of New Mexico Human Research Protections Office. All individuals provided written informed consent to participate.
Patient-reported measure of dysphagia severity
Dysphagia severity was assessed using a 14-item patient-reported dysphagia symptom battery (DSB) developed by McHorney et al.14 Summed scores (ranging from 14 to 70) were linearly transformed to a 0 to 100 scale with lower scores indicating worse symptoms. The DSB has previously been used to assess dysphagia severity in patients with spinobulbar muscular atrophy.15
Patient-reported measure of lower extremity function and mobility
Given that OPMD is known to cause proximal lower extremity weakness and can impair mobility16, the 8-item Neuro-QOL Lower Extremity Function (Mobility) – Short Form for adults was chosen to measure lower extremity dysfunction.17,18 Raw summed scores are converted to T-scores using conversion tables. T-score distributions produce standardized scores with a mean of 50 and a standard deviation of 10 and are referenced to the US general population. Higher scores indicate better self-reported health.17
Measurement of HRQoL
HRQoL was measured with the SF-36 (version 2), a generic health survey that includes 36 questions encompassing 8 domains of HRQoL (PF-physical functioning, RP-role limitation due to physical problems, BP-bodily pain, RE-role limitations due to emotional problems, VT-vitality, GH-general health perception, MH-mental health, and SF-social functioning). Two summary scores were derived: the physical component summary (PCS), which correlates most highly with PF and RP domains, and the mental component summary (MCS), which correlates most highly with the MH, RE and SF domains.19 The SF-36 (version 2) can be scored using standard scoring in which raw item scores are coded, summed, and transformed to a scale from 0 to 100 (“standard scores”), with higher scores indicating better health, or norm-based scoring that incorporates general U.S. population data from 2009 and generates T-scores (“norm-based scores”), such that the 8-subscale domain scores, and the PCS and MCS each have a mean of 50 and standard deviation of 10 in the general US population.19
Analyses
Descriptive statistics were used to summarize clinical and demographic characteristics of the sample. Internal consistency reliability of the DSB, Neuro-QOL, and each health domain scale of the SF-36 was assessed using the Cronbach alpha.
HRQoL in OPMD versus general US population
Mean PCS, MCS, and norm-based scores of the 8 domains of the SF-36 were compared to the general U.S. population mean using 1-sample t-tests.
Determinants of HRQoL in OPMD
Pearson pairwise correlations between PCS, MCS, and disease severity variables were first examined. I used multiple hierarchical regressions to investigate the relative contribution of dysphagia severity and lower extremity functional impairment (independent variables) to PCS and MCS scores (dependent variables), after allowing for the effects of the control variables (demographics and disease duration). The independent variables were entered into the regression analysis in blocks. The first block included the control variables, namely, age, gender, and disease duration. The second block included dysphagia severity as measured by the DSB, and the third block included lower extremity functional impairment as measured by the Neuro-QOL. To further evaluate the determinants of HRQoL in OPMD, I examined pairwise correlations between disease severity variables (DSB and Neuro-QOL) and each of the 8 domains of the SF-36 using Pearson correlation coefficients.
The level of significance for statistical tests was set at alpha=0.05, and all tests were 2-tailed. Analyses were performed using STATA 11.2 (StataCorp LP, College Station, TX).
RESULTS
Participants
Of 98 individuals enrolled in the US OPMD Registry during the study period, 89 met criteria for definite OPMD and were included in this study. All completed the SF-36 and the Dysphagia Symptom Battery. All but 1 (n=88) completed the Neuro-QOL. Table 1 shows the demographic and clinical characteristics of the study sample.
Table 1.
Demographic and clinical characteristics of the cohort (N=89).
| n (%) or mean (SD); range | |
|---|---|
| Demographic characteristics | |
| Men | 40 (44.9) |
| Age, yrs | 66.2 (8.9); (37-84) |
| Resident of New Mexico | 37 (41.6) |
| Ethnicity/Ancestry | |
| Hispanic or Latino | 49 (55.1) |
| French-Canadian | 9 (10.1) |
| Canadian | 7 (7.9) |
| Irish | 6 (6.7) |
| English/Scottish | 5 (5.6) |
| German | 4 (4.5) |
| Other European | 3 (3.4) |
| Middle Eastern | 1 (1.1) |
| Not reported | 5 (5.6) |
| Clinical characteristics | |
| Age at earliest symptom (ptosis, dysphagia, or limb weakness), yrs | 50.1 (8.1); (17-67) |
| Age at diagnosis, yrs | 56.9 (8.0); (35-79) |
| Ptosis | 83 (93.3) |
| Mean age at onset of ptosis, yrs | 53.1 (7.2); (37-75) |
| Dysphagia | 85 (95.5) |
| Mean age at onset of dysphagia, yrs | 53.4 (9.3); (20-75) |
| Lower limb weakness | 75 (84.3) |
| Mean age at onset of lower limb weakness, yrs | 56.9 (9.2); (17-77) |
| Upper limb weakness | 53 (59.6) |
| Mean age at onset of upper limb weakness, yrs | 58.2 (8.2); (36-74) |
| Positive family history | 88 (98.9) |
| Body mass index, kg/m2 | 24.9 (4.5); (18.1-46.8) |
| Therapeutic interventions | |
| Blepharoptosis surgery | 68 (76.4) |
| Esophageal dilatation | 28 (31.5) |
| Cricopharyngeal botulinum toxin | 9 (10.1) |
| Cricopharyngeal myotomy | 2 (2.3) |
| Gastrostomy for nutritional support | 0 (0.0) |
| PABPN1 Genotype | |
| GCN12, GCN10 | 4 (4.5) |
| GCN13, GCN10 | 43 (48.3) |
| GCN14, GCN10 | 7 (7.9) |
| GCN15, GCN10 | 1 (11) |
| Positive genetic test – genotype unknown | 2 (2.3) |
| Genetic test not performed; related to individual with positive gene test | 10 (11.2) |
| Genetic test not performed | 22 (24.7) |
Abbreviations: SD = standard deviation
Nearly half of respondents (44.9%; 40/89) reported use of an assistive device for ambulation (defined as a cane, walker, scooter, or wheelchair).
Thirty-nine individuals (43.8%) reported that OPMD symptoms had affected their employment status; 21 (23.6%) indicated that they needed to go on disability, and 18 (20.2%) reported that they had to retire early.
Dysphagia severity
The mean DSB score was 51.6 ± 20.4 (range 0-100). There was a negative correlation between DSB score and dysphagia duration (Pearson r=−0.22, P=0.04). Individuals who had undergone at least 1 therapeutic intervention for dysphagia had lower scores on the DSB compared with those who had not (45.8 ± 18.1 vs 54.7 ± 21.0, P=0.048, 2-sample t-test). These results support the construct validity of the DSB in OPMD.
Lower extremity function and mobility
Mean Neuro-QOL score was 41.6 ± 9.6 (range 25.1-58.6), which is statistically significantly lower than the mean of 50 ± 10 in the general US population (P<0.0001). There was a negative correlation between Neuro-QOL score and duration of leg weakness (Pearson r=−0.27, P=0.02). Individuals who used assistive devices for mobility had lower scores on the Neuro-QOL compared with those who did not (36.1 ± 8.2 vs 46.2 ± 8.3, P<0.0001, 2-sample t-test). These results support the construct validity of the Neuro-QOL in OPMD.
Scale reliability
Internal consistency reliability of the DSB, the Neuro-QOL, and all 8 domain scales of the SF-36 was excellent: DSB (α=0.92); Neuro-QOL (α=0.94); physical functioning (α=0.95); role physical (α=0.97); bodily pain (α=0.90); general health perception (α=0.83); vitality (α=0.87); social functioning (α=0.92); role emotional (α=0.93); and mental health (α=0.84).
HRQoL in OPMD versus general US population
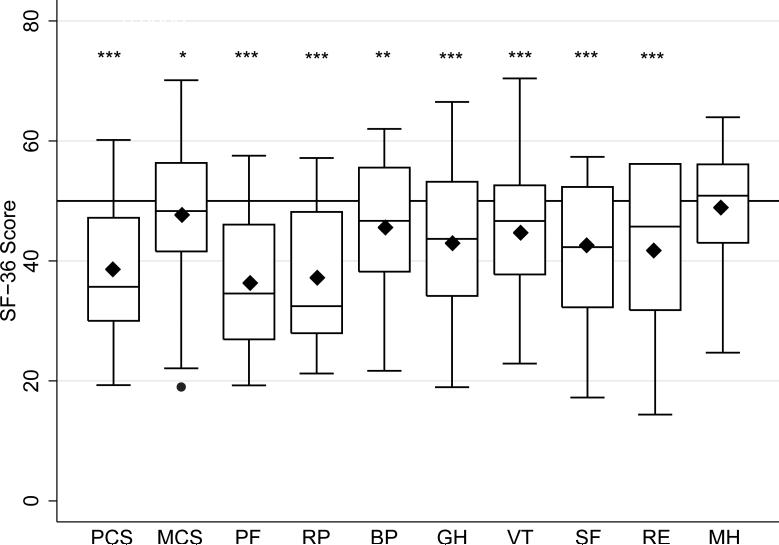
Figure 1 shows the SF-36 profile for the sample using boxplots of norm-based scores for the 2 summary measures (PCS and MCS) and the 8 domain scales. Mean PCS and mean MCS scores for OPMD patients were lower than the general population mean (mean PCS 38.4 ± 10.5, P<0.0001; mean MCS 47.6 ± 10.9, P=0.04; 1-sample t-tests). Mean scores for domains predominantly representing physical health (PF and RP) were lower than ones representing predominantly mental health (MH, RE, and SF). Table 2 shows mean scores for each SF-36 domain. Greatest impairments in HRQoL were in the physical functioning and role physical domains. The least impairment was in the mental health domain.
Figure 1.
SF-36 profile for the sample of OPMD patients (N=89 ). Box-and-whisker plots of norm-based scores. Medians (horizontal bars), 25th-75th percentiles (box), ranges (whiskers), means (black diamonds), and outliers (black dot) are shown. Horizontal reference line at a score of 50 represents the U.S. general population mean. Significance levels of comparisons between sample means and the US general population mean are shown.
* P<0.05, ** P<0.001, ***P<0.0001. (PCS=Physical Component Summary, MCS= Mental Component Summary, PF=physical functioning, RP=role limitation due to physical problems, BP=bodily pain, RE=role limitations due to emotional problems, VT=vitality, GH=general health perception, MH=mental health, and SF=social functioning.)
Table 2.
Mean norm-based scores on the 8 domains of the SF-36 version 2 and the summary scores (PCS, Physical Component Summary and MCS, Mental Component Summary). For reference, standard scores ±SD are provided in parentheses Norm-based scores were used in the analyses.
| Mean norm-based score ±SD | |
|---|---|
| PCS | 38.4±10.5 (NA*) |
| MCS | 47.6±10.9 (NA*) |
| Physical functioning | 36.5 ±11.9 (45.1±31.0) |
| Role physical | 37.1 ±12.5 (44.2 ±34.7) |
| Bodily pain | 45.6 ±11.7 (59.4 ±29.0) |
| General health perception | 42.9 ±11.8 (50.4 ±24.8) |
| Vitality | 44.5 ±10.8 (45.4 ±22.7) |
| Social functioning | 42.4 ±12.3 (62.8 ±30.7) |
| Role emotional | 41.6 ±13.6 (65.2 ±32.6) |
| Mental health | 48.7±9.8 (70.8 ±18.7) |
Summary scores are only calculated as norm-based scores.
Relationship between physical symptoms and HRQoL in OPMD
There were statistically significant correlations between disease severity variables and PCS, although the strength of the association was strongest for Neuro-QOL (r=0.77, P<0.0001) compared with DSB (r=0.27, P=0.01). Similarly, there were statistically significant correlations between disease severity variables and MCS, and the strength of the association was strongest for Neuro-QOL (r=0.46, P=<0.0001) compared with DSB (r=0.34, P=0.001). Multiple hierarchical regressions showed (Table 3) that the control variables (ΔR2 =0.06) and DSB (ΔR2 =0.03) did not contribute significantly to the proportion of explained variance in PCS. In contrast, Neuro-QOL explained a large proportion of the variance in PCS (ΔR2=0.50). For MCS, a modest amount of variance was explained by the control variables (ΔR2 =0.12), with significant amounts of additional variance explained by DSB (ΔR2 =0.10) and Neuro-QOL (ΔR2 =0.14). Among the control variables, shorter disease duration and advancing age were associated with higher MCS scores.
Table 3.
Multiple hierarchical regression results for PCS and MCS. Table shows changes in variance (ΔR2) and the associated F-statistic (F) for models resulting from addition of each block of variables. β is the regression coefficient.
| PCS | MCS | |||
|---|---|---|---|---|
| Independent variable | ΔR2 | β | ΔR2 | β |
| Control variables | 0.06 | 0.12 | ||
| Age, yrs | 0.07 | 0.50** | ||
| Gender | 1.34 | 3.88 | ||
| Disease duration, yrs | −0.32* | −0.46** | ||
| F | 1.65 | 3.90* | ||
| DSB score | 0.03 | 0.10 | 0.10 | 0.18** |
| F | 2.851 | 10.77** | ||
| Neuro-QOL score | 0.50 | 0.14 | 0.48*** | |
| F | 95.9*** | 0.835*** | 18.37*** | |
| Total R2 | 0.58 | 0.37 | ||
| Total F | 22.57*** | 9.38*** | ||
Significant association at P<0.05.
Significant association at P<0.01.
Significant association at P<0.001.
Table 4 shows the correlations between DSB, Neuro-QOL and the 8 domains of the SF-36. Neuro-QOL had moderate (.3≥r≤.5) to strong (r≥.5) associations with all domains of the SF-36. In contrast, DSB showed the strongest associations with general health perception and social functioning domains.
Table 4.
Pairwise correlations between DSB, Neuro-QOL, and the 8 domains of the SF-36. The Pearson correlation coefficient r is shown.
| Neuro-QOL | Dysphagia Symptom Battery | |||
|---|---|---|---|---|
| SF-36 domain | r | P-value | r | P-value |
| Physical functioning | 0.84 | <0.0001 | 0.25 | 0.02 |
| Role physical | 0.72 | <0.0001 | 0.22 | 0.03 |
| Bodily pain | 0.40 | 0.0001 | 0.14 | 0.20 |
| General health perception | 0.50 | <0.0001 | 0.45 | <0.0001 |
| Vitality | 0.59 | <0.0001 | 0.26 | 0.01 |
| Social functioning | 0.65 | <0.0001 | 0.42 | <0.0001 |
| Role emotional | 0.64 | <0.0001 | 0.26 | 0.01 |
| Mental health | 0.32 | 0.002 | 0.28 | 0.01 |
DISCUSSION
There are 2 key findings in this study. The first is that overall HRQoL is reduced in OPMD. Greatest reductions were found in the physical dimensions of HRQoL, while mental dimensions were relatively preserved. This result is consistent with prior research on HRQoL in muscular dystrophies, which shows that physical dimensions of HRQoL tend to be more affected than psychosocial dimensions.5 The second key finding is that lower extremity physical function is more strongly associated with the physical component of HRQoL in OPMD than is dysphagia severity. Dysphagia severity was an independent predictor of the mental component of HRQoL and appeared to impact the mental component of HRQoL in OPMD mainly by effects on the domains of general health perception and social functioning.
The observation that dysphagia severity is a determinant of the mental component of HRQoL is in line with prior work on OPMD which showed that individuals with dysphagia experience significant social and psychological effects, including a tendency to avoid social events, fear of choking, and embarrassment when eating in public.20,21 These findings underscore the fact that generic HRQoL measures such as the SF-36 are indispensable, since they permit comparisons of HRQoL across multiple studies and across various diseases; however, they will miss important domains that are disease-specific.22 Because a generic HRQoL measure such as the SF-36 may not capture all relevant aspects of QOL in OPMD, a complementary measure that is dysphagia-specific would likely improve assessment of HRQoL in OPMD. Examples of dysphagia-specific QOL measures include the SWAL-QOL tool and the M.D. Anderson Dysphagia Inventory.14,23 These scales, however, were developed and validated in populations weighted heavily toward patients with head and neck cancers. Future work should establish the psychometric properties of such dysphagia-specific QOL measures in OPMD and other muscle diseases in which dysphagia is an important symptom.
An unexpected observation is that mean bodily pain score in the OPMD sample was statistically significantly lower than the US population mean. Lower scores on this scale indicate higher levels of pain and/or greater impact of pain on normal activities. A case of exercise-induced proximal muscle pain has been reported in OPMD24, and pain is a known feature of other muscular dystrophies.25 Further studies of pain prevalence, location, and severity in OPMD should be considered, as they may lead to interventions that improve HRQoL.
Advancing age was associated with better MCS scores. This finding might be explained by “response shift.” In the context of QOL assessment, “response shift” refers to the phenomenon of stable QOL despite advancing disease, which may occur because an individual has changed his/her internal standards for judging QOL, changed his/her value system, or reconceptualized the meaning of QOL.26 It is possible that since OPMD is a late-onset, slowly progressive disease, patients may have adequate opportunity to adapt and cope, thereby maintaining a relatively stable psychological dimension of QOL. Interventions aimed at fostering such adaptive responses to muscle disease may help improve QOL even in the absence of cures.9 For example, a randomized controlled trial in facioscapulohumeral muscular dystrophy (FSHD) showed that cognitive-behavioral therapy reduced fatigue.27
A strength of this study is that it included a nationally representative sample of OPMD patients, spanning 23 US states. Application of the widely-used SF-36 survey will permit comparisons with published results for other neuromuscular diseases.
There are several limitations to this study. First, the majority of the variance (63%) in the mental component of the SF-36 was still unexplained after accounting for demographic variables, disease duration, and severity of major physical symptoms. This may be because this study did not measure psychological variables that influence HRQoL in muscular dystrophies, such as mood and illness perceptions.9,28 Second, this study included no clinician-reported measures of disease severity, only patient-reported outcomes. While I present preliminary data supporting the validity of DSB and Neuro-QOL in OPMD, future studies will need to further evaluate the validity of these patient-reported measures by assessing their correlation with clinical measures of dysphagia severity and lower limb function, respectively. Third, there were no individuals with gastrostomy in this sample. Gastrostomy is considered in patients with very severe dysphagia who are malnourished.16 Thus this sample likely did not include patients with the greatest severity of dysphagia. Fourth, other aspects of OPMD such as ptosis, voice changes, and upper extremity weakness were not assessed. It is not known to what extent such symptoms are independent predictors of HRQOL in OPMD. Further qualitative studies are needed to identify the symptoms of greatest impact from the OPMD patient's perspective, as has been done for other muscular dystrophies.29,30
Several important conclusions emerge from this study. Factors beyond dysphagia severity have significant impacts on HRQoL in OPMD, particularly lower limb weakness and associated functional impairments. Therefore, ameliorating dysphagia in OPMD, while necessary, may not be sufficient for improving overall HRQoL. HRQoL outcome measures in future trials of disease-modifying therapies in OPMD should consider incorporating both a generic measure and a dysphagia-specific measure. Moreover, to the extent that dysphagia severity appears to impact psychosocial domains of HRQoL, psychosocial interventions that target problems such as social withdrawal and fear related to eating could be explored as ways to improve QOL in OPMD.
Acknowledgments
This work was partly supported by a Muscular Dystrophy Association Clinical Research Training Grant (S.Y., #260590). I acknowledge the support from the Biostatistics core of the University of New Mexico Clinical and Translational Science Center (NIH/NCRR/NCATS, CTSA, 8UL1TR000041).
Abbreviations
- DSB
Dysphagia Symptom Battery
- HRQoL
health-related quality of life
- MCS
Mental Component Summary
- OPMD
oculopharyngeal muscular dystrophy
- PCS
Physical Component Summary
- QOL
Quality of life
Footnotes
FINANCIAL DISCOLURE: The author has no financial disclosures.
CONFLICTS OF INTEREST: The author has no conflicts of interest to declare.
References
- 1.Victor M, Hayes R, Adams RD. Oculopharyngeal muscular dystrophy. A familial disease of late life characterized by dysphagia and progressive ptosis of the evelids. N Engl J Med. 1962;267:1267–1272. doi: 10.1056/NEJM196212202672501. [DOI] [PubMed] [Google Scholar]
- 2.Brais B, Bouchard JP, Xie YG, Rochefort DL, Chretien N, Tome FM, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18(2):164–167. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 3.Blumen SC, Nisipeanu P, Sadeh M, Asherov A, Tome FM, Korczyn AD. Clinical features of oculopharyngeal muscular dystrophy among Bukhara Jews. Neuromuscul Disord. 1993;3(5-6):575–577. doi: 10.1016/0960-8966(93)90119-5. [DOI] [PubMed] [Google Scholar]
- 4.The WHOQOL Group Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28(3):551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 5.Graham CD, Rose MR, Grunfeld EA, Kyle SD, Weinman J. A systematic review of quality of life in adults with muscle disease. J Neurol. 2011;258(9):1581–1592. doi: 10.1007/s00415-011-6062-5. [DOI] [PubMed] [Google Scholar]
- 6.Wiklund I. Assessment of patient-reported outcomes in clinical trials: the example of health-related quality of life. Fundam Clin Pharmacol. 2004;18(3):351–363. doi: 10.1111/j.1472-8206.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 7.Dolan P. The measurement of health-related quality of life for use in resource allocation decisions in health care. In: Culyer AJ, Newhouse JP, editors. Handbook of Health Economics. 1 Part B. Elsevier Science B. V; 2000. pp. 1723–1760. [Google Scholar]
- 8.Bann CM, Abresch RT, Biesecker B, Conway KC, Heatwole C, Peay H, et al. Measuring quality of life in muscular dystrophy. Neurology. 2015;84(10):1034–1042. doi: 10.1212/WNL.0000000000001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose MR, Sadjadi R, Weinman J, Akhtar T, Pandya S, Kissel JT, et al. Role of disease severity, illness perceptions, and mood on quality of life in muscle disease. Muscle Nerve. 2012;46(3):351–359. doi: 10.1002/mus.23320. [DOI] [PubMed] [Google Scholar]
- 10.Perie S, Trollet C, Mouly V, Vanneaux V, Mamchaoui K, Bouazza B, et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol Ther. 2014;22(1):219–225. doi: 10.1038/mt.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer PM, Neel AT, Sprouls G, Morrison L. Swallow characteristics in patients with oculopharyngeal muscular dystrophy. J Speech Lang Hear Res. 2010;53(6):1567–1578. doi: 10.1044/1092-4388(2010/09-0068). [DOI] [PubMed] [Google Scholar]
- 12.Daneshvari S, Youssof S, Kroth PJ. The NIH Office of Rare Diseases Research Patient Registry Standard: A Report from the University of New Mexico's Oculopharyngeal Muscular Dystrophy Patient Registry. AMIA Annu Symp Proc. 2013;2013:269–277. [PMC free article] [PubMed] [Google Scholar]
- 13.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 14.McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17(2):97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 15.Mano T, Katsuno M, Banno H, Suzuki K, Suga N, Hashizume A, et al. Tongue pressure as a novel biomarker of spinal and bulbar muscular atrophy. Neurology. 2014;82(3):255–262. doi: 10.1212/WNL.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 16.Youssof S, Schrader R, Bear D, Morrison L. Hip flexion weakness is associated with impaired mobility in oculopharyngeal muscular dystrophy: a retrospective study with implications for trial design. Neuromuscul Disord. 2015;25(3):238–246. doi: 10.1016/j.nmd.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Neurological Disorders and Stroke (NINDS) [2015 Jan 10];User Manual for the Quality of Life in Neurological Disorders (Neuro-QOL) Measures, version 1.0. 2010 Sep; Available at http://www.neuroqol.org/HowDoI/UserManual/User%20Manual/Neuro-QOL_UserManual.pdf.
- 18.Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruish ME, editor. User's Manual for the SF-36v2 Health Survey. Third Edition QualityMetric Incorporated; Lincoln, RI: 2011. pp. 15–19.pp. 73–78. [Google Scholar]
- 20.Krause-Bachand J, Koopman W. Living with oculopharyngeal muscular dystrophy: a phenomenological study. Can J Neurosci Nurs. 2008;30(1):35–39. [PubMed] [Google Scholar]
- 21.Young EC, Durant-Jones L. Gradual onset of dysphagia: a study of patients with oculopharyngeal muscular dystrophy. Dysphagia. 1997;12(4):196–201. doi: 10.1007/PL00009536. [DOI] [PubMed] [Google Scholar]
- 22.Burns TM, Graham CD, Rose MR, Simmons Z. Quality of life and measures of quality of life in patients with neuromuscular disorders. Muscle Nerve. 2012;46(1):9–25. doi: 10.1002/mus.23245. [DOI] [PubMed] [Google Scholar]
- 23.Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–876. [PubMed] [Google Scholar]
- 24.Lampe JB, Schafer J, Gartner HJ, Reichmann H. [Proximal weakness and exercise-induced pain as initial symptom of oculopharyngeal muscular dystrophy]. Nervenarzt. 2001;72(8):652–655. doi: 10.1007/s001150170068. [DOI] [PubMed] [Google Scholar]
- 25.Miro J, Gertz KJ, Carter GT, Jensen MP. Pain location and intensity impacts function in persons with myotonic dystrophy type 1 and facioscapulohumeral dystrophy with chronic pain. Muscle Nerve. 2014;49(6):900–905. doi: 10.1002/mus.24079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 27.Voet N, Bleijenberg G, Hendriks J, de Groot I, Padberg G, van Engelen B, et al. Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology. 2014;83(21):1914–1922. doi: 10.1212/WNL.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 28.Graham CD, Weinman J, Sadjadi R, Chalder T, Petty R, Hanna MG, et al. A multicentre postal survey investigating the contribution of illness perceptions, coping and optimism to quality of life and mood in adults with muscle disease. Clin Rehabil. 2014;28(5):508–519. doi: 10.1177/0269215513511340. [DOI] [PubMed] [Google Scholar]
- 29.Heatwole C, Bode R, Johnson N, Quinn C, Martens W, McDermott MP, et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology. 2012;79(4):348–357. doi: 10.1212/WNL.0b013e318260cbe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson NE, Quinn C, Eastwood E, Tawil R, Heatwole CR. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2012;46(6):951–953. doi: 10.1002/mus.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]