Abstract
The purpose of this study was to identify key psychosocial characteristics of HIV-infected women who exhibit different levels of both ART adherence and risk behaviors. We analyzed baseline data from 193 predominately African American HIV-infected women participating in a behavioral clinical trial. Women were categorized into high/low groups based on levels of adherence and risky behaviors. There was a significant interaction effect for internal motivation for adherence. Women at high risk for poor health and transmitting HIV (low adherence/high risk group) had the lowest levels of internal motivation and also reported more difficult life circumstances. Gender roles, caretaking and reliance on men for economic and other support may promote external versus internal motivation as well as riskier behaviors in this group. The highest levels of internal motivation were found in those with High Adherence/High Risk behaviors. This group was highly knowledgeable about HIV and had the lowest VL. Compared to others, this group seems to tolerate risky behaviors given their high level of adherence. Adherence and risk reduction behaviors are key to individual and public health. Motivation and risk compensation should be addressed when providing interventions to women living with HIV.
Keywords: HIV/AIDS, ART, Antiretroviral medication adherence, Sexual risk behavior, Motivation, Difficult life circumstances, Self-efficacy
Introduction
Advances in HIV/AIDS research have revealed that undetectable viral loads are associated with lower risks of HIV transmission through horizontal and vertical routes [1–4]. As such, treatment to maintain viral loads at an undetectable level has become a primary focus for preventing transmission. Despite the increasing effectiveness of new antiretroviral drugs, relatively high levels of adherence to antiretroviral therapy (ART) are still needed to achieve this result [5, 6]. Sexual risk reduction behavior also continues to be critically important to reduce the spread of the virus—particularly in HIV-positive individuals with sub-optimal ART adherence, which can lead to the development of resistant virus and increased viral loads [7–9]. Without risk reduction behaviors, HIV and resistant strains can be spread. Safer sex is also necessary to prevent other sexually transmitted infections (STIs) that can heighten the risk of HIV transmission. Effective prevention of HIV, therefore, depends on both high levels of ART adherence and low levels of risky behaviors.
The literature suggests that ART adherence and sexual risk behavior are related behaviors. Among a diverse sample of 2849 HIV-infected adults, Remien et al. [10] found that individuals were 46 % more likely to report either ART non-adherence or higher sexual risk (unprotected vaginal or anal sex with partners of HIV-negative or unknown status) when the other behavior was present. Similarly, Kalichman and Rompa [11] showed that HIV-infected adults who were non-adherent to ART had greater rates of unprotected vaginal sex, more sex partners, and fewer protected sexual behaviors than those who were adherent to ART. In a national study of HIV-infected women, Wilson et al. [12] concluded that women who reported less than 95 % adherence to ART had twice the risk of inconsistent condom use as those with higher adherence levels. Finally, in examining the inverse relationship, Diamond et al. [13] found that ART adherence (≥95 %) and viral load suppression were associated with fewer episodes of unprotected vaginal or anal sex. All of the above studies were limited by cross-sectional research methods and self-reported measures of adherence. Nonetheless, these findings reveal that ART adherence and practice of risk-reduction behaviors appear to be directly associated.
Compared to men, women may experience more difficulty adhering to ART and practicing safer sex. Although Remien et al. [10] could not clearly identify a participant group that was both non-adherent and high risk based on psychosocial or demographic characteristics, their study did reveal that being female was associated with high-risk sexual behavior. Peretti-Watel et al. [14] found that heterosexual women were more likely to report unprotected vaginal or anal sex with an HIV-negative or HIV-unknown partner, as compared to heterosexual and homosexual men. Dependence on male partners for condom use is one gender-related factor that may lower sexual risk reduction behavior in women. Another factor is depression, which may be higher in HIV-infected women than in HIV-infected men and appears to diminish adherence to ART [15] and risk reduction behavior, as well as increase substance use [12, 16–22]. Fear of stigma and negative consequences resulting from disclosure of HIV-positive status to a male partner may also complicate ART adherence and sexual risk behavior [23–25], especially if a woman depends on this partner for economic or other support. Finally, lower levels of adherence may result from the caretaker role (i.e., caring for children, sick and elderly adults, housework, etc.) that many women assume [15]. The complex pathways by which these and other gender-related factors may shape women’s vulnerability to HIV exposure are comprehensively explored in Wingood and DiClemente’s adapted model of the theory of gender and power [26]. A recent evaluation of a conceptual model based on this adapted theory supports that both direct and indirect associations exist between specific psychosocial and behavioral risk factors and use of condoms among young African American women [27].
Previous research describes other psychosocial characteristics of HIV-infected adults who adhere or do not adhere to ART and who practice or do not practice safer sex, although rarely have these behaviors been examined together using the same study sample. Besides depression and fear of stigma, characteristics associated with non-adherence or high-risk behavior among women include perceived stress, stressful life events, and binge drinking [28–30]. Characteristics associated with ART adherence include social support and disclosure of HIV-positive status to close others [31–34]. The trait of conscientiousness has also been correlated with higher adherence and lower viral loads in adults living with HIV [35]. Despite these important knowledge gains, we could find no studies that have successfully identified any psychosocial characteristics of HIV-infected women who simultaneously practice or do not practice ART adherence and risk reduction behaviors.
The purpose of this study was to identify key psychosocial and behavioral characteristics of HIV-infected women who exhibit different levels of both ART adherence and risk behavior. In a previous study of 193 HIV-infected women, we found that those who were classified as being both low adherence and high risk-taking had the lowest mean CD4 counts and highest (detectable) mean viral loads, as compared to women in the other three categories examined (i.e., low adherence/low risk, high adherence/ low risk, and high adherence/high risk) [36]. Building on this work, we sought to provide a more complete understanding of the psychosocial characteristics of women who practice high and low levels of adherence and risk behaviors. This study was exploratory and relevant theoretical/conceptual applications were dependent on the results and will be discussed later in the paper. Better knowledge regarding psychosocial factors associated with these two behavioral outcomes may lead to the design of targeted interventions that improve the long-term health of women living with HIV and prevent HIV transmission.
Methods
Study Setting and Participants
This analysis used baseline data from the Keeping Healthy and Active with Risk Reduction and Medication Adherence (KHARMA) Project, a randomized clinical trial to test the efficacy of a nurse-led, motivational group intervention compared to a health promotion group on the outcomes of ART adherence and use of risk reduction behaviors. Conducted between January 2005 and January 2008, the goal of the trial was to increase levels of both behaviors in a sample of HIV-infected women. The study enrolled women from five sites in a large urban southeastern city. After completing a baseline assessment, women were randomized to an intervention or control group and participated in an 8-week intervention. Follow-up assessments were conducted at 2 weeks and at 3, 6, and 9 months post intervention. All assessments were conducted using audio computer assisted interview (ACASI) on laptops with touch screens. This study was approved by Emory University’s Institutional Review Board and by the research committees at the recruitment sites when required. The recruitment sites, eligibility criteria, intervention, control group, and study procedures are described in previous analyses [36, 37].
For the current analysis, we examined which psychosocial characteristics contributed to ART adherence, risky behaviors, and categories of combined ART adherence and risk behaviors. We used data from the 193 of 207 enrolled participants with available adherence data from electronic drug monitoring caps at study baseline. Only baseline data were used in this analysis.
Measures
Outcome: Adherence and Risk Categories
For the primary outcome, women were categorized into groups based on levels of adherence and risky behaviors. High adherence was based on the cut-point of ≥90 versus <90 % on the “Percent of Doses Taken” from the Medication Event Monitoring System (MEMS) Track Caps report. Ninety percent was chosen as a conservative cut point due to the wide range of adherence needed for the different types of antiretroviral medications (nucleosides, nonnucleosides, boosted protease inhibitors) the participants were taking during the study time period (2005–2008). MEMS caps data were downloaded monthly during the course of the study.
Risk behavior was measured with high-low split on an 11-item risk behavior index developed from items contained in two instruments used to measure risky behavior in the KHARMA Project [36, 37]. All items were chosen because they are commonly cited outcomes in risky behavior research [38–40]. Sex during menses was included due to the high risk related to contact with menstrual blood. Similar to Susser and colleagues [38, 39], we coded each risk behavior on a 3-point scale (0 = no risk, 1 = moderate risk, 2 = high risk) relative to the level of risk associated with the behavior and scores were summed. Risk items included: sexual activity in the last 3 months (no = 0; yes = 1); number of partners (none = 0; 1 = 1; ≥2 = 2); HIV status of partners (positive = 0; negative or unknown = 1); vaginal sex in past 3 months (no = 0; yes =1); anal sex in past 3 months (no = 0; yes = 2); frequency of sex during menstrual period (never/don’t have periods = 0; almost never/sometimes = 1; every month = 2); frequency of protection/condom use in past 3 months (always = 0; sometimes to never = 2); decided not to have sex at some time in past 3 months because no protection was available or you or your partner didn’t want to use it (yes = 0; no = 1); in past 3 months frequency of getting high or drinking before sex (never = 0; occasionally = 1; often/ all the time = 2); in past 3 months did substance use make it difficult to practice safer sex (never = 0; occasionally = 1; often/all the time = 2); in the past 3 months using a needle to inject drugs (no = 0; yes = 2). Scores ranged from 0 to 18 where higher scores indicated higher risk behaviors. Scores of ≤3 were classified as low risk and ≥4 were classified as high risk. Given that the final 11 risk items were either dichotomously coded (0 or 1) or ordinally scaled (0, 1 or 2), internal consistency and reliability (alpha) were computed based on polychoric correlations using the psych package (v 1.5.6) [41] in R (v.3.2.2) [42]. The 11 items from which the risk index was computed had good internal consistency and reliability (alpha = 0.89). Women were categorized into four groups based on adherence and risk scores: high adherence/low risk (HALR), high adherence/high risk (HAHR), low adherence/low risk (LALR), and low adherence/high risk (LAHR).
Predictor Variables
We included measures of established variables that have been previously associated with ART adherence and/or risk behaviors (i.e., HIV knowledge, depression, stigma, social support, self-efficacy, outcome expectancy, and disclosure), as well as several exploratory variables (difficult life circumstances, spirituality, religious coping, satisfaction with one’s health care, self-management, health care decision-making, and motivation). Variables specifically related to ART adherence and risk behavior were each measured in terms of three dimensions: motivation, self-efficacy, and outcome expectancy. We measured motivation for ART adherence and safer sex with two adapted instruments that asked about reasons for taking HIV medications and reasons for practicing safer sex [43, 44]. Both instruments had subscales that measured internal or autonomous motivation (“I take my HIV medicine because it is an important choice I really want to make” “I practice safer sex because it is an important choice I really want to make.”) and external or controlled motivation (“I take my HIV medicine because I want others to approve of me” “I practice safer sex because I want others to approve of me.”) and were theoretically based in self-determination theory (SDT) [45, 46]. Those with maximum scores possible on each instrument were reported as having ‘perfect’ scores for that scale. The scales used for self-efficacy (self-confidence to perform the behavior under specific circumstances), and outcome expectancy (attitudes concerning expectations if one performs the behavior) were based on Bandura’s Social Cognitive Theory [47, 48]. All instruments (including subscales) and corresponding reliability statistics are presented in Table 1.
Table 1.
Study instruments and measures of reliability and descriptive statistics
| # items | Alpha coefficient | n | Mean (SD) (%) | Median | [Min, max] | |
|---|---|---|---|---|---|---|
| HIV Knowledge [49, 50] (% correct out of 45) | 45 | 0.875a | 193 | 73.5 (15.6) | 75.6 | [4.4, 100.0] |
| CESD depression [51] | 20 | 0.899 | 193 | 16.7 (11.8) | 13.0 | [0, 48.0] |
| Personal stigma [52] | 24 | 0.869 | 190 | 46.0 (10.5) | 47.5 | [24.0, 76.0] |
| Spiritual wellbeing (EWB) [53, 54] | 10 | 0.812 | 188 | 46.3 (8.2) | 46.0 | [20.0, 60.0] |
| Spiritual wellbeing (RWB) [53, 54] | 10 | 0.838 | 188 | 51.6 (7.9) | 54.0 | [22.0, 60.0] |
| Spiritual wellbeing (SWB) [53, 54] | 20 | 0.885 | 189 | 97.4 (15.4) | 98.0 | [40.0, 120.0] |
| Difficult life circumstances [55] | 33 | 0.789 | 192 | 8.4 (4.5) | 8.0 | [0.0, 22.0] |
| PRQ social support [56] | 25 | 0.903 | 189 | 134.6 (22.9) | 138.0 | [45.0, 175.0] |
| PSQIII total satisfaction [57, 58] | 50 | 0.935 | 191 | 193.5 (30.0) | 198.0 | [87.2, 250.0] |
| HIV self-management | 22 | 0.809 | 191 | 71.8 (10.3) | 74.0 | [38.0, 88.0] |
| Brief RCOPE—positive [59, 60] (≤18 vs >18) | 7 | 0.920 | 191 | 17.3 (4.2) | 19.0 | [3.0, 21.0] |
| (% scored >18) | 99 | 51.8 % | ||||
| Brief RCOPE—negative [59, 60] (≤2 vs >2) | 7 | 0.847 | 180 | 4.0 (4.5) | 3.0 | [0.0, 21.0] |
| (% scored >2) | 93 | 51.7 % | ||||
| Condom self-efficacyb [47] | 34 | 0.949 | 154 | 4.0 (0.7) | 4.0 | [1.6, 5.0] |
| HIV medication self efficacy [48] | 19 | 0.860 | 192 | 164.2 (22.9) | 169.0 | [67.0, 190.0] |
| Outcome expectancy for HIV adherence [48] | 22 | 0.910 | 192 | 86.2 (14.0) | 86.0 | [30.0, 110.0] |
| Condom outcome expectancy [47] | ||||||
| Partner reaction | 7 | 0.808 | 187 | 26.8 (5.3) | 27.0 | [11.0, 35.0] |
| Physical hedonism | 5 | 0.821 | 192 | 17.8 (4.1) | 18.0 | [6.0, 25.0] |
| Positive self evaluation (≤30 vs >30) | 7 | 0.882 | 190 | 29.9 (4.9) | 31.0 | [11.0, 35.0] |
| (% scored >30) | 96 | 50.5 % | ||||
| Prevention efficacy (<10 vs =10) | 2 | 0.750 | 192 | 8.7 (1.8) | 9.5 | [2.0, 10.0] |
| (% score =10) | 96 | 50.0 % | ||||
| Social approval (≤ 15 vs >15) | 4 | 0.923 | 173 | 16.6 (3.6) | 16.0 | [4.0, 20.0] |
| (% scored >15) | 140 | 80.9 % | ||||
| Negative self evaluation (≤11 vs >11) | 3 | 0.828 | 189 | 11.6 (3.0) | 12.0 | [3.0, 15.0] |
| (% scored >11) | 115 | 60.8 % | ||||
| Multidimensional desire for control [61] | ||||||
| Internal | 7 | 0.813 | 190 | 18.8 (7.7) | 19.0 | [7.0, 35.0] |
| External (≤ 24 vs >24) | 6 | 0.813 | 190 | 23.6 (6.1) | 25.0 | [6.0, 30.0] |
| (% scored >24) | 100 | 52.6 % | ||||
| Shared (<20 vs =20) | 4 | 0.873 | 192 | 17.9 (3.8) | 20.0 | [4.0, 20.0] |
| (% score =20) | 110 | 57.3 % | ||||
| Safer sex motivation [43, 44] | ||||||
| Internal (<91 vs =91) | 13 | 0.860 | 188 | 86.1 (8.9) | 90.0 | [25.0, 91.0] |
| (% score =91) | 86 | 45.7 % | ||||
| External | 10 | 0.901 | 187 | 45.3 (17.9) | 46.0 | [10.0, 70.0] |
| HIV adherence motivation [43, 44] | ||||||
| Internal (≤84 vs =84) | 12 | 0.759 | 193 | 78.8 (7.5) | 82.0 | [44.0, 84.0] |
| (% score =84) | 76 | 39.4 % | ||||
| External | 13 | 0.889 | 193 | 53.8 (21.0) | 55.0 | [13.0, 91.0] |
| HIV status disclosure to main partner | ||||||
| No, don’t know, refused to answer, not applicable or missingc | 64 | (33.2 %) | ||||
| Yes | 129 | (66.8 %) |
Cα is Chronbach’s alpha reliability
Cα reported for HIV Knowledge is the Kuder-Richardon-20 (KR-20) statistics since the knowledge items are dichotomously scored (correct/ incorrect)
39 subjects did not respond completely to the Condom Self-Efficacy instrument
Only 1 subject did not respond to the HIV Status Disclosure question
Analysis
All data were reviewed for completeness and normal distribution assumptions. Some outcome measures which were highly skewed or had obvious ceiling effects with 30–50 % or more of the subjects obtaining the same maximum score were dichotomized either using a median split (≤median versus >median) or using the maximum value (<max versus =max). These are noted in Table 1 when applicable. High and low adherence was defined as subjects having taken 90 % or more of their prescribed doses or below 90 % respectively. High risk was defined as having a score of 4 or higher and low risk as having a score of 3 or lower on the risk index. Two-way (2-x-2) factorial ANOVA (analysis of variance) for the continuous outcomes and generalized linear models (GLM) with binomial responses and logit-link functions (i.e. logistic regression) for the dichotomous outcomes were performed to first test the adherence-by-risk interaction effects and then the main effects of adherence and risk when no interaction effect was detected. The goal of the analyses presented here is to highlight the potential significant differences and characteristics of participants at four levels of combined adherence and risk. Thus, all models are presented as stand-alone tests—no global multivariate ANOVA model was defined a priori—no Type I error adjustment applied. Reliability statistics were computed for all measurement questionnaires and instruments including their subscales using Cronbach’s alpha or the Kuder Richardson-20 (KR-20) for the dichotomously scored HIV-Knowledge test. All statistical analyses were performed using SPSS v.21 (IBM Corporation © 2012).
Results
The KHARMA Project enrolled and randomized 207 participants. The 193 participants in this analysis did not significantly differ from the original 207 on age, race, depression, years with HIV, and years on ART. The characteristics of the participants included in this analysis are presented in Table 2. Ninety-four percent were African American with an average age of 43 years. As can be seen from employment and income, the sample as a whole is socially and materially disadvantaged. On average, women had been HIV positive for almost 10 years and on ART for 6 years. Over half were sexually active in the past 3 months. Median adherence, based on the MEMS data percent of prescribed doses taken, was 89 % of doses taken per month and median VL (log) was in the undetectable range based on criteria at the time of study recruitment of <2.6 log (corresponding to <400 copies per ml). Table 2 also gives the proportion of women for each ART adherence, risk and combined adherence/risk category. Over half the participants had low ART adherence and almost 38 % practiced high-risk behaviors. Of the four adherence/risk categories, the largest proportion of women fell into the high adherence/low risk (HALR) group and the second largest proportion was women who had low adherence/low risk (LALR). It is also worth noting that adherence and risk are associated. Relative to risk level, there were 45 % low-risk participants with low adherence compared to 66 % of high-risk participants with low adherence (χ2(1) = 7.845, p = 0.005).
Table 2.
Demographics and sample characteristics (n = 193)
| Variable | n | Mean (SD) |
|---|---|---|
| Age | 193 | 43.4 (9.1) |
| Years HIV+ | 190 | 9.6 (6.3) |
| ARV years | 193 | 6.3 (5.0) |
| Variable | n | Median (range) |
| Income | 186 | $7494 ($0, $126,000) |
| CD 4 count | 138 | 285.5 (7, 2000) |
| CD 4 percent | 137 | 17.0 (1.0, 61.3) |
| Viral load (log10) | 148 | 2.16 (1.7, 5.5) |
| Risk index | 193 | 2.0 (0, 14) |
| Percent prescribed doses taken | 193 | 88.9 % (0, 100 %) |
| CESD | 193 | 13.0 (0, 48) |
| Variable | n | % |
| Ethnicity | ||
| Black | 181 | 93.8 |
| White | 7 | 3.6 |
| Other | 5 | 2.5 |
| Education | ||
| HS or less | 143 | 74.1 |
| College or more | 50 | 25.9 |
| Employment (%yes) | 30 | 15.5 |
| Marital status | ||
| Married/committed | 52 | 27.1 |
| Never married | 52 | 27.1 |
| Separated/div/wid | 88 | 45.8 |
| Children (% yes) (range 1–9) | 160 | 82.9 |
| Sexual identity | ||
| Straight/heterosexual | 153 | 79.3 |
| Gay/homosexual | 4 | 2.1 |
| Bisexual | 8 | 4.1 |
| None of above/unsure | 28 | 14.5 |
| Sexually active (last 3 months) (% yes) | 106 | 54.9 |
| Income | ||
| ≤$10 K | 125 | 67.2 |
| >$10 K | 61 | 32.8 |
| Variable | n | % |
| Adherence | ||
| Low adherence (LA) (<90 %) | 102 | 52.8 |
| High adherence (HA) (≥90 %) | 91 | 47.2 |
| Risk index | ||
| Low risk (LR) (≤3) | 120 | 62.2 |
| High risk (HR) (>4) | 73 | 37.8 |
| Adherence/risk groupsa | ||
| HALR | 66 | 34.2 |
| HAHR | 25 | 13.0 |
| LALR | 54 | 28.0 |
| LAHR | 48 | 24.9 |
Adherence and risk are associated—(percent conditional on risk) 45.0 % low risk subjects with low adherence compared to 65.8 % of high risk subjects with low adherence: χ2(1) = 7.845, p = 0.005
Combined Levels of Adherence and Risk Interaction Effects
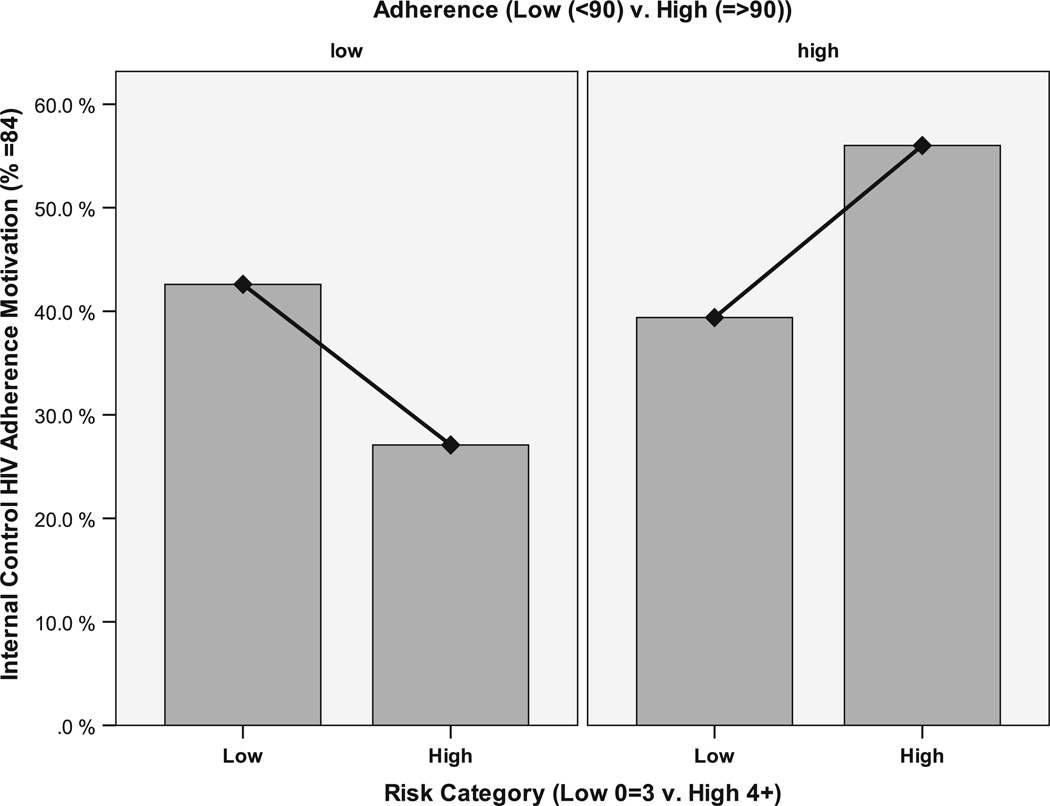
Following the procedures recommended by Dawson and Trapp, we first report the significant interaction effects and then report significant main effects for variables when no interaction effects are noted [62]. The results of comparisons by levels of adherence and risk are provided in Table 3. Significant interaction effects were seen for perfect internal adherence motivation. When we closely examined the drivers of these interaction results by adherence/risk category, the HAHR group had the largest proportion (56.0 %) of those with perfect internal motivation for adherence and the LAHR had the lowest (27.1.0 %) (Fig. 1).
Table 3.
Interaction and main effects for adherence and risk
| F-statistic (df1, df2); p value | df1 | df2 | Main effectsb |
Interaction effect | |
|---|---|---|---|---|---|
| Or Wald Chi square (df = 1)a, p value | Adherence | Risk | Adherence-x-risk | ||
| HIV knowledge (% correct out of 45) | 1 | 189 | 4.556, p = 0.034* | 10.461, p = 0.001*** | 0.393, p = 0.531 |
| CESD depression | 1 | 189 | 0.363, p = 0.547 | 2.580, p = 0.110 | 0.121, p = 0.729 |
| Personal stigma | 1 | 186 | 1.147, p = 0.286 | 0.000, p = 0.998 | 1.182, p = 0.278 |
| Spiritual wellbeing (EWB) | 1 | 184 | 0.501, p = 0.480 | 0.448, p = 0.504 | 0.090, p = 0.765 |
| Spiritual wellbeing (RWB) | 1 | 184 | 1.396, p = 0.239 | 0.047, p = 0.828 | 0.086, p = 0.770 |
| Spiritual wellbeing (SWB) | 1 | 185 | 1.132, p = 0.289 | 0.029, p = 0.865 | 0.004, p = 0.952 |
| Difficult life circumstances | 1 | 188 | 4.745, p = 0.031* | 8.317, p = 0.004** | 0.193, p = 0.661 |
| PRQ social support | 1 | 185 | 1.685, p = 0.196 | 0.020, p = 0.888 | 0.995, p = 0.320 |
| PSQIII total satisfaction | 1 | 187 | 1.403, p = 0.238 | 0.489, p = 0.485 | 1.526, p = 0.218 |
| HIV self-management | 1 | 187 | 12.954, p < 0.001*** | 0.094, p = 0.760 | 2.665, p = 0.104 |
| BRCOPE—positive (≤18 vs >18)a | − | 0.214, p = 0.643 | 0.665, p = 0.415 | 0.235, p = 0.628 | |
| BRCOPE—negative (≤2 vs>2)a | − | 0.032, p = 0.859 | 0.227, p = 0.634 | 1.194, p = 0.275 | |
| Condom self-efficacy | 1 | 150 | 0.002, p = 0.964 | 1.342, p = 0.249 | 0.922, p = 0.339 |
| HIV medication self efficacy | 1 | 188 | 10.702, p = 0.001*** | 0.000, p = 0.984 | 2.206, p = 0.139 |
| Outcome expectancy for HIV adherence | 1 | 188 | 0.026, p = 0.872 | 0.292, p = 0.589 | 0.993, p = 0.320 |
| Condom outcome expectancy | |||||
| Partner reaction | 1 | 183 | 2.847, p = 0.093∼ | 1.937, p = 0.166 | 0.182, p = 0.670 |
| Physical hedonism | 1 | 188 | 0.119, p = 0.730 | 5.082, p = 0.025* | 0.057, p = 0.812 |
| Positive self evaluation (≤30 vs >30)a | − | 0.367, p = 0.545 | 4.476, p = 0.034* | 0.065, p = 0.799 | |
| Prevention efficacy (<10 vs =10)a | − | 4.091, p = 0.043* | 0.653, p = 0.419 | 0.272, p = 0.602 | |
| Social approval (≤15 vs >15)a | − | 0.576, p = 0.448 | 1.446, p = 0.229 | 1.109, p = 0.292 | |
| Negative self evaluation (≤11 vs >11)a | − | 1.760, p = 0.185 | 12.682, p <0.001*** | 0.009, p = 0.924 | |
| Multidimensional desire for control | |||||
| Internal | 1 | 186 | 0.302, p = 0.583 | 3.030, p = 0.083∼ | 0.178, p = 0.674 |
| External (≤24 vs >24)a | − | 0.974, p = 0.324 | 0.345, p = 0.557 | 2.491, p = 0.115 | |
| Shared (<20 vs =20)a | − | 0.202, p = 0.653 | 0.351, p = 0.553 | 1.929, p = 0.165 | |
| Safer sex motivation | |||||
| Internal (<91 vs =91)a,c | − | 1.686, p = 0.194 | 5.998, p = 0.014* | 1.182, p = 0.277 | |
| External | 1 | 183 | 1.188, p = 0.277 | 0.125, p = 0.724 | 0.752, p = 0.387 |
| HIV adherence motivation | |||||
| Internal (<84 vs =84)a | − | 2.969, p = 0.085∼ | 0.001, p = 0.975 | 4.570, p = 0.033* | |
| External | 1 | 189 | 4.149, p = 0.043* | 0.663, p = 0.416 | 0.085, p = 0.771 |
| HIV status disclosure to main partnera (yes vs no, dk, rta, na) | − | 2.521, p = 0.112 | 7.183, p = 0.007** | 0.006, p = 0.936 | |
dk don’t know, rta refused to answer, na not applicable
p <0.10
p <0.05
p <0.01
p <0.001
2-x-2 factorial Generalized Linear Model was performed for these dichotomous outcome measures Using a Binomial Response with a Logit link function (i.e. logistic regression); Wald Chi square tests (df = 1) are reported
Adherence and risk are associated (χ2(1) = 7.845, p = 0.005). Both main effects and interaction effect reported for completeness
Model fit was uncertain for internal safer sex motivation (The maximum number of step-halvings was reached but the log-likelihood value cannot be further improved. Output for the last iteration was displayed)
Fig. 1.
Internal HIV adherence motivation (=84 %) by adherence and risk levels (low vs high)
Main Effects for Adherence and Risk Behaviors
Main effects associated with adherence are also shown in Table 3. Significant effects were found for HIV self-management, medication self-efficacy, external motivation for adherence, and prevention efficacy of condom use. We examined the scores of high and low adherers (data not shown in table) and note that high adherers had higher self-management (74.4, SD = 8.2, vs 69.4, SD = 11.4) and adherence self-efficacy (169.5, SD = 17.6, vs 159.5, SD = 26.0) scores. A larger proportion of high adherers had the strongest beliefs in the effectiveness of condoms to prevent HIV transmission (59.3 vs 41.6 %). Also, high adherers had lower mean scores of external motivation for adherence (50.0, SD = 21.3, vs 57.2, SD = 20.2).
Related to risk, there were significant main effects identified for several variables: physical hedonism for condom use, positive and negative self-evaluation for condom use, internal motivation for safer sex and disclosure to the main partner (see Table 3). We examined the mean scores of low and high risk women on these variables (data not shown in table). Low-risk women perceived less reduction in pleasure related to condom use (“sex doesn’t feel as good when you use a condom”) as evidenced by mean subscale scores of 18.3 (SD = 4.1) vs 16.9 (SD = 3.8). A greater proportion of the low-risk women had higher levels of negative self-evaluation outcomes for condom use (disappointed in self if didn’t use a condom; 70.3 vs 45.1 %) and positive self-evaluation (pleased with self for using condom; 57.3 vs 39.7 %). A larger proportion of low-risk women also had maximum scores on internal motivation for safer sex (53.8 vs 31.9 %). Surprisingly a significantly smaller proportion of low-risk women reported disclosing their HIV status to their main partner (60.0 vs 78.1 %).
Two variables, HIV knowledge and difficult life circumstances differed significantly by levels of adherence and risk respectively. Of interest is that significantly higher scores on HIV knowledge were seen in high risk takers compared to low (77.4, SD = 14.3, vs 71.1, SD = 15.9) and as one might expect in high adherers (75.1, SD = 4.7, vs 72.0, SD = 16.2) compared to low. A review of the data (not shown) by adherence/risk category revealed that those in the HAHR group had the highest mean knowledge scores (81.7, SD = 9.3) compared to the LALR group (69.1, SD = 16.0) which had the lowest scores.
We also found that significantly higher levels of difficult life circumstances were reported in both the low adherers (9.2, SD = 4.5, vs 7.5, SD = 4.4) and those with more risky behaviors (9.8, SD = 5.0, vs 7.5, SD = 4.0) (data not shown). When we examined this by adherence/risk category (data not shown) the HALR group reported the fewest (7.0, SD = 3.9) and the LAHR group reported the highest (10.4, SD = 4.7) occurrence of difficult circumstances.
Discussion
We sought to examine the characteristics associated with various levels of both adherence and risky behaviors in a group of highly vulnerable women (impoverished, HIV-infected, and predominantly African American) participating in a large behavioral clinical trial. Women who had low levels of adherence and reported high risk behaviors (the LAHR group) had the significantly lowest levels of internal motivation for adherence. This group was also plagued by difficult life situations.
An unexpected finding was that the HAHR group—and not the HALR group as expected—had the highest levels of internal motivation for adherence. They also had the highest HIV knowledge scores. This group also had the lowest median viral load log (1.81) putting them at lower, but not impossible risk for HIV transmission. Given the findings, the HAHR group may have consciously chosen to maintain high levels of ART adherence that resulted in low viral load, perhaps to compensate in some way for their high risk behaviors. In other words, the women may have practiced high adherence in an effort to compensate for not wanting to use condoms. The HAHR group may be less risk averse (i.e., more risk tolerant) than the HALR group. Kalichman has noted this phenomenon and describes a pathway that might describe how those highly adherent persons may perceive less infectivity and thus practice riskier behaviors [63].
Women who had low levels of adherence tended to score significantly lower on key characteristics associated with adherence such as HIV self-management, ART adherence self-efficacy, HIV knowledge, and were more externally motivated (i.e., influenced by others) to adhere. Women with higher risk behaviors also differed significantly from low-risk women on these variables; they also had significantly lower levels of internal motivation for safer sex and, though not statistically significant, higher levels of external motivation for safer sex. High-risk women tended to have more negative attitudes about various aspects of condom use, including a reduction in pleasure associated with condoms, less disappointment in themselves if didn’t use a condom, and fewer positive feelings if did use condoms. However, these women also had higher levels of HIV knowledge and disclosed their HIV status to their main partner more often than women practicing low-risk behaviors. The precise cause of this result is unknown, but perhaps it indicates that women in the high-risk category made educated decisions to practice risky behaviors, and/or were responding to demands from their partners (who were aware of their status).
Another interesting finding was the significant association of lower numbers of difficult life circumstances with those who had both better adherence and lower risk behaviors. There have been numerous reports describing particular difficult life circumstances of HIV infected women however, to the best of our knowledge, difficult life circumstances as a quantitative variable has not been examined with respect to adherence or safer sex behaviors in HIV infected women. In other samples, difficult life circumstances have been associated with abortion decision-making [64], poor pregnancy outcomes [65, 66] and negative depression coping in low income African American women [67]. Clinicians who care for HIV infected women and regularly listen to their stories will likely find this result to be understandable.
Surprisingly, depression was not significantly associated with either adherence or risk or adherence/risk group. A recent report found similar results in a diverse sample of men and women [68]. Depression has been consistently associated with poor adherence [15, 20], and in some studies higher risk behaviors among people with or at risk for HIV [69, 70]. One explanation for the non-significant finding in this study could be that depression was a common characteristic of the sample (i.e., a mean CES-D score of 16.4, just above the 16.0 cut point). Notably, the HALR group did have the lowest mean CES-D scores and their LAHR peers had the highest (15.3, SD = 12.6, vs 19.3, SD = 11.7).
Taken together, the findings suggest internal motivation for adherence plays an important role in behaviors associated with both adherence and risk. Also HIV-infected women with characteristics of low intrinsic motivation, low self-management, low self-efficacy, high external motivation, low HIV knowledge, negative attitudes about condoms, and more difficult life circumstances may be more likely to put their own health and/or that of others at risk. Theoretical concepts that might explain these results deserve more consideration. Self-determination theory (SDT) focuses on self-regulation and sources of motivation and may help to explain these motivations [45, 71–73]. SDT argues that conditions supporting individuals’ experiences of autonomy, competence, and relatedness promote high quality forms of intrinsic (internal) motivation that allow for sustained engagement in healthy behavior, including enhanced performance, perseverance, and creativity [73]. Internal or autonomous motivation, that which comes from one’s inner self, is driven by an intrinsic desire or value of the importance of a behavior. Whereas externally controlled motivation is driven by the expectations of others, or externally imposed pressures to partake in the behavior. Relying on external forces for motivation was associated with lower adherence in the LAHR group. Women in the HAHR group exhibited significantly higher levels of intrinsic motivation for adherence, which often stems from the inherent desire to do well in important behaviors. Those with ≥90 % adherence had lower levels of extrinsic motivation, higher levels of HIV self-management, and self-confidence in their ability to adhere (self-efficacy) compared to the low adherers. Self-efficacy has consistently been associated with higher levels of ART adherence.
Ego strength, another concept relevant to our findings and perhaps the inner source of intrinsic motivation, self-efficacy, and effective disease self-management, may also help explain our results. Conceptually, ego strength refers to an individual’s inner resource to cope and adapt, to be resilient in the face of adverse/stressful circumstances, to exert self-control and self-regulate, and to make appropriate choices relevant to the self [74]. Ego strength can be “measured” externally based upon one’s level of coping and adaptation (low versus high), the degree of self-control and self-regulation one is able to exert under given circumstances or conditions (depleted/decreased versus preserved/increased), and the quality of one’s choices (poor versus healthy).
For individuals with low adherence and/or high-risk behaviors, maladaptive coping and behavioral patterns are likely to emerge when ego strength and internal locus of control are diminished. In this case, coping and adaptation skills and mechanisms are more likely to be decreased, the capacity of self-control and self-regulation is more likely to fail, and poorer choices are more likely to be made, ultimately resulting in negative consequences/outcomes (such as poor adherence and engaging in high risk behaviors, and possibly more difficult life circumstances) [54]. Giola et al. [75] found that factors such as low ego strength and poor compulsion control (as well as depression and anxiety) were strongly associated with poor ART adherence. Furthermore, in patients with chronic kidney disease undergoing hemodialysis, Settineri et al. [76] found that the lower presence of assessed “ego strength” was linked to poor adherence with hemodialysis treatments, as well as the worsening of psychiatric symptoms such as demoralization and depressed mood. These authors concluded that ego strength should be increased to promote greater adherence. The maladaptive coping and behavioral patterns displayed in women in our sample with low adherence and/or high risk behaviors are consistent with low ego strength and poor intrinsic control mechanisms. Finally, risk compensation is another important issue that deserves attention and theoretically may explain the riskier behaviors despite high adherence in the high scoring intrinsically motivated HAHR group [77]. Risk compensation theory proposes that persons adjust their behavior based on their perceived level of risk. For example, behaviors that increase risk (such as unsafe sexual practices) may be offset by or compensated through behaviors intended to reduce risk (such as higher levels of ART adherence). In this manner, individuals may self-regulate to maintain a tolerable level of risk [78]. Women in the HAHR group of our study may have made a conscious, internally controlled decision to maintain high adherence (with resultant low viral loads) as a form of HIV prevention and forego other preventive behaviors.
Study Strengths and Weaknesses
The predominately African American sample, though reflective of the HIV epidemic among women in the U.S. South, may not be reflective of other HIV infected women and thus our results may not be generalizable to other non-African American HIV infected women. This study is limited by the potential for social desirability bias in reporting risky behaviors and to ameliorate this factor, we used ACASI to conduct interviews. There is also potential for ambiguity or measurement error in categorizing women as ‘high’ or ‘low’ in risk behaviors measured by self-report using the study developed risk index. Scores were visualized to avoid gross misclassification errors. The study is also limited by the cross sectional design and the fact that the original study was not designed and powered to specifically test for differences between participants in terms of their adherence and risk. Rather, the analyses presented here are observational based comparisons across a number of potential characteristics and measures in this population relative to their adherence and risk levels. Additionally, for this secondary data analysis, adherence and risk were significantly associated with each other. To fully explore the interaction effects (moderation effect) of adherence on risk, future studies might stratify for these effects during randomization.
Summary and Conclusion
We conducted a comprehensive assessment of factors related to ART medication adherence and risk behaviors in HIV infected women, both independently and combined. Based on the results presented, a pattern emerges. The women at high risk for poor health and transmitting HIV to others (LAHR) had the lowest levels of intrinsic motivation for adherence and reported more difficult life circumstances. Women’s gender roles and frequent social positioning, including caretaking and reliance on men for economic and other support [26] may promote or enhance external versus internal motivation as well as riskier behaviors.
There is also a subgroup of women (HAHR) who are highly internally motivated to adhere, highly knowledgeable about HIV transmission, yet report practicing risky behaviors. Compared to others, this group may be able to tolerate the high level of risk given their high level of adherence. Risk compensation is a persistent complication in HIV prevention and treatment as prevention efforts [77] and could be present in the HAHR group.
SDT offers an explanation for how sociocultural forces can facilitate or undermine one’s sense of motivation. SDT has been used to develop a number of interventions that assisted people to adopt healthier behaviors [44, 46, 79, 80] but only recently has it been applied to behaviors involving HIV/AIDS. Ego strength may also represent an important concept that reflects these behaviors. While it may not be possible to change an individual’s level of ego strength, it may certainly be possible to modify one’s behaviors and actions within the framework of his/her ego strength through modalities such as education, psychosocial support, counseling, psychotherapy, behavioral therapy, and motivational interviewing [75, 76]. Using these modalities to address motivations (i.e., motivational interviewing) as well as risk compensation is also possible. Based on these findings, motivation and ego strength should be emphasized and tested in future research interventions that promote adherence to ART and risk reduction behaviors for HIV infected women. Interventions could be tailored based on motivators (intrinsic vs extrinsic) and levels of key psychosocial variables. Managing potential risk compensation will also be important. Lastly, the effects of life events and circumstances can’t be ignored in this group of very vulnerable women. Interventions that include assistance with coping and social services are needed to help women cope with these significant barriers to adherence and safer behaviors.
Acknowledgments
This study was funded by the National Institutes of Nursing Research/National Institutes of Health (R01NR008094) and in part by the Emory Center for AIDS Research (P30 AI050409).
Footnotes
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J. HIV prevention. Halting HIV/AIDS epidemics. Science. 2011;334(6061):1338–1340. doi: 10.1126/science.334.6061.1338. [DOI] [PubMed] [Google Scholar]
- 3.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Effect of antiretroviral therapy on risk of sexual transmission of HIV infection and superinfection. 2009;2009:1–11. [Google Scholar]
- 5.Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197(Suppl 3):S272–S278. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- 6.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–1121. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Miller LG, Hays RD, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41(3):315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- 9.Parutti GML, Marani Toro P, et al. Long-term adherence to first-line highly active antiretroviral therapy in a hospital-based cohort: predictors and impact on virologic response and relapse. AIDS Patient Care STDs. 2006;20:48–57. doi: 10.1089/apc.2006.20.48. [DOI] [PubMed] [Google Scholar]
- 10.Remien RH, Exner TM, Morin SF, et al. Medication adherence and sexual risk behavior among HIV-infected adults: implications for transmission of resistant virus. AIDS Behav. 2007;11(5):663–675. doi: 10.1007/s10461-006-9201-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalichman SC, Rompa D. HIV treatment adherence and unprotected sex practices in people receiving antiretroviral therapy. Sex Transm Infect. 2003;79(1):59–61. doi: 10.1136/sti.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson TE, Barron Y, Cohen M, et al. Adherence to antiretroviral therapy and its association with sexual behavior in a national sample of women with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):529–534. doi: 10.1086/338397. [DOI] [PubMed] [Google Scholar]
- 13.Diamond C, Richardson JL, Milam J, et al. Use of and adherence to antiretroviral therapy is associated with decreased sexual risk behavior in HIV clinic patients. J Acquir Immune Defic Syndr. 2005;39(2):211–218. [PubMed] [Google Scholar]
- 14.Peretti-Watel P, Spire B, Schiltz MA, et al. Vulnerability, unsafe sex and non-adherence to HAART: evidence from a large sample of French HIV/AIDS outpatients. Soc Sci Med. 2006;62(10):2420–2433. doi: 10.1016/j.socscimed.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Tyer-Viola LA, Corless IB, Webel A, Reid P, Sullivan KM, Nichols P. Predictors of medication adherence among HIV-positive women in North America. J Obstet Gynecol Neonatal Nurs. 2014;43(2):168–178. doi: 10.1111/1552-6909.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayon C, Robertson K, Wolf E, et al. The prevalance of a positive screen for anxiety and/or depression in HIV-1 infected women across Western Europe and Canada-The CRANlum Study. Bethesda: 2nd international workshop on HIV & Women; 2012. Retrieved from http://regist2.virology-education.com/2012/2ndHIV&women/docs/02_vanwijk.pdf. [Google Scholar]
- 17.Mascolini M, editor. Differences between women and men on rilpivirine vs efavirenz for 96 weeks; 2nd international workshop on HIV & women; Bethesda. 2012. [Google Scholar]
- 18.Boarts JM, Sledjeski EM, Bogart LM, Delahanty DL. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253–261. doi: 10.1007/s10461-006-9069-7. [DOI] [PubMed] [Google Scholar]
- 19.Chin-Hong PV, Deeks SG, Liegler T, et al. High-risk sexual behavior in adults with genotypically proven antiretroviral-resistant HIV infection. J Acquir Immune Defic Syndr. 2005;40(4):463–471. doi: 10.1097/01.qai.0000162238.93988.0c. [DOI] [PubMed] [Google Scholar]
- 20.Starace F, Ammassari A, Trotta MP, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S136–S139. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- 21.Treisman G, Angelino A. Interrelation between psychiatric disorders and the prevention and treatment of HIV infection. Clin Infect Dis. 2007;45(Suppl 4):S313–S317. doi: 10.1086/522556. [DOI] [PubMed] [Google Scholar]
- 22.VanZile-Tamsen C, Testa M, Harlow LL, Livingston JA. A measurement model of women’s behavioral risk taking. Health Psychol. 2006;25(2):249–254. doi: 10.1037/0278-6133.25.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–482. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simbayi LC, Kalichman SC, Strebel A, Cloete A, Henda N, Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect. 2007;83(1):29–34. doi: 10.1136/sti.2006.019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wingood GM, DiClemente RJ. Application of the theory of gender and power to examin HIV-related exposures, risk factors, and effective interventions for women. Health Educ Behav. 2000;27(5):539–565. doi: 10.1177/109019810002700502. [DOI] [PubMed] [Google Scholar]
- 27.DePadilla L, Windle M, Wingood G, Cooper H, DiClemente R. Condom use among young women: modeling the theory of gender and power. Health Psychol. 2011;30(3):310–319. doi: 10.1037/a0022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottonari KA, Roberts JE, Ciesla JA, Hewitt RG. Life stress and adherence to antiretroviral therapy among HIV-positive individuals: a preliminary investigation. AIDS Patient Care STDS. 2005;19(11):719–727. doi: 10.1089/apc.2005.19.719. [DOI] [PubMed] [Google Scholar]
- 29.Golin C, Marks G, Wright J, et al. Psychosocial characteristics and sexual behaviors of people in care for HIV infection: an examination of men who have sex with men, heterosexual men and women. AIDS Behav. 2009;13(6):1129–1142. doi: 10.1007/s10461-009-9613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leserman J, Ironson G, O’Cleirigh C, Fordiani JM, Balbin E. Stressful life events and adherence in HIV. AIDS Patient Care STDS. 2008;22(5):403–411. doi: 10.1089/apc.2007.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyavaharkar M, Moneyham L, Tavakoli A, et al. Social support, coping, and medication adherence among HIV-positive women with depression living in rural areas of the southeastern United States. AIDS Patient Care STDS. 2007;21(9):667–680. doi: 10.1089/apc.2006.0131. [DOI] [PubMed] [Google Scholar]
- 32.Remien RH, Exner T, Kertzner RM, et al. Depressive symptomatology among HIV-positive women in the era of HAART: a stress and coping model. Am J Community Psychol. 2006;38(3–4):275–285. doi: 10.1007/s10464-006-9083-y. [DOI] [PubMed] [Google Scholar]
- 33.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25(1):74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stirratt MJ, Remien RH, Smith A, Copeland OQ, Dolezal C, Krieger D. The role of HIV serostatus disclosure in antiretroviral medication adherence. AIDS Behav. 2006;10(5):483–493. doi: 10.1007/s10461-006-9106-6. [DOI] [PubMed] [Google Scholar]
- 35.O’Cleirigh C, Ironson G, Weiss A, Costa PT. Conscientiousness predicts disease progression (CD4 number and viral load) in people living with HIV. Health Psychol. 2007;26(4):473–480. doi: 10.1037/0278-6133.26.4.473. [DOI] [PubMed] [Google Scholar]
- 36.Holstad MM, Diiorio C, McCarty F. Adherence, sexual risk, and viral load in HIV-infected women prescribed antiretroviral therapy. AIDS Patient Care STDS. 2011;25(7):431–438. doi: 10.1089/apc.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–896. doi: 10.1007/s10461-010-9865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susser E, Valencia E, Berkman A, et al. Reporting sexual risk behavior for hiv: a practical risk index and a method for improving risk indices. Am J Public Health. 1998;88(4):671–674. doi: 10.2105/ajph.88.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Susser EV, Valencia E, Berkman A, Sohler A, et al. Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Arch Gen Psychiatr. 1998;55:266–272. doi: 10.1001/archpsyc.55.3.266. [DOI] [PubMed] [Google Scholar]
- 40.Carmona J, Slesnick N, Guo X, Letcher A. Reducing high risk behaviors among street living youth: outcomes of an integrated prevention intervention. Child Youth Serv Rev. 2014;43:118–123. doi: 10.1016/j.childyouth.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revelle W. Psych: procedures for personality and psychological research, Version 1.5.6. Evanston: Northwestern University; 2015. [Google Scholar]
- 42.CoreTeam R. R: a language and environment for statistical computing. Vienna: Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 43.Resnicow K, Campbell MK, Carr C, et al. Body and soul a dietary intervention conducted through African-American churches. Am J Prev Med. 2004;27(2):97–105. doi: 10.1016/j.amepre.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Shaikh AR, Vinkur AD, Yaroch AL, Williams GC, Resnicow K. Direct and mediated effects of two theoretically based interventions to increase consumption of fruits and vegetables in the Healthy Body Healthy Spirit trial. Health Educ Behav. 2011;38(5):492–501. doi: 10.1177/1090198110384468. [DOI] [PubMed] [Google Scholar]
- 45.Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York: Plenum; 1985. [Google Scholar]
- 46.Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychol. 1998;17(3):269–276. doi: 10.1037//0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- 47.DiIorio C, Maibach E, O’Leary A, Sanderson CA, Celentano D. Measurement of condom use self-efficacy and outcome expectancies in a geographically diverse group of STD patients. AIDS Educ Prev. 1997;9(1):1–13. [PubMed] [Google Scholar]
- 48.Diiorio C, McCarty F, Depadilla L, et al. Adherence to antiretroviral medication regimens: a test of a psychosocial model. AIDS Behav. 2009;13(1):10–22. doi: 10.1007/s10461-007-9318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV knowledge questionnaire. AIDS Educ Prev. 2002;14(2):172–182. doi: 10.1521/aeap.14.2.172.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carey MP, Morrison-Beedy D, Johnson BT. The HIV-Knowledge Questionnaire: development and evaluation of a reliable, valid, and practical self-administered questionnaire. AIDS Behav. 1997;1(1):61–74. [Google Scholar]
- 51.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 52.Westbrook LE, Bauman LJ. Perceived stigma of HIV/AIDS scale. Bronx: Albert Einstein College of Medicine; 1996. [Google Scholar]
- 53.Ellison CW. Spiritual well-being: conceptualization and measurement. J Psychol Theol. 1983;11(4):330–340. [Google Scholar]
- 54.Paloutzian RF, Ellison C. Spiritual well-being scale. New York: Nyack; 1982. [Google Scholar]
- 55.Barnard KE. Difficult life circumstances scale. Seattle: NCAST Publications; 1994. [Google Scholar]
- 56.Brandt PA, Weinert C. The PRQ—a social support measure. Nurs Res. 1981;30(5):277–280. [PubMed] [Google Scholar]
- 57.Marshall GN, Hays RD. The patient satisfaction questionnaire short-form (PSQ-18) 1994 http://www.rand.org/content/dam/rand/pubs/papers/2006/P7865.pdf. [Google Scholar]
- 58.Marshall GN, Hays RD, Sherbourne CD, Wells KB. The structure of patient satisfaction with outpatient medical care. Psychol Assess. 1993;5(4):477. [Google Scholar]
- 59.Pargament KI, Smith BW, Koenig HG, Perez L. Patterns of positive and negative religious coping with major life stressors. J Sci Study Relig. 1998;37:710–724. [Google Scholar]
- 60.Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: Development and initial validation of the RCOPE. J Clin Psychol. 2000;56(4):519–543. doi: 10.1002/(sici)1097-4679(200004)56:4<519::aid-jclp6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Anderson LA, DeVellis RF, Boyles B, Feussner JR. Patients’ perceptions of their clinical interactions: development of the multidimensional desire for control scales. Health Educ Res. 1989;4:383–397. [Google Scholar]
- 62.Dawson B, Trapp RG. Basic & clinical biostatistics. 4th ed. New York: Lange Medical Books/McGraw-Hill; 2004. [Google Scholar]
- 63.Kalichman SC. Co-occurrence of treatment nonadherence and continued HIV transmission risk behaviors: implications for positive prevention interventions. Psychosom Med. 2008;70:593–597. doi: 10.1097/PSY.0b013e3181773bce. [DOI] [PubMed] [Google Scholar]
- 64.Jones RK, Frohwirth L, Moore AM. More than poverty: disruptive events among women having abortions in the USA. J Family Plan Reprod Health Care. 2013;39(1):36–43. doi: 10.1136/jfprhc-2012-100311. [DOI] [PubMed] [Google Scholar]
- 65.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 66.Wood L, France K, Hunt K, Eades S, Slack-Smith L. Indigenous women and smoking during pregnancy: knowledge, cultural contexts and barriers to cessation. Soc Sci Med. 2008;66(11):2378–2389. doi: 10.1016/j.socscimed.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 67.Oakley LD, Song MK, Debose-McQuirter M. Positive and negative depression coping in low-income African American women. Res Nurs Health. 2005;28(2):106–116. doi: 10.1002/nur.20061. [DOI] [PubMed] [Google Scholar]
- 68.Remien R, Dolezal C, Wagner GJ, et al. The association between poor antiretroviral adherence and unsafe sex: differences by gender and sexual orientation and implication for scale-up of treatment as prevention. AIDS Behav. 2014;18:1541–1547. doi: 10.1007/s10461-013-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. Am J Psychiatry. 2004;161(5):912–914. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- 70.Kelly JA, Murphy DA, Bahr GR, et al. Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with human immunodeficiency virus (HIV) infection. Health Psychol. 1993;12(3):215. doi: 10.1037//0278-6133.12.3.215. [DOI] [PubMed] [Google Scholar]
- 71.Deci EL, Ryan RM. The “What” and “Why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. 2009;11(4):227–268. [Google Scholar]
- 72.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 73.Ryan RM, Lynch MF, Vansteenkiste M, Deci EL. Motivation and autonomy in counseling, psychotherapy, and behavior change: a look at theory and practice. Couns Psychol. 2011;39:193. [Google Scholar]
- 74.Muraven M. Encyclopedia of social psychology. Thousand Oaks: Sage; 2007. Ego depletion. [Google Scholar]
- 75.Giola M, Quaranta B, Dalla Gasperina D, Basilico C, Tomasello L, Grossi P, editors. Adherence to antiretroviral therapy: underlying personality disorders can be more important than drugs convenience; 10th European AIDS clinical society conference.2005. [Google Scholar]
- 76.Settineri S, Mento C, Santoro D, et al. Ego strength and health: an empiric study in hemodialysis patients. Health. 2012;4(12):1328–1333. [Google Scholar]
- 77.Cassell M, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? Br Med J. 2006;332:605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinkerton SD. Sexual risk compensation and HIV/STD transmission: empirical evidence and theoretical considerations. Risk Anal. 2001;21(4):727–736. doi: 10.1111/0272-4332.214146. [DOI] [PubMed] [Google Scholar]
- 79.Hunter SD. Promoting intrinsic motivation in clients. Strength Cond J. 2008;30(1):52–54. [Google Scholar]
- 80.Neighbors C, Lewis MA, Fossos N, Grossbard JR. Motivation and risk behaviors: a self-determination perspective. In: Brown LV, editor. Psychology of motivation. Hauppauge: Nova Science Publishers; 2007. pp. 99–113. [Google Scholar]