Abstract
The worldwide epidemic of metabolic syndromes and the recognized burden of mental health disorders have driven increased research into the relationship between the two. A maladaptive stress response is implicated in both mental health disorders and metabolic disorders, implicating the hypothalamic-pituitary-adrenal (HPA) axis as a key mediator of this relationship. This review explores how an altered energetic state, such as hyper- or hypoglycemia, as may be manifested in obesity or diabetes, affects the stress response and the HPA axis in particular. We propose that changes in energetic state or energetic demands can result in “energetic stress” that can, if prolonged, lead to a dysfunctional stress response. In this review, we summarize the role of the hypothalamus in modulating energy homeostasis and then briefly discuss the relationship between metabolism and stress-induced activation of the HPA axis. Next, we examine seven mechanisms whereby energetic stress interacts with neuroendocrine stress response systems, including by glucocorticoid signaling both within and beyond the HPA axis; by nutrient-induced changes in glucocorticoid signaling; by impacting the sympathetic nervous system; through changes in other neuroendocrine factors; by inducing inflammatory changes; and by altering the gut-brain axis. Recognizing these effects of energetic stress can drive novel therapies and prevention strategies for mental health disorders, including dietary intervention, probiotics, and even fecal transplant.
Keywords: metabolic dysfunction, obesity, HPA axis, glucocorticoid, stress
1. Introduction
“If we break up a living organism by isolating its different parts it is only for the sake of ease in analysis and by no means in order to consider them separately. Indeed when we wish to ascribe to a physiological quality its value and true significance we must always refer it to this whole and draw our final conclusions only in relation to the effects in the whole.” – Claude Bernard, “An Introduction to the Study of Experimental Medicine,” 1927 (translated in Bernard (1980))
From “gut feelings” to “heart-broken” to “butterflies in your stomach,” the English language acknowledges the profound relationship between our physical and mental reactions to the external world. Though neuroscience has an historical tendency to separate its study of the nervous system and neurological disorders from the rest of the body, there is a growing awareness of the essential role that reciprocal interactions between systems may play in moderating physiology both in homeostasis and under stress. In particular, the worldwide epidemic of metabolic disorders (Swinburn et al., 2011, Ogden et al., 2014) and the recognized burden of mental health disorders (Ustun et al., 2004) have led to an increased interest in the potential relationship between the two. Multiple clinical studies have linked disorders such as depression and post-traumatic stress disorder to obesity and diabetes (Anderson et al., 2001, Weiss et al., 2011). Mechanistically, a maladaptive stress response is associated with both mental health disorders and metabolic disorders, implicating the hypothalamic-pituitary-adrenal (HPA) axis as a key mediator of this relationship. Recently, the International Diabetes Federation even highlighted the need for research into the role of the HPA axis in its review of Metabolic Syndrome (IDF, 2006).
As several other reviews (Bjorntorp and Rosmond, 2000, Rosmond, 2005, Reagan, 2012) and meta-analyses (Blaine, 2008) have focused on the potential role of stress and mental health disorders to induce alterations in metabolic function, this review will instead focus on the role of metabolic dysfunction and other disruptions to energy availability to alter the stress response and HPA axis function. Just as disruptions to the social and physical environment can promote psychosocial or mechanical stress, changes in energetic state or energetic demands can result in “energetic stress” as the body is faced with resource-based trade-offs leading to widespread effects at the cellular and systems levels (Thompson et al., 2010, Bland and Birnbaum, 2011, Miller and Hamilton, 2012). Thus, states of metabolic dysfunction, including obesity (excess energy storage), diabetes mellitus (altered usage and storage of energy), and irritable bowel syndrome (resulting in impaired uptake and usage of energy), all classify as energetic stressors. We will begin with an overview of the role of the hypothalamus in modulating energy homeostasis, and then briefly discuss the relationship between metabolism and stress-induced activation of the HPA axis. Next, we will discuss potential mechanisms whereby energetic alterations can moderate changes in the stress response (Figure 1; Table 1). Finally, we will conclude with a discussion aimed to guide future research and potential clinical therapy.
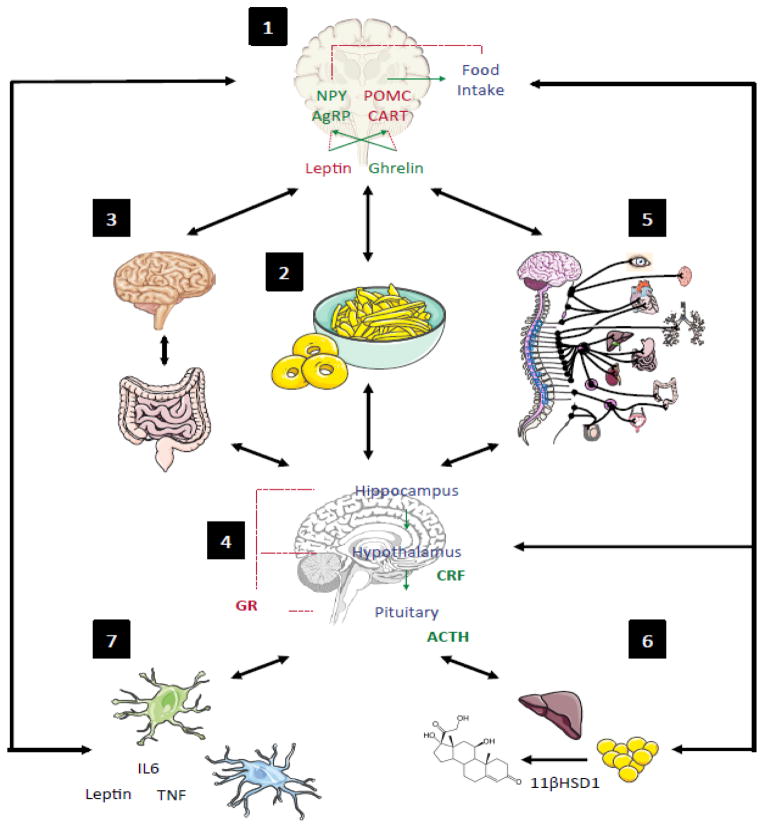
Figure 1. Disruptions to Energy homeostasis Alter the Stress Response through Multiple Mechanisms.
States of disrupted energy availability, such as hypoglycemia or obesity, can result in an impaired stress response in seven key areas. A dysfunctional response to the neuroendocrine stimulus to feed (1) may result states of energetic stress such as obesity or hypoglycemia (2), which further drives changes in the gut-brain axis (3), hypothalamic-pituitary-adrenal (HPA) axis activity (4), and sympathetic nervous system (SNS) drive (5). HPA axis SNS activity alters activity of glucocorticoids in adipose tissue (6), which feeds back to regulate feeding drive. In addition, both the HPA axis and gut-brain axis influence the immune system (7), which further alters stress reactivity and metabolic drive. Interactions among each of these elements are bidirectional and further clarified in Table 1.
Table 1.
Mediators of Energetic Stress
| Label in Fig 1 | Energetic Stress Response System | Section |
|---|---|---|
| 2 | Dietary Imbalance | 3.1 |
| 4 | Glucocorticoid Signaling within the HPA Axis | 3.2 |
| 6 | Glucocorticoid Signaling outside HPA Axis | 3.3 |
| 5 | Sympathetic Nervous System Activity | 3.4 |
| 1 | Additional Neuroendocrine and Neuropeptide Factors | 3.5 |
| 7 | Inflammation | 3.6 |
| 3 | Gut-Brain Axis | 3.7 |
2. Overview of the hypothalamic role in regulating energy and the stress response
2.1 The hypothalamus as regulator of energy availability
The responsibility for controlling energetic availability is shared by multiple peripheral organs as well as several brain regions. However, most research and interest in this field has centered on the hypothalamus. This region encompasses multiple nuclei that communicate using over 50 neurochemicals (Williams et al., 2000, Williams et al., 2001), many of which are involved in the control of energy and feeding. Historical views of the control of food intake based on lesion models have traditionally labeled the ventromedial nucleus of the hypothalamus (VMH) as the “satiety center,” while labeling the lateral hypothalamus (LH) the “hunger center” (Saper et al., 2002). However, research in the 1990s paved the way for a more nuanced understanding of energy homeostasis and body weight regulation (Zhang et al., 1994, Tartaglia et al., 1995, Fan et al., 1997, Schwartz et al., 2000, Saper et al., 2002). These advances began with identification of the gene for the adipose-derived hormone leptin and its receptor in 1994 and 1995 (Tartaglia et al., 1995) and continued as scientists gained insight into the role of ghrelin and other gut hormones as well as extended knowledge of hypothalamic peptides, such as Neuropeptide Y (NPY) and Agouti-Related Peptide (AgRP), and their receptors.
Though current knowledge is far from complete, we now understand the multiple drives that can result in feeding. These can include circadian drives, mediated through the suprachiasmatic nucleus (SCN); sensory drives, mediated by olfactory, gustatory, and mechanical stimuli; hedonic drives, mediated by the mesolimbic system and its connections with hypothalamic nuclei; and homeostatic drives, mediated by inputs from the periphery, including plasma leptin, insulin, and glucose levels, as well as other gut hormones and peptides (Saper et al., 2002). These drives converge at the hypothalamus, where they are synthesized to elicit various outputs, including endocrine outputs, primarily through the paraventricular nucleus (PVN); motor outputs, through the LH; and autonomic outputs, through the LH, PVN and arcuate nucleus (ARC) (Saper et al., 2002). In a simplified model of energy homeostasis, adiposity and satiety signals, such as leptin and insulin, signal to the arcuate nucleus to inhibit anabolic NPY/AgRP pathways while stimulating catabolic proopiomelanocortin/cocaine-and-amphetamine-related-peptide (POMC/CART) pathways relayed to the PVN and LH (Schwartz et al., 2000). These signals are simultaneously integrated with signals from additional drives mentioned above. Output from the PVN and LH converges at the nucleus of the solitary tract (NTS), where information is integrated with additional ascending input from the gut. The net output results in a signal to eat or not (Schwartz et al., 2000). However, this multi-level system of inputs and outputs lends itself easily to modulation by external factors, such as stress.
2.2 Relationship between metabolism and stress-induced activation of the HPA axis
The HPA axis is a key effector of the stress response; however, it is worth considering the numerous additional roles played by the primary output of the HPA axis, glucocorticoids. Hans Selye named glucocorticoids for their effects on liver gluconeogenesis (Szabo et al., 2012). Glucocorticoids play multiple important metabolic roles including regulation of gluconeogenesis, insulin sensitivity, glucose uptake, preadipocyte differentiation, and lipolysis (Carroll et al., 2011). Glucocorticoids, along with adrenocorticotropic hormone (ACTH), regulate the HPA axis by negative feedback via the glucocorticoid receptor (GR), transcription factors that exist on nearly every cell of the body (Panagiotakopoulos and Neigh, 2014). This negative feedback helps attenuate adrenal output in response to stressors (reviewed in Herman and Cullinan (1997), Bourke et al. (2012)).
In response to a stressor, the PVN of the hypothalamus releases corticotropin-releasing factor (CRF), which is transported through the hypophyseal portal system to the pars distalis of the anterior pituitary to stimulate secretion of adrenocorticotropic hormone (ACTH), a cleavage product of POMC. ACTH travels through the bloodstream to the adrenal cortex to stimulate glucocorticoid production, and in turn, glucocorticoids travel in the bloodstream bound to corticosteroid binding globulin (CBG). The availability of glucocorticoids in target tissues depends on the activity of 11-β hydroxysteroid dehydrogenase (11βHSD), which converts inactive forms of glucocorticoids to their active forms. Active glucocorticoids mediate their effects via GR both within the HPA axis and in additional tissues, including liver and adipose tissue (Schimmer and Funder, 2011). Exposure to chronic stress can alter GR expression (Chiba et al., 2012) or expression of GR co-chaperones that modulate feedback (Bourke et al., 2013) in key limbic brain regions to promote depressive- and anxiety-like behavior. When GR activity is altered or impaired, it can have important ramifications for metabolism as well, as will be discussed in the next sections.
3. Energetic stressors alter the HPA axis
Just as the hypothalamus and HPA axis are critical regulators of energy availability and the stress response, metabolic dysfunction, a type of energetic stressor, is associated with HPA axis impairment on multiple levels, including excessive forward drive; distorted sensitivity to negative feedback; and altered sensitivity of peripheral tissues to glucocorticoid activity (reviewed in Seimon et al. (2013)). These HPA axis disruptions can be modulated by changes in energy intake as well as changes in weight and fat distribution. In addition, numerous additional neuroendocrine mediators, including insulin, leptin, and several neuropeptides (Saper et al., 2002), as well as inflammatory factors, can both be affected by metabolic dysfunction and impact HPA axis activity. Collectively, this review will address seven key areas of research that explore the effects of energetic stressors on the HPA axis. These are: 1) effects of diet-based energetic stressors on the HPA axis; 2) energetic stress effects on HPA axis signaling; 3) energetic stress effects on glucocorticoid signaling outside the HPA axis; 4) energetic stress effects on sympathetic nervous system hyperactivity; 5) energetic stress effects on additional neuroendocrine and neuropeptide factors; 6) energetic stress effects on inflammation in the HPA axis; and 7) metabolic influences on the gut-brain axis. We will now turn our attention to explore these mechanisms. Further, we highlight some key aspects of these topics Figure 1 and in Table 1.
3.1 Dietary imbalance disrupts HPA axis signaling
Disruptions in energy balance may result in critical changes to an organism’s HPA axis signaling, and vice versa. Indeed, the insulin-hypoglycemia test, which normally stimulates CRF release, increases ACTH, and promotes cortisol secretion, has been proposed by some as “the best indicator of the integrity of the response of the HPA axis to stress” (Erturk et al., 1998). This HPA axis activation is part of the counterregulatory response to hypoglycemia, which promotes release of glucagon via glucosensitive neurons in the VMH (Borg et al., 1994).
However, situations less extreme than hypoglycemia can also induce changes in HPA axis output. In healthy subjects, food consumption alone is associated with an increase in plasma cortisol (Rosmond et al., 2000) and CRF-induced cortisol is strongly associated with increases with food intake (George et al., 2010). A study of abdominally-obese vs. peripherally-obese or healthy control women demonstrated that abdominally obese women have an attenuated cortisol response to protein & lipid-dense meals but an elevated cortisol response to carbohydrate-dense meals (Vicennati et al., 2002). In contrast, peripherally obese and control women had an elevated cortisol response to the protein and lipid-dense meals but little change in response to the carbohydrate-dense meals, indicating that the HPA axis response to macronutrients may depend in part on body fat distribution.
Diet can also alter the HPA axis mediated output in response to stress, though the literature is mixed with respect to the effects of diet on HPA axis output as well as the effects of stress on feeding. In rodent models, weight loss and reduced food intake are commonly used as markers of stressor efficacy and activation of the HPA axis, but these same stressors and/or HPA axis activation can increase intake of palatable food and visceral obesity in particular (reviewed in Dallman et al. (2003), Dallman et al. (2005), Adam and Epel (2007)). Corticosterone replacement in adrenalectomized rats promotes sucrose consumption, as does prior exposure to sucrose compared to observations of sucrose consumption in sucrose-naïve intact rats (Bell et al., 2000). Male rats exposed to restraint stress after five to seven days of access to lard, sucrose, or both have a reduced plasma ACTH and corticosterone response as well as increased food consumption (Pecoraro et al., 2004, Foster et al., 2009), indicating the potential for energy-dense foods to dampen HPA axis output in the short term. A similar effect has been observed in humans, as two weeks of thrice daily consumption of sucrose-sweetened beverages blunts the cortisol response to naltrexone in adult women (Tryon et al., 2015). However, six weeks of high-fat consumption in adult male mice increases plasma corticosterone in response to acute stress (Sharma et al., 2013), and eight weeks of a high-fat diet in combination with 21 days of chronic social defeat stress increases both basal and stress-induced plasma corticosterone (Balsevich et al., 2014). Female rhesus macaques given the choice of an energy-dense high-fat high-sugar diet for a two-week time period also have an increased cortisol response to an acute stressor (Michopoulos et al., 2012). “Low quality” food intake (high consumption of meat, saturated fat, and refined sugar) is additionally associated with high basal cortisol and impaired negative feedback in the dexamethasone suppression test in humans with type 2 diabetes mellitus (T2DM) (Duong et al., 2012). Consistent with this impaired negative feedback, eight weeks of high-fat diet exposure can reduce mRNA expression of GR and increase expression of the negative GR co-chaperone FK binding protein 51 (FKBP51) in the VMH of male mice (Balsevich et al., 2014). Upregulated FKBP51 and reduced GR in the hypothalamus may be one mechanism leading to glucocorticoid resistance after consumption of an energy-dense diet.
The GR is not the only modulator of HPA axis output affected by (and affecting) food intake. CRF appears to have dual roles with respect to food intake. Intracerebroventricular infusion of CRF can result in upregulation of pituitary POMC and concomitant weight loss or delayed weight gain in both normal weight (Hotta et al., 1991, Buwalda et al., 1997) and obese rats (Arase et al., 1989). The effects of CRF infusion on weight also appear sex-dependent, reducing food intake and promoting weight loss in male animals but not females (Rivest et al., 1989). In contrast to CRF infusion, however, transgenic overexpression of CRF can promote increased food intake and weight gain (Stenzel-Poore et al., 1996, Nakayama et al., 2011, Dedic et al., 2012), indicating receptor downregulation/desensitization or mediation of ACTH release by factors other than CRF, such as arginine vasopressin (Inui, 2000).
CRF can act on either of two receptors, CRF1 or CRF2, which are differentially sensitive to CRF and appear to play separate roles in feeding and stress regulation (Cullen et al., 2001, Bale and Vale, 2004). In mice, CRF1 receptor agonists urocortin 1 (UCN1) and stressin-A reduce food intake while increasing corticosterone, while urocortin 2 (UCN2) reduced food intake without an effect on the stress phenotype by way of a CRF2 receptor-dependent mechanism (Fekete et al., 2011). The CRF2 receptor system may be primarily responsible for anorexigenic effects of CRF or UCNs (Stengel and Tache, 2014) whereas the CRF1 receptor may be responsible for the stress-induced increase in palatable food consumption or binge-eating (Koob, 2010, Parylak et al., 2011). In support of this role for the CRF1 receptor, antagonism of CRF1 receptors in socially subordinate female rhesus macaques blocks the increase in palatable food consumption observed with access to a diverse dietary environment (Moore et al., 2015). Manipulation of a biological factor such as CRF or corticosterone can have diverse effects depending on the site of action and given different environmental exposures, such as diet.
3.2 Energetic stress alters glucocorticoid signaling at the level of the HPA axis
In addition to the specific effects of diet, changes in energy availability, notably in the form of obesity and other metabolic dysfunction, can result in significantly altered signaling within the HPA axis. Evaluation of plasma or salivary cortisol and ACTH after ACTH stimulation and dexamethasone suppression tests is used to pinpoint the location of dysfunctionality within the HPA axis (Woodworth et al., 2014), and such tests have been used to determine changes in HPA axis functionality in the context of metabolic change. Relative to women with more peripheral gluteofemoral fat distribution, viscerally obese women have elevated salivary cortisol after an ACTH stimulation test as well as attenuated salivary cortisol after a dexamethasone suppression test (Duclos et al., 2001). These data indicate a pattern of HPA axis hypersensitivity, with hyperresponsiveness of the adrenal gland to exogenous ACTH, as well as likely hyperresponsiveness at the level of the pituitary, given that it is the preferential site of action for dexamethasone (Cole et al., 2000). A similar pattern of HPA axis hypersensitivity has also been observed in men, as evening serum cortisol levels in men correlate negatively with waist-to-hip ratio (WHR), and a WHR of greater than one is associated with reduced serum cortisol in the dexamethasone suppression test (Ljung et al., 1996). In adolescent girls, elevated BMI is associated with HPA axis hypersensitivity and depression, though such a link was not observed in adolescent boys (Dockray et al., 2009). However, the literature is not entirely consistent with respect to HPA axis hypersensitivity. Premenopausal adult women with visceral obesity have been observed to have increased morning ACTH pulsatility but reduced ACTH pulse amplitude without total changes in plasma cortisol relative to healthy controls, indicating disruption at the level of the pituitary (Pasquali et al., 1998). In addition, as previously mentioned, T2DM is linked to high basal cortisol and dexamethasone non-suppression (Duong et al., 2012), and obese men have shown non-suppression of ACTH and cortisol after evening exposure to hydrocortisone (Jessop et al., 2001), indicative, in both cases, of glucocorticoid resistance and not hypersensitivity.
Given the lack of clarity achieved by examining the effects of metabolic factors on glucocorticoid signaling at a single time point, other studies have sought to examine temporal patterns of glucocorticoid signaling. As part of a series of studies led by Bjorntorp and Rosmond, researchers evaluated the association between HPA axis activity and anthropometric measures in a sample of middle-aged male subjects, reduced salivary cortisol diurnality, characterized by low morning cortisol and a blunted cortisol surge in response to a midday meal, was associated with a wide spectrum of cardiometabolic risk factors, including elevated body mass index (BMI), increased WHR, elevated fasting glucose and insulin, increased triglycerides and cholesterol, and elevated blood pressure (Bjorntorp and Rosmond, 1999). Further stratification of diurnal salivary cortisol and stress reactivity in this cohort of men indicated that three groups existed: group one which demonstrated high diurnal variability of cortisol, a typical cortisol response to food intake, and low stress reactivity; group two which also showed high diurnal variability of cortisol but in contrast to group one had elevated cortisol after food intake and demonstrated high stress reactivity (approximately a third of the cohort); and finally group three which was characterized by low diurnal cortisol variability, reduced cortisol response to food intake, and low stress reactivity (approximately a tenth of the cohort) (Bjorntorp and Rosmond, 2000). This last group (group three) with low adaptability has been termed a “burnout” condition that may follow prolonged stress. Indeed, this low adaptability is sometimes observed in individuals who have experienced prolonged severe stress (McEwen, 1999), in some cases of depression (Burke et al., 2005), and in some patients with post-traumatic stress disorder (PTSD) (Meewisse et al., 2007). While psychosocial environmental factors may have contributed to HPA axis variability in the studies performed by Bjorntorp and Rosmond, it is notable that changes in energy availability additionally reflect the pattern of HPA axis activity. The pattern of HPA axis variability associated with changes in energy availability may reflect the chronicity of disruption and correlate with alterations in the stress response. Further research will be necessary to disentangle the relative contributions of energetic and psychosocial stress on the HPA axis. Moreover, additional factors, including changes in other neuroendocrine signals or in the sympathetic nervous system, may also contribute to the HPA axis variability associated with altered metabolic status, as will be discussed subsequently.
3.3 Energetic stress alters glucocorticoid activity outside the HPA axis
In addition to its multiple effects on glucocorticoid activity within the HPA axis, disrupted energy availability can profoundly alter glucocorticoid signaling in non-neural tissue such as adipose tissue and liver. This change, in turn, may reflect back on the HPA axis by altering other neuroendocrine targets, inflammatory mediators, and the gut-brain axis (discussed subsequently). To understand the interplay between energetic stress and glucocorticoid signaling outside the HPA axis, one must first examine the metabolic role of glucocorticoids. As reviewed in Pasquali et al. (2006) and Carroll et al. (2011), glucocorticoids mobilize sources of energy to different tissues, specifically increasing lipolysis in adipose tissue and gluconeogenesis in liver while inhibiting glucose uptake and insulin receptor function. This has the end result of increasing post-absorptive and circulating glucose and free fatty acids. Additional actions of glucocorticoids include reducing calcium reabsorption; regulating gonadotrophic and growth hormone axes; immunosuppression; increasing contractility and vascular reactivity; and increasing appetite. In humans, active cortisol is synthesized from metabolism of cholesterol, and specifically through hydroxylation of 11-deoxycortisol by 11β-hydroxylase. Cortisol can then be inactivated by conversion to cortisone by 11β-hydroxysteroid dehydrogenase-2 (11βHSD2) and cortisone can be reconverted to cortisol by 11β-hydroxysteroid dehydrogenase-1 (11βHSD1). 11βHSD1 can be considered an amplifier of glucocorticoid action, and its action in liver, fat, and skeletal muscle, as well as neurons, may play an important role in the feedback of the HPA axis (reviewed in Sandeep and Walker (2001)).
Lipid accumulation and alterations in lipolytic pathways are associated with changes in glucocorticoid signaling, with tissue-specific variation in glucocorticoid-mediated effects (Xu et al., 1990, Joyner et al., 2000). Visceral adipose tissue has a four times greater density of GR than subcutaneous adipose tissue, and typically has a greater activity of GR-mediated lipoprotein lipase (LPL) (Pedersen et al., 1994). Given the role of LPL in adipocyte differentiation, this is likely one mechanism whereby glucocorticoids preferentially promote accumulation of visceral fat (reviewed in McCarty (2001)). Sex hormones interact with glucocorticoids and LPL to differentially impact fat distribution as well; estrogen enhances activity in subcutaneous adipocytes while testosterone enhances activity in visceral fat, resulting in the sex differences observed in central obesity (Rebuffe-Scrive et al., 1987, Bjorntorp, 1997, Ramirez et al., 1997). Tissue-specific changes in 11βHSD1 expression and activity also appear to play a role in obesity-induced actions on glucocorticoid signaling. While obesity is associated with a reduction in liver 11βHSD1, it is linked with an increase in adipose tissue 11βHSD1 in both humans (Rask et al., 2002) and animal models (Masuzaki et al., 2001). Transgenic overexpression of 11βHSD1 results in visceral obesity and insulin resistance (Wamil and Seckl, 2007). Conversely, 11βHSD1 knockout mice are resistant to hyperglycemia induced by either high-fat diet or psychosocial stress (Kotelevtsev et al., 1997). Moreover, 11βHSD1 may play a role in the resolution of inflammation; cytokines upregulate 11βHSD1 in adipocytes (Tomlinson et al., 2001), potentially as a counter-regulatory mechanism to induce the production of anti-inflammatory cortisol. However, this could theoretically result in a feed forward loop leading to upregulation of cortisol, which could enhance adipocyte differentiation, which may in turn result in elevations in adipokine production, and further enhancement of 11βHSD1 conversion of inactive cortisone to cortisol. Though the mechanisms remain to be elucidated, the current literature points to a role of visceral obesity resulting in elevated glucocorticoid actions, which have the potential to further disrupt HPA axis signaling.
3.4 Energetic stress induces hyperactivity of the sympathetic nervous system
The other arm of the stress axis, the sympathetic nervous system (SNS), is also modulated by disruptions to energy availability. For example, the counterregulartory response to hypoglycemia is partially mediated by release of epinephrine and norepinephrine via the SNS, which promote hepatic and renal gluconeogenesis and glycogenolysis (Cryer and Davis, 2015). In addition, SNS hyperactivity underlies the relationship between weight and essential hypertension (Landsberg, 1986, Esler et al., 2001). The majority of essential hypertension risk can be explained by obesity in both men and women (Garrison et al., 1987). Obesity results in high rates of spillover of norepinephrine from both the heart and kidneys, which are likely attributable to increases in sympathetic nerve firing rates (Esler et al., 2001). Animal models have had a key role in shaping our present understanding of pathophysiology of essential hypertension. These include the diet-induced obesity models (Dobrian et al., 2000), the obese Zucker rat (Kurtz et al., 1989), and the spontaneously hypertensive rat (Jacob et al., 1991). Increased sympathetic neural activity (SNA) is a feature common to these animal models of obesity (Rahmouni et al., 2005) and is associated with increased levels of circulating leptin and insulin occurring in parallel with decreased levels of ghrelin and adiponectin which may also contribute to SNA (Vaneckova et al., 2014). Recent work demonstrates that leptin mediates essential hypertension through its actions on hypothalamic POMC neurons leading to activation of melanocortin 4 receptors (MC4R) and stimulation of SNA (da Silva et al., 2013). Leptin also mediates SNA in peripheral tissues, including the adrenal glands, kidneys, and muscle (Dunbar et al., 1997). Selective leptin resistance may develop in obesity such that increased leptin fails to incite satiety and weight reduction, but continues to increase SNA (Mark, 2013). For example, evidence from obesity in agouti yellow mice (Correia et al., 2002) and diet-induced obesity in C57Bl/6 mice (Rahmouni et al., 2005) indicates that both exogenously administered or endogenously elevated leptin will continue to stimulate renal SNA to promote hypertension while failing to reduce food intake or sympathetically stimulate adipose tissue.
This SNS hyperactivity in obesity may have significant crosstalk with the HPA axis. Sympathetic innervation of the adrenal cortex via preganglionic cholinergic neurons in the greater splanchnic nerve enhances sensitivity to ACTH, which promotes glucocorticoid secretion (Edwards and Jones, 1987, 1993, Engeland and Arnhold, 2005). In addition, HPA axis activity augments sympathetic effects in a feed-forward manner, such as promoting vasoconstriction for maintenance of blood pressure (Ulrich-Lai and Herman, 2009). SNS hyperactivity in obesity can result in an exacerbated response to acute stress or a CRF/AVP challenge, and can occur in conjunction with HPA axis hyperactivity (Pasquali et al., 1996). These changes in SNA can have important health outcomes, as autonomic imbalance, measured as reduced heart rate variability due to increased SNA in the context of vagal activity withdrawal, is associated with increased risk of myocardial infarction (MI) in obese patients (Karason et al., 1999). Weight loss can reverse this imbalance in some cases and may be able to decrease risk of adverse cardiac outcomes (Karason et al., 1999). Intriguingly, similar changes in heart rate variability and increased risk of MI occur in the context of depression (Carney et al., 1995) and depressive symptoms increase the risk of mental-stress induced myocardial ischemia after a prior MI (Wei et al., 2014), though these risks can occur even controlling for metabolic risk factors. Taken together, disruptions to energy availability alter signaling of both axes of the stress response, and sympathetic hyperactivity may modulate the patterns of HPA axis activity observed in obesity, resulting in potentially fatal pathology.
3.5 Energetic stress disrupts signaling of neuroendocrine and neuropeptide factors that interact with the HPA axis
The previous several sections have made evident that metabolic effects on the HPA axis are caused not only through direct interactions with components of the axis, but also through indirect mediators. For example, we have discussed the manner in which adipocyte production of leptin can promote excess sympathetic nerve activity (da Silva et al., 2013), and hyperactive SNA can induce hyperactive HPA activity (Pasquali et al., 1996). Leptin is additionally an adipokine, or cytokine produced by adipose tissue and therefore has inflammatory impacts as well. The effects of inflammatory factors on the HPA axis will be further discussed in the next section. However, several other neuroendocrine and neuropeptide factors play important modulatory roles impacting the HPA axis in the context of disrupted energy homeostasis.
One category of such factors includes the melanocortins and their receptors. The melanocortins are the group of ligands derived from the precursor POMC (ACTH, α, β, and γ-melanocyte-stimulating hormone (MSH)) that bind G-protein coupled receptors that activate adenylyl cyclase (Huszar et al., 1997). MC2R is the receptor for ACTH and is primarily expressed in adrenal cortex (Mountjoy et al., 1992), while MC3R and MC4R are neural melanocortin receptors activated by agouti-related protein (AgRP) and melanocyte-stimulating hormones. Both MC4R knockout mice and POMC knockout mice display hyperphagia and weight gain (Zemel and Shi, 2000), and this pathway is believed to be the mechanism whereby the agouti gene induces obesity (Huszar et al., 1997). MC4R is also implicated in human obesity, as several mutations in the gene are associated with inherited forms of obesity (Farooqi et al., 2000). Given this relationship, there has been growing interest in the potential of MC4R agonists as potential weight loss aids; however, clinical trials have so far proven inefficacious due to lack of weight loss and non-serious adverse events (Krishna et al., 2009, Royalty et al., 2014). Interestingly, rats heterozygous for a loss-of-function mutation in MC4R have a blunted response to stress in terms of plasma ACTH and corticosterone release as well as reduced activation of the paraventricular nucleus of the hypothalamus and the medial amygdala (Ryan et al., 2014). Moreover, exposure to the intranasal MC4R antagonist HS014 prevents the development of anxiety- and depressive-like behaviors in rats (Serova et al., 2013). Thus, the melanocortins and MC4R in particular appear to play a key role in regulating the bidirectional relationship between obesity and mood disorders that remains to be further explored.
Neuropeptide Y (NPY), a 36 amino acid residue peptide of the pancreatic polypeptide family, is another modulator of the relationship between metabolic dysfunction and mood. CNS administration of NPY results in a multi-fold increase in food intake in rats (Morley et al., 1987). NPY acts on hypocretin/orexin neurons in the arcuate nucleus to promote feeding. However, the precise mechanism remains somewhat unclear because NPY appears to inhibit the activity of orexigenic hypocretin neurons while still inducing food consumption (Fu et al., 2004). Diet-induced obesity reduces hypothalamic NPY (Levin, 1999, Lin et al., 2000), seemingly part of a negative feedback loop. NPY promotes HPA axis activity, as microinjections of NPY into the PVN can stimulate ACTH and corticosterone release (Wahlestedt et al., 1987). Conversely, corticosterone is necessary for NPY elicited food intake (Stanley et al., 1989). In spite of this HPA axis activation, NPY produces anxiolytic effects in rodent models, though its behavioral effects may be due to effects on the basolateral amygdala (Heilig et al., 1993, Sajdyk et al., 2008). With respect to neuropsychiatric conditions, depression and PTSD are associated with reduced cerebrospinal NPY (Heilig et al., 2004). Conversely, administration of antidepressants (Heilig et al., 1988) increases hippocampal NPY, though hypothalamic expression of NPY in depression is less well understood. Collectively, these data suggest the possibility that obesity-induced alterations in NPY signaling contribute to the depressive- and anxiety-like behavioral changes often seen in obesity.
Obesity is not the only type of metabolic dysfunction that impacts these neuroendocrine factors; hypoglycemia can also have a significant impact of neuroendocrine peptide signaling and crosstalk with the HPA axis. For example, during a rodent model of 24-hour fasting, corticosterone levels rose significantly and correlated inversely with both plasma leptin and leptin mRNA in fat, while neuropeptide Y (NPY) mRNA in the arcuate nucleus increased across the 24-hour period (Dallman et al., 1999). These starvation-induced reductions in leptin and insulin signaling not only drive the increase in NPY, but potentially also feedback to reduce the catabolic CRF (reviewed in (Schwartz and Seeley, 1997). Likewise, glucocorticoid-driven negative-feedback similarly reduces the catabolic signals from CRF while promoting its own anabolic drive (Schwartz and Seeley, 1997), which is critical in the counterregulation to fasting.
Though a complete examination of neuropeptide and neuroendocrine factors that are altered in metabolic dysfunction is beyond the scope of this review, it should additionally be noted that factors such as thyroid hormone and growth hormone have impaired signaling in obese states (Douyon and Schteingart, 2002), and these same factors are also inhibited with hypothalamic arousal (Chrousos and Gold, 1992). Furthermore, altered signaling of both thyroid (Musselman and Nemeroff, 1996, Joffe and Marriott, 2000, Fountoulakis et al., 2004) and growth hormone (Linkowski et al., 1994) can be depressogenic. The changes in melanocortins, NPY, and additional neuropeptide and neuroendocrine factors may represent a broader state of hypothalamic remodeling that occurs in response to disruptions of energetic homeostasis to alter HPA axis activity and behavior.
3.6 Energetic stress alters the HPA axis response to inflammatory stimuli
Inflammatory stimuli, whether bacterial or viral challenges or even psychosocial stressors, are known to activate the HPA axis to potentiate hypothalamic secretion of CRF and AVP as well as adrenal glucocorticoid secretion via the actions of cytokines and chemokines (Charmandari et al., 2005, Pace and Miller, 2009). This HPA axis stimulation is evolutionarily adaptive, as the GR functions as an immune modulator not only through its activity as a transcription factor but also through direct protein-protein interactions with other transcription factors such as NfκB and AP-1 (Pariante and Lightman, 2008). However, chronic inflammation can result in long-term disruption of the HPA axis including impaired glucocorticoid negative feedback.
Like inflammatory stimuli, changes in energy availability, such as hyper- and hypoglycemia, can also trigger alterations in the immune response. While hyperglycemia is more typically associated with proinflammatory outcomes and increases plasma cytokines (Esposito et al., 2002), hypoglycemia can also impact the stress response through its effects on inflammatory mediators. Hypoglycemia not only elevates circulating cytokines such as IL-6 (Dotson et al., 2008), but it also increases expression of pro-inflammatory vascular factors including vascular cell adhesion molecule (VCAM) and intracellular adhesion molecule (ICAM)(Briscoe et al., 2010, Gogitidze Joy et al., 2010).
Similarly, states such as obesity and diabetes (Schmidt et al., 1999, Arkan et al., 2005, Hotamisligil, 2005, Herder et al., 2007), as well as in malnutrition or anorexia (Nakai et al., 1999, Kalantar-Zadeh et al., 2004), are associated with chronic inflammation. Giving recognition to the pivotal role of inflammation, the International Diabetes Federation introduced the new worldwide definition for metabolic syndrome, it highlighted the pro-inflammatory state as a key area of research to determine the predictive power of such factors as C-reactive protein (CRP), tumor necrosis factor-α (TNF), and interleukin-6 (IL6) for cardiovascular disease or diabetes (Alberti et al., 2006, IDF, 2006). These recommendations come from clinical research findings that demonstrate consistent elevations in circulating CRP, TNF, and IL6 in obesity and/or diabetes (Schmidt et al., 1999, Brunner et al., 2002, Lamers et al., 2013). At least 16 different adipokines, or chemokines or cytokines secreted by adipose tissue, are over-secreted in human obesity, including IL1, IL6, IL8, monocyte-chemotactic protein-1 (MCP-1), and leptin (Maury et al., 2007). Basic research supports the association between metabolic dysfunction and inflammation and has been able to directly link increased adipose tissue expression of TNF in particular to development of insulin resistance via TNF-mediated serine phosphorylation of insulin related substrate-1 (IRS1) (Hotamisligil et al., 1993).
This research linking metabolic dysfunction to inflammation has arisen from a growing literature demonstrating that adipose tissue is not only metabolically active, but moreover a major secretor of bioactive peptides and proteins that play a central role in energy balance and immunity (Maury and Brichard, 2010). Adiponectin, leptin, and resistin are adipokines primarily produced in adipose tissue and changes in these factors are associated with metabolic dysfunction. Adiponectin, commonly reduced in obesity, interacts with its receptors to suppress the nuclear factor κB-dependent synthesis of TNF and interferon-γ (IFNγ) while promoting production of anti-inflammatory mediators including IL-10 and IL-1Ra (Tilg and Moschen, 2006). In addition to the previously discussed effects of leptin on the sympathetic nervous system, leptin also promotes production of the pro-inflammatory cytokines TNF, IL-6, and IL-12 as well as reactive oxygen species through some of the same molecular pathways involved in SNA, included MAPK, ERK, and STAT3 signaling (Tilg and Moschen, 2006). In contrast to adiponectin and leptin, the effects of resistin on immune function and metabolism are less clear. Diet-induced models of obesity increase circulating levels of resistin in parallel with increased expression of adhesion molecules, and antibody-mediated neutralization of resistin enhances blood sugar regulation, potentially linking diabetes and obesity mechanistically (Steppan et al., 2001).
In terms of understanding the relevance of metabolically-mediated immune alterations to mental health, Lamers et al. (2013) suggest that one might be able to use biological correlates in metabolic, inflammatory, and endocrine systems to differentiate between depressive subtypes. In their study of 122 atypical depressed patients, 111 melancholic depressed patients, and 543 controls, the authors found that atypical depressed patients had increased plasma levels of the inflammatory markers CRP, TNF, and IL-6 along with a negative cardiometabolic profile characterized by high BMI, large waist circumference, elevated triglycerides, elevated fasting glucose, and a concomitant reduction in the diurnal cortisol slope (Lamers et al., 2013), perhaps indicative of the HPA axis burnout described earlier (McEwen, 1998, Bjorntorp and Rosmond, 2000, Meewisse et al., 2007). Conversely, the melancholic depressed patients showed reduced BMI and increased area under the curve for diurnal cortisol and an increased diurnal cortisol slope, indicating HPA axis hyperactivity, but did not differ from controls in any of the inflammatory markers measured (CRP, IL-6, TNF; (Lamers et al., 2013)). As a consequence of the cross-sectional design of the study, one cannot draw conclusions about the causal or directional nature of these findings; nonetheless, these findings do point to important relationships among metabolic, inflammatory, and endocrine factors that can impact behavioral phenotype. Taken together, however, it is clear that changes in energy availability are linked to chronic low-grade inflammation, and such inflammation adversely affects HPA axis function and mental health.
3.7 Energetic stress modulates the gut-brain axis to dysregulate the HPA axis
Though a recent resurgence of attention has brought it to the forefront of stress physiology, the reciprocal interaction between the brain and the gastrointestinal tract has been described since the mid-nineteenth and early twentieth century through the work of classical physiologists including Claude Bernard, Ivan Pavlov, William James, Carl Lange, and Walter Cannon (Cryan and Dinan, 2012). In “The Emotions” (Lange and James, 1922), James and Lange developed the “James-Lange theory of emotion” arguing that emotions followed the visceral reaction to a stimulus. To explain differences in susceptibility to emotional expression, James wrote, “the visceral and organic part of the expression can be suppressed in some men, but not in others, and on this it is probably that the chief part of the felt emotion depends” (p. 116) (Lange and James, 1922). Cannon alternatively refuted this primacy of the viscera, arguing that the brain principally regulated such reactions due to factors such as the slow timing of the visceral response and the similarity of visceral reactions across a wide spectrum of emotions (Cannon, 1927).
We now recognize the bidirectional nature of this interaction and thus term it the “gut-brain axis” (Cryan and Dinan, 2012). During homeostasis, the brain signals to the gut through both branches of the autonomic nervous system; the HPA axis; the sympatho-adrenal axis; and descending monoaminergic projections (Mayer, 2011). The gut, in turn, signals the brain through primary afferent neurons; immune cells, and enteroendocrine cells (Mayer, 2011). Enteroendocrine cells may communicate with the hypothalamus via either endocrine or paracrine (often vagal) signaling. Primary vagal and spinal afferent signals are integrated via the NTS and lamina I and subsequently through the thalamus and insula, and can communicate nutritional and inflammatory state information to cortical regions (Mayer, 2011).
Changes in energetic state can perturb this homeostatic relationship. A well-characterized example of this perturbation involves the CRF system in irritable bowel syndrome (IBS). Patients with IBS exhibit exacerbated intestinal motility, enhanced mast cell response, and increased gut permeability in response to systemic CRF administration (Tache and Million, 2015). In addition, IBS patients have lower basal circulating CRF relative to healthy controls but an enhanced circulating CRF and ACTH response to mental stress (Posserud et al., 2004). A rodent study identifying the differential effects of CRFR1 and CRFR2 in the gut demonstrated that peripherally-administered CRF and urocortin act via both CRFR1 and CRFR2 to inhibit gastric emptying and stimulate colonic transit and defecation, whereas urocortin 2 and 3 act through CRFR2 only to inhibit gastric emptying (Martinez et al., 2002). While in this study experimental compounds of CRF and the urocortins were administered for study, it is notable that CRF (Yuan et al., 2010) as well as the urocortins and the CRF receptors (Muramatsu et al., 2000) are produced in colonic tissue. In animal models of acute stress, both peripheral and central CRFR1 stimulate gastric emptying (Tache and Perdue, 2004), and early life stress increases both gut permeability and this CRF-mediated response to acute stress (Soderholm et al., 2002). Anxiety and depression are highly comorbid with IBS, and the enhanced neuroendocrine responses to stress may play a key role in this comorbidity (Mayer et al., 2001).
More recent literature has implicated the gut microbiome in both metabolic dysfunction and mood disorders. The human gastrointestinal tract comprises between 1013 and 1014 microorganisms that collectively give rise to more than 100 times the number of genes in the human genome (Gill et al., 2006). The mammalian microbiome is composed primarily of the gram-positive Bacteroidetes and Proteobacteria as well as the gram-negative Firmicutes and Actinobacteria phyla with lower abundance of Fusobacteria, and Verrucomicrobia (Eckburg et al., 2005, Gill et al., 2006, Tilg and Kaser, 2011). As humans are born with a relatively sterile gut, the microbiome is developed throughout childhood and into adulthood (Koenig et al., 2011). This development can be influenced by such factors as diet, illness, antibiotic treatment, and other environmental exposures. Notably, a high-fat diet can shift the mouse microbiome towards a greater predominance of Firmicutes, and this shift is both reversible (Turnbaugh et al., 2008) and can occur in the absence of obesity (Hildebrandt et al., 2009). In the absence of a high-fat diet, obesity in ob/ob mice is also associated with an increase in Firmicutes with a relative decrease in Bacteroidetes compared with lean mice (Ley et al., 2005). The microbiome in obese mice enables greater energy harvest than in lean mice, and transfer of caecal microbiota from ob/ob mice to germ-free mice is sufficient to produce an obese phenotype (Turnbaugh et al., 2008). In humans, obesity is associated with a decrease in phylogenetic diversity of the microbiome, with approximately 75% of obesity-associated genes derived from Actinobacteria (vs. 0% of lean-associated genes) and 25% from Firmicutes (Turnbaugh et al., 2009). In contrast, 42% of lean-associated genes in humans derive from Bacteroidetes, compared with 0% of obesity-associated genes (Turnbaugh et al., 2009). Though research is in the nascent stage and evidence is most convincing for its efficacy to treat inflammatory bowel disease (Anderson et al., 2012), fecal microbiota transplantation has shown some promise in increasing insulin sensitivity in obese men (Vrieze et al., 2012). While much work remains to be done with respect to the regulatory role of diet, genetic background, and other environmental factors, it is clear that the gut microbiome has a strong influence on metabolic phenotype.
In addition to its impact on energy availability, the microbiome has a bidirectional relationship with the development of the stress response and behavioral outcomes. Stress can alter the microbiome, like high-fat diet, to decrease the relative abundance of Bacteroidetes and increase circulating levels of the inflammatory cytokines IL-6 and MCP-1 (Bailey et al., 2011). These effects are blocked by administration of antibiotics, indicating the necessity of microbiota in moderating the response to social disruption stress (Bailey et al., 2011). However, just as stress can alter the species composition of the microbiome, the composition of the microbiome may also influence behavior and the stress response (Bailey et al., 2011). Germ-free (GF) mice exhibit reduced anxiety-like behaviors relative to specific-pathogen free (SPF) mice with normal gut microbiota, and colonization of adult germ-free mice fails to reverse their behavior (Diaz Heijtz et al., 2011). However, offspring of GF mice exposed to gut microbiota exhibit anxiety-like behavior in the elevated plus maze similar to SPF mice (Diaz Heijtz et al., 2011), indicating the presence of a critical period for the microbiome to influence behavior. This critical period of microbiotic influence has also been demonstrated with respect to HPA axis development. Intriguingly in light of their behavior, GF mice exhibit greater ACTH and corticosterone responses to restraint stress than SPF mice (Sudo et al., 2004). Colonization of GF mice with Bifidobacterium infantis reduces this stress response while Escherichia coli enhances it. Colonization with gut microbiota from SPF mice can also reduce this enhancement in the HPA axis response, and colonization earlier in development is more effective (Sudo et al., 2004).
Thus, it appears that the gut microbiome may be a crucial link in the bidirectional gut-brain axis, both by determining levels of low-grade inflammation (Bailey 2011) and in altering HPA axis reactivity (Sudo 2004). While the precise mechanisms remain unclear, it is possible that stress increases in intestinal permeability, allowing gut bacteria direct access to the enteric nervous system and immune cells (reviewed in (Foster and McVey Neufeld, 2013)); Alternatively, certain bacteria may alter the signaling of the vagus nerve through the nucleus of the tractus solitarus to key limbic regions that modulate stress reactivity. This hypothesis is supported by data indicating that ingestion of L. rhamnosus alters GABA-ergic signaling in the hippocampus, amygdala, and prefrontal cortex, which correlates with changes to stress-induced corticosterone levels (Bravo et al., 2011). Continued efforts to manipulate the gut microbiome to combat obesity and alter the HPA axis and stress response, such as by using probiotics and fecal transplants will shed more light on the relative importance of these proposed mechanisms (Dinan et al., 2013). In combination with progressively expanding knowledge of the gut microbiome’s role in regulating energy balance, such research may result in novel treatments to target disorders associated with both metabolic and mental health outcomes.
4. Discussion
Throughout this review, we have discussed the potential for perturbations in energy availability (primarily in obesity and diabetes) to alter the stress response. Though we have analyzed this relationship between energy availability and the stress response and mechanisms for disruption in separate sections, we must heed Claude Bernard’s advice in drawing our final conclusions with respect to the effects on the whole organism. Clearly, energy balance is maintained through multiple organ systems, not only through actions of the hypothalamus. These systems include the digestive system, the immune system, the musculoskeletal system (including adipose tissue), the endocrine system, and several arms of the nervous system. Two arms, in turn, regulate the stress response: the swift-acting sympathetic nervous system, and the longer acting HPA axis. Given the wealth of literature examining the role of the HPA axis in energy homeostasis (Dallman et al., 2005, Rosmond, 2005), the bulk of this review has focused on interactions of metabolic dysfunction with this axis.
We began by exploring three potential mechanisms relating directly to the HPA axis that mediate the relationship between changes in energy availability and the stress response. First, diet has a reciprocal relationship with the HPA axis, as palatable diet can both dampen and enhance reactivity while HPA axis activity can alternatively promote or inhibit consumption of palatable or non-palatable food (Section 3.1). However, this relationship is altered depending on the energetic status of the individual, and metabolic dysfunction can impair this balance. Next, such energetic stress may not only alter HPA axis activity at a single time point, but may also shift whole signaling patterns as well (Section 3.2). Changes in diurnal cortisol variability are linked to changes in the cortisol response to food as well as stress reactivity, and different patterns may represent different stages of allostatic load to “burnout” (McEwen, 1998, Rosmond et al., 2000). Glucocorticoid signaling outside the HPA axis is also altered by disrupted energy homeostasis, resulting in the potential for glucocorticoid-mediated shifts toward fuel storage in visceral adipose tissue, which can in turn feedback to affect HPA axis activity (Section 3.3).
We subsequently examined several other systems that mediate the relationship between energy availability and the stress response. Energetic stress results in SNS hyperactivity, changing the crosstalk between the SNS and the HPA axis (Section 3.4). Further, multiple neuroendocrine and neuropeptide factors in addition to glucocorticoids and the SNS respond to energetic stressors, particularly POMC/melanocortins and NPY. In turn, this altered neuropeptide and neuroendocrine signaling can promote excessive ACTH and glucocorticoid stimulation (Section 3.5). Energetic stress also stimulates low-grade inflammation through some of these neuroendocrine factors, such as leptin, as well as other through other adipokines and cytokines. This inflammation can subsequently have adverse effects on HPA axis function and mental health (Section 3.6). Finally, energetic stress alters the gut-brain axis by altering activity of peripheral CRF receptors as well as changing the gut microbiome, which feeds back to affect brain function (Section 3.7).
Collectively, these multiple mechanisms represent areas of future research and target areas for potential therapeutics. The need for better treatments is high; with respect to depression, 50–60% of patients fail to achieve an adequate response to antidepressant treatment (Fava, 2003). Indeed, treatment of mental health disorders may be complicated by comorbid disorders such as obesity or diabetes (Fava, 2003), and some classes of medications, such as atypical antipsychotics, can have unfavorable effects on metabolism (Nasrallah, 2003). If direct links can be established between failures in energy homeostasis and mental health outcomes, then targeting the disruption in these links is a potential avenue to improved therapy. For example, dietary shifts such as adopting a “Mediterranean” diet, have been proposed to assist in mental health therapy (Psaltopoulou et al., 2013). The “Mediterranean” diet may be effective in the context of depression because of its high content of omega-3 fatty acids, which have been shown to alleviate depressive symptoms in some patients (Freeman and Rapaport, 2011). Interestingly, omega-3 fatty acid therapy may be most effective in patients with a higher inflammatory profile, linking the role of diet to systemic inflammation and mental health (Rapaport et al., 2015). L-methylfolate (Papakostas et al., 2012) is a compound administered adjunctively with conventional antidepressant therapy to remediate deficits in micronutrient status that may otherwise contribute to treatment resistance in depressed patients.
Of course, the best cure is prevention. Understanding the role of modifiable risk factors in energy availability and stress reactivity could ultimately lead to better policies and prevention strategies that might reduce the burden of metabolic dysfunction and mental health disorders from the start. Diet may not only play a role as an adjuvant to therapy, but it may be essential to preventing the exacerbation of mood disorders as well as metabolic disorders. The most recent nutritional guidelines from the United States Department of Agriculture and the Department of Human Health and Services called for significant limitations to dietary added sugars. While multiple dietary patterns, from low-carbohydrate to low-protein, can be effective at inducing weight loss in the short term with mixed results beyond one year (USDA), particular macronutrients may have specific effects on other aspects of metabolism or stress reactivity. For example, carbohydrates and proteins or fats elicit opposing effects on HPA axis reactivity in women with different body morphologies (Vicennati et al., 2002). Simple sugars, and fructose in particular, are known to induce a spike in plasma corticosterone in rodent models (Brindley et al., 1981, Brindley et al., 1985). However, there is a dearth of research into the specific effects of macro- and micronutrient status on brain function. Bridging the gap between nutritional research and the neuroscientific understanding of the relationship between energy availability and the stress response may lead to novel strategies for prevention to guide policy-makers and clinicians.
Recently, growing interest in caloric restriction (CR), brought about by the possible beneficial role of CR in aging (reviewed in (Heilbronn and Ravussin, 2003)), have shown that CR may help reduce expression of circulating (Wang et al., 2013) and hypothalamic cytokines (Diane et al., 2015). The mechanism of CR-mediated reductions in inflammation remains unclear, though sirtuins, NAD-dependent deacetylases, may play a role (reviewed in (Haigis and Guarente, 2006, Kitada and Koya, 2013)). Intriguingly, recent research has also demonstrated that sirtuins Sirt1, Sirt2, and Sirt6 were reduced in peripheral white blood cells of patients with major and bipolar depression (Abe et al., 2011), but the mechanistic link between sirtuins and mood disorders remains unclear. Sirt1 can alter GR signaling in muscle cells by inhibiting GR-induced transcription of uncoupling protein-3, which occurs in the context of LPS-stimulation (Amat et al., 2007). However, the relationship among CR, sirtuins, and inflammation in the context of HPA axis dysregulation and mood disorders remains unstudied. Further research into the specific actions of sirtuins and SIRT1 in particular may bring novel therapeutic targets into view.
In moving forward, researchers should pay special attention to the potential sex differences that can affect both energy availability and stress reactivity. Body morphology differs significantly between males and females, with females typically carrying greater gluteofemoral and subcutaneous fat (Westerbacka et al., 2004, Lovejoy et al., 2009). While there is generally a slightly lower population prevalence of obesity in men, obese men are at a higher risk of obesity-related chronic disease, due, in part, to the visceral accumulation of fat (Lovejoy et al., 2009). Women generally have a better cholesterol profile and greater insulin sensitivity than men (Cnop et al., 2003), though age-related changes in hormonal status (such as menopause) may moderate this effect (Lovejoy et al., 2009). At the same time, women are at a roughly two-fold risk of lifetime depression (Kessler et al., 2003). Men, however, are not protected from psychiatric disorders and are two to three times more likely than women to abuse drugs (Becker and Hu, 2008). In this review, significant sex differences were noted in the effects of CRF on weight (Rivest et al., 1989) as well as in the actions of LPL and glucocorticoids on adipose tissue (Rebuffe-Scrive et al., 1989). In addition, several disorders that alter metabolism, such as IBS (Saito et al., 2002) and autoimmune disorders like Hashimoto’s and Graves’ Disease (Whitacre, 2001), affect women at substantially higher rates than men. Greater research is needed to understand the specifics of how these sex differences in metabolic and mental health outcomes manifest, and to determine if these potential relationships offer new avenues for therapies.
The role that development plays in regulating the relationship between energy availability and the stress response is also understudied. Developmental growth must, by definition, alter energy homeostasis, as the organism is in a state of flux. The current obesity epidemic is not limited to adults, as today over 20% of American adolescents are obese (Ogden et al., 2010, Ogden et al., 2014) and rates of type 2 diabetes mellitus are increasing among youth (Nadeau and Dabelea, 2008). Adolescence appears to be a “critical period” for determining energy homeostasis; evidence shows that high BMI during adolescence predicts overweight and obesity in adulthood; for example, the probability that a15-year-old with a BMI at the 95th percentile will be overweight at age 35 is 0.93 and, if male, the probability that he will be obese is 0.50 or, if female, 0.60 (Guo et al., 2002). The concept of “critical periods” of development with respect to stress reactivity has also been examined across developmental periods, including both prenatal (Pankevich et al., 2009, Tamashiro et al., 2009) and adolescent (Bourke and Neigh, 2011, McCormick et al., 2013) periods. Adolescence may represent a critical period for crosstalk between energetic regulatory systems, the HPA axis, and the hypothalamic-pituitary-gonadal axis, as each of these systems undergo extensive change during adolescence. Expanding research to include these key developmental stages when examining energy availability and stress reactivity will be essential toward understanding the emergence of a disrupted relationship and developing targeted therapies.
With such diverse effects stemming from changes in energetic availability, it would be overly simplistic to imply that the mechanistic relationships among each component are fully understood. However, better understanding the multiple links among these factors will enable better design of future experiments to target key areas that should be explored in more depth. Figure 1 partially outlines a possible series of relationships among the various components. A dysfunctional response to neuroendocrine signals to stimulate eating results in an energetic stressor (for example, hyper- or hypoglycemia). This energetic stressor activates the HPA axis and the sympathetic nervous system (Erturk et al., 1998); and may also stimulate stress responsive neurons in the enteric nervous system (Tache and Perdue, 2004), or perhaps affect the gut microbiome (Dinan and Cryan, 2012). Glucocorticoid activity alters metabolism as well as the distribution of adipose tissue (Pasquali et al., 2006), which then feeds back to alter both the HPA axis and stress reactivity as well as the original neuroendocrine stimulus to feed. In addition, diet-induced changes in the gut microbiome may interact with HPA axis dysfunction to promote low-grade inflammation, which is implicated in both changes in stress reactivity and in metabolic outcomes (Bailey et al., 2011, Lamers et al., 2013). As such, energetic stress has widespread effects across multiple systems that interact to modulate both stress reactivity and metabolism.
In conclusion, changes to energy availability induce energetic stress, which alter the stress response through a multifaceted approach. Energetic stress disrupts HPA axis activity and glucocorticoid signaling outside the HPA axis; promotes hyperactivity of the sympathetic nervous system; changes neuroendocrine and neuropeptide signaling; alters inflammatory responses; and perturbs the gut-brain axis. Greater research is needed to clarify the roles that sex and sex hormones, as well as developmental status, play in modulating these effects. Addressing these areas of research will provide new avenues for potential therapy. Similar to thoughts expressed by Thomas A. Edison nearly 100 years ago, “The doctor of the future will give no medication, but will interest his patients in the care of the human frame, diet and in the cause and prevention of disease,” we propose that by researching the effects of modifiable risk factors, such as diet, we may be able to circumvent the need for therapy by promoting prevention.
Table 2.
Effects of Energetic Stress on the HPA Axis and Stress Response
| Feature of Energetic Stress | Effects with Respect to the HPA Axis and the Stress Response |
|---|---|
| Dietary Imbalance | Visceral obesity blunts the cortisol response post-ingestion of protein and lipids, but increases the response post-ingestion of carbohydrates (Vicennati et al., 2002). Excess fat or sugar consumption blunts the glucocorticoid response to stress (Pecoraro et al., 2004, Foster et al., 2009, Tryon et al., 2015). Excess fat or sugar consumption elevates the glucocorticoid response to stress (Duong et al., 2012, Michopoulos et al., 2012, Sharma et al., 2013, Balsevich et al., 2014). |
| Central Glucocorticoid Signaling | Visceral obesity is linked with HPA axis hypersensitivity (Ljung et al., 1996, Duclos et al., 2001, Dockray et al., 2009). Visceral obesity is linked to glucocorticoid resistance (Jessop et al., 2001, Duong et al., 2012). Negative cardiometabolic profile is associated with reduced cortisol variability, low morning cortisol, and blunted cortisol response to midday meal (Bjorntorp and Rosmond, 1999). |
| Peripheral Glucocorticoid Signaling | Testosterone enhances lipoprotein lipase activity in visceral fat, resulting in sex differences in visceral obesity (Rebuffe-Scrive et al., 1987, Bjorntorp, 1997, Ramirez et al., 1997). Obesity increases adipose 11β-hydroxysteroid dehydrogenase-1 (11βHSD1) (Masuzaki et al., 2001, Rask et al., 2002), which converts inactive cortisone to active glucocorticoids. Increased 11βHSD1 may increase insulin resistance (Kotelevtsev et al., 1997, Wamil and Seckl, 2007) and promote inflammation (Tomlinson et al., 2001). |
| Sympathetic Nervous System (SNS) | SNS hyperactivity links obesity to essential hypertension (Landsberg, 1986, Garrison et al., 1987, Kurtz et al., 1989, Jacob et al., 1991, Dobrian et al., 2000, Esler et al., 2001, Rahmouni et al., 2005). Obesity-associated SNS hyperactivity may be caused by increased circulating leptin and insulin alongside reduced ghrelin and adiponectin (Vaneckova et al., 2014). Leptin resistance fails to incite satiety but continues to promote SNS activity (Mark, 2013). SNS hyperactivity stimulates the adrenal cortex and can exacerbate the response to stress (Pasquali et al., 1996). |
| Additional Neuroendocrine and Neuropeptide Factors | MC4R knockout mice are hyperphagic (Zemel and Shi, 2000) and have a blunted response to stress (Ryan et al., 2014). Diet-induced obesity reduces NPY, which promotes feeding (Levin, 1999, Lin et al., 2000). NPY is anxyiolytic in rodent models (Heilig et al., 1993, Sajdyk et al., 2008) though glucocorticoids activate NPY to elicit food intake (Stanley et al., 1989). Obesity alters thyroid and growth hormone signaling (Douyon and Schteingart, 2002), and which can be depressogenic (Linkowski et al., 1994, Musselman and Nemeroff, 1996, Joffe and Marriott, 2000, Fountoulakis et al., 2004). |
| Inflammation | Obesity or diabetes (Schmidt et al., 1999, Arkan et al., 2005, Hotamisligil, 2005, Herder et al., 2007) as well as malnutrition or anorexia (Nakai et al., 1999, Kalantar-Zadeh et al., 2004) are proinflammatory and elevate CRP, TNF, and IL6. At least 16 different adipokines are oversecreted in obesity (Maury et al., 2007). Increased adipose TNF is directly linked to insulin resistance (Hotamisligil et al., 1993). Atypical depression is linked to a negative cardiometabolic profile, altered diurnal cortisol, and increased CRP, TNF, and IL6 (Lamers et al., 2013). |
| Gut-Brain Axis | IBS patients have lower basal CRF but increased CRF response to stress (Posserud et al., 2004). High-fat diet (Turnbaugh et al., 2008) and obesity (Hildebrandt et al., 2009) increase gut Firmicutes and reduce gut Bacteroidetes in rodents. In humans, obesity reduces phylogenetic diversity of the gut (Turnbaugh et al., 2009). Stress also alters the mouse gut microbiome and reduces Bacteroidetes (Bailey et al., 2011) and exposure of germ-free mice to gut microbiota induces anxiety-like behavior (Diaz Heijtz et al., 2011). |
Acknowledgments
GNN was supported by K18MH105098. CSH was supported by the American Heart Association Training Grant 14PRE18910002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Uchida S, Otsuki K, Hobara T, Yamagata H, Higuchi F, Shibata T, Watanabe Y. Altered sirtuin deacetylase gene expression in patients with a mood disorder. Journal of psychiatric research. 2011;45:1106–1112. doi: 10.1016/j.jpsychires.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology & behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine : a journal of the British Diabetic Association. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2012;36:503–516. doi: 10.1111/j.1365-2036.2012.05220.x. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Arase K, Shargill NS, Bray GA. Effects of corticotropin releasing factor on genetically obese (fatty) rats. Physiology & behavior. 1989;45:565–570. doi: 10.1016/0031-9384(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain, behavior, and immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Balsevich G, Uribe A, Wagner KV, Hartmann J, Santarelli S, Labermaier C, Schmidt MV. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. The Journal of endocrinology. 2014;222:15–26. doi: 10.1530/JOE-14-0129. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. Journal of neuroendocrinology. 2000;12:461–470. doi: 10.1046/j.1365-2826.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- Bernard C. An Introduction to the Study of Medicine. Birmingham, AL: Classics of Medicine Library; 1980. [Google Scholar]
- Bjorntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic syndrome X. Annals of the New York Academy of Sciences. 1999;892:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. The metabolic syndrome--a neuroendocrine disorder? The British journal of nutrition. 2000;83(Suppl 1):S49–57. doi: 10.1017/s0007114500000957. [DOI] [PubMed] [Google Scholar]
- Blaine B. Does depression cause obesity?: A meta-analysis of longitudinal studies of depression and weight control. Journal of health psychology. 2008;13:1190–1197. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- Bland ML, Birnbaum MJ. Cell biology. ADaPting to energetic stress. Science. 2011;332:1387–1388. doi: 10.1126/science.1208444. [DOI] [PubMed] [Google Scholar]
- Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. The Journal of clinical investigation. 1994;93:1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Cooling J, Glenny HP, Burditt SL, McKechnie IS. Effects of chronic modification of dietary fat and carbohydrate on the insulin, corticosterone and metabolic responses of rats fed acutely with glucose, fructose or ethanol. Biochem J. 1981;200:275–283. doi: 10.1042/bj2000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Saxton J, Shahidullah H, Armstrong M. Possible relationships between changes in body weight set-point and stress metabolism after treating rats chronically with D-fenfluramine. Effects of feeding rats acutely with fructose on the metabolism of corticosterone, glucose, fatty acids, glycerol and triacylglycerol. Biochem Pharmacol. 1985;34:1265–1271. doi: 10.1016/0006-2952(85)90504-0. [DOI] [PubMed] [Google Scholar]
- Briscoe VJ, Griffith ML, Davis SN. The role of glimepiride in the treatment of type 2 diabetes mellitus. Expert Opin Drug Metab Toxicol. 2010;6:225–235. doi: 10.1517/17425250903512955. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Van Kalkeren AA, Koolhaas JM. Physiological and behavioral effects of chronic intracerebroventricular infusion of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1997;22:297–309. doi: 10.1016/s0306-4530(97)00032-2. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The James-Lange Theory of Emtions: A Critical Examination and an Alternative Theory. The American Journal of Psychology. 1927;39:106–124. [PubMed] [Google Scholar]
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- Carroll T, Aron D, Findling J, Tyrrell B. Glucocorticoids and Adrenal Androgens. In: Gardner D, Shoback D, editors. Greenspan’s Basic & Clinical Endocrinology. New York, NY: McGraw-Hill; 2011. [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA : the journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–167. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cryer P, Davis S. Hypoglycemia. In: DK, et al., editors. Harrison’s Principles of Internal Medicine. New York, NY: McGraw-Hill; 2015. [Google Scholar]
- Cullen MJ, Ling N, Foster AC, Pelleymounter MA. Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology. 2001;142:992–999. doi: 10.1210/endo.142.3.7989. [DOI] [PubMed] [Google Scholar]
- da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Current opinion in nephrology and hypertension. 2013;22:135–140. doi: 10.1097/MNH.0b013e32835d0c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain, behavior, and immunity. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dedic N, Touma C, Romanowski CP, Schieven M, Kuhne C, Ableitner M, Lu A, Holsboer F, Wurst W, Kimura M, Deussing JM. Assessing behavioural effects of chronic HPA axis activation using conditional CRH-overexpressing mice. Cellular and molecular neurobiology. 2012;32:815–828. doi: 10.1007/s10571-011-9784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diane A, Pierce WD, Mangat R, Borthwick F, Nelson R, Russell JC, Heth CD, Jacobs RL, Vine DF, Proctor SD. Differential expression of hypothalamic, metabolic and inflammatory genes in response to short-term calorie restriction in juvenile obese- and lean-prone JCR rats. Nutr Diabetes. 2015;5:e178. doi: 10.1038/nutd.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biological psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. J Adolesc Health. 2009;45:344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson S, Freeman R, Failing HJ, Adler GK. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes care. 2008;31:1222–1223. doi: 10.2337/dc07-2243. [DOI] [PubMed] [Google Scholar]
- Douyon L, Schteingart DE. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol Metab Clin North Am. 2002;31:173–189. doi: 10.1016/s0889-8529(01)00023-8. [DOI] [PubMed] [Google Scholar]
- Duclos M, Gatta B, Corcuff JB, Rashedi M, Pehourcq F, Roger P. Fat distribution in obese women is associated with subtle alterations of the hypothalamic-pituitary-adrenal axis activity and sensitivity to glucocorticoids. Clinical endocrinology. 2001;55:447–454. doi: 10.1046/j.1365-2265.2001.01384.x. [DOI] [PubMed] [Google Scholar]
- Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- Duong M, Cohen JI, Convit A. High cortisol levels are associated with low quality food choice in type 2 diabetes. Endocrine. 2012;41:76–81. doi: 10.1007/s12020-011-9527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. The effect of splanchnic nerve stimulation on adrenocortical activity in conscious calves. The Journal of physiology. 1987;382:385–396. doi: 10.1113/jphysiol.1987.sp016373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. Autonomic control of adrenal function. J Anat. 1993;183(Pt 2):291–307. [PMC free article] [PubMed] [Google Scholar]
- Engeland WC, Arnhold MM. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28:325–332. doi: 10.1385/ENDO:28:3:325. [DOI] [PubMed] [Google Scholar]
- Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. The Journal of clinical endocrinology and metabolism. 1998;83:2350–2354. doi: 10.1210/jcem.83.7.4980. [DOI] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. American journal of hypertension. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. The Journal of clinical investigation. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biological psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Szucs A, Sabino V, Cottone P, Rivier J, Vale WW, Koob GF, Zorrilla EP. Systemic urocortin 2, but not urocortin 1 or stressin1-A, suppresses feeding via CRF2 receptors without malaise and stress. British journal of pharmacology. 2011;164:1959–1975. doi: 10.1111/j.1476-5381.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Iacovides A, Grammaticos P, St Kaprinis G, Bech P. Thyroid function in clinical subtypes of major depression: an exploratory study. BMC psychiatry. 2004;4:6. doi: 10.1186/1471-244X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Rapaport MH. Omega-3 fatty acids and depression: from cellular mechanisms to clinical care. The Journal of clinical psychiatry. 2011;72:258–259. doi: 10.4088/JCP.11ac06830. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Preventive medicine. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- George SA, Khan S, Briggs H, Abelson JL. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology. 2010;35:607–612. doi: 10.1016/j.psyneuen.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes care. 2010;33:1529–1535. doi: 10.2337/dc09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. The American journal of clinical nutrition. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes & development. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. The American journal of clinical nutrition. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Wahlestedt C, Ekman R, Widerlov E. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur J Pharmacol. 1988;147:465–467. doi: 10.1016/0014-2999(88)90182-3. [DOI] [PubMed] [Google Scholar]
- Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M, Asberg M, Ekman R, Wahlestedt C, Agren H. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. Journal of psychiatric research. 2004;38:113–121. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, Bredahl R, Hahnloser E, Martin S. Low-grade inflammation, obesity, and insulin resistance in adolescents. The Journal of clinical endocrinology and metabolism. 2007;92:4569–4574. doi: 10.1210/jc.2007-0955. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1711–1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Yamauchi N, Ohno H, Benoit R, Ling N, Demura H. The effects of chronic central administration of corticotropin-releasing factor on food intake, body weight, and hypothalamic-pituitary-adrenocortical hormones. Life Sci. 1991;48:1483–1491. doi: 10.1016/0024-3205(91)90186-f. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Alberti G, et al., editors. IDF. The IDF Worldwide Consensus Definition of Metabolic Syndrome. Brussels, Belgium: 2006. p. 24. [Google Scholar]
- Inui A. Transgenic approach to the study of body weight regulation. Pharmacological reviews. 2000;52:35–61. [PubMed] [Google Scholar]
- Jacob HJ, Lindpaintner K, Lincoln SE, Kusumi K, Bunker RK, Mao YP, Ganten D, Dzau VJ, Lander ES. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell. 1991;67:213–224. doi: 10.1016/0092-8674(91)90584-l. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Dallman MF, Fleming D, Lightman SL. Resistance to glucocorticoid feedback in obesity. The Journal of clinical endocrinology and metabolism. 2001;86:4109–4114. doi: 10.1210/jcem.86.9.7826. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Marriott M. Thyroid hormone levels and recurrence of major depression. The American journal of psychiatry. 2000;157:1689–1691. doi: 10.1176/appi.ajp.157.10.1689. [DOI] [PubMed] [Google Scholar]
- Joyner JM, Hutley LJ, Cameron DP. Glucocorticoid receptors in human preadipocytes: regional and gender differences. The Journal of endocrinology. 2000;166:145–152. doi: 10.1677/joe.0.1660145. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19:1507–1519. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity Survey R. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kitada M, Koya D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab J. 2013;37:315–325. doi: 10.4093/dmj.2013.37.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R, Gumbiner B, Stevens C, Musser B, Mallick M, Suryawanshi S, Maganti L, Zhu H, Han TH, Scherer L, Simpson B, Cosgrove D, Gottesdiener K, Amatruda J, Rolls BJ, Blundell J, Bray GA, Fujioka K, Heymsfield SB, Wagner JA, Herman GA. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: energy intake predicts lack of weight loss efficacy. Clinical pharmacology and therapeutics. 2009;86:659–666. doi: 10.1038/clpt.2009.167. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Morris RC, Pershadsingh HA. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13:896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular psychiatry. 2013;18:692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. The Quarterly journal of medicine. 1986;61:1081–1090. [PubMed] [Google Scholar]
- Lange CG, James W. The Emotions. Baltimore, MD: 1922. [Google Scholar]
- Levin BE. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am J Physiol. 1999;276:R382–387. doi: 10.1152/ajpregu.1999.276.2.R382. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain research. 2000;875:89–95. doi: 10.1016/s0006-8993(00)02580-4. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L’Hermite-Baleriaux M, Leclercq R, Brasseur M, Mendlewicz J, Van Cauter E. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Archives of general psychiatry. 1994;51:616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- Ljung T, Andersson B, Bengtsson BA, Bjorntorp P, Marin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obesity research. 1996;4:277–282. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Sainsbury A Stock Conference Working G. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- Mark AL. Selective leptin resistance revisited. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305:R566–581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier JE, Vale W, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM. Adipokines oversecreted by omental adipose tissue in human obesity. American journal of physiology Endocrinology and metabolism. 2007;293:E656–665. doi: 10.1152/ajpendo.00127.2007. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nature reviews Neuroscience. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. American journal of physiology Gastrointestinal and liver physiology. 2001;280:G519–524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Modulation of adipocyte lipoprotein lipase expression as a strategy for preventing or treating visceral obesity. Medical hypotheses. 2001;57:192–200. doi: 10.1054/mehy.2001.1317. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR, Cameron NM, Nixon F, Levy MJ, Clark RA. Deficits in male sexual behavior in adulthood after social instability stress in adolescence in rats. Horm Behav. 2013;63:5–12. doi: 10.1016/j.yhbeh.2012.11.009. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual review of neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012;37:1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Hamilton KL. A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2012;302:E496–499. doi: 10.1152/ajpendo.00578.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Johnson ZP, Higgins M, Toufexis D, Wilson ME. Antagonism of Corticotrophin-Releasing Factor Type 1 Receptors Attenuates Caloric Intake of Free Feeding Subordinate Female Rhesus Monkeys in a Rich Dietary Environment. Journal of neuroendocrinology. 2015;27:33–43. doi: 10.1111/jne.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol. 1987;252:R599–609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Nemeroff CB. Depression and endocrine disorders: focus on the thyroid and adrenal system. Br J Psychiatry Suppl. 1996:123–128. [PubMed] [Google Scholar]
- Nadeau K, Dabelea D. Epidemiology of type 2 diabetes in children and adolescents. Endocrine research. 2008;33:35–58. doi: 10.1080/07435800802080138. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Hamagaki S, Takagi R, Taniguchi A, Kurimoto F. Plasma concentrations of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in patients with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1999;84:1226–1228. doi: 10.1210/jcem.84.4.5589. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Nishiyama M, Iwasaki Y, Shinahara M, Okada Y, Tsuda M, Okazaki M, Tsugita M, Taguchi T, Makino S, Stenzel-Poore MP, Hashimoto K, Terada Y. Corticotropin-releasing hormone (CRH) transgenic mice display hyperphagia with increased Agouti-related protein mRNA in the hypothalamic arcuate nucleus. Endocrine journal. 2011;58:279–286. doi: 10.1507/endocrj.k10e-370. [DOI] [PubMed] [Google Scholar]
- Nasrallah H. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology. 2003;28(Suppl 1):83–96. doi: 10.1016/s0306-4530(02)00114-2. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA : the journal of the American Medical Association. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA : the journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Annals of the New York Academy of Sciences. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN. Development of the HPA axis: where and when do sex differences manifest? Front Neuroendocrinol. 2014;35:285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Mueller BR, Brockel B, Bale TL. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiology & behavior. 2009;98:94–102. doi: 10.1016/j.physbeh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. The American journal of psychiatry. 2012;169:1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in neurosciences. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiology & behavior. 2011;104:149–156. doi: 10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Anconetani B, Chattat R, Biscotti M, Spinucci G, Casimirri F, Vicennati V, Carcello A, Labate AM. Hypothalamic-pituitary-adrenal axis activity and its relationship to the autonomic nervous system in women with visceral and subcutaneous obesity: effects of the corticotropin-releasing factor/arginine-vasopressin test and of stress. Metabolism: clinical and experimental. 1996;45:351–356. doi: 10.1016/s0026-0495(96)90290-5. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Biscotti D, Spinucci G, Vicennati V, Genazzani AD, Sgarbi L, Casimirri F. Pulsatile secretion of ACTH and cortisol in premenopausal women: effect of obesity and body fat distribution. Clinical endocrinology. 1998;48:603–612. doi: 10.1046/j.1365-2265.1998.00458.x. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Annals of the New York Academy of Sciences. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Jonler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. The Journal of clinical endocrinology and metabolism. 1994;78:1354–1359. doi: 10.1210/jcem.78.6.8200937. [DOI] [PubMed] [Google Scholar]
- Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Annals of neurology. 2013;74:580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- Ramirez ME, McMurry MP, Wiebke GA, Felten KJ, Ren K, Meikle AW, Iverius PH. Evidence for sex steroid inhibition of lipoprotein lipase in men: comparison of abdominal and femoral adipose tissue. Metabolism: clinical and experimental. 1997;46:179–185. doi: 10.1016/s0026-0495(97)90299-7. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. The Journal of clinical endocrinology and metabolism. 2002;87:3330–3336. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications. Experimental neurology. 2012;233:68–78. doi: 10.1016/j.expneurol.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Andersson B, Olbe L, Bjorntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism: clinical and experimental. 1989;38:453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Lonnroth P, Marin P, Wesslau C, Bjorntorp P, Smith U. Regional adipose tissue metabolism in men and postmenopausal women. International journal of obesity. 1987;11:347–355. [PubMed] [Google Scholar]
- Rivest S, Deshaies Y, Richard D. Effects of corticotropin-releasing factor on energy balance in rats are sex dependent. Am J Physiol. 1989;257:R1417–1422. doi: 10.1152/ajpregu.1989.257.6.R1417. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Holm G, Bjorntorp P. Food-induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24:416–422. doi: 10.1038/sj.ijo.0801173. [DOI] [PubMed] [Google Scholar]
- Royalty JE, Konradsen G, Eskerod O, Wulff BS, Hansen BS. Investigation of safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of a long-acting alpha-MSH analog in healthy overweight and obese subjects. Journal of clinical pharmacology. 2014;54:394–404. doi: 10.1002/jcph.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Mul JD, Clemmensen C, Egan AE, Begg DP, Halcomb K, Seeley RJ, Herman JP, Ulrich-Lai YM. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. doi: 10.1016/j.psyneuen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. The American journal of gastroenterology. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep TC, Walker BR. Pathophysiology of modulation of local glucocorticoid levels by 11beta-hydroxysteroid dehydrogenases. Trends in endocrinology and metabolism: TEM. 2001;12:446–453. doi: 10.1016/s1043-2760(01)00499-4. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Schimmer B, Funder J. ACTH, Adrenal Steroids, and Pharmacology of the Adrenal Cortex. In: Brunton L, et al., editors. Goodman & Gillman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2011. [Google Scholar]
- Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ. Seminars in medicine of the Beth Israel Deaconess Medical Center. Neuroendocrine responses to starvation and weight loss. The New England journal of medicine. 1997;336:1802–1811. doi: 10.1056/NEJM199706193362507. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seimon RV, Hostland N, Silveira SL, Gibson AA, Sainsbury A. Effects of energy restriction on activity of the hypothalamo-pituitary-adrenal axis in obese humans and rodents: implications for diet-induced changes in body composition. Hormone molecular biology and clinical investigation. 2013;15:71–80. doi: 10.1515/hmbci-2013-0038. [DOI] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Sabban EL. Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behavioural brain research. 2013;250:139–147. doi: 10.1016/j.bbr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes (Lond) 2013;37:1183–1191. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. American journal of physiology Gastrointestinal and liver physiology. 2002;283:G1257–1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Lanthier D, Chin AS, Leibowitz SF. Suppression of neuropeptide Y-elicited eating by adrenalectomy or hypophysectomy: reversal with corticosterone. Brain research. 1989;501:32–36. doi: 10.1016/0006-8993(89)91023-8. [DOI] [PubMed] [Google Scholar]
- Stengel A, Tache Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front Neurosci-Switz. 2014:8. doi: 10.3389/fnins.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Duncan JE, Rittenberg MB, Bakke AC, Heinrichs SC. CRH overproduction in transgenic mice: behavioral and immune system modulation. Annals of the New York Academy of Sciences. 1996;780:36–48. doi: 10.1111/j.1749-6632.1996.tb15110.x. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Szabo S, Tache Y, Somogyi A. The legacy of Hans Selye and the origins of stress research: a retrospective 75 years after his landmark brief “letter” to the editor# of nature. Stress. 2012;15:472–478. doi: 10.3109/10253890.2012.710919. [DOI] [PubMed] [Google Scholar]
- Tache Y, Million M. Role of Corticotropin-releasing Factor Signaling in Stress-related Alterations of Colonic Motility and Hyperalgesia. J Neurogastroenterol Motil. 2015;21:8–24. doi: 10.5056/jnm14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thompson ME, Muller MN, Kahlenberg SM, Wrangham RW. Dynamics of social and energetic stress in wild female chimpanzees. Hormones and behavior. 2010;58:440–449. doi: 10.1016/j.yhbeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. The Journal of clinical investigation. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Moore J, Cooper MS, Bujalska I, Shahmanesh M, Burt C, Strain A, Hewison M, Stewart PM. Regulation of expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue: tissue-specific induction by cytokines. Endocrinology. 2001;142:1982–1989. doi: 10.1210/endo.142.5.8168. [DOI] [PubMed] [Google Scholar]
- Tryon MS, Stanhope KL, Epel ES, Mason AE, Brown R, Medici V, Havel PJ, Laugero KD. Excessive Sugar Consumption May Be a Difficult Habit to Break: A View From the Brain and Body. The Journal of clinical endocrinology and metabolism. 2015:jc20144353. doi: 10.1210/jc.2014-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Vaneckova I, Maletinska L, Behuliak M, Nagelova V, Zicha J, Kunes J. Obesity-related hypertension: possible pathophysiological mechanisms. The Journal of endocrinology. 2014;223:R63–78. doi: 10.1530/JOE-14-0368. [DOI] [PubMed] [Google Scholar]
- Vicennati V, Ceroni L, Gagliardi L, Gambineri A, Pasquali R. Comment: response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and high-carbohydrate meals in women with different obesity phenotypes. The Journal of clinical endocrinology and metabolism. 2002;87:3984–3988. doi: 10.1210/jcem.87.8.8718. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Skagerberg G, Ekman R, Heilig M, Sundler F, Hakanson R. Neuropeptide Y (NPY) in the area of the hypothalamic paraventricular nucleus activates the pituitary-adrenocortical axis in the rat. Brain Res. 1987;417:33–38. doi: 10.1016/0006-8993(87)90176-4. [DOI] [PubMed] [Google Scholar]
- Wamil M, Seckl JR. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug discovery today. 2007;12:504–520. doi: 10.1016/j.drudis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Vanegas SM, Du X, Noble T, Zingg JM, Meydani M, Meydani SN, Wu D. Caloric restriction favorably impacts metabolic and immune/inflammatory profiles in obese mice but curcumin/piperine consumption adds no further benefit. Nutrition & metabolism. 2013;10:29. doi: 10.1186/1743-7075-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PloS one. 2014;9:e102986. doi: 10.1371/journal.pone.0102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, Ressler KJ. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. General hospital psychiatry. 2011;33:135–142. doi: 10.1016/j.genhosppsych.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen AM, Fredriksson J, Yki-Jarvinen H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiology & behavior. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59:385–396. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- Woodworth A, Lakhani V, Aleryani SL, Laposata M. The Endocrine System. In: Laposata M, editor. Laboratory Medicine: The Diagnosis of Disease in the Clinical Laboratory. McGraw-Hill; 2014. [Google Scholar]
- Xu X, De Pergola G, Bjorntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229–1234. doi: 10.1210/endo-126-2-1229. [DOI] [PubMed] [Google Scholar]
- Yuan PQ, Wu SV, Wang L, Tache Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322–331. doi: 10.1016/j.peptides.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel MB, Shi H. Pro-opiomelanocortin (POMC) deficiency and peripheral melanocortins in obesity. Nutrition reviews. 2000;58:177–180. doi: 10.1111/j.1753-4887.2000.tb01857.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]