Abstract
Studies of infants at risk for autism spectrum disorder (ASD) have proliferated, but few of these samples have been followed longer-term. We conducted a follow-up study, at age 5.5-9 years, of younger siblings of children with ASD (high-risk group, n=79) or typical development (low-risk group, n=60), originally recruited as infants. Children with ASD were excluded because of the focus on understanding the range of non-ASD outcomes among high-risk siblings. Using examiner ratings, parent ratings, and standardized assessments, we evaluated differences in clinical outcomes, psychopathology symptoms, autism symptoms, language skills, and nonverbal cognitive abilities. After adjusting for covariates, the high-risk group had increased odds of any clinically elevated/impaired score across measures relative to the low-risk group (43% vs. 12%, respectively). The high-risk group also had increased odds of examiner-rated Clinical Concerns (CC) outcomes (e.g., ADHD concerns, broader autism phenotype, speech-language difficulties, anxiety/mood problems, learning problems) relative to the low-risk group (38% vs. 13%, respectively). The high-risk group with CC outcomes had higher parent-reported psychopathology and autism symptoms, and lower directly-assessed language skills, than the Low-Risk Typically Developing (TD) and High-Risk TD groups, which did not differ. There were no differences in nonverbal cognitive skills. For some in the high-risk group, clinical concerns persisted from early childhood, whereas for others clinical concerns were first evident at school-age. Results suggest continued vulnerability in at least a subgroup of school-age children with a family history of ASD and suggest that this population may benefit from continued screening and monitoring into the school-age years.
Keywords: Autism spectrum disorder, broader autism phenotype, psychopathology, siblings, school-age
In an effort to enhance early detection efforts, investigations of infant siblings of children with autism spectrum disorder (ASD) have proliferated in recent years. These studies follow infants with (high-risk) and without (low-risk) an older sibling with ASD from before the first birthday through around 3 years of age, with the goal of identifying early signs of ASD. Such studies have revealed recurrence rates of ASD of nearly 20% in younger siblings (Ozonoff et al., 2011), and have also found that those siblings who do not develop ASD are at increased risk for the development of other atypical outcomes in the toddler years (Messinger et al., 2013; Ozonoff et al., 2014). Yet very few of these samples have been followed into school-age, making it difficult to determine whether differences found during the toddler years are time-limited, or whether they persist later in development. This has also made it difficult to determine whether new difficulties might emerge in additional domains (e.g., psychopathology) that become increasingly relevant or apparent during later developmental periods.
The study of infant siblings of children with ASD who do not go on to develop ASD themselves is important for several reasons. First, a focus on these children has potential to inform screening and intervention needs of a broader group of children at risk for suboptimal outcomes, with the goal of reducing the number who display later difficulties. Second, research focused on characterizing the longer-term outcomes of this group could provide important data for genomic and neurobiological studies of both the broader autism phenotype (BAP) and ASD, as well as studies of individuals at risk for a variety of neurodevelopmental and mental health conditions.
A range of non-typical developmental outcomes has been documented during the toddler years among younger siblings of children with ASD who do not develop ASD themselves. These outcomes span multiple developmental domains including language, cognition, social communication, and broader aspects of behavioral functioning (see, for a review, Jones, Gliga, Bedford, Charman, & Johnson, 2014). Collectively, difficulties in these areas are consistent with the BAP, a constellation of subclinical ASD-like characteristics (e.g., social difficulties, language delays, rigidity in personality or behavior) seen at elevated rates in family members of individuals with ASD (see Bailey, Palferman, Heavey, & LeCouteur, 1998; Sucksmith, Roth, & Hoekstra, 2011).
As a construct, the BAP has been recognized for many years, but only recently have there been efforts to identify its earliest manifestations. A recent study found that 28% of a high-risk infant sibling sample (who did not, themselves, develop ASD) demonstrated non-typical development in cognitive, motor, receptive/expressive language, and social domains at 36 months of age (Ozonoff et al., 2014). Similarly, in a partially overlapping sample, 35% of children in the high-risk group who did not develop ASD were identified with pragmatic language impairment at 36 months of age using a parent report measure (Miller et al., 2015). Recently, a large, international, multi-site infant sibling study also found that high-risk siblings who did not develop ASD showed higher levels of autism symptoms and lower developmental functioning than low-risk siblings at 36 months of age (Messinger et al., 2013). Additionally, 21% of the high-risk siblings were classified into latent classes that were characterized by high levels of autism symptoms and/or lower developmental abilities (Messinger et al., 2013), consistent with the BAP. Thus it appears that, in addition to the 18.7% of infants who develop ASD themselves (Ozonoff et al., 2011), a substantial proportion of the remaining high-risk infant siblings also go on to develop other non-typical outcomes, spanning multiple developmental domains, by 36 months of age.
Although these studies highlight the increased risk of the development of non-typical outcomes among younger siblings of children with ASD, whether such early difficulties show developmental continuity or discontinuity has largely remained unclear. To address this question, longer-term follow-up studies of infant sibling samples are necessary, but only a few such studies have been conducted. Of those that have, Gamliel, Yirmiya, Jaffe, Manor, and Sigman (2009) found, in a sample of 37 high-risk and 47 low-risk children, that 40% of the high-risk group (versus 16% of the low-risk group) showed difficulties in cognitive, language, or academic domains at age 7. This study also found that, for some of the high-risk children, these difficulties showed continuity from early in life, whereas for others, early difficulties resolved over time, and for still others, new difficulties emerged at age 7 (Gamliel et al., 2009). Ben-Yizhak and colleagues (2011) followed this same sample further into early preadolescence (ages 9-12), finding lower pragmatic language skills in a small subgroup of the high-risk sample characterized by BAP-related difficulties. Additionally, Drumm and colleagues (2015) evaluated language abilities in a small sample of high-risk siblings without ASD followed into the school-age years and found poorer performance in phonological memory and awareness relative to the standardized tests’ normative samples. However, a recent study of an independent sample focused on early joint attention predictors of school-age pragmatic and structural language skills found no differences in these language measures between the high- and low-risk groups at school-age (Gillespie-Lynch et al., 2013).
Approaches to evaluating outcomes in such samples have differed, with some focusing on comparisons between the high-risk and low-risk groups, and others focusing on particular subgroups within high-risk samples (e.g., those characterized by BAP traits). This may result, to some degree, in inconsistencies of findings, which are also evident in the larger literature focusing on siblings and other first-degree relatives of children with ASD outside of the context of the infant sibling study design. For example, some studies have found lower cognitive or language skills in siblings of individuals with ASD relative to siblings of typically developing children (for a review specific to language, see Drumm & Brian, 2014), some have found no differences (Gizzonio, Avanzini, Fabbri-Destro, Campi, & Rizzolatti, 2014; Pilowsky, Yirmiya, Shalev, & Gross-Tsur, 2003), and some have even raised the possibility of higher cognitive and language skills in siblings of children with ASD (Fombonne et al., 1997). Similarly, some studies have found higher levels of behavioral problems in siblings of children with ASD relative to siblings of children without ASD (Hastings, 2003; Ross & Cuskelly, 2006) and some have found no differences (Quintero & McIntyre 2010; Verté et al., 2003). The impact of different methodological strategies on results is clearly demonstrated in a recent study of siblings of individuals with ASD in adulthood (Howlin et al., 2015). They found that, when measured as a group, siblings were functioning in the average range across most domains, with few group differences. However, when subgroups were examined separately, the adults who had been characterized by the BAP in earlier in life showed difficulties in social relationships, lower occupational attainment, and significant mental health problems as adults (Howlin et al., 2015), with two-thirds rated as having poor or very poor mental health outcomes, including moderate-to-severe depression and moderate-to-severe ADHD.
Overall, the prior literature suggests that at least a subgroup of younger siblings of children with ASD, who do not develop ASD themselves, are at heightened risk for a variety of developmental difficulties, and that this vulnerability may extend into the school-age years and potentially even beyond. However, most of the later follow-up studies of infant siblings have consisted of smaller samples and have not included assessments of domains beyond those more directly related to ASD symptomatology (i.e., social behavior, language skills). Thus, we sought to extend these prior findings in a larger, two-site infant sibling sample using a multidimensional, multi-informant approach, evaluating performance across key domains of functioning during the school-age years (autism symptoms, psychopathology, language skills, and cognitive ability). Notably, there are various approaches to characterizing functioning among children. Empirical-quantitative approaches tend to capitalize on the use of standardized rating scales and normed tests to inform group categorization – a “bottom up” approach – whereas clinical-diagnostic approaches use the expertise of highly trained clinicians to categorize functioning in a “top down” manner (see Kasius et al., 1997). Prior studies have suggested that, although there is convergence between these approaches, each provides unique information (Bellina et al., 2013; Ferdinand et al., 2004; Kasius et al., 1997). Thus, we employed both approaches in this study.
The present investigation had three primary goals, addressed in a sample of school-age children originally ascertained as infants, 79 of whom had an older sibling diagnosed with ASD (high-risk group), and 60 of whom had typically developing older siblings (low-risk group). First, we took the empirical-quantitative approach, using standardized assessments and rating scales and published cutoffs, to determine the rates of dysfunction in the domains of cognitive and language skills, psychopathology, and ASD symptoms. Second, taking a clinically-driven approach, we examined the rates of expert-defined clinical concerns (CC) outcomes among children at high and low risk for ASD and the domains in which those with and without CC outcomes differed. Finally, to address the question of continuities and discontinuities over time, we examined correspondence between CC outcomes at 36-months and school-age. We hypothesized that: (1) using an empirical approach, the high-risk group would evidence higher rates of any clinically elevated or impaired score across measures; and (2) using a clinical approach, the high-risk group would evidence higher rates of CC outcomes relative to the low-risk group, and that the high-risk group with CC outcomes would demonstrate greater impairment across each domain assessed relative to the low-risk and high-risk groups without CC outcomes. We did not make specific hypotheses regarding correspondence between 36-month and school-age outcomes given that such data were examined descriptively.
Method
Participants
The sample was drawn from a larger prospective longitudinal study of younger siblings of children with ASD (high-risk group) or typical development (low-risk group) conducted at two sites. For the purposes of this study, and in line with multiple prior investigations taking a similar approach (Ben-Yizhak et al., 2011; Drumm et al., 2015; Gamliel et al., 2009; Messinger et al., 2013), we excluded the small group of children with school-age ASD outcomes because the research questions motivating this study focus on the range of non-typical outcomes in high-risk siblings beyond ASD, given the potential implications for screening and intervention1. Additionally, inclusion of the small number of children with ASD outcomes could bias risk-group contrasts. For the high-risk group, diagnosis of the affected older sibling was confirmed by meeting ASD criteria on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and the Social Communication Questionnaire (SCQ; Rutter et al., 2003); exclusion criteria included birth before 32 weeks gestation or a known genetic disorder in the older sibling. Low-risk status was confirmed by an intake screening questionnaire and older sibling SCQ scores below the ASD range. Exclusion criteria for this group included birth before 37 weeks gestation; developmental, learning, or medical conditions in older siblings; and ASD in first-, second-, or third-degree relatives.
Participants were originally enrolled before 18 months of age (mean age at enrollment=7.5 months, SD=5.5 months; 71% enrolled by 6 months). When participants reached the ages of between 4 and 9 years (mean=6.62 years, SD=1.01), they were invited back for a follow-up visit. Of the originally-recruited sample (n=327), 188 participants (57%) were seen at school-age (n=74/100 [74%] of the original low-risk group, n=114/227 [50%] of the original high-risk group). Reasons for missing school-age data include lack of interest in further participation, inability to contact (e.g., due to moving residence), etc. Of the 188 children tested at follow-up, 49 were subsequently excluded from the present analyses due to having an ASD diagnosis at school-age (n=14 high-risk) or not meeting minimum age requirements for the present analyses (i.e., school-age, defined as age 5.5 years or greater). This resulted in a final sample of 79 high-risk children and 60 low-risk children between the ages of 5.5 and 9 years (mean=6.93 years, SD=0.84) whose data was used in all subsequent analyses. We examined whether any systematic differences existed at 36 months of age between participants whose data were analyzed and those whose data were not analyzed at school-age (i.e., those who were not seen as well as those who were seen but excluded based on the criteria described above), to determine whether the analyzed sample was more affected on any measure, thus biasing the results toward greater impairment at school-age. The analyzed school-age follow-up sample did not significantly differ from the non-analyzed sample with respect to 36-month rates of CC vs. TD outcomes (p = .44), developmental quotient (p = .06; non-analyzed sample with marginally significantly lower scores), or socioeconomic status (p = .28); the non-analyzed sample had significantly higher Autism Diagnostic Observation Schedule (ADOS) scores at 36 months than did the analyzed sample (p < .001) which is not unexpected given that participants with school-age ASD outcomes were intentionally excluded from the analyzed sample. Thus, there is no evidence that the analyzed sample was more impaired at 36 months than the non-analyzed sample and, to the contrary, these comparisons reflect a conservative bias (i.e., analyzed sample less impaired).
At each enrollment stage, informed consent was obtained from parents; children provided verbal assent for the school-age follow-up assessment. Participants were assessed by examiners who had extensive experience in assessment of child psychopathology (i.e., masters or doctoral level), were clinically licensed or supervised by someone with a clinical license, and were unaware of group membership. Ongoing administration and scoring fidelity procedures were in place to ensure minimal cross-examiner and cross-site differences, including regular exchanges of protocols and videos of assessments for administration and scoring reliability checks within and across sites. The study was conducted under the approval of both universities’ Institutional Review Boards.
Measures
Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000)
This is a semi-structured standardized interaction and observation that measures autism symptoms. It has two empirically-derived cutoffs, one for ASD and one for Autistic Disorder. Psychometric studies report high inter-rater reliability and agreement in diagnostic classification. The ADOS was used to confirm older sibling diagnosis and determine participant diagnostic outcomes at the school-age follow-up assessment.
Child Behavior Checklist (CBCL 6-18; Achenbach & Rescorla, 2001)
The school-age form of this standardized rating scale was used to assess children’s behavior problems. Parents rate items based on the child’s behavior for the prior six months. T-scores above 70 are considered to be clinically elevated. Although T-scores can be obtained for all CBCL subscales, continuous raw scores for the eight empirically-derived syndrome scales (Anxious/Depressed, Somatic Complaints, Withdrawn/Depressed, Attention Problems, Aggressive Behavior, Social Problems, Thought Problems, and Rule Breaking Behavior) were used in analyses focused on differences among group means; the CBCL manual (Achenbach & Rescorla, 2001) recommends using raw scores instead of truncated T-scores for such purposes in order to account for the full range of variation. The CBCL has good internal consistency (0.78-0.97) and test-retest reliability (0.68-0.92). Some children were just under the lower age limit for the school-age version of this measure at the time of the follow-up assessment, thus this measure is missing for those 13 participants.
Clinical Best Estimate (CBE) outcome classification and Clinical Concerns (CC) categorization
At the end of the school-age visit, examiners classified each child into one of seven outcome categories: ASD (excluded from analyses in the present study), Broader Autism Phenotype (BAP), Attention-Deficit/Hyperactivity Disorder (ADHD) Concerns, Speech-Language Problems, Learning Difficulties, Anxiety or Mood Problems, or Typically Developing (TD). Outcome categories other than ASD were not intended to correspond with specific DSM diagnoses, but instead reflected clinical concerns based on expert clinical judgment. Children classified with BAP displayed social communication difficulties below the ASD threshold. Children classified with ADHD Concerns displayed developmentally atypical levels of hyperactivity, inattention, and/or disruptive behavior. Children classified with Speech-Language Problems displayed immature speech patterns or low language levels based on standardized testing. Children classified with Learning Difficulties had low nonverbal cognitive scores and/or a reported history of academic difficulties. Finally, children classified with Anxiety or Mood Problems displayed anxious, depressed, or emotionally dysregulated behavior, confirmed by parent report. All outcomes other than TD were collapsed to form a Clinical Concerns (CC) group; that is, any child who received an outcome of BAP, ADHD Concerns, Speech-Language Problems, Learning Difficulties, or Anxiety/Mood Problems was included in the CC group. All other children were classified as TD. A licensed clinical psychologist observed (either live or via video) testing sessions for all CC cases before such outcomes were finalized.
A similar procedure took place at the 36-month outcome visit. Categories were the same as those used at the school-age follow-up with one exception: Rather than “Learning Difficulties,” the outcome of “Global Developmental Delay” was used at 36 months. For the purposes of the present analyses, any cases of ASD at 36-months were considered “CC” and classified as such.
Differential Abilities Scale – Second Edition (DAS-II; Elliott, 2007)
The DAS-II is a measure of intelligence and consists of both verbal and nonverbal subtests. For the purposes of the present investigation, only nonverbal intellectual abilities were evaluated through the administration of the Picture Similarities, Picture Construction, Matrices, and Copy subtests. Scores from these subtests can be combined to yield an overall measure of nonverbal ability, the Special Nonverbal Composite (SNC), which we selected as the primary outcome measure from the DAS-II. The DAS-II has excellent internal consistency (0.83 to 0.95 for subtests administered; 0.94 for SNC) and test re-test reliability (0.81 to 0.92). Standard Scores greater than 1.5 SD below the mean were considered impaired.
Clinical Evaluation of Language Fundamentals – Fourth Edition (CELF-4; Semel, Wiig, & Secord, 2003)
The CELF-4 is a measure of language skills in individuals ages 5 through 21 years. It consists of 19 total subtests evaluating skills including receptive language, expressive language, and working memory. Participants in the present study were administered the following subtests: Concepts and Following Directions, Word Structure, Recalling Sentences, Formulated Sentences, Word Classes, and Sentence Structure. Scores from these subtests are combined to yield two composite scores: the Expressive Language index, which is an overall measure of expressive language skills, and the Receptive Language index, which is a measure of listening and auditory comprehension; these two composite scores were selected as dependent variables. The CELF-4 has good internal consistency (0.87 to 0.95 for composite scores) and test-retest reliability (0.88 to 0.92 for composite scores). Standard Scores greater than 1.5 SD below the mean were considered impaired.
Social Responsiveness Scale (SRS; Constantino & Gruber, 2005)
The SRS is a parent report measures that provides information about ASD symptoms via various dimensions of social, communication, and repetitive/stereotyped behaviors. The SRS is reported to have good psychometric properties (test-retest reliability of 0.83). T-scores above 60 are considered elevated. Because T-scores are truncated and do not capture the full range of variation, continuous raw SRS Total scores were used in analyses focused on differences among group means.
Data Analytic Plan
As described previously, participants with school-age ASD outcomes were excluded from all analyses. Table 1 contains descriptive statistics (mean, SD) by risk group for age, ADOS severity scores, and all outcome measures; T-scores are presented for the CBCL and SRS for ease of interpretation, although we reiterate that raw scores were used in analyses focused on differences among group means. All tests were two-sided, with α = 0.05. All analyses were implemented in SAS Version 9.4 (SAS Institute, Inc.).
Table 1.
Descriptive statistics for overall sample.
| Low-Risk (n = 60) | High-Risk (n = 79) | |||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Age (months) | 82.97 (10.33) | 69.72-118.70 | 83.29 (9.87) | 66.30-116.11 |
| ADOS severity | 1.47 (0.91) | 1-6 | 1.99 (1.70) | 1-8 |
| DAS-II SNC | 109.72 (12.28) | 84-138 | 106.58 (15.36) | 74-161 |
| CELF-4 | ||||
| Receptive Language | 110.03 (12.23) | 49-129 | 102.74 (16.92) | 45-129 |
| Expressive Language | 110.37 (14.51) | 55-136 | 103.73 (17.34) | 47-136 |
| CBCLT-scores | ||||
| Anxious/Depressed | 52.27 (3.71) | 50-64 | 54.94 (7.38) | 50-82 |
| Somatic Complaints | 52.93 (4.25) | 50-67 | 55.19 (6.86) | 50-76 |
| Withdrawn/Depressed | 51.39 (3.21) | 50-68 | 53.30 (5.58) | 50-79 |
| Attention Problems | 52.14 (3.89) | 50-70 | 54.66 (6.21) | 50-75 |
| Aggressive Behavior | 51.68 (3.20) | 50-64 | 55.06 (7.18) | 50-81 |
| Social Problems | 51.79 (2.32) | 50-59 | 53.99 (4.37) | 50-67 |
| Thought Problems | 51.95 (2.67) | 50-64 | 54.11 (7.27) | 50-82 |
| Rule-Breaking | 52.55 (3.62) | 50-67 | 54.16 (5.57) | 50-70 |
| SRS TotalT-score | 42.69 (5.04) | 36-58 | 47.34 (9.26) | 34-74 |
Note: ADOS = Autism Diagnostic Observation Schedule; DAS-II SNC = Differential Ability Scale, 2nd Edition Special Nonverbal Composite; CELF-4 = Clinical Evaluation of Language Fundamentals, 4th Edition; CBCL = Child Behavior Checklist; SRS = Social Responsiveness Scale.
First, taking an empirically-driven approach, we established the proportion of children in the low-risk and high-risk groups who exhibited any clinically elevated or impaired score, based on established cutoffs, across any of the measures examined (i.e., any CBCL subscale ≥70, any SRS subscale ≥60, CELF-4 Receptive or Expressive Language or DAS-II SNC <78). Multivariate logistic regression models were used to assess the relationship between risk group (high vs. low) and having any clinically elevated or impaired score (yes/no), adjusting for site, enrollment age, age at follow-up, and sex. Odds ratios (OR) and 95% confidence intervals (CI) were derived from these logistic regression models.
Next, employing a clinically-driven approach, multivariate logistic regression models were used to assess the relationship between risk group (high vs. low) and the dependent variable of examiner-rated outcome (TD vs. CC), adjusted for site, enrollment age, age at follow-up, and sex, obtaining OR and 95% CI.
We then examined group differences in continuous scores on selected CBCL, SRS, DAS-II and CELF-4 variables via linear models based on these CC classifications (Low-Risk TD, High-Risk TD, High-Risk CC), adjusting for site, enrollment age, age at follow-up, and sex (note that age was not included as a covariate in the CELF-4 and DAS-II analyses as age is already accounted for in Standard Scores); the low-risk participants with CC outcomes (n=8) were excluded from these analyses, given the small size of this group. This approach was flexible and allowed the variances to vary by group for dependent variables for which the assumption of homogeneity of variances across group was not met (DAS-II and CELF-4). Raw CBCL and SRS scores were square root transformed for these analyses in order to meet normality assumptions. Following a significant main effect of group, subsequent planned contrasts examined differences between the Low-Risk TD and High-Risk TD groups, the Low-Risk TD and High-Risk CC groups, and the High-Risk TD and High-Risk CC groups.
Results
Empirically-defined impairment
Across all measures, the proportion of any clinically elevated or impaired score on the CBCL, SRS, CELF-4, or DAS-II was higher in the high-risk group (43%) than in the low-risk group (12%). After adjusting for site, enrollment age, age at follow-up, and sex, the high-risk group had a higher likelihood of clinically elevated/impaired scores than the low-risk group (OR = 5.71, 95% CI = 2.25-14.50, p < .001).
Examiner-rated CC outcomes
As described previously, examiner-rated outcomes were classified as either TD (i.e., those who received Typically Developing outcomes) versus CC (i.e., those who received any of the five non-ASD clinical concerns outcome classifications). Table 2 presents outcomes by risk group. The proportion of CC outcomes was higher in the high-risk group (38%) than in the low-risk group (13%). After adjusting for site, enrollment age, age at follow-up, and sex, the children in the high-risk group were more likely to have CC outcomes than those in the low-risk group (OR = 4.31, 95% CI = 1.72-10.77, p = .002).
Table 2.
Examiner-rated clinical best estimate outcome by risk group.
| Low-Risk (n = 60) | High-Risk (n = 79) | |
|---|---|---|
| BAP | 0 (0%) | 12 (15.2%) |
| ADHD Concerns | 5 (8.3%) | 10 (12.7%) |
| Speech-Language Problems | 1 (1.7%) | 4 (5.1%) |
| Learning Problems | 2 (3.3%) | 3 (3.8%) |
| Anxiety or Mood Problems | 0 (0%) | 1 (1.3%) |
| Typically Developing | 52 (86.7%) | 49 (62.0%) |
Note: BAP = Broader Autism Phenotype; ADHD = Attention-Deficit/Hyperactivity Disorder.
ASD symptoms
Differences in SRS Total scores among the Low-Risk TD, High-Risk TD, and High-Risk CC groups were evaluated. The overall model revealed a significant main effect of group, F(2, 114) = 13.50, p < .001. Follow-up planned contrasts are presented in Table 3 and indicate that the High-Risk CC group had significantly higher scores than the Low-Risk TD and High-Risk TD groups, which did not differ. Although raw transformed scores revealed differences on the SRS total score, T-scores were, at a group level, still in the average range for all groups (see Table 4).
Table 3.
Estimated group differences for planned contrasts.
| High-Risk TD vs. Low-Risk TD | High-Risk CC vs. Low-Risk TD | High-Risk CC vs. High-Risk TD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate (SE) | p | Estimate (SE) | p | Estimate (SE) | p | |
| SRS Totala | 0.18 (0.32) | .57 | 1.85 (0.38) | < .001 | 1.67 (0.38) | < .001 |
| CBCLa | ||||||
| Withdrawn/Depressed | 0.21 (0.15) | .15 | 0.62 (0.17) | < .001 | 0.40 (0.18) | .03 |
| Attention Problems | 0.31 (0.21) | .14 | 1.09 (0.25) | < .001 | 0.78 (0.25) | .002 |
| Aggressive Behavior | 0.28 (0.24) | .24 | 1.33 (0.28) | < .001 | 1.04 (0.29) | < .001 |
| Rule-Breaking | -0.09 (.17) | .58 | 0.41 (.20) | .04 | 0.51 (.20) | .01 |
| Social Problems | 0.27 (0.17) | .11 | 0.58 (0.20) | .004 | 0.30 (0.20) | .14 |
| CELF-4b | ||||||
| Receptive Language | -3.52 (2.10) | .10 | -16.67 (4.09) | < .001 | -13.14 (4.06) | .003 |
| Expressive Language | -2.74 (2.52) | .28 | -15.46 (4.00) | < .001 | -12.72 (4.05) | .003 |
Note. TD = Typically Developing; CC = Clinical Concerns; SRS = Social Responsiveness Scale; CBCL = Child Behavior Checklist; CELF-4 = Clinical Evaluation of Language Fundamentals, 4th Edition.
Estimated from linear models adjusted for age at enrollment, age at school-age follow-up, sex, and site fitted to raw, square root transformed data.
Estimated from linear models adjusted for age at enrollment, sex, and site and allowing variances to vary by group.
Table 4.
Descriptive statistics for examiner-rated CC groups.
| Low-Risk TD (n = 52)a | High-Risk TD (n = 49) | High-Risk CC (n = 30) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Age (months) | 82.76 (10.63) | 69.72-118.70 | 82.98 (10.76) | 66.30-116.11 | 83.79 (8.35) | 66.56-102.60 |
| ADOS severity | 1.44 (.94) | 1-6 | 1.43 (.91) | 1-6 | 2.90 (2.23) | 1-8 |
| DAS-II SNC | 111.26 (11.73) | 88-138 | 108.87 (12.60) | 89-151 | 103.03 (18.56) | 74-161 |
| CELF-4 | ||||||
| Receptive Language | 111.35 (12.02) | 49-129 | 108.65 (9.40) | 82-129 | 92.97 (21.67) | 45-123 |
| Expressive Language | 111.12 (14.21) | 55-136 | 109.75 (11.88) | 83-136 | 94.10 (20.34) | 47-124 |
| CBCL T-scoresb | ||||||
| Anxious/Depressed | 52.33 (3.53) | 50-62 | 53.98 (6.90) | 50-76 | 56.58 (7.86) | 50-82 |
| Somatic Complaints | 53.00 (4.40) | 50-67 | 55.02 (7.07) | 50-76 | 55.46 (6.62) | 50-72 |
| Withdrawn/Depressed | 51.54 (3.41) | 50-68 | 52.25 (4.84) | 50-79 | 55.08 (6.36) | 50-70 |
| Attention Problems | 51.17 (1.88) | 50-59 | 53.18 (5.21) | 50-69 | 57.15 (7.04) | 50-75 |
| Aggressive Behavior | 51.35 (2.95) | 50-64 | 53.45 (6.36) | 50-81 | 57.77 (7.77) | 50-75 |
| Social Problems | 51.71 (2.14) | 50-59 | 53.36 (4.21) | 50-67 | 55.04 (4.52) | 50-64 |
| Thought Problems | 51.90 (2.66) | 50-64 | 53.48 (6.76) | 50-82 | 55.19 (8.08) | 50-80 |
| Rule-Breaking | 52.23 (3.20) | 50-67 | 53.18 (4.76) | 50-66 | 55.81 (6.51) | 50-70 |
| SRS Total T-scorea | 43.00 (5.32) | 36-58 | 44.39 (7.66) | 34-66 | 52.37 (9.71) | 34-74 |
Note: ADOS = Autism Diagnostic Observation Schedule; DAS-II SNC = Differential Ability Scale, 2nd Edition Special Nonverbal Composite; CELF-4 = Clinical Evaluation of Language Fundamentals, 4th Edition; CBCL = Child Behavior Checklist; SRS = Social Responsiveness Scale.
The 8 low-risk children with CC outcomes were excluded from these analyses resulting in n=52 Low-Risk TD children.
Raw scores were used in analyses; T-scores are presented for descriptive purposes and ease of clinical interpretation.
Parent-rated psychopathology symptoms
The overall models for the CBCL empirically-derived subscales indicated a significant main effect of group (Low-Risk TD, High-Risk TD, High-Risk CC) on Withdrawn/Depressed, F(2, 111) = 6.22, p = .003; Attention Problems, F(2, 111) = 9.95, p < .001; Aggressive Behavior, F(2, 111) = 11.39, p < .001; Rule-Breaking Behavior, F(2, 111) = 3.29, p = .04; and Social Problems, F(2, 111) = 4.34, p = .02. Planned contrasts for these subscales are displayed in Table 3. On the Withdrawn/Depressed, Attention Problems, Aggressive Behavior, and Rule-Breaking Behavior subscales, the High-Risk CC group had significantly higher scores than the Low-Risk TD and High-Risk TD groups, which did not differ. The High-Risk CC group had significantly higher scores on the Social Problems subscale relative to Low-Risk TD group, but did not differ from the High-Risk TD group; the Low-Risk TD and High-Risk TD groups also did not differ. As with the SRS, although analyses revealed differences on these subscales, T-scores were, at a group level, still in the average range for all groups (see Table 4).
The main effect of group approached statistical significance for the Anxious/Depressed, F(2, 111) = 2.73, p = .07, and was not significant for the Somatic Complaints, F(2, 111) = 1.87, p = .16, or Thought Problems subscales, F(2, 111) = 1.23, p = .30.
Language skills
The overall model for CELF-4 Receptive Language scores indicated a significant main effect of group, F(2, 60.2) = 8.37, p < .001. Follow-up planned contrasts are presented in Table 3 and indicate that the High-Risk CC group had significantly lower scores than the Low-Risk TD and High-Risk TD groups, which did not differ themselves. A similar pattern emerged for CELF-4 Expressive Language scores, with a significant main effect of group, F(2, 63.9) = 7.40, p < .001, and lower scores in the High-Risk CC group relative to the other two groups, which did not differ. Similar to scores on the SRS and CBCL, standard scores on the Receptive and Expressive Language composites were, at a group level, still in the average range for all groups (see Table 4).
Nonverbal cognitive ability
The main effect of group for DAS-II SNC did not reach statistical significance, F(2, 62.9) = 2.49, p = .09. See Table 4 for mean/SD Standard Scores stratified by group.
Correspondence between 36-month and school-age CC outcomes
CBE outcome data were available at 36-months for 58 of the 60 low-risk school-age participants and 76 of the 79 high-risk school-age participants. Correspondence between CC outcomes at the two time points for the low- and high-risk groups are summarized in Figure 1. Correspondence for the high-risk group was variable; 23.7% exhibited persistent CC outcomes from 36 months to school-age, 17.1% had 36-month CC outcomes that resolved by the school-age visit, 11.8% had CC outcomes at the school-age visit but not at 36 months, and 47.4% of this group were considered TD at both time points.
Figure 1.
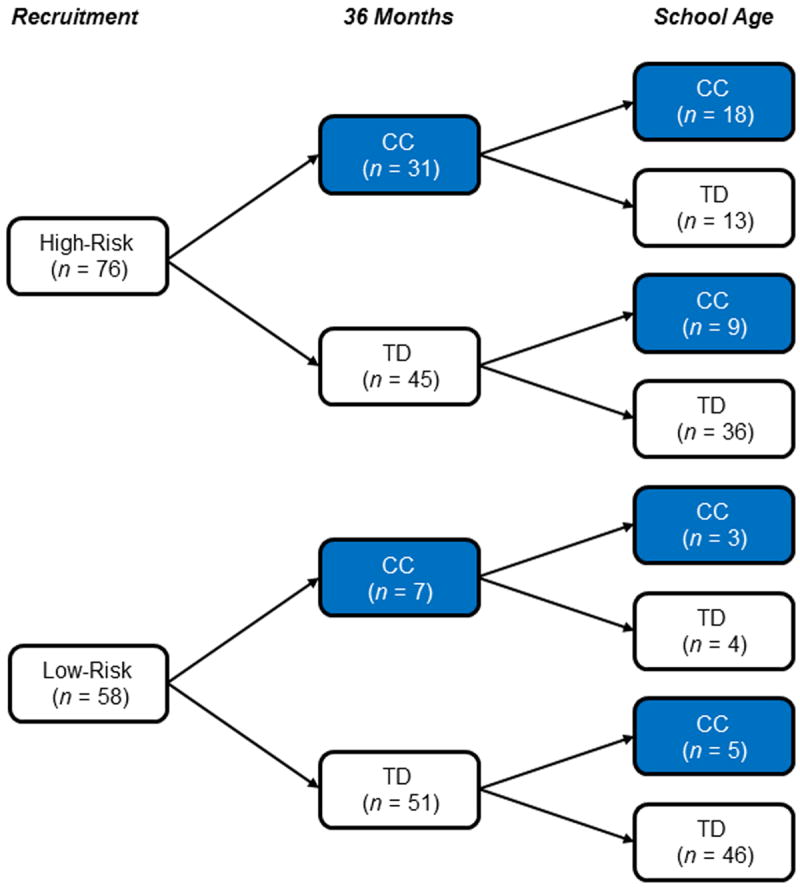
Correspondence between 36-month and school-age examiner-rated Clinical Concerns (CC) and Typically Developing (TD) outcome classifications for school-age participants with available 36-month outcome data. Any cases of ASD at 36 months were considered “CC” and classified as such (n = 3); all 3 were classified into the CC group (non-ASD) at school-age follow-up by examiners unaware of risk group or prior diagnoses.
Discussion
In the present investigation, we examined school-age outcomes of younger siblings of children with ASD, all of whom had been followed prospectively from infancy, via both empirical/quantitative (standard cutoffs on standardized tests and rating scales) and clinical (examiner global impressions) approaches. We also sought to evaluate correspondence between early (36-months) and later (school-age) clinical outcomes. In evaluating categorically-defined impairment by examining the frequency of scores in the clinically elevated or impaired ranges on each measure, we found that 43% of the high-risk group had at least one clinically elevated or impaired score on any of the measures evaluated, versus 12% of the low-risk group. It appears that scores on the SRS and CBCL drove this effect; 32% of the high-risk group had elevated scores on at least one SRS subscale (versus 5% of the low-risk group), and 17% of the high-risk group had clinically elevated scores on at least one CBCL subscale (versus 2% of the low-risk group). Lower proportions (14% and 1%) of the high-risk group scored greater than 1.5 SD below the mean on the CELF-4 and DAS-II (vs. 5% and 0% of the low-risk group).
We also took a clinically-oriented approach by examining examiner-rated clinical best estimate outcomes, finding significantly higher odds of CC outcomes among the high-risk school-age siblings, with 38% of this group receiving such outcomes, versus only 13% of the low-risk group; the most common outcomes in our high-risk school-age sample were BAP and ADHD Concerns. Although we did not conduct formal diagnostic evaluations based on DSM categories, but rather relied on examiner clinical judgment, the rate of CC outcomes in our low-risk group are consistent with prior studies examining the overall prevalence of any DSM-defined disorder in children/adolescents. Specifically, in a large, population-based sample of children and adolescents, the prevalence of any DSM-IV disorder was found to be 9.5% (Ford, Goodman, & Meltzer, 2003). In another representative population sample of children and adolescents, the 3-month prevalence of any DSM-IV diagnosis was 13.3% (Costello et al., 2003). These estimates are substantially lower than the rate of CC outcomes in the high-risk group. The proportion of the high-risk group receiving CC outcomes is also generally consistent with the prior literature focusing on infant siblings at age 3 (Messinger et al., 2013; Ozonoff et al., 2014), as well as the smaller existing literature on school-age outcomes of infant siblings (e.g., Gamliel et al., 2009). In fact, findings from Gamliel and colleagues (2009) are strikingly similar, with 40% of their high-risk group identified during the school-age years with atypical developmental outcomes spanning cognition, language, and academic skills versus 16% of their low-risk sample.
The proportion of the high-risk group receiving CC outcomes is also consistent with the proportion of this group who exhibited elevated scores on the CBCL or SRS, based on our empirically-oriented analyses using standard cutoffs. In contrast, only a relatively small number of children scored below CELF-4 or DAS-II cutoffs. It may be that nonverbal cognitive and language abilities are not primary areas of impairment among school-age siblings of children with ASD. It is also possible that direct assessment approaches may not always pick up on the most clinically relevant challenges of this population and that parent reports are more sensitive. This is clinically important since schools are more likely to evaluate cognitive and language functioning, and are less likely to evaluate mental health symptoms, thus missing the difficulties most relevant to this group. Indeed, a recent study of adult siblings of individuals with ASD found evidence of significant mental health problems in at least one third of this population (Howlin et al., 2015), suggesting that this is an area of great clinical importance. It will be critical to evaluate the presence and nature of clinically and functionally impaired subgroups in future studies.
We also aimed to evaluate the domains in which those high-risk siblings with examiner-determined CC outcomes were impaired, finding differences among groups across multiple domains, on both parent report and direct assessment measures. With respect to autism symptoms, we found that the High-Risk CC group had significantly higher total SRS scores than the other two groups, consistent with the notion of the BAP and with prior research on siblings of children with ASD (Constantino et al., 2006). In line with the literature reporting greater behavioral and psychiatric concerns in family members of individuals with ASD (e.g., Petalas, Hastings, Nash, Lloyd, & Dowey, 2009; Piven & Palmer, 1999), we also found that the High-Risk CC group had significantly higher CBCL scores than the Low-Risk TD and High-Risk TD groups spanning internalizing, externalizing, and social domains. These findings fit well within the broader literature examining similar dimensions in first-degree relatives of individuals with ASD (for a review, see Sucksmith et al., 2011) as well as adult siblings (Howlin et al., 2015), and are also consistent with research on infant sibling samples at younger ages (e.g., Schwichtenberg et al., 2013). The present findings are, to our knowledge, the first to extend this research on broad-based psychopathology to an infant sibling sample during the school-age years.
We also examined language and nonverbal cognitive skills in our sample via standardized assessments, finding significantly lower receptive and expressive language scores in the High-Risk CC group, which is somewhat in contrast to Ben-Yizhak et al. (2011), who found impaired pragmatic language skills but no differences in general linguistic abilities in their school-age sample. This may be due to the fact that our CC group was comprised of additional outcomes beyond just BAP. This will be important to parse in future studies consisting of larger groups of school-age children with BAP outcomes. The only domain in which we did not find differences was nonverbal cognitive ability, consistent with the limited existing literature focused on school-age outcomes of infants at risk for ASD (e.g., Gamliel et al., 2009).
It is important to consider the multitude of potential mechanisms underlying the higher rates of CC outcomes and higher levels of psychopathology symptoms found in our sample of high-risk siblings, including interactive effects among each of the domains examined in the present investigation. For example, given well-documented co-occurrence of communication and behavioral disorders (for a relevant review, see Carpenter & Drabick, 2011), it is possible that communication difficulties underlie the expression of higher levels of psychopathology symptoms and CC outcomes among the high-risk siblings. Similarly, difficulties with executive function might mediate the higher rates of psychopathology at school-age (e.g., Tannock & Schachar, 1996). It will be of great interest to examine early predictors of, and longitudinal mediational mechanisms underlying, the development of these outcomes in future investigations.
We lastly sought to address the question of continuities versus discontinuities over time, examining correspondence between examiner-rated CC outcomes at the 36-month and school-age assessments. Our findings indicated some variability within the high-risk group: Some evidenced CC outcomes at school-age but not earlier (11.8%), and some had earlier CC outcomes that appear to have resolved by school-age (17.1%). The majority of the high-risk sample, however, evidenced either persistent CC outcomes from 36-months to school-age (23.7%), or persistent lack of CC outcomes (47.4%). These findings highlight the immense heterogeneity among siblings of children with ASD and reiterate the need to further track developmental progress in this population.
Although examiners classified 15% of the high-risk group with the BAP, the remaining CC outcomes – an additional 23% of the high-risk group – were distributed among other categories. This raises the question: What really constitutes the BAP during the school-age years? Are the other CC categories (e.g., ADHD concerns, speech-language delays, learning problems, anxiety or mood problems) truly separable from the BAP, or are they part and parcel of this construct? Traditionally, the BAP has been defined as being comprised of subclinical ASD traits (i.e., social communication problems, language delays, repetitive behaviors), but it has long been noted that additional features also occur more frequently in siblings of children with ASD than in siblings of TD children, including significant levels of inattention, hyperactive-impulsive behavior, and anxiety (reviewed in Sucksmith, 2011). This is an important conceptual issue for the field to continue to consider.
Overall, our findings suggest continued vulnerability in a subset of school-age children with family histories of ASD, whether measured using empirical/quantitative (i.e., via cutoffs on standardized measures) or clinical approaches. In examining group means on continuous measures, we note that vulnerabilities were exclusively found in the subgroup of high-risk children with expert examiner-identified CC outcomes. This approach of identifying subgroups within high-risk cohorts, which has been used and recommended by others (see Ben-Yizhak et al., 2011; Sucksmith et al., 2011), may help to begin to resolve some of the inconsistences in the literature. That is, some studies have not found differences between siblings of children with and without ASD during the school-age years, but this may be because only a subset of high-risk children are characterized by lower scores. Indeed, similar to the findings of Ben-Yizhak and colleagues (2011), in our sample, the High-Risk TD group was remarkably similar to the Low-Risk TD group on both parent-reported and objective standardized measures. It should also be noted that although group differences were significant across many of the variables evaluated, on average, all groups (including the High-Risk CC group) still performed within the normative range in terms of mean scores on measures; this, coupled with our findings of significantly higher rates of categorically-defined impairment (whether based on numerical cutoffs or clinical judgment) highlights the point that group differences in mean scores on individual measures do not necessarily equate to, or fully capture, impairment (see Lee, Lahey, Owens, & Hinshaw, 2008), necessitating the use of additional approaches to understanding functioning. Taken together, our findings indicate that at least a subgroup of siblings of children with ASD will benefit from continued screening and monitoring into the school-age years.
The present study features, to our knowledge, the largest school-age follow-up sample of infants at risk of ASD to date, but it is not without limitations. First, we excluded the children with ASD outcomes from analyses for both conceptual and practical reasons. However, as similar samples are followed over time, it will be of great interest to evaluate school-age outcomes of these diagnosed children. Second, we included only a broadband screening measure of child psychopathology; it will likely be of value to conduct more in-depth evaluation in this area in future follow-up investigations. Finally, we relied on examiner CBE ratings to create our clinically-defined groups which consists of a range of heterogeneous outcomes (e.g., BAP, ADHD concerns, speech-language problems, etc.); this may limit generalizability of these findings. However, given the small numbers within any one of these categories, and given our objective of maintaining clinical relevance, this was a necessary approach.
Future investigations should continue to follow infant sibling samples into the school-age and early adolescent years, further evaluating correspondence among clinical outcomes over time. Additionally, future investigations would likely benefit from expanding assessment batteries to examine additional domains beyond those evaluated in the present study, such as learning disorders, more fine-grained assessment of ADHD symptoms and mental health more broadly, and more detailed evaluations of BAP characteristics and peer relationships. Finally, future work in infant sibling samples should aim to capitalize on these unprecedented samples of children followed from infancy by seeking to identify early predictors of school-age outcomes, including determining what factors very early in life predict competence and what factors predict persistent difficulty.
Scientific Summary for Families.
Recent studies have focused on infants at risk for autism spectrum disorder (ASD), but few have followed these samples into school-age. We conducted a follow-up study, during the school-age years (ages 5.5-9 years), of younger siblings of children with ASD (high-risk group, n=79) or typical development (low-risk group, n=60), originally recruited as infants, to determine whether the high-risk group without ASD showed difficulties during this developmental period. The high-risk group was more likely to have clinically elevated/impaired scores in the domains of mental health symptoms, autism symptoms, language skills, and nonverbal cognitive abilities relative to the low-risk group (43% vs. 12%, respectively). The high-risk group was also more likely to have been classified by an examiner as having Clinical Concerns (CC; e.g., ADHD concerns, subclinical ASD symptoms, speech-language difficulties, anxiety/mood problems, learning problems) relative to the low-risk group; 38% of the high-risk group received a CC outcome versus 13% of the low-risk group. The high-risk group with CC outcomes had higher levels of mental health and autism symptoms, as well as lower language skills, than both the Low-Risk Typically Developing (TD) and High-Risk TD groups, which did not differ. For some of the high-risk children, clinical concerns persisted from early childhood, whereas for others clinical concerns were first evident at school-age. Overall, results suggest continued vulnerability in at least a subgroup of school-age children with a family history of ASD and suggest that this population may benefit from continued screening and monitoring into the school-age years.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health: R01 MH068398 (Ozonoff), U54 MH068172 (Sigman), and K01 MH 096961 (Hutman); and the National Institute of Child Health and Human Development: P50HD055784 (Bookheimer) and Intellectual and Developmental Disabilities Research Center U54 HD079125 (Abbeduto).
Footnotes
The small number of children with ASD is insufficient to address questions focused on how this group fares over time; future studies will benefit from multi-site collaborations that provide larger samples, such as the Baby Siblings Research Consortium.
The authors have no biomedical financial disclosures or potential conflicts of interest to report.
References
- Achenbach TM, Rescorla L. Child Behavior Checklist for ages 1½-5. Burlington, VT: University of Vermont; 2000. [Google Scholar]
- Bailey A, Palferman S, Heavey L, LeCouteur A. Autism: The phenotype in relatives. Journal of Autism & Developmental Disorders. 1998;28:369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Bellina M, Brambilla P, Garzitto M, Negri GAL, Molteni M, Nobile M. The ability of CBCL DSM-oriented scales to predict DSM-IV diagnoses in a referred sample of children and adolescents. European Child & Adolescent Psychiatry. 2013;22:235–246. doi: 10.1007/s00787-012-0343-0. [DOI] [PubMed] [Google Scholar]
- Ben-Yizhak N, Yirmiya N, Seidman I, Alon R, Lord C, Sigman M. Pragmatic language and school related linguistic abilities in siblings of children with autism. Journal of Autism & Developmental Disorders. 2011;41:750–760. doi: 10.1007/s10803-010-1096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JL, Drabick DAG. Co-occurrence of linguistic and behavioural difficulties in early childhood: A developmental psychopathology perspective. Early Child Development & Care. 2011;181:1021–1045. doi: 10.1080/03004430.2010.509795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorders. American Journal of Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanil A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Drumm E, Brian J. The developing language abilities and increased risks of ‘unaffected’ siblings of children with autism spectrum disorder. Neuropsychiatry. 2014;3:513–524. [Google Scholar]
- Drumm E, Bryson S, Zwaigenbaum L, Brian J. Language-related abilities in ‘unaffected’ school-age siblings of children with ASD. Research in Autism Spectrum Disorders. 2015;18:83–96. [Google Scholar]
- Elliott CD. Differential Ability Scales – Second Edition (DAS-II) San Antonio, TX: Harcourt; 2007. [Google Scholar]
- Ferdinand RF, Visser JH, Hoogerheide KN, van der Ende J, Kasius MC, Koot HM, Verhulst FC. Improving estimation of the prognosis of child psychopathology; combination of DSM-III-R-/DISC diagnoses and CBCL scores. Journal of Child Psychology & Psychiatry. 2004;45:599–608. doi: 10.1111/j.1469-7610.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: Cognitive patterns and levels in parents and siblings. Journal of Child Psychology & Psychiatry. 1997;38:667–683. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Ford T, Goodman R, Meltzer H. The British child and adolescent mental health survey 1999: The prevalence of DSM-IV disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:1203–1211. doi: 10.1097/00004583-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Jaffe DH, Manor O, Sigman M. Developmental trajectories in siblings of children with autism: Cognition and language from 4 months to 7 years. Journal of Autism & Developmental Disorders. 2009;39:1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Khalulyan A, Del Rosario M, McCarthy B, Gomez L, Sigman M, Hutman T. Is early joint attention associated with school-age pragmatic language among siblings of children with autism and low-risk controls? Autism. 2013;19:168–177. doi: 10.1177/1362361313515094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizzonio V, Avanzini P, Fabbri-Destro M, Campi C, Rizzolatti G. Cognitive abilities in siblings of children with autism spectrum disorders. Experimental Brain Research. 2014;232:2381–2390. doi: 10.1007/s00221-014-3935-8. [DOI] [PubMed] [Google Scholar]
- Hastings RP. Brief report: Behavioral adjustment of siblings of children with autism. Journal of Autism & Developmental Disorders. 2003;33:99–104. doi: 10.1023/a:1022290723442. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, Bolton P, Rutter M. Outcomes in adult life among siblings of individuals with autism. Journal of Autism & Developmental Disorders. 2015;45:707–718. doi: 10.1007/s10803-014-2224-5. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasius MC, Ferdinand RF, van den Berg H, Verhulst FC. Associations between different diagnostic approaches for child and adolescent psychopathology. Journal of Child Psychology & Psychiatry. 1997;38:625–632. doi: 10.1111/j.1469-7610.1997.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Owens EB, Hinshaw SP. Few preschool boys and girls with ADHD are well-adjusted during adolescence. Journal of Abnormal Child Psychology. 2008;36:373–383. doi: 10.1007/s10802-007-9184-6. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M, et al. The Autism Diagnostic Observation Schedule – Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, Sigman M, et al. Beyond autism: A Baby Siblings Research Consortium study of high-risk children at three years of age. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Young GS, Hutman T, Johnson S, Schwichtenberg AJ, Ozonoff S. Early pragmatic language difficulties in siblings of children with autism: Implications for DSM-5 Social Communication Disorder. Journal of Child Psychology & Psychiatry. 2015;56:774–781. doi: 10.1111/jcpp.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, Iosif AM, et al. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petalas MA, Hastings RP, Nash S, Lloyd T, Dowey A. Emotional and behavioural adjustment in siblings of children with intellectual disability with and without autism. Autism. 2009;13:471–483. doi: 10.1177/1362361309335721. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Shalev RS, Gross-Tsur V. Language abilities of siblings of children with autism. Journal of Child Psychology & Psychiatry. 2003;44:914–925. doi: 10.1111/1469-7610.00175. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry. 1999;156:557–563. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- Ross P, Cuskelly M. Adjustment, sibling problems and coping strategies of brothers and sisters of children with autistic spectrum disorder. Journal of Intellectual & Developmental Disabilities. 2006;31:77–86. doi: 10.1080/13668250600710864. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire: Manual. Western Psychological Services; 2003. [Google Scholar]
- SAS Institute, Inc. SAS/STAT Version 9.3. Cary, NC, USA: 2002-2010. [Google Scholar]
- Schwichtenberg AJ, Young GS, Hutman T, Iosif A, Sigman M, Rogers SJ, Ozonoff S. Behavior and sleep problems in children with a family history of autism. Autism Research. 2013;6:169–176. doi: 10.1002/aur.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4) Toronto, Canada: Psychological Corporation; 2003. [Google Scholar]
- Sucksmith E, Roth I, Hoekstra RA. Autistic traits below the clinical threshold: Re-examining the broader autism phenotype in the 21st century. Neuropsychology Reviews. 2011;21:360–389. doi: 10.1007/s11065-011-9183-9. [DOI] [PubMed] [Google Scholar]
- Tannock R, Shachar R. Executive dysfunction as an underlying mechanism of behavior and language problems in attention deficit hyperactivity disorder. In: Beitchman JH, Cohen NJ, Konstanarea MM, Tannock R, editors. Language, Learning, & Behavior Disorders: Developmental, Biological, & Clinical Perspectives. New York, NY: Cambridge University Press; 1996. pp. 128–155. [Google Scholar]
- Quintero N, McIntyre LL. Sibling adjustment and maternal well-being: An examination of families with and without a child with an autism spectrum disorder. Focus on Autism & Other Developmental Disabilities. 2010;25:37–46. doi: 10.1177/1088357609350367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verté S, Roeyers H, Buysse A. Behavioural problems, social competence and self- concept in siblings of children with autism. Child: Care, Health, & Development. 2003;29:193–205. doi: 10.1046/j.1365-2214.2003.00331.x. [DOI] [PubMed] [Google Scholar]


