Abstract
Rationale
Bronchopulmonary dysplasia is the most common chronic respiratory disease in premature infants. Genetic factors might contribute to bronchopulmonary dysplasia susceptibility.
Objectives
To identify genetic variants involved in bronchopulmonary dysplasia through a genome-wide association study.
Methods
We prospectively evaluated 418 premature neonates (gestational age below 28 weeks), of whom 22% developed bronchopulmonary dysplasia. Two discovery series were created, using a DNA pooling strategy in neonates from Caucasian and African ancestry. Polymorphisms associated with the disease were confirmed in an independent replication population. Genes were then explored by fine mapping and associations were replicated in an external Finnish population of 213 neonates. Validated genes expression patterns were studied in rat lung, following air or hyperoxia exposure.
Measurements and Main Results
SPOCK2 gene was identified by both discovery series. The most significant polymorphism (rs1245560, p=1.66×10−7) was confirmed by individual genotyping, and in the replication population (p=0.002). Fine mapping confirmed the association of rs1245560 with bronchopulmonary dysplasia in both Caucasian and African populations with adjusted odds ratios of 2.96 (95% CI [1.37–6.40]) and 4.87 [1.88–12.63] respectively. In Caucasian neonates, rs1049269 was also associated with the disease (OR=3.21 [1.51–6.82]). These associations were replicated in the Finnish population. In newborn rat lungs, SPOCK2 mRNA levels markedly increased during the alveolar stage of lung development. After rat exposure to hyperoxia, SPOCK2 expression increased relative to air-exposed controls.
Conclusions
We identified SPOCK2 as a new possible candidate susceptibility gene for bronchopulmonary dysplasia. Its lung expression pattern points towards a potential role in alveolarization.
Keywords: African Continental Ancestry Group; Animals; Genotype; Humans; Infant, Newborn; Infant, Premature; Lung; Male; Polymorphism, Single Nucleotide; Proteoglycans; Rats; Animals, Newborn; Bronchopulmonary Dysplasia; European Continental Ancestry Group; Female; Finland; Gene Expression; Genetic Predisposition to Disease; Genome-Wide Association Study
INTRODUCTION
Bronchopulmonary dysplasia (BPD), defined as a requirement for oxygen supplementation at 36 weeks of postmenstrual age (PMA), is the most common chronic respiratory disease in premature infants, and its treatment places major demands on health services (1). Despite considerable obstetric and neonatal advances in the care of very-low-birth-weight (VLBW) infants, BPD continues to occur among 20% to 40% of survivors (2). BPD appears to result from multiple factors that can injure the immature lung and interrupt normal alveolar and distal vascular development (3). Besides the recognized detrimental effects of environmental factors, VLBW twin concordance studies recently suggested a role of genetic factors (4–6). After controlling for covariates, genetic factors accounted for 53% to 82% of the variance in BPD. A few genetic association studies have attempted to identify candidate BPD susceptibility genes (7–11). Genome-wide association studies (GWAS) have the potential to identify genetic factors underlying complex traits and diseases. DNA pooling-based strategies have recently been used to screen for major genetic associations (12), providing the same efficiency and power as individual genotyping of cases and controls (13, 14). This strategy has been successfully used for initial prioritizing of single nucleotide polymorphisms (SNPs) for validation by individual genotyping in several complex diseases (15–18). Moreover, DNA pooling studies can accurately estimate allelic frequencies, even in pools of only about 50 individuals (19). This is of particular interest when studying a disorder such as BPD that affects preterm infants. To identify genetic variants influencing BPD susceptibility, we conducted a GWAS of a multicenter population, combining pooling-based genome-wide case-control analysis, fine scale mapping of gene, and animal experiments.
METHODS
Detailed methods are available in the online supplement.
Study design and population
The study was approved by the local ethics committee (CPP Île-de-France IX). Written informed consent was obtained from the parents. We prospectively included 418 neonates, with a gestational age below 28 weeks, from three French neonatal intensive care units between 2002 and 2009 (Figure E1). BPD definition was based on the physiologic test validated by Walsh (20). Our population was divided into two discovery series and an independent replication series. We performed a GWAS based on a DNA pooling strategy in the two discovery series. SNPs associated with BPD were validated by individual genotyping in these two populations. Validated SNPs were then genotyped in two different replication populations: the internal replication population and an external population composed of 213 Finnish neonates born before 30 weeks of gestation and included between 1997 and 2010 from the neonatal intensive care unit of the Oulu University Hospital in Finland. Selected genes were also investigated in neonatal rats, focusing on lung developmental gene expression patterns and on changes in gene expression during distal lung development in healthy controls and animals exposed to hyperoxia.
DNA pooling
The first discovery series consisted of 22 Caucasian cases and 76 Caucasian controls, and the second of 21 black African cases and 86 black African controls. Pooled DNA samples were created in quadruplicate for case and control groups in each series. Genotyping was performed with the Infinium II Illumina HumanHap300 Genotyping BeadChip array for the Caucasian population (318,237 SNPs) and the Illumina HumanHap650Y array for the African population (660,918 SNPs). Raw and transformed data sets for each microarray are available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi (Accession Number GSE22284).
We used two methods to select SNPs for further individual genotyping. First, SNPs were ranked according to their allelic frequency differences between cases and controls, and the top-ranked 1% was selected. The second method was based on a combined Z-score analysis (15) and association signals reaching a threshold of p≤7.2×10−8 in Africans (21) and a less stringent threshold of p≤5×10−7 in Caucasians (22), since this population was genotyped with less SNPs, were retained. We considered that the identification of at least two significant SNPs within 30kb reflected a single locus (23). Finally, we selected for replication loci identified by both statistical methods in both series.
Fine mapping of the SPOCK2 region
Using HapMap data, we identified 70 SNPs with a minimum minor allele frequency (MAF) of 0.05 in a region spanning 20 kb upstream and downstream of the SPOCK2 gene. We identified 29 tagging SNPs that allowed capturing the 70 polymorphisms with r2=1 using the pair-wise approach implemented in Haploview4.1. The genotyping of our case-control samples was performed by Integragen with Golden Gate Illumina technology. SNPs showing deviation from Hardy-Weinberg equilibrium in controls (p≤0.05) or with genotyping success rates of less than 90% were excluded from analysis, as were individual DNA samples for which the genotyping success rate across all SNPs was less than 70%. Twenty-two SNPs passed this quality control and three DNA samples were excluded.
Animal experiments
Rat lung tissues from controls and animals exposed to hyperoxia were collected to perform RNA extraction, cell isolation and frozen tissue sections. mRNA expression profiles were determined by qPCR and location of the SPOCK2 protein was studied by immunofluorescence.
Statistical analysis
Individual genotyping
In order to assess the number of cases and controls required for the replication process, a power computation was applied and showed that a sample size of at least 120 controls and 30 cases allowed to detect a mean allelic frequency difference of at least 10% between cases and controls with a type I risk error of 5% and a power of 80%. Our internal replication set was composed of 49 cases and 163 controls (30 and 112 Europeans, 15 and 32 Africans and 4 and 19 infants with one Caucasian parent and one black African parent). We carried out separate analyses in neonates from Caucasian and African ancestry to avoid population stratification problems. Odds ratios (ORs) and 95% confidence intervals (CIs) for association between SNPs and BPD were estimated by logistic regression. Adjustment on relevant clinical factors associated to bronchopulmonary dysplasia in univariate analyses (p≤0.05) was also performed. The effect of a single SNP on disease was tested under additive and dominant genetic models for the minor allele. In the fine scale mapping process, to correct for the effect of testing multiple SNPs (n=22), we estimated the effective number of independent SNPs (Meff) using Li and Ji’s method (24), which is based on the pairwise LD measure r2 between SNPs. A Bonferroni correction was then applied using the Meff estimates leading to p-value thresholds of 0.0056 and 0.0036 for Caucasian and African subjects respectively. Additional analysis was performed to assess whether SPOCK2 polymorphisms were also associated with mild disease, defined by the need for oxygen supply at 28 days but room air breathing at 36 weeks, using the same statistical models. In the Finnish population, the same statistical models were also used. All statistical analyses were performed using Stata10.
Animal studies
Differences between treatment groups were evaluated with the Kruskal-Wallis non-parametric test for multiple group comparisons and the Mann-Whitney U test for two-group comparisons; p≤0.05 indicated statistical significance.
RESULTS
Study populations
The 418 French neonates had a mean gestational age and birth weight of 26.4 ± 0.1 weeks (range 23.6–27.9) and 845 ± 9 g (range 460–1410), respectively. Ninety-one infants (22%) were diagnosed with BPD (22% in Caucasian and 23% in African samples). In the whole sample, the following five clinical factors were significantly associated with BPD: birth weight (OR [95% CI] = 0.996 [0.995–0.998] per g birth weight, p<0.001), male gender (1.60 [0.99–2.56], p=0.05), the need for a second dose of surfactant (2.60 (1.59–4.22], p<0.001), persistent ductus arteriosus receiving surgical treatment (6.62 [3.19–13.74], p<0.001), and postnatal sepsis (2.40 [1.49–3.98], p<0.001). Thus, these factors were used as adjustment covariates in genetic analyses. In the Finnish population mean gestational age was 27.8 ± 0.1 weeks (range 23–29.9) and mean birth weight was 1017 ± 20 g (range 370–1755). Fifty five infants (26%) were diagnosed with BPD. Available adjustment covariates in this population were birth weight, gender and the occurrence of neonatal respiratory distress syndrome (RDS).
DNA pooling
The allele frequency difference method selected respectively 583 SNPs belonging to 388 genes and 1458 SNPs belonging to 933 genes in the Caucasian and African series of pools respectively. Among these genes, 105 were identified in both populations. Identical SNPs belonging to four genes were detected in the two populations (IRF2, KIT, SPOCK2 and LRRC4C) and 25 genes with SNPs less than 30kb apart in the two populations were selected (Table E1).
Using the combined Z-score analysis, we selected 51 SNPs in 45 genes and 134 SNPs in 108 genes in the Caucasian and African series of pools respectively. Among these genes, three were detected in both populations (SPOCK2, AGBL1 and IL1RAPL1). SPOCK2 was the single gene exhibiting association signals with SNPs less than 30kb apart in the two populations (Table E2).
SPOCK2 (Entrez Gene ID: 9806) was the only gene identified in both populations using both selection methods. Comparison of MAF between each control pool and corresponding HapMap data showed high similarity for the four SNPs belonging to SPOCK2 region identified by pooling analyses (Table E3). As rs1245560 was the only SNP showing similar MAF in Caucasian and African control pools (0.463 and 0.497 respectively), this genetic variant was chosen for validation by individual genotyping in the two discovery series and in an independent replication set including neonates from Caucasian and/or African ancestry.
Individual genotyping of rs1245560 in the discovery series and in independent replication sample
Individual genotyping of rs1245560 in DNA samples used for pooling studies confirmed the results obtained by pooling analyses. The C allele was significantly associated with the risk of BPD in both Caucasian (p=0.003) and African (p=0.01) series. C allele frequencies were 0.479 and 0.512 in the Caucasian and African controls, respectively, compared to 0.75 and 0.737 in the Caucasian and African BPD neonates, respectively. Results were also significant for risk genotype effects with ORs for having the CC genotype of 3.88 [1.39–10.94 (p=0.01) and of 5.22 [1.82–15.00] p=0.002) in Caucasian and African series respectively. In the replication sample composed of 49 cases and 163 controls, allele C of rs1245560 was also significantly associated with an increased risk of BPD (adjusted OR= 3.57 [1.19–10.76], p=0.02) as was CC genotype (adjusted OR=3.75 [1.61–8.76], p=0.002).
Fine mapping of SPOCK2 region
Fine mapping was performed separately in the whole Caucasian sample (51 cases and 185 controls) (Figure 1) and in the whole African sample (36 cases and 118 controls) (Figure E2). In univariate analysis, one SNP (rs1245560) was significantly associated with BPD in both populations with p value for the association between the risk of BPD and the CC genotype equal to 0.004 and 0.0007 in the Caucasian sample and in the African sample respectively (Table 1 and see Table E4 for detailed genotypes counts and frequencies). These associations remained statistically significant after correction for multiple testing. Carrying the CC genotype remained a significant risk factor for BPD after adjustment for perinatal factors with OR [95% CI] equal to 2.96 [1.37–6.40] for Caucasian subjects and 4.87 [1.88–12.63] for African subjects respectively (Table 1). These associations remained also statistically significant after correction for multiple testing. Since the allele frequencies of rs1245560 did not differ among controls of the two ethnic groups (0.489 for Caucasian neonates and 0.479 for African neonates), a logistic regression analysis was applied to the whole population (91 cases and 322 controls). The overall ORs was equal to 3.71 (CI= [2.07–6.64]; p=10−5) with subjects carrying CC genotype being at risk of BPD. Among the subjects from Caucasian ancestry, four supplementary SNPs were significantly associated with BPD (rs1245509, rs1245576, rs1049269 and rs1245558, p values ranging from 0.001 to 0.05). As shown in Figure 1, all significant SNPs were in strong linkage disequilibrium. After correction for multiple comparisons, a single association signal remained significant (rs1049269: OR=3.21 [1.51–6.82] after adjustment for perinatal factors) (Table 1 and see Table E4 for detailed genotypes counts and frequencies). Among African subjects, one SNP was also associated with the disease but this association did not remain significant after correction for multiple comparisons.
Figure 1. Fine scale mapping of SPOCK2 locus in neonates of Caucasian ancestry.
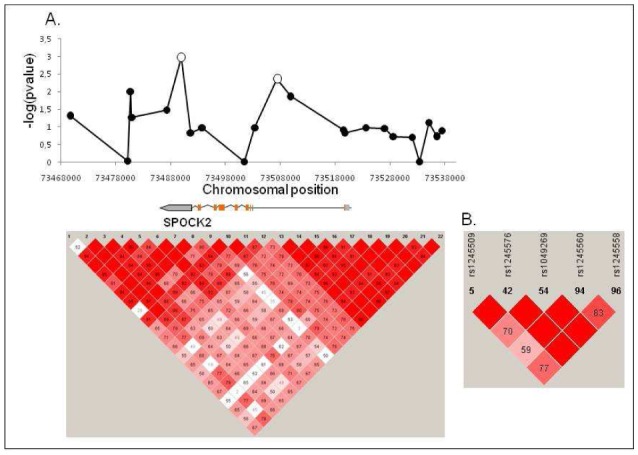
Individual genotyping of tag-SNPs in the Caucasian population (51 cases and 185 controls) confirmed the results obtained in DNA pooling studies. Statistical analyses identified 5 SNPs as being significantly associated with BPD (B). Only rs1245560 and rs1049269 remained significant after correction for multiple testing and adjustment for major clinical risk factors (A, white spots). LD plots were constructed using Haploview version 4.1. Red squares indicate regions of strong linkage disequilibrium (D′ given).
Table 1.
Associations of the SPOCK2 locus with BPD in Caucasian (51 cases and 185 controls) and in African populations (36 cases and 118 controls).
| Caucasian Population | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Risk Allele Effect | Risk Genotype Effect | ||||||||||||
|
| |||||||||||||
| SNP | Risk Allele | AF in cases | AF in controls | OR [95% CI] | p value | Adjusted OR† [95% CI] | p value | Risk Genotype | Genotype counts (%)‡ in cases / controls | OR [95% CI] | p value | Adjusted OR† [95% CI] | P value |
| rs1049269 | G | 0.7 | 0.541 | 1.76 [1.11–2.79] | 0.015 | 1.79 [1.08–2.99] | 0.025 | GG | 29 (58) / 56 (31) | 2.96 [1.55–5.68] | 0.001 | 3.21 [1.51–6.82] | 0.003 |
| rs1245560 | C | 0.64 | 0.489 | 1.75 [1.11–2.78] | 0.017 | 1.85 [1.10–3.11] | 0.020 | CC | 23 (46) / 43 (23) | 2.62 [1.35–5.09] | 0.004 | 2.96 [1.37–6.40] | 0.005 |
|
| |||||||||||||
| African Population | |||||||||||||
|
| |||||||||||||
| Risk Allele Effect | Risk Genotype Effect | ||||||||||||
|
| |||||||||||||
| rs1245560 | C | 0.676 | 0.479 | 2.23 [1.26–3.94] | 0.006 | 2.43 [1.28–4.62] | 0.007 | CC | 18 (53) / 26 (22) | 4.01 [1.79–8.97] | 0.0007 | 4.87 [1.88–12.63] | 0.001 |
Abbreviations: AF, allele frequency; OR, odds ratio; CI, confidence interval; BPD, bronchopulmonary dysplasia
This table shows SNPs significantly associated with BPD in Caucasians and Africans before and after adjustment for clinical covariates. Each SNP was tested under an additive model (risk allele effect) and a dominant model for the minor allele (risk genotype effect).
Adjusted ORs were computed with a logistic regression model including clinical significant risk factors for BPD i.e. birth weight, gender, persistent ductus arteriosus, postnatal sepsis, and the need for a second dose of surfactant.
For rs1049269, genotyping failed for 2 Caucasian samples (1 case and 1 control) and for 5 African samples (1 case and 4 controls). For rs1245560, genotyping failed for 3 Caucasian samples (1 case and 2 controls) and for 3 African samples (2 cases and 1 control).
Regarding mild disease, fifty percent of included children had mild BPD. No significant association was found with the CC genotype of rs1245560 (p=0.63) for mild BPD. C allele frequency was 47.9%, 49.2%, and 65.9% in infants with no BPD, mild BPD, and moderate-to-severe BPD respectively, leading to p values of 0.75 for mild BPD versus no BPD and of 0.0003 for moderate-to-severe BPD versus no BPD for the risk allele effect. Those results strengthened the finding that rs1245560 was only associated with moderate-to-severe BPD.
Genotyping of rs1245560 and rs1049269 in the Finnish population
In the Finnish replication population, rs1245560 CC and rs1049269 GG genotypes were significantly associated with an increased risk of BPD in univariate analysis (Table 2). After adjustment for available covariates, results remained significant with OR=2.38 [1.15–4.98] for rs1245560 and OR=2.10 [1.03–4.28] for rs1049269 (Table 2). Detailed genotype counts and frequencies are given in Table E4.
Table 2.
Individual genotyping of rs1245560 and rs1049269 in the Finnish population (55 cases and 158 controls)
| Risk Allele Effect | Risk Genotype Effect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| SNP | Risk Allele | AF in cases | AF in controls | OR [95% CI] | p value | Adjusted OR† [95% CI] | p value | Risk Genotype | Genotype counts (%)in cases / controls | OR [95% CI] | p value | Adjusted OR† [95% CI] | p value |
| rs1049269 | G | 0.564 | 0.494 | 1.77 [0.73–4.30] | 0.2 | 1.84 [0.77–4.68] | 0.2 | GG | 20 (36) / 35 (22) | 2.01 [1.03–3.91] | 0.040 | 2.10 [1.03–4.28] | 0.040 |
| rs1245560 | C | 0.546 | 0.471 | 1.87 [0.76–4.59] | 0.17 | 1.99 [0.78–5.09] | 0.15 | CC | 19 (35) / 30 (19)‡ | 2.30 [1.16–4.56] | 0.017 | 2.38 [1.15–4.98] | 0.019 |
Abbreviations: AF, allele frequency; OR, odds ratio; CI, confidence interval.
Each SNP was tested under an additive model (risk allele effect) and a dominant model for the minor allele (risk genotype effect).
OR adjusted for birth weight, gender, and neonatal respiratory distress syndrome.
For rs1245560, genotyping failed for one case and one control
SPOCK2 expression in rat lung
mRNA expression studies showed that SPOCK2 is expressed in the developing lung. SPOCK2 mRNA in whole lung tissue increased gradually after birth, reaching levels about tenfold higher on postnatal day 18, remaining high until postnatal day 28, and returning to the neonatal level in adulthood (Figure 2A). SPOCK2 was expressed in both fibroblasts and airway epithelial cells (AECs). In isolated fibroblasts, mRNA levels were low until postnatal day 7 and then increased sharply from day 14 to day 21, following a pattern similar to that observed in the whole lung (Figure 2A and 2C). No major change in SPOCK2 expression was seen in AECs (Figure 2D). The SPOCK2 protein was strongly expressed throughout the extracellular matrix (ECM) (Figure 3). When rat pups were exposed to hyperoxia for 5 days (P5 to P10), SPOCK2 gene expression increased by about 60% relative to untreated controls (Figure 2B).
Figure 2. SPOCK2 mRNA expression patterns.
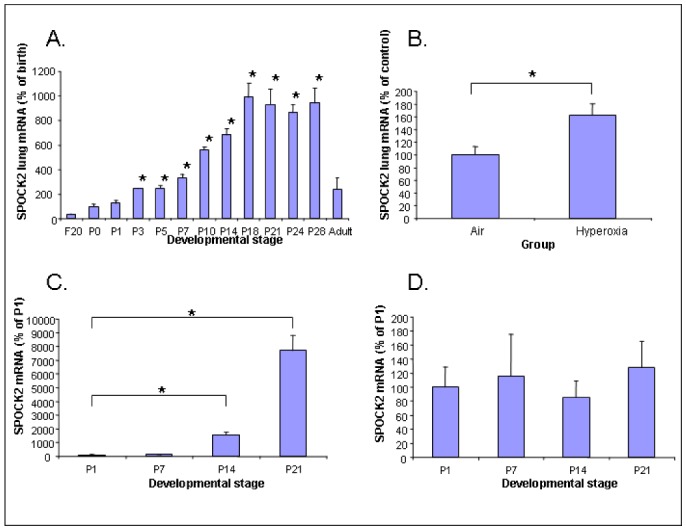
Developmental SPOCK2 mRNA expression patterns in rat whole-lung tissue (A), lung fibroblasts (C) and AECs (D). Expression was quantified by real-time PCR from fetal life to adulthood, in three to six individual lung samples per stage. The birth level in whole lung tissue and the day-1 level in isolated cells were arbitrarily attributed a value of 100. Values are mean ± SEM. * p<0.05 versus reference level. B. Changes in lung SPOCK2 mRNA expression in newborn rats exposed to hyperoxia from day 5 to day 10. Values are mean ± SEM and results are expressed as a percentage of the control value. * p<0.05.
Figure 3. SPOCK2 immunofluorescence studies in rat lung tissue on postnatal day 14.
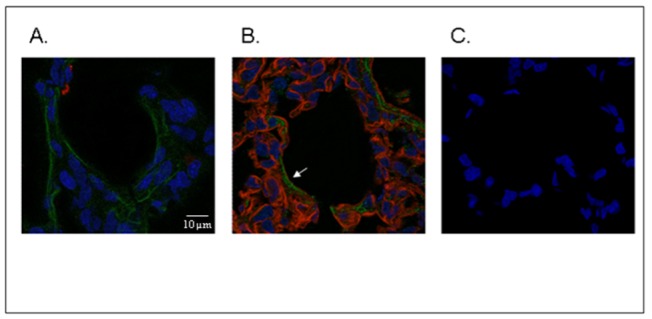
Immunocolocalization of SPOCK2 (green labelling) and SP-B (red labelling, A) or collagen IV (red labelling, B) showed the presence of SPOCK2 throughout the ECM, including the basement membrane, as shown by superimposing the 2 fluorescent signals in some areas (B, white arrow). Negative control lung incubated with mouse and rabbit polyclonal IgG, showing fluorescence restricted to the nucleus (C.) (original magnification x126).
DISCUSSION
Recent studies highlighted the contribution of genetic factors to BPD susceptibility (4–6). We conducted a GWAS based on DNA pooling in two different ethnic populations. SPOCK2 was the only BPD-associated gene that emerged in all analyses. Fine scale mapping of the SPOCK2 region validated its association with BPD. These results were replicated in an independent external population for the two associated SNPs belonging to SPOCK2, with genotype effects in the same direction and of the same order of magnitude as in the discovery panels. In addition, SPOCK2 expression increased during normal rat lung alveolarization. GWAS have identified a large number of robust associations between specific loci and complex human diseases. However, regarding genetic susceptibility to BPD, differences in clinical practice across neonatal intensive care units may hamper analyses, and environmental stressors must be tightly controlled in order to detect genetic associations in this setting. The relatively low rate of moderate to severe BPD (22%) in our high risk study population suggests good control of these external factors. Furthermore, all potential risk factors for BPD were systematically collected in our study population, which allowed accurate multivariate analyses. We found that birth weight, male gender, persistant ductus arteriosus and postnatal sepsis were independent risk factors for BPD in our population. These factors are fully concordant with those recently identified in an epidemiological study including 3,629 subjects (25), arguing for the well representativeness of our population. The need for supplemental oxygen at 36 weeks PMA was chosen as the main outcome for analysis, and was determined with the same standardized test (20) in all the participating centres. Indeed, the relative contributions of genetic and environmental effects were demonstrated to depend on the severity of BPD (5). Variations in 28-day oxygen need-based BPD were previously shown attributable completely to environmental effects whereas dependence on supplemental oxygen at 36 weeks seems to better reflect underlying genetic susceptibility (5). In agreement with these previous findings, the genetic factors identified in the present study were associated to moderate or severe BPD, but not to mild BPD. The Finnish population used for external validation included less premature neonates (gestational age below 30 weeks). However, BPD phenotype was also determined by the standardized oxygen reduction test, and the BPD rate (26%) was very similar as in our population. Although confounding factors were not all systematically collected, we were able to adjust results on birth weight, gestational age and RDS in this population.
SPOCK2 (SPARC/osteonectin, CWCV, and Kazal-like domains proteoglycan 2), also known as Testican-2, is a member of the testican group of extracellular chondroitin/heparan sulfate proteoglycans. SPOCK2 was originally cloned from a human cDNA library (26). Its role has mainly been explored in the central nervous system (26, 27). It has been shown in mice that SPOCK2 is expressed in lung (26). In humans, available data concern analyses of EST databases which detect human SPOCK2 ESTs in brain, ovary, testis, retina, lung, prostate and kidney (26). More recent studies evidenced hypermethylation of SPOCK2 regions in cell lines derived from human prostate, breast and colon cancer (28, 29).
We noted that SPOCK2 was expressed in the developing rat lung and that mRNA levels changed with stages of lung development. Alveolarization takes place between days 4 and 21 in rats. We found that SPOCK2 expression was low during the canalicular and saccular stages of rat lung development and started to increase significantly very close to the beginning of alveolarization. The highest expression was found on day 18, i. e. at termination of alveolarization, and remained elevated until day 28, before falling to the neonatal level in adulthood. This pattern of expression may be consistent with the involvement of SPOCK2 in the progress of septation and/or in its termination, rather than in its triggering. Immunoflurorescent experiments confirmed the expression of SPOCK2 during lung development and the protein was found to be expressed throughout the ECM. Both fibroblasts and AECs may contribute to the expression of SPOCK2 during lung development. We also found that SPOCK2 mRNA expression increased in rat pups exposed to hyperoxia which induces alveolar growth disorders in newborn rats (30). According to the hypothetical roles of SPOCK2 during alveolarization discussed above, the hyperoxia-induced increase of SPOCK2 could be interpreted either as a deleterious effect triggering a premature termination of septation, either as a beneficial response attempting to counteract the effects of environmental injuries on lung development. Further work is required to determine the role of SPOCK2 in lung development.
SPOCK2 is known to interact with matrix metalloproteinase (MMP) 14 (31), whose key role in lung development has been previously established (32, 33). SPOCK2 interacts also with MMP16, and we recently brought arguments for the role of this protease in BPD (7). In particular, we found a pattern of expression very similar to that observed here with SPOCK2 (7).
The fine scale mapping identified several SNPs associated with BPD, and in close linkage disequilibrium with rs1245560. Among these, rs1049269’s association with BPD was replicated in the Finnish population. rs1049269 is located in the 3′UTR region of SPOCK2 and could thus modify micro-RNA (miRNA) binding. MiRNAs are small non-coding RNAs known to regulate gene expression at the post-transcriptional level (34, 35). Computational analysis of SPOCK2 3′UTR region identified three miRNAs (mir-194*, mir-939 and mir 449b) for which binding to their target sequence could be altered by the polymorphic site rs1049269. Further experiments are required to explore the interaction between miRNAs and this polymorphism, and to determine the potential consequence on SPOCK2 expression.
Other susceptibility genes have been previously suggested in candidate gene studies in BPD (36). Among these, two were identified in our genome-wide DNA pooling studies: MMP16 and L Selectin that were selected by the allele frequency difference method in the Caucasian series. However, none were confirmed by the combined Z-score analysis, or were selected in the African series.
Thus, a genome-wide association study of a multicenter population of preterm neonates identified SPOCK2 as a new candidate susceptibility locus for BPD. Its pattern of expression during lung development points to a potential role in alveolarization.
At a Glance Commentary.
Scientific Knowledge on the Subject
Recent studies highlighted the contribution of genetic factors to bronchopulmonary dysplasia susceptibility. Few candidate-gene association studies have attempted to identify BPD susceptibility genes.
What This Study Adds to the Field
By performing a genome-wide association study, we found an association between the SPOCK2 gene and bronchopulmonary dysplasia susceptibility. In rats, we observed that SPOCK2 was highly expressed during lung development. These data suggest SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Its pattern of lung expression points to a potential role in alveolarization.
Supplementary Material
Figure E1: Flowchart of the study population
* 1 case and 1 control were excluded after DNA pooling quality control (QC) steps. † 3 controls were excluded from individual genotyping data after QC procedure.
Figure E2: Fine mapping of the SPOCK2 locus in African neonates
Individual genotyping of tag-SNPs in the African population (36 cases and 118 controls) confirmed the results obtained by DNA pooling studies. Analyses identified rs1245560 and rs1245540 as being significantly associated with BPD (B). Only rs1245560 (A, white spot), remained significant after multiple testing and adjustment for major clinical risk factors (p<0.05). LD plot were constructed using Haploview version 4.1. Red squares indicate regions of strong linkage disequilibrium (D′ given).
Table E1: Genes and SNPs selected by the allele frequency difference method in the Caucasian and African pools*.
Table E2: Genes and SNPs selected by the combined Z-score analysis in the Caucasian and African pools*.
Table E3: Comparison of minor allele frequencies between controls pools and HapMap data for four SNPs belonging to SPOCK2 and evidenced by DNA pooling
Table E4. Genotype counts for rs1245560 and rs1049269 in the different study populations.
Acknowledgments
Funding/Support: Programme Hospitalier de Recherche Clinique AOR 07 018, Assistance Publique - Hôpitaux de Paris. Agence Nationale de la Recherche ANR-09-GENO-037. Hadchouel was funded by INSERM.
Footnotes
Author contributions: Dr Hadchouel had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hadchouel, Danan, Delacourt.
Acquisition of data: Hadchouel, Durrmeyer, Jarreau, Lenclen, Layouni, Patkai, Danan, Huusko, Hallman.
Analysis and interpretation of data: Hadchouel, Incitti, Bouzigon, Franco-Montoya, Demenais, Bourbon, Delacourt.
Drafting the article: Hadchouel, Delacourt, Incitti, Bouzigon, Franco-Montoya.
Critical revision of the manuscript for important intellectual content: Hadchouel, Durrmeyer, Bouzigon, Jarreau, Demenais, Layouni, Patkai, Bourbon, Danan, Delacourt.
Final approuval of the version to be published: Hadchouel, Durrmeyer, Incitti, Bouzigon, Jarreau, Demenais, Franco-Montoya, Layouni, Patkai, Bourbon, Danan, Delacourt.
Financial disclosures: none reported.
This article has an online data supplement, which is accessible from this issue’s table of content online at www.atsjournals.org.
References
- 1.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147, e141–148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:179–184. doi: 10.1053/j.semperi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 5.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122:479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker RA, Lindstrom DP, Cotton RB. Evidence from twin study implies possible genetic susceptibility to bronchopulmonary dysplasia. Semin Perinatol. 1996;20:206–209. doi: 10.1016/s0146-0005(96)80049-8. [DOI] [PubMed] [Google Scholar]
- 7.Hadchouel A, Decobert F, Franco-Montoya ML, Halphen I, Jarreau PH, Boucherat O, Martin E, Benachi A, Amselem S, Bourbon J, Danan C, Delacourt C. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: Identification of mmp16 as a new player in lung development. PLoS One. 2008;3:e3188. doi: 10.1371/journal.pone.0003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazzi SN, Kim UO, Quasney MW, Buhimschi I. Polymorphism of tumor necrosis factor-alpha and risk and severity of bronchopulmonary dysplasia among very low birth weight infants. Pediatrics. 2004;114:e243–248. doi: 10.1542/peds.114.2.e243. [DOI] [PubMed] [Google Scholar]
- 9.Kwinta P, Bik-Multanowski M, Mitkowska Z, Tomasik T, Legutko M, Pietrzyk JJ. Genetic risk factors of bronchopulmonary dysplasia. Pediatr Res. 2008;64:682–688. doi: 10.1203/PDR.0b013e318184edeb. [DOI] [PubMed] [Google Scholar]
- 10.Manar MH, Brown MR, Gauthier TW, Brown LA. Association of glutathione-s-transferase-p1 (gst-p1) polymorphisms with bronchopulmonary dysplasia. J Perinatol. 2004;24:30–35. doi: 10.1038/sj.jp.7211020. [DOI] [PubMed] [Google Scholar]
- 11.Rova M, Haataja R, Marttila R, Ollikainen V, Tammela O, Hallman M. Data mining and multiparameter analysis of lung surfactant protein genes in bronchopulmonary dysplasia. Hum Mol Genet. 2004;13:1095–1104. doi: 10.1093/hmg/ddh132. [DOI] [PubMed] [Google Scholar]
- 12.Pearson JV, Huentelman MJ, Halperin RF, Tembe WD, Melquist S, Homer N, Brun M, Szelinger S, Coon KD, Zismann VL, Webster JA, Beach T, Sando SB, Aasly JO, Heun R, Jessen F, Kolsch H, Tsolaki M, Daniilidou M, Reiman EM, Papassotiropoulos A, Hutton ML, Stephan DA, Craig DW. Identification of the genetic basis for complex disorders by use of pooling-based genomewide single-nucleotide-polymorphism association studies. Am J Hum Genet. 2007;80:126–139. doi: 10.1086/510686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardon LR, Bell JI. Association study designs for complex diseases. Nat Rev Genet. 2001;2:91–99. doi: 10.1038/35052543. [DOI] [PubMed] [Google Scholar]
- 14.Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]
- 15.Abraham R, Moskvina V, Sims R, Hollingworth P, Morgan A, Georgieva L, Dowzell K, Cichon S, Hillmer AM, O’Donovan MC, Williams J, Owen MJ, Kirov G. A genome-wide association study for late-onset alzheimer’s disease using DNA pooling. BMC Med Genomics. 2008;1:44. doi: 10.1186/1755-8794-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunckley T, Huentelman MJ, Craig DW, Pearson JV, Szelinger S, Joshipura K, Halperin RF, Stamper C, Jensen KR, Letizia D, Hesterlee SE, Pestronk A, Levine T, Bertorini T, Graves MC, Mozaffar T, Jackson CE, Bosch P, McVey A, Dick A, Barohn R, Lomen-Hoerth C, Rosenfeld J, O’Connor DT, Zhang K, Crook R, Ryberg H, Hutton M, Katz J, Simpson EP, Mitsumoto H, Bowser R, Miller RG, Appel SH, Stephan DA. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N Engl J Med. 2007;357:775–788. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- 17.Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, Kendler KS, Li T, O’Donovan M, O’Neill FA, Owen MJ, Walsh D, Weinberger DR, Sun C, Flint J, Darvasi A. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steer S, Abkevich V, Gutin A, Cordell HJ, Gendall KL, Merriman ME, Rodger RA, Rowley KA, Chapman P, Gow P, Harrison AA, Highton J, Jones PB, O’Donnell J, Stamp L, Fitzgerald L, Iliev D, Kouzmine A, Tran T, Skolnick MH, Timms KM, Lanchbury JS, Merriman TR. Genomic DNA pooling for whole-genome association scans in complex disease: Empirical demonstration of efficacy in rheumatoid arthritis. Genes Immun. 2007;8:57–68. doi: 10.1038/sj.gene.6364359. [DOI] [PubMed] [Google Scholar]
- 19.Craig DW, Huentelman MJ, Hu-Lince D, Zismann VL, Kruer MC, Lee AM, Puffenberger EG, Pearson JM, Stephan DA. Identification of disease causing loci using an array-based genotyping approach on pooled DNA. BMC Genomics. 2005;6:138. doi: 10.1186/1471-2164-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K, Newman N, Rowan G, Grisby C, Arnell K, Miller L, Ball B, McDavid G. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 21.Dudbridge F, Gusnanto A. Estimation of significance thresholds for genomewide association scans. Genet Epidemiol. 2008;32:227–234. doi: 10.1002/gepi.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy J, Singleton A. Genomewide association studies and human disease. N Engl J Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 25.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, Stoll BJ, Buchter S, Laptook AR, Ehrenkranz RA, Cotten CM, Wilson-Costello DE, Shankaran S, Meurs KP, Davis AS, Gantz MG, Finer NN, Yoder BA, Faix RG, Carlo WA, Schibler KR, Newman NS, Rich W, Das A, Higgins RD, Walsh MC. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannahme C, Schubel S, Herud M, Gosling S, Hulsmann H, Paulsson M, Hartmann U, Maurer P. Molecular cloning of testican-2: Defining a novel calcium-binding proteoglycan family expressed in brain. J Neurochem. 1999;73:12–20. doi: 10.1046/j.1471-4159.1999.0730012.x. [DOI] [PubMed] [Google Scholar]
- 27.Schnepp A, Komp Lindgren P, Hulsmann H, Kroger S, Paulsson M, Hartmann U. Mouse testican-2. Expression, glycosylation, and effects on neurite outgrowth. J Biol Chem. 2005;280:11274–11280. doi: 10.1074/jbc.M414276200. [DOI] [PubMed] [Google Scholar]
- 28.Chung W, Kwabi-Addo B, Ittmann M, Jelinek J, Shen L, Yu Y, Issa JP. Identification of novel tumor markers in prostate, colon and breast cancer by unbiased methylation profiling. PLoS One. 2008;3:e2079. doi: 10.1371/journal.pone.0002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordgard SH, Johansen FE, Alnaes GI, Bucher E, Syvanen AC, Naume B, Borresen-Dale AL, Kristensen VN. Genome-wide analysis identifies 16q deletion associated with survival, molecular subtypes, mrna expression, and germline haplotypes in breast cancer patients. Genes Chromosomes Cancer. 2008;47:680–696. doi: 10.1002/gcc.20569. [DOI] [PubMed] [Google Scholar]
- 30.Boucherat O, Franco-Montoya ML, Thibault C, Incitti R, Chailley-Heu B, Delacourt C, Bourbon JR. Gene expression profiling in lung fibroblasts reveals new players in alveolarization. Physiol Genomics. 2007;32:128–141. doi: 10.1152/physiolgenomics.00108.2007. [DOI] [PubMed] [Google Scholar]
- 31.Nakada M, Yamada A, Takino T, Miyamori H, Takahashi T, Yamashita J, Sato H. Suppression of membrane-type 1 matrix metalloproteinase (mmp)-mediated mmp-2 activation and tumor invasion by testican 3 and its splicing variant gene product, n-tes. Cancer Res. 2001;61:8896–8902. [PubMed] [Google Scholar]
- 32.Atkinson JJ, Holmbeck K, Yamada S, Birkedal-Hansen H, Parks WC, Senior RM. Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Dev Dyn. 2005;232:1079–1090. doi: 10.1002/dvdy.20267. [DOI] [PubMed] [Google Scholar]
- 33.Boucherat O, Bourbon JR, Barlier-Mur AM, Chailley-Heu B, D’Ortho MP, Delacourt C. Differential expression of matrix metalloproteinases and inhibitors in developing rat lung mesenchymal and epithelial cells. Pediatr Res. 2007;62:20–25. doi: 10.1203/PDR.0b013e3180686cc5. [DOI] [PubMed] [Google Scholar]
- 34.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by micrornas: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 35.Rana TM. Illuminating the silence: Understanding the structure and function of small rnas. Nat Rev Mol Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie PM, Dube MP. Genetics of bronchopulmonary dysplasia in the age of genomics. Curr Opin Pediatr. 2010;22:134–138. doi: 10.1097/MOP.0b013e328336eb85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1: Flowchart of the study population
* 1 case and 1 control were excluded after DNA pooling quality control (QC) steps. † 3 controls were excluded from individual genotyping data after QC procedure.
Figure E2: Fine mapping of the SPOCK2 locus in African neonates
Individual genotyping of tag-SNPs in the African population (36 cases and 118 controls) confirmed the results obtained by DNA pooling studies. Analyses identified rs1245560 and rs1245540 as being significantly associated with BPD (B). Only rs1245560 (A, white spot), remained significant after multiple testing and adjustment for major clinical risk factors (p<0.05). LD plot were constructed using Haploview version 4.1. Red squares indicate regions of strong linkage disequilibrium (D′ given).
Table E1: Genes and SNPs selected by the allele frequency difference method in the Caucasian and African pools*.
Table E2: Genes and SNPs selected by the combined Z-score analysis in the Caucasian and African pools*.
Table E3: Comparison of minor allele frequencies between controls pools and HapMap data for four SNPs belonging to SPOCK2 and evidenced by DNA pooling
Table E4. Genotype counts for rs1245560 and rs1049269 in the different study populations.


