Abstract
Background
Little is known about the relations of magnesium intake with risk of heart failure (HF) hospitalizations, particularly in African-Americans. We hypothesize that magnesium intake relates to HF hospitalization in African-Americans.
Methods and Results
From the Jackson Heart Study cohort (n=5,301), we studied 4,916 African-Americans recruited during 2000–2004 in Jackson (MS), who completed an 158-item Food-Frequency Questionnaire which included dietary supplements. Total daily magnesium intake derived from the questionnaire was divided by the body weight (Kg) to account for body storage, and stratified by quartiles (0.522–2.308, 2.309–3.147, 3.148–4.226 and ≥4.227mg magnesium intake/Kg). Cox proportional hazards modeling assessed the association between quartiles of magnesium intake/Kg and hospitalizations for HF adjusting for HF risk, energy intake and dietary factors affecting magnesium uptake. The cohort had a mean age=55.3 (SD=12.7yrs), and composed of 63.4% women, 21.6% diabetes, 62.7% hypertension, 7.1% coronary disease and 2.8% with known HF. When compared to participants in the first quartile of magnesium intake/Kg, those with higher magnesium intake (>2.308 mg/kg) had decreased risk of HF admission, with adjusted hazard ratios of 0.66(95%CI: 0.47–0.94) in the second quartile to 0.47 (95% CI: 0.27–0.82) in the highest quartile. Results were similar when individuals with previous diagnosed HF (2.8%) were excluded or when the analysis was repeated using quartiles of magnesium intake without accounting for body weight.
Conclusions
Magnesium intake below 2.3mg/Kg (~181 mg/day) was related to increased risk for subsequent HF hospitalizations. Future studies are needed to test whether serum Magnesium levels and/or intake predict risk of heart failure.
Keywords: magnesium intake, heart failure, African Americans, echocardiographic parameters, biomarker
Approximately 5.1 million people in the United States are currently diagnosed with heart failure (HF) 1. The prevalence of HF is projected to increase 25% by 2030 with the cost of its hospitalizations increase by 120% to nearly 70 billion dollars/year. African-Americans are at a greater risk for HF and also have higher HF related morbidity and mortality compared to Caucasians 2–4. However, the underlying reason for such disparity is not clearly understood. Aside from a higher prevalence of medical comorbidities such as hypertension and diabetes in African-Americans5, nutritional factors, such as magnesium intake, may be related to HF incidence or recurrence, but yet unproven; which would be of paramount importance for prevention of HF in the population.
Low serum and/or dietary magnesium intake are established risk factors for stroke6, coronary disease7, hypertension8, metabolic syndrome9 and type 2 diabetes mellitus10, but little is known about the relation between magnesium intake and HF. Unlike HF patients with known systolic dysfunction, evidence is lacking on the effective management of individuals who are at risk of HF or have been diagnosed with HF but with preserved left ventricular ejection fraction. Thus, identifying dietary risk factors such as low magnesium intake, could serve as basis for design of non-pharmacological strategies for HF prevention. Since African-Americans are known to have a low dietary magnesium intake and have a greater burden of cardiovascular risk factors and HF compared to the general population11, the primary purpose of this study was to examine magnesium intake as it relates to HF hospitalizations in African-Americans in the Jackson Heart Study (JHS), the largest African-American cohort to date for the study of cardiovascular disease.
Methods
We conducted a longitudinal analysis to evaluate the relationship of magnesium intake (including dietary and oral supplements) and HF hospitalizations in African-American participants of the JHS. We also sought to determine whether magnesium intake is related to left ventricular systolic or diastolic function, as determined by echocardiography, as potential mechanisms in the development of HF. The JHS protocol was approved by the University of Mississippi Medical Center Institutional Review Board and the participants provided written informed consent. The current analysis of the JHS data was approved by the JHS Publication and Presentations Subcommittee and exempted from ongoing review by the Providence VA Medical Center Institutional Review Board given de-identified data hosted at the institution.
Population
The JHS is a population-based, prospective study designed to evaluate the etiology of cardiovascular disease among African-Americans residing in a tri-county metropolitan area (Hinds, Madison, Rankin Counties) in central Mississippi 12. The JHS study sample (n=5,301) was recruited between 2000 and 2004 and was drawn from predominantly three sources that targeted adults aged 21 to 95 years 13. The first recruitment sample (age 55–74 years) accounted for approximately 30% of the full JHS cohort and was from the Jackson Field Center of the Atherosclerosis Risk in Communities (ARIC) Study. The second recruitment source, 39% of the full JHS cohort was a community sample (age 35–84 years) from a commercially available list of all residents and volunteers identified through participant referral or outreach activities for which recruitment was distributed among defined demographic cells in proportions designed to mirror those in the overall population. The third recruitment source, approximately 31% of the full JHS cohort was from relatives of JHS index participants (age >21 years).
During the baseline visit, subjects answered questionnaires and underwent clinical examination and echocardiographic evaluation. Individuals were included in this analysis if they completed a validated 158-item food frequency questionnaire (FFQ) during the baseline visit (n=5,133)14.
Exposure
Elements of oral intake were assessed by means of a validated 158-item FFQ administered face-to-face by trained African-American interviewers14. The FFQ was adapted and validated14, 15 from a longer, 283-item FFQ developed using data from 2 individual, 24-hour recalls collected from a representative sample of adults living in the Mississippi Delta region16 (r= 0.60 for men and 0.55 for women between the 158 item FFQ and the mean of four 24-hour dietary recalls). Total daily magnesium intake was calculated from the contributions of each of the 158 food items and from the use of common vitamin and mineral supplement formulations. Daily magnesium intake was divided by body weight (Kg) to account for individual’s distinct magnesium body storage capacity (bones and soft tissue) and stratified by quartiles (0.522 to 2.308, 2.309 to 3.147, 3.148 to 4.226 and ≥4.227 mg magnesium intake/Kg).17
Outcomes
The main outcome was time to first adjudicated probable or definite HF hospitalization or death during 2005–201018. The definition of HF hospitalization was developed by the CHF working group at the JHS based on the modification of the Multiethnic Study of Atherosclerosis19, Framingham20, 21 and Cardiovascular Health Study (patient symptoms, physician physical findings and diagnostic procedures)22. Adjudication of HF hospitalizations was performed by trained and certified HF abstractors and began on January 1, 2005. The criteria for HF hospitalization were 1) a discharge diagnosis ICD-9 code of 428 and/or underlying cause of death of I50; and 2) radiographic findings consistent with congestive HF, physical findings of increased venous pressure >16mmHg, or dilated ventricle/left ventricular function <40% by echocardiogram/multiple gated acquisition (MUGA) scan; or 3) autopsy finding of pulmonary edema/congestive HF.
Echocardiographic parameters
Given that echocardiographic parameters reflect potential underlying pathophysiologic pathways linking magnesium intake to HF, these parameters were analyzed separately from the demographic and clinical variables to explore the association of echocardiographic parameters (Left ventricular (LV) systolic and diastolic function) with quartiles of total magnesium intake/Kg of body weight. Detailed echocardiography procedures are available online23. Briefly, echocardiograms were recorded by trained sonographers and interpreted by experienced cardiologists in the Echocardiography Reading Center located at the University of Mississippi Medical Center, who were blinded to the participants’ clinical data 24. All measurements were performed on 2D images and no Tissue Doppler was performed. LV mass was indexed to height to adjust for body habitus. LV systolic function was assessed with ejection fraction (EF), and in the absence of availability of contemporary Doppler indices to grade diastolic dysfunction 25, we chose trans-mitral early (E) filling velocity that reflect the LA-LV pressure gradients in early diastole as a surrogate for diastolic function. Tricuspid regurgitation peak velocity was used to estimate Pulmonary Artery Systolic Pressures.
Covariates
Coronary heart disease was considered present when the participant reported a history of myocardial infarction, abnormal stress test, prior coronary artery bypass graft surgery or prior coronary angioplasty. The presence of diabetes was defined as a self-reported history of diabetes, use of diabetes medications, HgbA1c ≥6.5, or a fasting blood glucose ≥ 7mmol/L (126mg/dL). Heart rate was measured at the baseline EKG. History of HF was considered present if the patient responded affirmatively to the question “Has a doctor ever said you had HF or congestive HF?” at the time of first annual telephone follow up between 2001–2005 prior to the HF hospitalization outcome ascertainment. Cigarette smoking was categorized as never smoker (having smoked less than 400 cigarettes in one’s life), former smoker (smoked >400 cigarettes but not currently smoking), and current smoker. Blood pressure lowering medication use was ascertained at the baseline clinical examination. Antihypertensive medications were categorized into the following classes: alpha adrenergic blockers (AAB), angiotensin converting enzyme inhibitors (ACE), angiotensin II receptor blockers (ARB), beta adrenergic blockers (BB), calcium channel blockers (CCB), thiazide diuretics, loop diuretics, and potassium-sparing diuretics. Infrequently prescribed medications classified as ‘Others’ comprised of vasodilators, centrally-acting alpha agonists, and Rauwolfia derivatives. Total daily protein, phosphorous, alcohol, calcium, potassium and caloric intake were calculated from the contributions of each of the 158 food items and dietary supplements.
Statistical Analysis
We excluded participants missing magnesium intake information from the FFQ (n = 164) and baseline weight (n = 7). We also excluded participants with improbable daily energy intakes (≤ 400 kcal or ≥10,000 kcal [n = 15]). Patients were also removed from the analysis if they were lost to follow up or died before the first adjudicated HF date on January 1st 2005 (n=199), leaving a total of 4,916 (92.7%) participants for analysis (Figure 1). We computed descriptive statistics and tested for linearity trend for demographic characteristics, health behaviors, baseline co-morbidities and echocardiographic parameters across dietary magnesium intake/Kg quartiles.
Figure 1.
Consort Study Flow Diagram
We used Cox proportional hazards modeling to study the association between quartiles of dietary magnesium intake/Kg with HF hospitalization adjusting for variables used in the ARIC HF prediction model (age, sex, coronary heart disease, body mass index, diabetes, systolic blood pressure, blood pressure medication use, heart rate, smoking status)26 and dietary measures influencing intestinal magnesium absorption and excretion such as intake of phosphorus 27, protein28, 29, and alcohol30 using the lowest dietary magnesium/Kg quartile as the reference category (0.522 to 2.308mg/Kg). Adjustment for alcohol intake was made as chronic alcohol consumption increases urinary magnesium excretion and reduces total muscle magnesium content.30
Race was part of the ARIC model but was dropped since our population was exclusively African-American. Our result model has excellent discrimination for the study population (Harrell’s C =0.84) and adequate calibration. Kaplan-Meier curves were created to estimate freedom from HF hospitalization according to dietary magnesium intake/Kg quartiles. We tested the proportionality of hazards assumption for the Kaplan–Meier curves by visual inspection and analysis of the Schoenfeld Residuals 31. The proportional hazards assumption was confirmed for the primary analysis (p=0.66). Subjects during the study period that died prior to a HF event were censored. In order to confirm that censoring participants that died did not significantly alter the hazards of HF hospitalization (main outcome), we repeated our analysis by estimating the sub-hazard ratios using the competing-risks regression model, according to the method of Fine and Gray 32.
We repeated our analyses excluding individuals with previously diagnosed HF (n= 138) or did not report HF status (n= 254) to ensure that our estimates are not driven mostly by individuals with diagnosed HF. Since HF hospitalization risk may vary by gender, diabetes status and systolic function (≤55% vs. >55%), effect modification analyses using multiplicative terms were conducted to determine if subgroup analyses should be performed. In addition, as magnesium intake may just be a marker for intake of healthy foods that also contain nutrients such as potassium and calcium content, for total amount of caloric intake or for a distinct annual income status, we conducted sensitivity analyses adjusting for these variables. Furthermore, because as much as 10% of dietary intake may be derived from the hardness of water and water magnesium concentrations were not available, we conducted an analysis adjusting for the total amount of daily water intake. Sensitivity analyses were also conducted using both quartiles of magnesium intake (without Kg of body mass) and the logarithmically transformed magnesium intake, with and without division for Kg of body mass, as the main exposures, to obtain non-weight adjusted and continuous estimates of magnesium intake, respectively, and their association with hospitalization risk. Given that significant differences in dietary patterns and family income status were found between subjects from the three sources of JHS recruitment strategy (ARIC participants, n = 1,749; community volunteers, n = 2,059 and JHS participant’s relatives, n =1,108; Supplemental Table 1), sensitivity analyses of subgroups stratified by recruitment strategy were also conducted. Further analyses adjusting for clustering by family were conducted for JHS participants who were identified as being family members (includes the relatives cohort and participants from other recruitment sources, n=1535), as family members may share similar genetic predisposition, dietary habits and/or health behaviors.
Data were 75.9% complete for all covariates included in the model. Missing data ranged from 0.18% (heart rate) to 18.4% (blood pressure medication use). Missing data were imputed based on 5 sets of simulated values generated from non-missing variables using the multiple imputation method in STATA (StataCorp, College Station, TX)33. Analyses were performed on each of the 5 data sets completed with imputed values, and then combined using Rubin’s combination rules to consolidate the individual estimates into a single set of estimates using the MI estimate command in STATA34. Sensitivity analyses were also repeated without imputation of missing variables. All analyses were performed using STATA/SE version 11.2 software (StataCorp LP, College Station, TX). A 2-sided p-value of <0.05 was considered significant.
Results
Baseline Characteristics of the Study Cohort
Of the 5,155 subjects that completed the 158-item FFQ, we have follow-up information on HF admission starting on January 1, 2005 on 4,916 participants. Excluded participants (7.3% of the original sample) had higher intakes of total daily energy, protein, alcohol, phosphorous water, potassium, calcium and magnesium. Excluded participants were also more likely to be a current smoker and to have a history of COPD, stroke, coronary heart disease or diabetes (Supplemental Table 2). The median gap from the baseline visit to initiation of HF adjudication was 816 days (275–1,526 days). The median follow up time starting January 1st 2005 was 1,837 days (4–2,190 days). A total of 270 individuals had a confirmed HF hospitalization during the follow-up period. The incidence of HF admission during follow-up was 1.1% per person-year during the adjudication period.
The baseline clinical characteristics of the study population, both overall and according to magnesium intake/Kg quartiles, are shown in Table 1. The overall study cohort had a mean age of 55.4 (SD 12.7 years), of which 63.8% were women, 21.7% had diabetes, 61.4% hypertension, and 7.1% had coronary heart disease. Compared with the participants in the lowest quartile, participants in the higher quartiles of dietary magnesium intake/Kg were more likely to be male, to have higher HDL-cholesterol and total daily energy intake despite having lower BMI. They also had higher intake of protein, alcohol, water, calcium, potassium and phosphorus; and were more likely to be a current smoker. Participants in the lowest dietary magnesium intake/Kg quartile, on the other hand, were more likely to have diabetes mellitus, hypertension and higher glycosylated hemoglobin (HBA1c), and high-sensitivity-C-reactive protein (hsCRP). Those in the lowest quartile were also more likely to report taking antihypertensive or diuretic medications than those participants in the higher quartiles.
Table 1.
Baseline Characteristics of Quartiles of Total Magnesium Intake/Kg of Total Body Weight in African American Women and Men
| Quartiles of Total Magnesium Intake/Kg of Total Body Weight | ||||||
|---|---|---|---|---|---|---|
| Overall | 0.522–2.308 | 2.309–3.147 | 3.148–4.267 | >=4.227 | P value for linearity trend across quartiles | |
| N | 4916 | 1233 | 1240 | 1236 | 1207 | |
| Total Daily Energy Intake ±SD, Kcal | 2146.6±1161.2 | 1329.7±526.5 | 1782.0±620.0 | 2248.3±781.0 | 3251.3±1463.3 | <0.001 |
| Total Daily Magnesium Intake ±SD, mg | 305.2±140.4 | 181.0±50.4 | 252.5±56.9 | 317.0±68.5 | 474.0±149.4 | <0.001 |
| Weight ±SD, Kg | 90.5±21.4 | 102.2±24.0 | 92.9±19.5 | 86.7±17.7 | 80.1±17.1 | <0.001 |
| Total Daily Magnesium mg/Kg of Body Weight ±SD, | 3.5±1.8 | 1.8±0.4 | 2.7±0.2 | 3.7±0.3 | 6.0±1.9 | <0.001 |
| Age ±SD, years | 55.3±12.7 | 55.4±12.0 | 56.2±12.1 | 55.6±12.8 | 54.2±13.8 | 0.008 |
| Male, % | 36.2 | 29.9 | 35.9 | 37.4 | 41.8 | <0.001 |
| BMI ±SD, Kg/M2 | 31.8±7.2 | 36.0±8.4 | 32.6±6.5 | 30.3±5.7 | 28.1±5.6 | <0.001 |
| Current Smoker, % | 12.4 | 7.7 | 10.6 | 13.1 | 18.6 | <0.001 |
| Former Smoker, % | 18.7 | 17.8 | 19.8 | 19.1 | 18.1 | |
| Never Smoker, % | 68.4 | 74.4 | 69.1 | 66.7 | 63.0 | |
| Type 1 or 2 Diabetes Mellitus, % | 21.7 | 27.7 | 23.6 | 21.6 | 13.5 | <0.001 |
| Hypertension, % | 61.4 | 66.3 | 65.4 | 59.3 | 54.3 | <0.001 |
| COPD, % | 8.5 | 7.2 | 8.5 | 8.9 | 9.5 | 0.048 |
| Hyperlipidemia, % | 30.9 | 32.8 | 32.4 | 28.9 | 29.5 | 0.036 |
| Stroke, % | 4.0 | 3.6 | 4.4 | 4.6 | 3.5 | 0.993 |
| Myocardial Infarction, % | 5.0 | 5.6 | 5.2 | 5.0 | 4.2 | 0.122 |
| Coronary Heart Disease, % | 7.1 | 7.8 | 7.2 | 7.0 | 6.3 | 0.153 |
| Heart Failure, % | 2.8 | 3.7 | 2.4 | 2.8 | 2.5 | 0.152 |
| Systolic Blood Pressure ±SD, mmHg | 126.9±18.3 | 127.4±18.2 | 127.6±18.2 | 126.4±17.9 | 126.4±18.9 | 0.015 |
| Diastolic Blood Pressure ±SD, mmHg | 78.9±10.5 | 78.9±10.4 | 79.3±10.5 | 78.3±10.3 | 78.9±10.9 | 0.420 |
| Heart Rate ±SD, bpm | 64.4±10.6 | 65.9±11.1 | 64.5±10.8 | 63.7±10.1 | 63.5±10.0 | <0.001 |
| HBA1c ±SD, % | 6.0 ± 1.3 | 6.1 ± 1.3 | 6.1 ± 1.3 | 6.0 ± 1.3 | 5.7 ± 1.1 | <0.001 |
| Fasting Plasma Glucose ±SD, mmol/L | 5.5±1.8 | 5.8±2.1 | 5.6±1.8 | 5.5±1.8 | 5.2±1.8 | <0.001 |
| Total Cholesterol, mmol/L | 5.2±1.0 | 5.2±1.0 | 5.2±1.0 | 5.2±1.0 | 5.1±1.0 | 0.108 |
| LDL-Cholesterol ±SD, mmol/L | 3.3±0.9 | 3.3±1.0 | 3.3±0.9 | 3.3±1.0 | 3.2±0.9 | 0.023 |
| HDL-Cholesterol ±SD, mmol/L | 1.3±0.4 | 1.3±0.4 | 1.3±0.4 | 1.4±0.4 | 1.4±0.4 | <0.001 |
| Triglycerides ±SD, mmol/dL | 1.2±0.9 | 1.2±0.9 | 1.3±0.9 | 1.2±0.8 | 1.1±1.0 | <0.001 |
| Calibrated Scr ±SD, umol/dL | 76.3±38.1 | 83.9±45.8 | 76.3±38.1 | 76.3±30.5 | 76.3±38.1 | 0.587 |
| hsCRP ±SD, mg/L | 4.8±7.6 | 5.7±7.6 | 4.8±6.7 | 4.8±7.6 | 3.8±7.6 | <0.001 |
| Blood Pressure Medication, % | 61.4 | 67.6 | 66.2 | 60.2 | 50.7 | <0.001 |
| Diuretic Medication, % | 39.5 | 46.4 | 43.2 | 37.4 | 30.4 | <0.001 |
| Total Protein ±SD, g | 77.2±44.5 | 47.2±20.3 | 64.8±26.4 | 81.7±33.1 | 115.9±56.8 | <0.001 |
| Alcohol ±SD, g | 4.0±15.7 | 1.4±5.7 | 3.0±9.3 | 3.8±11.5 | 7.8±26.9 | <0.001 |
| Phosphorous ±SD, mg | 1270.1±674.6 | 757.5±299.1 | 1061.5±373.2 | 1345.6±446.4 | 1930.9±812.3 | <0.001 |
| Water ±SD, g | 3870.5±2288.1 | 2969.8±1804.7 | 3598.9±2001.8 | 4037.2±2153.2 | 4898.8±2674.4 | <0.001 |
| Potassium ±SD, mg | 2750.9±1353.4 | 1746.1±670.7 | 2328.1±734.0 | 2872.6±917.5 | 4087.4±1603.5 | <0.001 |
| Calcium ±SD, mg | 857.3±500.8 | 522.9±196.5 | 735.1±360.4 | 895.4±382.2 | 1285.4±578.7 | <0.001 |
Abbreviations: BMI = Body mass index; COPD: chronic obstructive pulmonary disease; HBA1c = hemoglobin A1c; hsCRP = high-sensitivity C-reactive protein
Heart Failure Hospitalizations
When compared with participants in the first quartile of magnesium intake/Kg, those in the higher quartiles of magnesium intake/Kg had lower unadjusted hazards of HF admission starting with intake levels in the second quartile (2.308 mg magnesium/kg of weight) and higher. Figure 2 shows the unadjusted Kaplan Meier survival curves of participants grouped by the quartiles of dietary magnesium/Kg. After adjustment for potential confounding (Model 1, Table 2), the adjusted hazard ratios of HF admission for subjects with magnesium intake in the second quartile or higher were lower, ranging from 0.66 (95% CI: 0.47–0.94) in the second quartile to 0.47 (95% CI: 0.27–0.82) in the fourth quartile, compared to individuals in the lowest quartile of magnesium intake/Kg (Table 2). Results were not significantly changed after further adjustment for baseline ejection fraction (Model 2), daily water intake (Model 3), daily potassium intake (Model 4), daily calcium intake (Model 5), daily energy intake (Model 6) and family annual income (Model 7)(Table 2). Confirmatory analyses using the competing-risks regression model by estimating the sub-hazard ratios of HF in presence of a competing event (death) showed very similar results.
Figure 2.
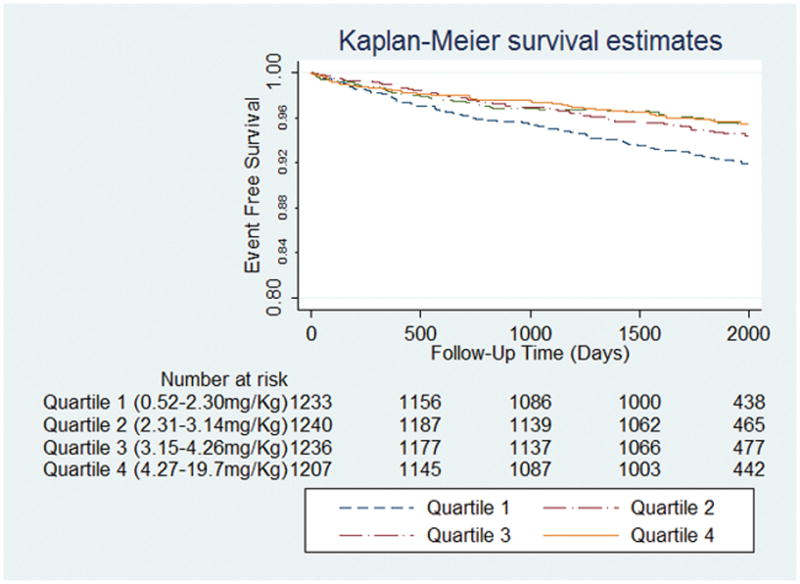
Kaplan Meier curves showing heart failure admission free survival in quartiles of dietary magnesium intake/Kg of body weight. The events are heart failure admissions and the follow-up data starts at 1/1/2005, when the adjudication for heart failure admissions began.
Table 2.
Hazard Ratios of Heart Failure Admission per Quartile of Total Magnesium Intake/Kg of Total Body Weight
| Quartiles of Total Magnesium Intake/Kg of Total Body Weight (mg/Kg) | |||||
|---|---|---|---|---|---|
| N | 0.522–2.308 | 2.309–3.147 | 3.148–4.226 | ≥4.227 | |
| Unadjusted | 4916 | Referent | 0.66(0.48–0.90) | 0.56(0.40– 0.78) | 0.54(0.39– 0.76) |
| aModel 1 | 4916 | Referent | 0.66(0.47–0.94) | 0.55(0.36–0.84) | 0.47(0.27–0.82) |
| bModel 2 | 4698 | Referent | 0.71(0.50–1.02) | 0.59(0.38–0.91) | 0.54(0.30–0.95) |
| cModel 3 | 4916 | Referent | 0.63(0.45–0.90) | 0.51(0.34–0.78) | 0.41(0.23–0.74) |
| dModel 4 | 4916 | Referent | 0.65(0.45–0.92) | 0.53(0.35–0.81) | 0.44(0.24–0.78) |
| eModel 5 | 4916 | Referent | 0.65(0.46–0.92) | 0.54(0.36–0.83) | 0.45(0.25–0.79) |
| fModel 6 | 4916 | Referent | 0.66(0.46–0.93) | 0.54(0.36–0.83) | 0.45(0.25–0.79) |
| gModel 7 | 4916 | Referent | 0.67(0.47–0.94) | 0.56(0.37–0.85) | 0.47(0.27–0.82) |
| Excluding Individuals with Previously Diagnosed Heart Failure or did not Report Heart Failure Status | |||||
| Model 1 | 4524 | Referent | 0.71 (0.48–1.04) | 0.52 (0.32–0.84) | 0.50 (0.27–0.93) |
| Quartiles of Total Magnesium Intake (mg) (not per Kg) | |||||
| N | 12.7–210.2 | 210.3–278.1 | 278.2–367.3 | ≥367.3 | |
| Model 1 | 4916 | Referent | 0.62 (0.44–0.87) | 0.81 (0.55–1.18) | 0.53 (0.30–0.91) |
| Model 6 | 4916 | Referent | 0.62 (0.44–0.87) | 0.80 (0.55–1.17) | 0.51 (0.29–0.90) |
Model 1: Adjusted for age, gender, heart rate, systolic blood pressure, smoking status, body mass index, blood pressure medication, history of diabetes, history of coronary heart disease, history of heart failure, total daily intake of protein, alcohol and phosphorous
Model 2: Model 1 and baseline ejection fraction levels (220 subjects with missing ejection fraction data)
Model 3: Model 1 and total daily water intake
Model 4: Model 1 and total daily potassium intake
Model 5: Model 1 and total daily calcium intake
Model 6: Model 1 and total daily energy intake
Model 7: Model 1 and socioeconomic status
Effect modification analyses using multiplicative terms for gender, ejection fraction and diabetes diagnosis were non-significant, for which subgroup analyses were not pursued.
Sensitivity analyses
Using Cox regression modeling to study the relation between quartiles of magnesium intake without accounting for Kg of body weight and HF hospitalizations yielded similar results even when adjusted for daily energy intake (Model 6) (Table 2). Results remained unchanged when individuals with previous diagnosed HF (2.8%) and those who did not report HF status were excluded (Table 2). Analyses using the logarithmically transformed dietary magnesium intake (with and without division for Kg) as a continuous variable did not show a significant linear relationship between magnesium intake and HF hospitalizations (Adjusted hazard ratio for log magnesium intake/kg = 0.73, 95%CI 0.45–1.20, p=0.21; and for log magnesium intake accounting for caloric intake = 0 .65, 95%CI 0.39–1.10, p=0.11). Both non-imputed and imputed sensitivity analyses results were similar. Analyses in subgroups stratified by recruitment strategy and amongst JHS participants who are identified as family members, adjusting for clustering by the family identification variable, showed very similar results (Supplemental Table 3).
Echocardiographic parameters
Table 3 shows the echocardiographic parameters of the study cohort. The mean EF of the study cohort was 61.7 ± 7.7%, with a relatively high overall prevalence of a normal EF (92.5% with EF ≥55%). Compared to the participants in the lowest quartile of magnesium intake/Kg, participants across quartiles of magnesium intake/Kg had similar LV mass and ejection fraction values. However, Doppler peak mitral E wave velocity (surrogate for left ventricular filling pressures) and tricuspid regurgitation peak velocity (to estimate pulmonary systolic pressures) were significantly lower in participants with higher quartiles of magnesium intake/Kg.
Table 3.
Echocardiographic parameters per Quartile of Total Magnesium Intake/Kg of Total Body Weight (Unadjusted)
| Quartiles of Total Magnesium Intake/Kg of Total Body Weight (mg/Kg) | P-Value for linear trend across quartiles | ||||||
|---|---|---|---|---|---|---|---|
| N | Overall | 0.522–2.308 | 2.309–3.147 | 3.148–4.267 | ≥4.227 | ||
| Left Ventricular Mass indexed by BSA, g/m2 | 3176 | 74.4±18.5 | 74.6±18.3 | 74.6±18.0 | 73.7±18.8 | 74.8±18.8 | 0.472 |
| Doppler Peak Mitral E Wave Velocity, m/sec | 4422 | 0.84±0.19 | 0.86±0.20 | 0.85±0.20 | 0.84±0.19 | 0.83±0.18 | <0.001 |
| Tricuspid Regurgitation Peak Velocity, cm/s | 3052 | 22.9±7.3 | 23.8±7.6 | 22.9±7.5 | 22.6±7.3 | 22.0±6.6 | <0.001 |
| LV Ejection Fraction, % | 4698 | 61.8±7.5 | 61.6±7.7 | 61.9±7.4 | 61.8±7.4 | 61.9±7.7 | 0.451 |
Abbreviations: BSA = Body surface area; LV= left ventricular
Discussion
In the largest cardiovascular cohort of African-Americans to date, magnesium intake was inversely related to future HF hospitalization in both men and women followed for 5 years. To our knowledge, these findings are the first to relate magnesium intake to incident HF events in a cohort of mostly non-HF patients (<3% of our baseline population) with normal left ventricular systolic function. Results remained consistent when adjusting for dietary factors and when individuals with previously diagnosed HF were excluded. Quartiles of magnesium intake/Kg were also inversely related to Doppler peak mitral E wave velocity (a surrogate for diastolic function) and tricuspid regurgitation peak velocity (an estimate of pulmonary systolic pressures), but unrelated to systolic function.
Potential mechanisms of how magnesium intake is related to HF hospitalizations remain to be clarified but are likely multi-factorial. Magnesium is a principal intracellular cation that affects muscle relaxation,17, 35, 36 and may improve diastolic dysfunction as shown previously in intravenous supplementation,37 and in the current study we found that magnesium intake was related to lower peak mitral E wave velocity. Equally important, we also observed that magnesium intake was inversely related to estimates of pulmonary systolic pressures. These findings support the notions that magnesium intake may have either a direct effect on the pulmonary vasculature or an indirect effect as a result of improvement in diastolic function, which should be pursued further in future mechanistic work. In contrast, we did not find a significant relation between magnesium intake and systolic function. This can be due to a lack of statistical power because 92.5% of our study population had a normal left ventricular ejection fraction of >55%. Additionally, magnesium intake may also be related to lower risk of HF hospitalizations due to improvement of blood pressure and glycemic control and/or lower LDL cholesterol levels which may result in lower prevalence of coronary heart disease.10, 11, 38 As shown in Table 1, individuals with higher magnesium intake had lower blood pressure, LDL cholesterol and hemoglobin A1c levels as well as lower prevalence of hypertension and diabetes. Previous studies have demonstrated that magnesium exerts beneficial effects on the cardiovascular system by enhancing endothelium-dependent vasodilation, improving lipid and glucose metabolism, reducing inflammation, and inhibiting platelet aggregation9, 39–41. Indeed, higher levels of magnesium intake were associated with lower hsCRP levels as also seen in Table 1.
The magnesium intake of 2.7–3.7mg magnesium/kg of body weight or 252.5–317.0 mg per day in the middle quartiles of our study cohort are slightly higher than the average consumption of magnesium for African-Americans of 237mg/day shown in 200411, yet still slightly below the US recommended daily allowances (RDA) values of greater than 320mg of intake for women and far below the 420mg for men aged 31–70 years old. However, even at these below RDA values of magnesium intake from the second quartile and thereafter, we found an association with less HF hospitalization risk when compared with the magnesium intake/kg at the lowest quartile (1.8 mg/kg of body weight or 181mg per day). Although an important finding, these results should not be surprising since RDA values were developed for the planning and procurement of food supplies at the population level in order to provide adequate intake for those with the highest requirements and not necessarily as a threshold below which disease will develop with inadequate intake42. As such, RDA levels do not take into account biological variability or efficiency of utilization of the nutrient in food sources. In addition, the lack of a significant linear relationship between magnesium intake and HF hospitalization risk in our study may suggest that there is a threshold effect, after which higher magnesium intake levels did not translate into less risk. In our population with a mean age of 55±12 years, we did not see further lowering of the hazards of HF hospitalization after the third quartile of magnesium intake (mean 3.7±0.3 mg/Kg or 317±68.5 mg per day).
There are limitations to our study. First, inherent to all observational studies and despite our efforts to adjust for potential confounding, our results may still be biased by residual confounding and by exclusion of a small group of sicker participants for various reasons. However, our study sample still has adequate representation of participants with distinct comorbidities. More importantly, the exclusion of the sicker participants is likely to bias toward the null hypothesis given less likelihood of incident events. We tried to conduct sensitivity analyses accounting for calcium, potassium, total daily water intake, in addition to total energy intake (Table 2, models 3–6) and found the results to be consistent with the main findings. Second, serum magnesium levels were not available and the general limitations of the FFQ as a dietary assessment tool cannot be overlooked, including its reliance on memory, reporting completeness and over- or under-estimation of nutrients and of total energy intake, especially in obese subjects.43 Ideally, calibration of nutrient/caloric intake with biomarkers to validate the questionnaire findings would be valuable to overcome these limitations.16, 44 Finally, detailed data involving clinical presentation of HF and mode of death were unavailable to allow us to elucidate potential mechanisms, and adjudication of HF events did not start until 2005; therefore we could have underestimated the relationship between dietary magnesium and HF hospitalizations. Given these limitations, our results should be considered as hypothesis generating.
In conclusion, we report that quartiles of magnesium intake/Kg body mass predicted future HF events and an intake below 2.3 mg/Kg weight (~181mg/day) may represent a risk factor for future HF hospitalizations. Additional studies are needed to test whether serum magnesium concentration or intake (or both) predict risk of heart failure.
Supplementary Material
Clinical Perspective.
In an observational study of the largest cardiovascular cohort of African-Americans to date, the Jackson Heart study, quartiles of magnesium intake was inversely related to future heart failure hospitalization in 4916 men and women who were followed for 5 years. These findings are the first to relate magnesium intake to incident heart failure events in a cohort of mostly non-HF patients (<3% of our baseline population) with normal left ventricular systolic function. Results remained consistent when adjusting for energy intake and dietary factors, and when individuals with previously diagnosed heart failure were excluded. Quartiles of magnesium intake/Kg of body mass were also inversely related to Doppler peak mitral E wave velocity (a surrogate for diastolic function) and tricuspid regurgitation peak velocity (an estimate of pulmonary systolic pressures), but unrelated to systolic function. In conclusion, we report that total magnesium intake below 2.3 mg/Kg weight (~181mg/day) may be a risk factor for future HF hospitalizations. Additional studies are needed to test whether serum magnesium concentration or intake (or both) predict risk of heart failure.
Acknowledgments
Sources of Funding
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration.
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities
Footnotes
Disclosures
None of the authors have any relationship with industry to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.Go AS, Mozaffarian D, Roger VrL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics - 2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams KF, Jr, Dunlap SH, Sueta CA, Clarke SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L, Koch G. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol. 1996;28:1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, Sueta CA, Gheorghiade M, O’Connor CM, Schwartz TA, Koch GG, Uretsky B, Swedberg K, McKenna W, Soler-Soler J, Califf RM. Gender differences in survival in advanced heart failure. Insights from the first study. Circulation. 1999;99:1816–1821. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- 4.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 5.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA International Society on Hypertension in B. Management of high blood pressure in blacks: An update of the international society on hypertension in blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: A meta-analysis of prospective studies. Am J Clin Nutr. 2012;95:362–366. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 7.Abbott RD, Ando F, Masaki KH, Tung KH, Rodriguez BL, Petrovitch H, Yano K, Curb JD. Dietary magnesium intake and the future risk of coronary heart disease (the honolulu heart program) Am J Cardiol. 2003;92:665–669. doi: 10.1016/s0002-9149(03)00819-1. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson HO, Nicolson DJ, Campbell F, Cook JV, Beyer FR, Ford GA, Mason J. Magnesium supplementation for the management of essential hypertension in adults. Cochrane database of systematic reviews (Online) 2006:CD004640. doi: 10.1002/14651858.CD004640.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, c-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older u.S. Women. Diabetes care. 2005;28:1438–1444. doi: 10.2337/diacare.28.6.1438. [DOI] [PubMed] [Google Scholar]
- 10.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: Meta-analysis of prospective cohort studies. Diabetes Care. 2011;34:2116–2122. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of u.S. Adults. The Journal of Nutrition. 2003;133:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 12.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in african americans: Design and methods of the jackson heart study. Ethn Dis. 2005;15:S6-4–17. [PubMed] [Google Scholar]
- 13.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting african-american research participation in the jackson heart study: Methods, response rates, and sample description. Ethn Dis. 2005;15:S6-18–29. [PubMed] [Google Scholar]
- 14.Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA, Tucker KL. Validity and calibration of food frequency questionnaires used with african-american adults in the jackson heart study. Journal of the American Dietetic Association. 2009;109:1184–1193. e1182. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talegawkar SA, Johnson EJ, Carithers TC, Taylor HA, Jr, Bogle ML, Tucker KL. Serum carotenoid and tocopherol concentrations vary by dietary pattern among african americans. J Am Diet Assoc. 2008;108:2013–2020. doi: 10.1016/j.jada.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, Zaghloul S, Carithers T, Bogle ML. A regional food-frequency questionnaire for the us mississippi delta. Public Health Nutr. 2005;8:87–96. [PubMed] [Google Scholar]
- 17.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5:i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in african americans: Jackson heart study. Circ Heart Fail. 2014;7:558–564. doi: 10.1161/CIRCHEARTFAILURE.114.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: The multi-ethnic study of atherosclerosis (mesa) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB Framingham Heart S. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The framingham heart study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM. The risk of congestive heart failure: Sobering lessons from the framingham heart study. Curr Cardiol Rep. 2001;3:184–190. doi: 10.1007/s11886-001-0021-1. [DOI] [PubMed] [Google Scholar]
- 22.Schellenbaum GD, Heckbert SR, Smith NL, Rea TD, Lumley T, Kitzman DW, Roger VL, Taylor HA, Psaty BM. Congestive heart failure incidence and prognosis: Case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–122. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Jackson Heart Study Coordinating Center. [Accessed on 3/7/2016 at];Jackson Heart Study Manual 6 Echocardiography Visit 1 v1.0. 2001 Feb 15; http://www.cscc.unc.edu/jhs/docs/manuals/m6_echocardiography_02152001.pdf.
- 24.Samdarshi TE, Taylor HA, Edwards DQ, Liebson PR, Sarpong DF, Shreenivas SS, Howard G, Garrison RJ, Fox ER. Distribution and determinants of doppler-derived diastolic flow indices in african americans: The jackson heart study (jhs) Am Heart J. 2009;158:209–216. doi: 10.1016/j.ahj.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: The atherosclerosis risk in communities (aric) study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franz K. Magnesium in health and disease. London: John Libbey & Co; 1989. Influence of phosphorus on intestinal absorption of calcium and magnesium. [Google Scholar]
- 28.Hunt SM, Schofield FA. Magnesium balance and protein intake level in adult human female. Am J Clin Nutr. 1969;22:367–373. doi: 10.1093/ajcn/22.3.367. [DOI] [PubMed] [Google Scholar]
- 29.Wong NL, Quamme GA, Dirks JH. Effects of acid-base disturbances on renal handling of magnesium in the dog. Clin Sci (Lond) 1986;70:277–284. doi: 10.1042/cs0700277. [DOI] [PubMed] [Google Scholar]
- 30.Abbott L, Nadler J, Rude RK. Magnesium deficiency in alcoholism: Possible contribution to osteoporosis and cardiovascular disease in alcoholics. Alcoholism, clinical and experimental research. 1994;18:1076–1082. doi: 10.1111/j.1530-0277.1994.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 31.Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika. 1982;69:239–241. [Google Scholar]
- 32.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 33.Rubin D. Multiple imputation for nonresponse in surveys. Hoboken, NJ: 1987. [Google Scholar]
- 34.Marchenko Y. Multiple-imputation analysis using Stata’s mi command; Stata Corp LP. Stata Conference; Boston. 7/16/2010; [Accessed on 3/7/2016 at]. http://www.stata.com/meeting/boston10/boston10_marchenko.pdf. [Google Scholar]
- 35.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: A review. Molecular and cellular biochemistry. 2002;238:163–179. doi: 10.1023/a:1019998702946. [DOI] [PubMed] [Google Scholar]
- 36.Wacker WE, Parisi AF. Magnesium metabolism. N Engl J Med. 1968;278:658–663. doi: 10.1056/NEJM196803212781205. [DOI] [PubMed] [Google Scholar]
- 37.Kraus F. Reversal of diastolic dysfunction by intravenous magnesium chloride. Can J Cardiol. 1993;9:618–620. [PubMed] [Google Scholar]
- 38.Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2013;2:e000114. doi: 10.1161/JAHA.113.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shechter M. Magnesium and cardiovascular system. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2010;23:60–72. doi: 10.1684/mrh.2010.0202. [DOI] [PubMed] [Google Scholar]
- 40.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 41.Song Y, Hsu YH, Niu T, Manson JE, Buring JE, Liu S. Common genetic variants of the ion channel transient receptor potential membrane melastatin 6 and 7 (trpm6 and trpm7), magnesium intake, and risk of type 2 diabetes in women. BMC Med Genet. 2009;10:4. doi: 10.1186/1471-2350-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaton GH. Uses and limits of the use of the recommended dietary allowances for evaluating dietary intake data. Am J Clin Nutr. 1985;41:155–164. doi: 10.1093/ajcn/41.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Johnson RK, Soultanakis RP, Matthews DE. Literacy and body fatness are associated with underreporting of energy intake in us low-income women using the multiple-pass 24-hour recall: A doubly labeled water study. J Am Diet Assoc. 1998;98:1136–1140. doi: 10.1016/S0002-8223(98)00263-6. [DOI] [PubMed] [Google Scholar]
- 44.Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, Beresford SA, Caan B, Thomson C, Satterfield S, Kuller L, Heiss G, Smit E, Sarto G, Ockene J, Stefanick ML, Assaf A, Runswick S, Prentice RL. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the women’s health initiative. Am J Epidemiol. 2008;167:1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.