Abstract
Background
Left atrial size is an established marker of risk for adverse outcomes in heart failure with preserved ejection fraction (HFpEF). However, the independent prognostic importance of LA function in HFpEF is not known.
Methods and Results
We assessed LA function measured by speckle tracking echocardiography in 357 HFpEF patients enrolled in the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial who were in sinus rhythm at the time of echocardiography. Lower peak LA strain, indicating LA dysfunction, was associated with older age, higher prevalence of atrial fibrillation and LV hypertrophy, worse LV and RV systolic function, and worse LV diastolic function. At a mean follow-up of 31 months (IQR 18 – 43months), 91 patients (25.5%) experienced the primary composite endpoint of CV death, HF hospitalization, and aborted sudden death. Lower peak LA strain was associated with a higher risk of the composite endpoint (HR 0.96 per unit of reduction in strain, 95% CI 0.94-0.99; p=0.009) and of HF hospitalization alone (HR 0.95 per unit of reduction in strain, 95% CI 0.92-0.98; p=0.003). The association of LA strain with incident HF hospitalization remained significant after adjustment for clinical confounders, but not after further adjustment for LV global longitudinal strain and the E/E′ ratio, parameters of LV systolic and diastolic function respectively.
Conclusions
LA dysfunction in HFpEF is associated with a higher risk of HF hospitalization independent of potential clinical confounders, but not independent of LV strain and filling pressure. Impairment in LV systolic and diastolic function largely explain the association between impaired LA function and higher risk of HF hospitalization in HFpEF.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00094302.
Keywords: diastolic heart failure, echocardiography, atrial strain, prognosis
Heart failure with preserved ejection fraction (HFpEF) is common, accounting for up to half of patients with heart failure (HF),1,2 and for an increasing proportion of patients hospitalized with acute decompensated heart failure.3,4 Although the pathophysiologic mechanisms underlying HFpEF remain unclear, these patients are more likely to have left ventricular (LV) hypertrophy or concentric remodeling, LV diastolic dysfunction, and left atrial (LA) enlargement.5 LA size is independently associated with increased risk of morbidity and mortality in HFpEF.6,7 Previous studies have demonstrated impairment of LA function in HFpEF,8-10 with LA reservoir function measured by peak LA strain emerging as a particularly robust measure of LA dysfunction in these patients.11 However, data regarding the prognostic relevance of LA dysfunction in HFpEF is limited,12 and the prognostic value of LA dysfunction beyond measures of LV function is not known.
We used baseline data from the echocardiographic study of the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial5 to determine the correlates of LA dysfunction measured by peak LA strain in HFpEF patients, and to define the prognostic importance of LA dysfunction for the composite endpoint of cardiovascular death, HF hospitalization and aborted sudden death, and its individual components.
Methods
Patient Population
The TOPCAT trial was designed to determine the efficacy of the aldosterone antagonist spironolactone to reduce cardiovascular morbidity and mortality in patients with HFpEF.13 3,445 adults at least 50 years old with symptomatic HF, an LVEF ≥45% per local site reading, controlled systolic blood pressure, and a serum potassium level of less than 5 mmol/liter were enrolled. Eligible patients had at least one hospitalization in the prior 12 months for which HF was a major component or, if no qualifying hospitalization, a B-type natriuretic peptide (BNP) in the prior 60 days ≥100 pg/ml or N-terminal pro-BNP (NT-proBNP) ≥360 pg/ml. Baseline demographics and clinical characteristics of the trial population were previously described in detail.14
For quality control purposes, each enrolling site was required to submit echocardiographic images obtained within 6 months prior enrollment from at least the first 2 randomized patients to the echocardiographic core laboratory for verification of LVEF as previously described in detail.5 At 27 sites, patients consenting to participation in the overall TOPCAT trial were separately consented for participation in the echocardiographic sub-study. The current analysis pooled all baseline echocardiograms with quality suitable for quantitative analysis from the echo sub-study and the quality assurance studies. All patients provided written informed consent, and the study was approved by the local Institutional Review Board at each site.
Echocardiographic methods
Standard echocardiographic and Doppler parameters were analyzed using an offline analysis workstation at a dedicated core laboratory blinded to clinical information as previously described. 5 All measurements were made in accordance with the recommendations of the American Society of Echocardiography15,16 and included LV and LA dimensions and volumes, RV areas, LV wall thickness, LV mass, LVEF, RV fractional area change, mitral inflow propagation and mitral annular relaxation velocities, and tricuspid regurgitation (TR) jet velocity. LV wall thickness was defined as the average between interventricular septal wall and posterior wall thickness, and relative wall thickness (RWT) was calculated as 2 * (posterior wall thickness) / LV end-diastolic diameter. LV hypertrophy (LVH) was defined as LV mass indexed to BSA >95 g/m2 in women and >115 g/m2 in men. Normal geometry was defined as RWT ≤ 0.42 and no LVH; concentric remodeling as RWT >0.42 and no LVH; concentric hypertrophy as RWT >0.42 and LVH; and eccentric hypertrophy as RWT ≤ 0.42 and LVH).
LA and LV deformation were measured using a B-mode speckle-tracking vendor-independent software with algorithms designed for the LV (TomTec Imaging Systems, Unterschleissheim, Germany). This software is angle independent and identifies cardiac motion by tracking multiple reference points over time.17,18 The LA and LV endocardial borders were traced at the end-diastolic frame of 2D images acquired from the apical 2- and 4-chamber views.17 End-diastole was defined by the QRS complex or as the frame after mitral valve closure. Speckles were tracked by the software frame by frame over the course of one cardiac cycle. Semi-quantitative segment tracking was carefully inspected for each image and manually adjusted as needed. For patients in atrial fibrillation (Supplemental data) strain values were averaged over three cardiac cycles.11
For LV deformation, global longitudinal strain was calculated as the average LV longitudinal strain across the 12 segments obtained using apical 4- and 2-chamber views as previously described.19 As LV myocardial contraction results in ventricular shortening in systole, global longitudinal strain results in a negative strain value. From LA speckle tracking analysis, LA phasic function was measured using volumes and strain indices calculated as the average of the 12 segments obtained using apical 4- and 2-chamber views. LA time-volume curves were generated by calculating LA volume at each phase of the cardiac cycle (LA maximal, LA pre-A, and LA minimum volumes) using the Simpson's method. From these LA volumes, LA phasic function was estimated as:
-LA emptying fraction (reservoir function) = [(LA maximum volume – LA minimal volume)/LA maximum volume]*100
-LA passive emptying fraction (conduit function) = [(LA maximum volume – LA pre-A volume)/LA maximum volume]*100
-LA active emptying fraction (pump function) = [(LA pre-A volume – LA minimal volume)/LA pre-A volume]*100
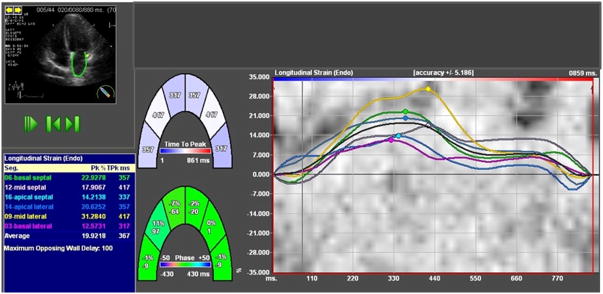
From LA strain analysis, LA reservoir function was estimated using peak strain during ventricular systole (peak LA strain) which represents LA filling during LV systole (Figure 1). As the LA expands during ventricular systole, peak LA strain is a positive strain value. If more than 2 segments could not be tracked or there was a lack of a full cardiac cycle, missing view, non- DICOM images, or significant foreshortening of the cavity, the measurements were considered unreliable and the patient was excluded from the analysis. As dedicated software for LA strain analysis has not yet been released, we used the current software for LV analysis to study the LA strain. Of the 935 studies suitable for conventional echocardiographic measures, images were not in DICOM format in 278 patients and image quality was inadequate in another 191 patients, leaving 466 patients with image quality sufficient for LA speckle-tracking. As atrial fibrillation has been strongly associated with LA dysfunction,20 the main analysis was performed restricted to patients in sinus rhythm at the time of echocardiography (357 patients; Supplemental Figure 1). All LV strain analysis was performed by a single investigator, as was LA strain analysis. Intraobserver variability for peak LA strain was assessed in a sample of 20 randomly selected TOPCAT studies. The coefficient of variation was 7.7% and the intraclass correlation coefficient was 0.96 (95% confidence interval (CI) 0.93-0.99). Reproducibility measures for conventional echocardiographic measures and for the LV strain have been previously published.5,19
Figure 1.
Two-dimensional speckle tracking imaging in the apical four-chamber view in a HFpEF patient with impairment of LA function (decreased peak LA strain).
Outcomes
The primary outcome for this analysis was the same as for the overall TOPCAT trial, the composite of CV death, HF hospitalization, and aborted sudden death. HF hospitalization alone and CV death alone were secondary outcomes. All events were reported by the primary site investigator and independently adjudicated by the Clinical Endpoints Center as previously described.13
Statistical Analysis
All normally distributed data were presented as mean and standard deviation (continuous data) or as count and proportion (categorical data). To compare patients in the TOPCAT trial not included in this LA strain analysis with patients included (Supplement Table 1), and to compare patients in the TOPCAT echo study who were included in this analysis with those not included (Supplemental Table 2), we performed X2 tests for categorical variables and two sided t-test with unequal variance for continuous variables. The prevalence of impaired LA deformation was based on thresholds obtained from data in a healthy population with similar age.11 Smaller numeric values of peak LA strain denote worse LA reservoir function. Values of peak LA strain lower than 26% represented 2 standard deviation below the mean value for this healthy group and were considered abnormal. Analysis was performed for the population overall and stratified by LA size (normal versus enlarged based on the threshold of 34 ml/m2).16 To assess the association between LA dysfunction and demographics, clinical characteristics, and echocardiographic measures of cardiac structure and function, HFpEF patients were categorized according to quartiles of peak LA strain with trend tests across ordered groups, using linear regression. As the available natriuretic peptide levels in TOPCAT were a mixture of BNP and NT-proBNP, to assess the association of peak LA strain with natriuretic peptide level, we combined these data by calculating the Z score of the log-transformed BNP or NT-proBNP level for each patient with available data (n=197).
The association of peak LA strain with the outcome variables of interest was assessed using a time to event analysis with univariate and multivariable Cox proportional hazards models, adjusting for prognostic demographic and clinical prognostic covariates (age, sex, race, randomization strata, enrollment region [Americas versus Russia/Georgia], randomized treatment assignment, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, and hematocrit) as previously described,7 as well as LA volume and core lab LVEF. The proportional hazards assumption was tested for all analyses, and there was no evidence of violation of the proportional-hazards assumption by LA strain. Similar analyses were performed for LA phasic volumes (reservoir, conduit, and pump function). Additionally, we investigated whether prognostic characteristics of the LV in HFpEF,6,21 including LV hypertrophy, LV systolic function measured by global longitudinal strain (GLS), and filling pressure measured by E/E′ ratio, may explain the association of LA functional measures with clinical outcomes due to shared physiological mechanisms. To evaluate if the rhythm at the time of echocardiography may affect the prognostic utility of LA strain, we repeated the analysis including all participants with adequate image quality for LA speckle-tracking (n=466), including 109 patients in atrial fibrillation at the time of echocardiography. We also tested for effect modification of rhythm at the time of echocardiography on the relationship between LA strain and clinical outcomes. Finally, we performed two sensitivity analyses: (1) one restricted to patients with normal LA size as LA enlargement has been associated with LA dysfunction (10); (2) a second restricted to patients enrolled in the Americas as marked differences in patient characteristics and outcomes were noted by enrollment region in TOPCAT.22
All statistical analyses were performed with STATA 12.0 (Stata Corp, College Station, Texas). All tests were two-sided and p-values of <0.05 were considered statistically significant.
Results
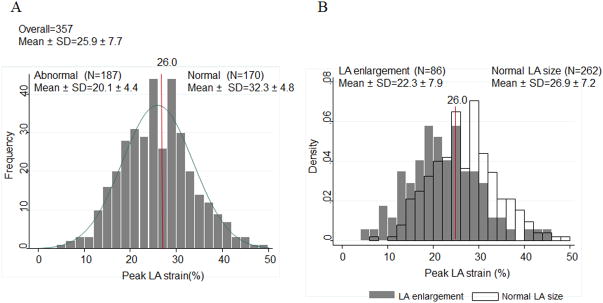
Among the 357 HFpEF patients with measurable peak LA strain, the mean value was 25.9±7.7% and 52% had abnormal peak LA strain based on data in a healthy population with similar age. 11 The LA strain was abnormal in 47% in patients with normal LA size and 71% patients with LA enlargement (Figure 2). Compared with the 3088 patients in the TOPCAT trial not included in this LA strain analysis, patients included in this analysis were more frequently female, less frequently white, less frequently enrolled in Russia or Georgia, and less frequently in the prior hospitalization randomization stratum. Diabetes was more prevalent and, partially by design, a clinical history of atrial fibrillation was less prevalent in the included patients (Supplement Table 1). Similarly, several differences in clinical and echocardiographic measures were noted between patients in the TOPCAT echo study who were included, compared to those not included (n=578) in this analysis (Supplemental Table 2), most notably younger age, greater proportion of women and non-white patients, lower heart rate and hematocrit, greater LV volumes, higher LVEF, and smaller LA size.
Figure 2.
Distribution of peak LA strain in the HFpEF study population (A) overall and (B) by LA size.
Baseline correlates of LA strain
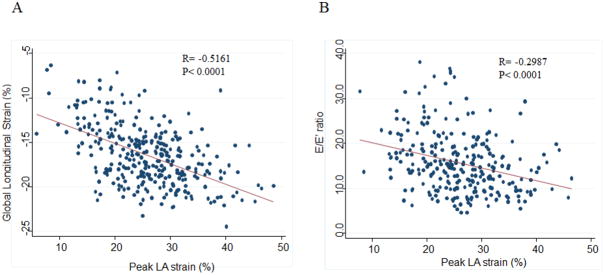
Patients with lower peak LA strain were older and had a higher prevalence of atrial fibrillation when compared to patients with higher peak LA strain (Table 1). LA strain was not associated with NYHA class. Lower peak LA strain was associated with greater LA size, greater LV and RV systolic dimensions, greater LV mass index, LV wall thickness, and prevalence of hypertrophy (Table 2, Supplemental Figure 2). Lower peak LA strain was also associated with worse LV systolic function, measured by LVEF and LV GLS, and higher LV diastolic filling pressure (Figure 3). Worse LA strain was also associated with worse RV systolic function by RVFAC and higher pulmonary artery systolic pressure by the TR jet velocity. Among the 197 patients with natriuretic peptide levels measured, worse LA strain was associated with higher natriuretic peptide levels (Supplemental Table 3).
Table 1.
Baseline clinical characterstics in the study population overall and by quartile of LA strain.
| Peak LA strain Overall (n=357) | Quartile 1 <20.7 16.3 ± 3.3 (n=90) | Quartile 2 20.7 to 25.5 23.3 ± 1.5 (n=89) | Quartile 3 25.5 to 30.9 28.2 ± 1.4 (n=89) | Quartile 4 30.9 to 48.6 35.8 ± 4.2 (n=89) | p for trend | |
|---|---|---|---|---|---|---|
| Age (years) | 68.9 ± 9.7 | 70.6 ± 10.0 | 69.8 ± 9.4 | 68.3 ± 9.9 | 67.1 ± 9.1 | 0.008 |
| Female | 202 (56.6%) | 52 (57.8%) | 52 (58.4%) | 48 (53.9%) | 50 (56.2%) | 0.69 |
| White | 272 (76.2%) | 69 (76.7%) | 70 (78.7%) | 67 (75.3%) | 66 (74.2%) | 0.59 |
| Randomized treatment assignment | 187 (52%) | 51 (57%) | 46 (52%) | 48 (54%) | 42 (47%) | 0.27 |
| Enrollment in Russia/Georgia | 101 (28.3%) | 19 (21.1%) | 32 (36.0%) | 27 (30.3%) | 23 (25.8%) | 0.68 |
| Enrollment Strata: Prior Hospitalization | 233 (65.3%) | 46 (51.1%) | 65 (73.0%) | 61 (68.5%) | 61 (68.5%) | 0.033 |
| Co-morbidities | ||||||
| Hypertension | 331(93.0%) | 81 (90.0%) | 82 (92.1%) | 83 (93.3%) | 85 (96.6%) | 0.09 |
| Myocardial Infarction | 108 (30.3%) | 31 (34.4%) | 31 (34.8%) | 23 (25.8%) | 23 (26.1%) | 0.12 |
| Coronary Revascularization | 52 (14.6%) | 17 (18.9%) | 13 (14.6%) | 6 (6.7%) | 16 (18.2%) | 0.54 |
| Stroke | 36 (10.1%) | 10 (11.1%) | 10 (11.2%) | 5 (5.6%) | 11 (12.5%) | 0.91 |
| Atrial Fibrillation | 77 (21.6%) | 27 (30.0%) | 21 (23.6%) | 16 (18.0%) | 13 (14.8%) | 0.009 |
| Diabetes | 148 (41.6%) | 44 (48.9%) | 29 (32.6%) | 38 (42.7%) | 37 (42.1%) | 0.65 |
| Obesity | 213 (59.8%) | 47 (52.2%) | 55 (61.8%) | 51 (57.3%) | 60 (68.2%) | 0.06 |
| NYHA Functional Class | 0.09 | |||||
| 1 | 27 (7.6%) | 4 (4.5%) | 3 (3.4%) | 9 (10.1%) | 11 (12.6%) | |
| 2 | 198 (55.9%) | 54 (60.7%) | 46 (51.7%) | 48 (53.9%) | 50 (57.5%) | |
| 3 | 126 (35.6%) | 31 (34.8%) | 38 (42.7%) | 32 (36.0%) | 25 (28.7%) | |
| 4 | 3 (0.9%) | 0 (0.0%) | 2 (2.2%) | 0 (0.0%) | 1 (1.2%) | |
| Physical Characteristics | ||||||
| BMI (kg/m2) | 32.9 ± 7.1 | 32.2 ± 7.4 | 32.8 ± 6.8 | 32.1 ± 6.4 | 34.6 ± 7.7 | 0.05 |
| Heart rate (bpm) | 67 ± 11 | 68 ± 12 | 67 ± 11 | 67 ± 10 | 65 ± 9 | 0.06 |
| Systolic blood pressure (mmHg) | 129 ± 15 | 127 ± 16 | 129 ± 16 | 129 ± 15 | 129 ± 14 | 0.37 |
| Diastolic blood pressure (mmHg) | 73 ± 11 | 72 ± 11 | 73 ± 11 | 73 ± 11 | 73 ± 9 | 0.41 |
| Laboratory Values | ||||||
| eGFR (mL/min per 1.73 m2) | 67 ± 21 | 67 ± 21 | 69 ± 22 | 70 ± 22 | 65 ± 21 | 0.63 |
| Creatinine (mg/dL) | 1.11 ± 0.33 | 1.11 ± 0.30 | 1.10 ± 0.30 | 1.10 ± 0.38 | 1.17 ± 0.35 | 0.22 |
| Hematocrit (%) | 38.6 ± 4.8 | 38.9 ± 4.6 | 38.4 ± 5.1 | 38.8 ± 4.7 | 38.4 ± 4.8 | 0.67 |
| Incident CV events | ||||||
| CV composite | 91 (25.5%) | 31 (34.4%) | 19 (21.4%) | 27 (30.3%) | 14 (15.7%) | 0.022 |
| CV death | 43 (12.0%) | 14 (15.6%) | 12 (13.5%) | 10 (11.2%) | 7 (7.9%) | 0.101 |
| HF hospitalization | 62 (17.4%) | 23 (25.6%) | 14 (15.7%) | 18 (20.2%) | 7 (7.9%) | 0.007 |
Numbers represent mean ± S.D. for continuous variables and N (%) for categorical variables.
Table 2.
Echocardiographic measurements in the study population overall and by quartile of LA strain.
| N | Peak LA strain Overall (n=357) | Quartile 1 <20.7 16.3 ± 3.3 (n=90) | Quartile 2 20.7 to 25.5 23.3 ± 1.5 (n=89) | Quartile 3 25.5 to 30.9 28.2 ± 1.4 (n=89) | Quartile 4 30.9 to 48.6 35.8 ± 4.2 (n=89) | p for trend | |
|---|---|---|---|---|---|---|---|
| LV Structure | |||||||
| LVEDVi (ml/m2) | 352 | 52.0 ± 16.2 | 54.7 ± 19.6 | 52.3 ± 16.7 | 52.4 ± 14.0 | 48.7 ± 13.5 | 0.022 |
| LVESVi (ml/m2) | 352 | 21.3 ± 10.4 | 24.7 ± 13.5 | 21.7 ± 10.8 | 20.8 ± 8.0 | 18.0 ± 7.3 | <0.001 |
| Mean wall thickness (cm) | 354 | 1.16 ± 0.19 | 1.22 ± 0.25 | 1.14 ± 0.16 | 1.15 ± 0.16 | 1.14 ± 0.16 | 0.023 |
| LV mass index (mg/m2) | 353 | 109 ± 30 | 119 ± 32 | 109 ± 30 | 107 ± 26 | 100 ± 28 | <0.001 |
| Relative wall thickness | 354 | 0.48 ± 0.10 | 0.51 ± 0.16 | 0.46 ± 0.07 | 0.47 ± 0.07 | 0.49 ± 0.09 | 0.40 |
| LV Hypertrophy | 353 | 176 (49.9%) | 55 (61.8%) | 50 (56.2%) | 41 (46.6%) | 30 (34.5%) | <0.001 |
| LV geometry | 353 | 0.004 | |||||
| Normal | 56 (15.9%) | 15 (16.9%) | 13 (14.6%) | 13 (14.8%) | 15 (17.2%) | ||
| Concentric remodeling | 121 (34.3%) | 19 (21.4%) | 26 (29.2%) | 34 (38.6%) | 42 (48.3%) | ||
| Eccentric hypertrophy | 33 (9.3%) | 10 (11.2%) | 12 (13.5%) | 6 (6.8%) | 5 (5.8%) | ||
| Concentric hypertrophy | 143 (40.5%) | 45 (50.6%) | 38 (42.7%) | 35 (39.8%) | 25 (28.7%) | ||
| LV Systolic Function | |||||||
| LVEF (%) | 353 | 60.4 ± 7.7 | 56.7 ± 9.1 | 59.9 ± 7.3 | 61.2 ± 6.3 | 63.9 ± 6.3 | <0.001 |
| LV GLS (%) | 307 | -16.4 ± 3.4 | -13.7 ± 3.5 | -16.5 ± 3.0 | -17.1 ± 2.7 | -18.3 ± 2.6 | <0.001 |
| LV Diastolic Function | |||||||
| E/A ratio | 332 | 1.2 ± 0.6 | 1.5 ± 0.8 | 1.2 ± 0.5 | 1.1 ± 0.5 | 1.0 ± 0.5 | <0.001 |
| DT (ms) | 334 | 211 ± 57 | 198 ± 59 | 215 ± 57 | 217 ± 59 | 215 ± 53 | 0.06 |
| TDI E' (septal) (cm/sec) | 269 | 5.6 ± 1.9 | 4.8 ± 1.4 | 5.4 ± 1.8 | 5.7 ± 2.1 | 6.5 ± 1.9 | <0.001 |
| E/E′ (septal) | 267 | 15.7 ± 6.7 | 18.9 ± 6.8 | 16.4 ± 7.5 | 14.1 ± 5.6 | 13.5 ± 5.3 | <0.001 |
| LAV (ml) | 349 | 55.8 ± 23.3 | 64.5 ± 35.2 | 55.0 ± 17.1 | 51.3 ± 15.6 | 52.0 ± 17.2 | <0.001 |
| LAVi (ml/m2) | 348 | 28.4 ± 11.4 | 33.6 ± 16.9 | 28.3 ± 8.8 | 26.1 ± 7.6 | 25.5 ± 7.6 | <0.001 |
| LA diameter (cm) | 354 | 4.19 ± 0.59 | 4.40 ± 0.63 | 4.18 ± 0.51 | 4.11 ± 0.57 | 4.06 ± 0.61 | <0.001 |
| Diastolic Dysfunction Grade (Olmsted) | 277 | <0.001 | |||||
| Normal | 28 (10.1%) | 1 (1.6%) | 8 (10.4%) | 8 (11.9%) | 11 (15.5%) | ||
| Mild | 82 (29.6%) | 16 (25.8%) | 17 (22.1%) | 27 (40.3%) | 22 (31.0%) | ||
| Moderate | 116 (41.9%) | 23 (37.1%) | 39 (50.7%) | 23 (34.3%) | 31 (43.7%) | ||
| Severe | 51 (18.4%) | 22 (35.5%) | 13 (16.9%) | 9 (13.4%) | 7 (9.9%) | ||
| Pulmonary Vascular and RV | |||||||
| TR jet velocity (m/sec) | 185 | 2.81 ± 0.46 | 2.89 ± 0.51 | 2.82 ± 0.42 | 2.82 ± 0.44 | 2.64 ± 0.46 | 0.024 |
| RVFAC | 281 | 0.50 ± 0.08 | 0.47 ± 0.08 | 0.51 ± 0.08 | 0.50 ± 0.07 | 0.51 ± 0.07 | 0.020 |
| RVEDA (cm2) | 281 | 19.7 ± 5.9 | 20.3 ± 6.4 | 19.7 ± 6.6 | 19.8 ± 5.1 | 18.8 ± 5.5 | 0.16 |
| RVESA (cm2) | 281 | 9.9 ± 3.5 | 10.8 ± 3.8 | 9.6 ± 3.7 | 10.0 ± 3.4 | 9.2 ± 3.0 | 0.027 |
| Valvular Disease | |||||||
| ≥Moderate Mitral Regurgitation | 281 | 33 (11.7%) | 8 (10.7%) | 15 (21.7%) | 5 (7.3%) | 5 (7.3%) | 0.17 |
| Aortic Valve Peak Velocity (m/sec) | 283 | 1.51 ± 0.46 | 1.47 ± 0.52 | 1.59 ± 0.46 | 1.50 ± 0.41 | 1.51 ± 0.47 | 0.87 |
| Valve Disease | 342 | 44 (12.9%) | 13 (14.8%) | 16 (18.6%) | 7 (8.3%) | 8 (9.5%) | 0.11 |
| LA function | |||||||
| LA conduit function | |||||||
| LA passive emp fraction | 357 | 27.3 ± 10.3 | 21.4 ± 8.0 | 25.1 ± 8.3 | 28.4 ± 9.1 | 34.5 ± 10.7 | <0.001 |
| LA pump function | |||||||
| LA active emp fraction | 357 | 36.6 ± 13.2 | 24.9 ± 10.0 | 35.4 ± 9.7 | 41.0 ± 11.7 | 45.1 ± 11.5 | <0.001 |
Numbers represent mean ± S.D. for continuous variables and N (%) for categorical variables.
Figure 3.
Scatter plot and Pearson's correlation of peak LA strain and (A) global longitudinal strain and (B) E/E′ in the study population overall.
Peak LA strain and Incident Cardiovascular events
During a median follow up of 31 (25th and 75th percentile limits 18 – 43) months, 91 patients (25.5%) experienced the primary composite endpoint, including 43 (12.0%) CV deaths and 62 (17.4%) patients with HF hospitalization. Lower peak LA strain was associated with a heightened risk for the primary composite endpoint (p=0.009) and HF hospitalization alone (p=0.003) in the unadjusted analysis (Table 3). This association was independent of demographic and clinical characteristics, LV ejection fraction, and LA size for HF hospitalization, but not for the composite outcome. The association of peak LA strain with incident HF hospitalization remained significant after additional adjustment for LVH, but not after further adjustment for LV GLS or E/E′ (Table 4). Similar results were observed in analysis including patients in atrial fibrillation at the time of echocardiography (Supplemental Tables 4 and 5). No significant interaction was noted between LA strain and rhythm at time of echocardiography for the composite endpoint; CV death; or HF hospitalization (all p for interaction >0.05). In contrast, these associations were only observed in unadjusted analysis when the analysis was restricted to patients with normal LA size (Supplemental Table 6). Among patients enrolled in the Americas, peak LA strain remained associated with the primary composite endpoint and HF hospitalization alone in multivariable analysis adjusting for demographic and clinical characteristics, LVEF, LA size, and LVH, although these associations were not independent of LV GLS and E/E′ (Supplemental Tables 7 and 8).
Table 3. Association of peak LA strain with cardiovascular outcomes in univariate and multivariate analysis.
| Unadjusted | Adjusted* | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Events/person-time at risk | HR (95% CI) | p-value | N | Events/person-time at risk | HR (95% CI) | p-value | |
| Composite Endpoint | 357 | 91/11510 | 0.96 (0.94-0.99) | 0.009 | 335 | 87/10773 | 0.97 (0.94-1.00) | 0.07 |
| CV Death | 357 | 43/12487 | 0.97 (0.93-1.01) | 0.13 | 335 | 41/11749 | 0.98 (0.93-1.03) | 0.50 |
| HF Hospitalization | 357 | 62/11510 | 0.95 (0.92-0.98) | 0.003 | 335 | 59/10773 | 0.95 (0.91-0.99) | 0.009 |
Hazard ratios are per unit reduction in peak LA strain.
Adjusted for age, sex, race, randomization strata, enrollment region [Americas versus Russia/Georgia], randomized treatment assignment, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, LVEF, left atrial volume index; CV=Cardiovascular; HF= Heart Failure; ACA= Aborted Cardiac Arrest.
Table 4. Potential contributors to the association of peak LA strain with cardiovascular outcomes.
| N | Events/person-time at risk | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Composite Endpoint | ||||
| Model 1 | 335 | 87/10773 | 0.97 (0.94-1.00) | 0.07 |
| Model 1+ LVMi | 332 | 87/10676 | 0.97(0.94-1.00) | 0.10 |
| Model 1+ GLS | 289 | 73/9300 | 1.00 (0.96-1.04) | 0.81 |
| Model 1+ E/E′ | 250 | 67/7927 | 0.99 (0.95-1.04) | 0.69 |
| CV Death | ||||
| Model 1 | 335 | 41/11749 | 0.98 (0.93-1.03) | 0.50 |
| Model 1+ LVMi | 332 | 41/11653 | 0.98 (0.93-1.04) | 0.52 |
| Model 1+ GLS | 289 | 34/10081 | 1.03 (0.97-1.10) | 0.40 |
| Model 1+ E/E′ | 250 | 32/8735 | 1.00 (0.94-1.07) | 0.87 |
| HF Hospitalization | ||||
| Model 1 | 335 | 59/10773 | 0.95 (0.91-0.99) | 0.009 |
| Model 1+ LVMi | 332 | 59/10676 | 0.95 (0.91-0.99) | 0.015 |
| Model 1+ GLS | 289 | 49/9300 | 0.98 (0.93-1.03) | 0.39 |
| Model 1+ E/E′ | 250 | 44/7927 | 0.96 (0.91-1.02) | 0.19 |
Hazard ratios are per unit reduction in peak LA strain.
Model 1 age, sex, race, randomization strata, enrollment region [Americas versus Russia/Georgia], randomized treatment assignment, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, LVEF, left atrial volume index LVMi= Left Ventricular Mass index; GLS= Global Longitudinal Strain; CV=Cardiovascular; HF= Heart Failure; ACA= Aborted Cardiac Arrest.
LA phasic volumes and Incident Cardiovascular events
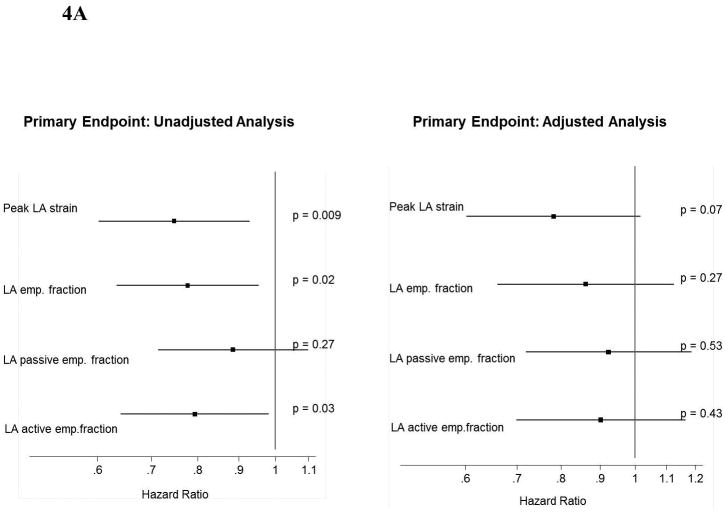
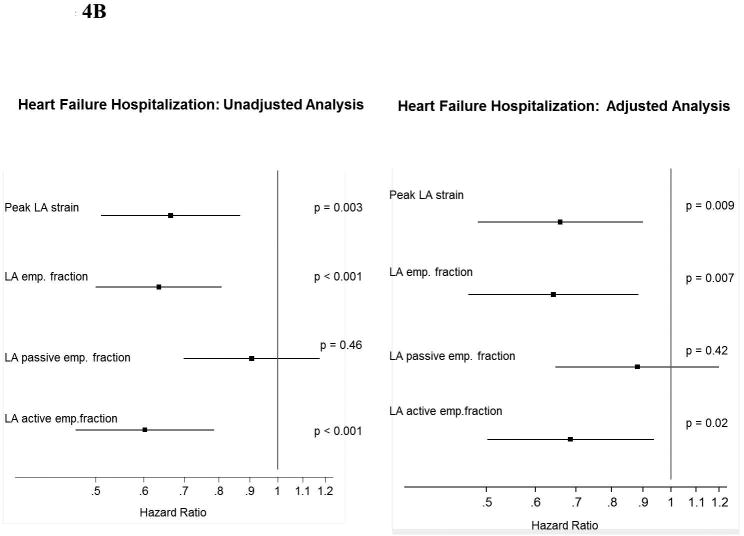
Lower LA strain was associated with worse measures of LA conduit and pump function (Table 1). Concordant with our findings with peak LA strain, LA emptying fraction – also a measure of LA reservoir function – was associated with a heightened risk for the primary composite endpoint in unadjusted analysis and for HF hospitalization alone in unadjusted and adjusted analyses (Figure 4). Worse LA pump function, measured by the LA active emptying fraction, was also associated with higher risk of the primary composite endpoint and HF alone, and remained significantly associated with HF hospitalization in adjusted analysis. LA conduit function, reflected in the LA passive emptying fraction, was not prognostic of outcomes.
Figure 4.
Forest plot graph demonstrating the association of LA function (reservoir, conduit and pump) with (A) the primary endopoint and (B) HF hospitalization.
*Multivariable analysis is adjusted for age, sex, race, enrollment region (Americas versus Russia/Georgia), randomization strata, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, LVEF, left atrial volume index, and randomized treatment assignment.
*Hazard Ratio: per unit of SD
Discussion
Our study is the largest, to our knowledge, to assess the prognostic implications of LA dysfunction in HFpEF and the first to evaluate the association of LA dysfunction with clinical outcomes beyond clinical predictors and LV features in HFpEF. Worse LA strain was associated with a higher risk of HF hospitalization independent of potential clinical confounders, but not after adjusting for LV systolic deformation and filling pressure. These findings suggest that impairments in LV systolic and diastolic function largely explain the association between impaired LA function and worse outcomes in HFpEF.
LA function by strain analysis using speckle tracking is a direct measurement of intrinsic LA myocardial deformation. While not load independent, LA strain appears to be less dependent on loading conditions and geometric assumptions than traditional parameters,23,24 and has high feasibility and reproducibility.17 However, this measure still lacks clear standardization and validation. The presence of LA dysfunction by speckle tracking in HFpEF has been demonstrated in relatively small populations, and the reported prevalence of LA reservoir dysfunction was around 30%.9-11 We found a high prevalence of LA reservoir dysfunction, independent of LA dilation. Patients with worse LA strain were older and the association between lower LA strain and older age may be related to the age-related loss or hypertrophy of myocytes, interstitial fibrosis, and impaired cellular calcium uptake that occurs in the LV25,26 and may affect the LA in parallel.27 Indeed, we found that lower LA strain was related to more LVH and greater impairment of LV systolic and diastolic function. This association may therefore be due to shared risk factors impairing LV and LA function in parallel. Alternatively, or concomitantly, primary impairments in LV performance may result in abnormal LA performance. Worse LV longitudinal systolic function may contribute to LA dysfunction due to the influence of downward motion of the mitral plane during ventricular systole, leading to reduced systolic expansion of the LA.28 Indeed, LA strain was highly correlated with LV longitudinal strain in our population (Figure 3). Additionally, diastolic dysfunction with resulting elevation in LV filling pressure may contribute to LA dysfunction through increasing LA afterload and wall tension.29-31 We noted significant associations of worse LA strain with higher E/E′ ratio and E/A ratio and lower E'. However, we cannot exclude the possibility that a primary abnormality of LA function, with reduced LA compliance, may result in a higher LA pressure for any given LV diastolic pressure, with resulting higher E wave velocity and E/E′ ratio. The low prevalence of significative mitral regurgitation (12%) in our population may indicate that mitral valvular disease is not an major factor responsible for LA dysfunction in HFpEF patients included in this analysis.
LA reservoir dysfunction is a predictor of cardiovascular outcomes in population-based studies32,33, in patients with HF with reduced ejection fraction,34 and in patients with stable coronary heart disease and preserved ejection fraction.35 Two studies have demonstrated the prognostic value of LA strain after acute myocardial infarction, but the larger of these studies (n=843 patients) showed that this association was not independent of global longitudinal strain and LA size.36,37 There is little data regarding the prognostic relevance of LA dysfunction in HFpEF. A recent study of 101 highly symptomatic patients with HFpEF who were followed for a median of 350 days (31 events) demonstrated an association between reduced LA reservoir function (measuring by volumes) and increased risk of death after adjusting for age and sex. A similar trend was also seen for LA active function (p=0.05).12
In our study, we found that peak LA strain is associated with a risk of the composite endpoint and of HF hospitalization, but not CV mortality, after adjusting for clinical variables, LA size, LVEF and LV mass. HF hospitalization may be more sensitive to changes in LA strain than CV mortality. Impaired LA function may contribute to clinical symptoms and decompensation in HFpEF through elevated pulmonary venous pressure and associated right heart dysfunction.12 Indeed, we observed a significant association of impaired LA strain with LV filling pressure (E/E′ ratio), pulmonary pressure (TR velocity), and RV systolic function (RVFAC). In contrast to a recent study by Melenovsky et al., we did not find LA dysfunction to be a predictor of mortality in HFpEF. This discrepancy may be due to differences in severity of illness of the study populations, with HFpEF patients in our study at a less advanced stage of the syndrome (56% patients NYHA II) than the previous studied group (74% patients NYHA III-IV). 12 Importantly, peak LA strain no longer predicted adverse events after adjusting for LV GLS or LV filling pressure. Given the coupling of LA strain with LV longitudinal function due to the downward motion of the mitral plane in systole, and with LV filling pressure due to impact on LA wall tension, this finding suggests that LV systolic and diastolic dysfunction largely explain the association of LA dysfunction with adverse events in HFpEF. Atrial fibrillation is common in HFpEF and is associated with adverse outcomes.38 Atrial fibrillation is also associated with worse LA function.39 Importantly, however, LA strain was prognostic among patients in sinus rhythm, suggesting that impaired LA strain is not simply a marker of risk associated with atrial fibrillation. In contrast, we did not observe the same prognostic value of LA function in patients with normal LA size. We are unable to conclusively determine whether this is a consequence of the lower prevalence of LA dysfunction in patients with normal LA size compared to the overall population or if impaired LA function is a weaker predictor among patients with less advanced disease.
We observed an association of worse LA pump function with heightened risk of the composite endpoint and of HF alone. The prognostic importance of LA pump function in HFpEF is not well known. LA pump function is associated with risk of AF in patients with aortic stenosis40 and in the general population,41 but has also been associated with adverse cardiovascular events in hypertensive populations.42,43 Impaired LA contractile reserve secondary to progressive elevation of LV filling pressure may contribute to HFpEF symptoms, particularly during exercise.44 Concomitantly, reduced LV longitudinal function, with associated impairment in LA systolic expansion and reservoir function, may result in increased reliance on late-diastolic LA pump function for adequate LV filling.
Several limitations of this analysis should be noted. We analyzed only a subset of the patients enrolled in the overall TOPCAT trial, with some notable differences between the patients included and excluded from this analysis. The generalizability of these findings to the overall TOPCAT population, and to the HFpEF syndrome more broadly, may therefore be limited. Significant differences in patient characteristics, event rates, and treatment response have been noted by region of enrollment (Americas versus Russia/Georgia) in TOPCAT.22 However, we found similar findings in a sensitivity analysis restricted to the patients enrolled in the Americas (Supplemental Tables 5 and 6). Although the analysis of three-dimensional images may be a more accurate measurement of LA function, such images were not acquired in the TOPCAT trial.45 Limited data are available regarding the stability of LA strain measures over time. In a subset of patients in this study, echocardiography was performed up to 6 months prior randomization, possibly resulting in misclassification. In addition, the generalizability of these findings to HFpEF patients in the community may be limited because of the inclusion and exclusion criteria of the TOPCAT trial.
In summary, LA dysfunction is associated with a higher risk of HF hospitalization in HFpEF, independent of potential clinical confounders, but not independent of LV systolic deformation and diastolic filling pressure. Concomitant impairments in LV systolic and diastolic function largely explain the association of LA dysfunction with adverse clinical outcomes in HFpEF. Our data suggested that the assessment of LA function does not have additional clinical utility beyond the assessment of LV function in the prognostic evaluation of HFpEF patients. Future studies are indicated to investigate the mechanisms responsible for coupled LA and LV dysfunction in HFpEF
Supplementary Material
Clinical Perspective.
Heart failure with preserved ejection fraction (HFpEF) is common, accounting for an increasing proportion of patients hospitalized with acute decompensated heart failure. Left atrial size is an established marker of risk for adverse outcomes in HFpEF. However, the independent prognostic importance of LA function in HFpEF is not known. We assessed LA function measured by speckle tracking echocardiography in 357 HFpEF patients enrolled in the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. At a mean follow-up of 31 months, 91 patients (25.5%) experienced the primary composite endpoint of CV death, HF hospitalization, and aborted sudden death. Lower peak LA strain, indicating LA dysfunction, was associated with a higher risk of the composite endpoint (HR 0.96 per unit of reduction in strain, 95% CI 0.94-0.99; p=0.009) and of HF hospitalization alone (HR 0.95 per unit of reduction in strain, 95% CI 0.92-0.98; p=0.003). The association of LA strain with incident HF hospitalization remained significant after adjustment for clinical confounders, but not after further adjustment for LV global longitudinal strain and the E/E′ ratio, parameters of LV systolic and diastolic function respectively. Our findings suggest that quantification of LA function does not provide additional clinical utility beyond the assessment of LV function in the prognostic evaluation of HFpEF patients. Future studies are indicated to investigate the mechanisms responsible for coupled LA and LV dysfunction in HFpEF.
Acknowledgments
Sources of Funding: Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist was funded by National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, contract number HHSN268200425207C. The content of this article does not necessarily represent the views of the sponsor or of the Department of Health and Human Services. The work for this manuscript was also supported by NHLBI grant 1K08HL116792 (A.M.S.) and AHA grant 14CRP20380422 (A.M.S.).
Disclosures: Dr A. Shah reports receiving research support from Novartis, Gilead, and Actelion. Dr. S. Shah reports receiving research support from Actelion; consulting fees from the American Board of Internal Medicine, AstraZeneca, DC Devices, Novartis, Bayer, and Alnylam; and speaker fees from the Pulmonary Hypertension Association and the American Society of Echocardiography. Dr Pitt reports serving as a consultant for Pfizer, Bayer, Elli-Lilly, Novartis, and DaVinci therapeutics, and has a patent pending on site specific delivery of Eplerenone to the myocardium.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC Get With the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function in Heart Failure With Preserved Ejection Fraction: Baseline Findings From the Echocardiographic Study of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial. Circ Heart Fail. 2014;7:104–115. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE I-PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 7.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 10.Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:651–662. doi: 10.1016/j.echo.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, Voors AA, Lefkowitz M, Bransford T, Shi V, Packer M, McMurray JJ, Shah AM, Solomon SD. PARAMOUNT Investigators. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:1096–103. doi: 10.1002/ejhf.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left Atrial Remodeling and Function in Advanced Heart Failure With Preserved or Reduced Ejection Fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 13.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial: A randomized controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O'Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;8:7, 6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–243. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132:402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 21.Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography. 2015;32:71–78. doi: 10.1111/echo.12613. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner J, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer N, McKinlay S, Pitt B. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 23.Boyd AC, Richards DA, Marwick T, Thomas L. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart. 2011;97:1513–1519. doi: 10.1136/heartjnl-2011-300134. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Yip GW, Yu CM. Approaching regional left atrial function by tissue Doppler velocity and strain imaging. Europace. 2008;10:62–69. doi: 10.1093/europace/eun237. [DOI] [PubMed] [Google Scholar]
- 25.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 27.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 28.Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100:427–436. doi: 10.1161/01.cir.100.4.427. [DOI] [PubMed] [Google Scholar]
- 29.D'Andrea A, De Corato G, Scarafile R, Romano S, Reigler L, Mita C, Allocca F, Limongelli G, Gigantino G, Liccardo B, Cuomo S, Tagliamonte G, Caso P, Calbrò R. Left atrial myocardial function in either physiological or pathological left ventricular hypertrophy: a two-dimensional speckle strain study. Br J Sports Med. 2008;42:696–702. doi: 10.1136/bjsm.2007.041210. [DOI] [PubMed] [Google Scholar]
- 30.Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol. 1998;82:756–761. doi: 10.1016/s0002-9149(98)00452-4. [DOI] [PubMed] [Google Scholar]
- 31.Guan Z, Zhang D, Huang R, Zhang F, Wang Q, Guo S. Association of left atrial myocardial function with left ventricular diastolic dysfunction in subjects with preserved systolic function: a strain rate imaging study. Clin Cardiol. 2010;33:643–649. doi: 10.1002/clc.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, Mondillo S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–269. doi: 10.1016/j.amjcard.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, Drazner MH. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278–285. doi: 10.1093/eurheartj/ehs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motoki H, Borowski AG, Shrestha K, Troughton RW, Martin MG, Tang WH, Klein AL. Impact of left ventricular diastolic function on left atrial mechanics in systolic heart failure. Am J Cardiol. 2013;112:821–826. doi: 10.1016/j.amjcard.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. 2012;59:673–680. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoni ML, ten Brinke EA, Atary JZ, Marsan NA, Holman ER, schalij MJ, Bax JJ, Delgado V. Left atrial strain is related to adverse events in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2011;97:1332–1337. doi: 10.1136/hrt.2011.227678. [DOI] [PubMed] [Google Scholar]
- 37.Ersbøll M, Andersen MJ, Valeur N, Mogensen UM, Waziri H, Møller JE, Hassager C, Søgaard P, Køber L. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging. 2013;6:26–33. doi: 10.1161/CIRCIMAGING.112.978296. [DOI] [PubMed] [Google Scholar]
- 38.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal Relationship and Prognostic Significance of Atrial Fibrillation in Heart Failure Patients with Preserved Ejection Fraction: A Community-Based Study. Cirulation. 2013;128:e465. doi: 10.1161/CIRCULATIONAHA.113.001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon YE, Kim HJ, Kim SA, Kim SH, Park JH, Park KH, Choi S, Kim MK, Kim HS, Cho GY. Left Atrial Mechanical Function and Stiffness in Patients with Paroxysmal Atrial Fibrillation. J Cardiovasc Ultrasound. 2012;20:140–145. doi: 10.4250/jcu.2012.20.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imanishi J, Tanaka H, Sawa T, Motoji Y, Miyoshi T, Mochizuki Y, Fukuda Y, Tatsumi K, Matsumoto K, Okita Y, Hirata K. Left atrial booster-pump function as a predictive parameter for new-onset postoperative atrial fibrillation in patients with severe aortic stenosis. Int J Cardiovasc Imaging. 2014;30:295–304. doi: 10.1007/s10554-013-0346-z. [DOI] [PubMed] [Google Scholar]
- 41.Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ. National Heart, Lung, and Blood Institute, National Institutes of Health. Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study) Am J Cardiol. 2003;91:1079–1083. doi: 10.1016/s0002-9149(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 42.Chinali M, de Simone G, Roman MJ, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Devereux RB. Left atrial systolic force and cardiovascular outcome. The Strong Heart Study. Am J Hypertens. 2005;18:1570–1576. doi: 10.1016/j.amjhyper.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 43.Kaminski M, Steel K, Jerosch-Herold M, Khin M, Tsang S, Hauser T, Kwong RY. Strong cardiovascular prognostic implication of quantitative left atrial contractile function assessed by cardiac magnetic resonance imaging in patients with chronic hypertension. J Cardiovasc Magn Reson. 2011;13:42. doi: 10.1186/1532-429X-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang F, Lee AP, Yu CM. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr Opin Cardiol. 2014;29:430–436. doi: 10.1097/HCO.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 45.Nagaya M, Kawasaki M, Tanaka R, Onishi N, Sato N, Ono K, Watanabe T, Minatoguchi S, Miwa H, Goto Y, Hirose T, Arai M, Noda T, Watanabe S, Minatoguchi S. Quantitative validation of left atrial structure and function by two-dimensional and three-dimensional speckle tracking echocardiography: a comparative study with three-dimensional computed tomography. J Cardiol. 2013;62:188–194. doi: 10.1016/j.jjcc.2013.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.