Abstract
Hypoproliferative anemia results from the inability of bone marrow to produce adequate numbers of red blood cells. The list of conditions that cause hypoproliferative anemia is long, starting from common etiologies as iron deficiency to rarer diagnoses of constitutional bone marrow failure syndromes. There is no perfect diagnostic algorithm, and clinical data may not always clearly distinguish “normal” from “abnormal”, yet it is important for practicing clinicians to recognize each condition so that treatment can be initiated promptly. This review describes diagnostic approaches to hypoproliferative anemia, with particular emphasis on bone marrow failure syndromes.
INTRODUCTION
Anemia of central origin, or hypoproliferative anemia, broadly refers to anemia resulting from underproduction of red blood cells by the bone marrow. Hypoproliferative anemia is characterized by an inappropriately low reticulocyte count and is distinguished from anemia secondary to blood loss or peripheral erythrocytes destruction, which are accompanied by elevated reticulocyte counts from a bone marrow regenerative response. Table 1 lists a classification of hypoproliferative anemia. The most common etiology worldwide is iron deficiency, followed by the anemia of chronic disease and inflammation, and the anemia of renal disease (1) (the anemia of chronic disorders is discussed by Weiss in this issue).
Table 1.
Classification of hypoproliferative anemia
| Nutritional deficiency |
| • Iron |
| • Vitamin B12 |
| • Folate |
| Anemia of chronic disease and inflammation |
| Anemia of renal disease |
| Exposure to toxins or drugs |
| • Alcohol |
| • Drugs |
| Endocrine abnormality |
| • Hypothyroidism |
| • Hyperthyroidism |
| • Panhypopituitalism |
| • Primary or secondary hyperparathyroidism |
| • Androgen deficiency (male) |
| Hemophagocytic lymphohistiocytosis |
| Secondary to hematologic malignancies |
| • Leukemia |
| • Lymphoma |
| • Multiple myeloma |
| • Myelodysplastic syndrome (MDS) |
| • Myeloproliferative neoplasms |
| Bone marrow failure syndromes (see Table 2) |
| Myelophthisic anemia |
| • Metastatic cancers |
| • Hematologic malignancies |
| • Infections |
| • Autoimmune diseases |
| • Granulomatous diseases |
| • Renal osteodystrophy |
| • Hypo- and hyperparathyroidism |
Clinical reasoning toward a diagnosis often employs at least two methods: pattern recognition or clinical gestalt and algorithms that use decision trees to differentiate causes or associations. Clinical gestalt can be described as pattern recognition of a patient's presentation as a whole, and physicians use it to quickly reach a tentative clinical decision. For example, a low hemoglobin in a young woman with no known medical issues except for menorrhagia and recent pregnancy “looks like iron deficiency”, based on a coherent conception of a typical iron deficiency patient. Pattern recognition improves with clinical experience (2). However, clinical gestalt is subject to bias and error, as in “path-determination”, in which inconsistent or incompatible features are ignored to the patient's peril. Parallel to the process of the pattern recognition is a review of a nonheuristic range of didactic aids, from lists of differential diagnoses to decision trees and formal algorithms, some now computer assisted. Differential diagnoses can be dynamically modified and shortened in an efficient manner by systematically incorporating preliminary clinical data, as well as clinical gestalt.
This review aims to provide helpful information for practicing physicians to refine their both pattern recognition and differential diagnosis of hypoproliferative anemia, particularly of bone marrow failure syndromes. We start with a brief overview of general diagnostic considerations followed by sections dedicated to representative bone marrow failure syndromes. Detailed pathophysiologies and treatments for specific bone marrow failure syndromes have recently been reviewed (3-8) and are not discussed in detail here. Myelodysplastic syndrome (MDS), another important category of bone marrow failure, is discussed by Santini in this issue.
DIAGNOSTIC APPROACH TO HYPOPROLIFERATIVE ANEMIA
History and Physical Examination
A careful history is critical to develop insight into both the underlying causes of anemia and the potential role of concurrent illnesses. Any history suggestive of blood loss should be elicited by asking specific pertinent questions. Social history should be reviewed, especially for exposure to toxic substances (occupational exposure, smoking, alcohol, etc.) and risk of chronic infection such as human immunodeficiency virus (HIV), hepatitis viruses, and tuberculosis. A dietary history may suggest nutritional deficiencies are the dominant cause of hypoproliferative anemia. Family history is important, not only in pediatric patients but also in adults, as some inherited anemias can present late in life without apparent physical anomalies, and an affected pedigree may be the only clue to a constitutional origin of blood abnormalities. Physical findings of anemia are usually non-specific, such as pallor and systolic murmurs. However, a systematic physical examination can provide valuable clues to underlying etiologies: neurologic abnormalities in vitamin B12 deficiency, hepatosplenomegaly and lymphadenopathy in lymphoproliferative disease, spoon-shaped nails (koilonychia) and angular stomatitis in iron deficiency, and specific physical anomalies associated with constitutional bone marrow failure syndromes (see below).
Laboratory Evaluation
Considerable information is contained in the initial laboratory tests that led to the detection of anemia: complete blood count (CBC) with white blood cell (WBC) differential, mean corpuscular volume (MCV), and reticulocyte count. Further basic laboratory tests are performed as appropriate depending on the clinical context: complete metabolic panel, lactate dehydrogenase (LDH), haptoglobin, iron study, occult blood, vitamin B12, folate, and thyroid function tests. If inherited hemoglobinopathies are suspected based on ethnicity and family history, electrophoresis of hemoglobin should be performed. The presence of abnormalities in other hematopoietic cell lines alters the differential diagnosis of hypoproliferative anemia, and identification of red blood cell size is also useful (see Iolascon – microcytic anemia, Green – macrocytic anemia, in this issue). More than one etiology may coexist, leading to a mixed picture, so overreliance on preliminary categorizations based on the MCV and other initial test results should be avoided. Examination of the peripheral blood smear confirms the findings of automated counts (CBC), and can reveal schistocytes, teardrop cells, target cells, nucleated red blood cells, as well as morphological changes in WBC and platelets.
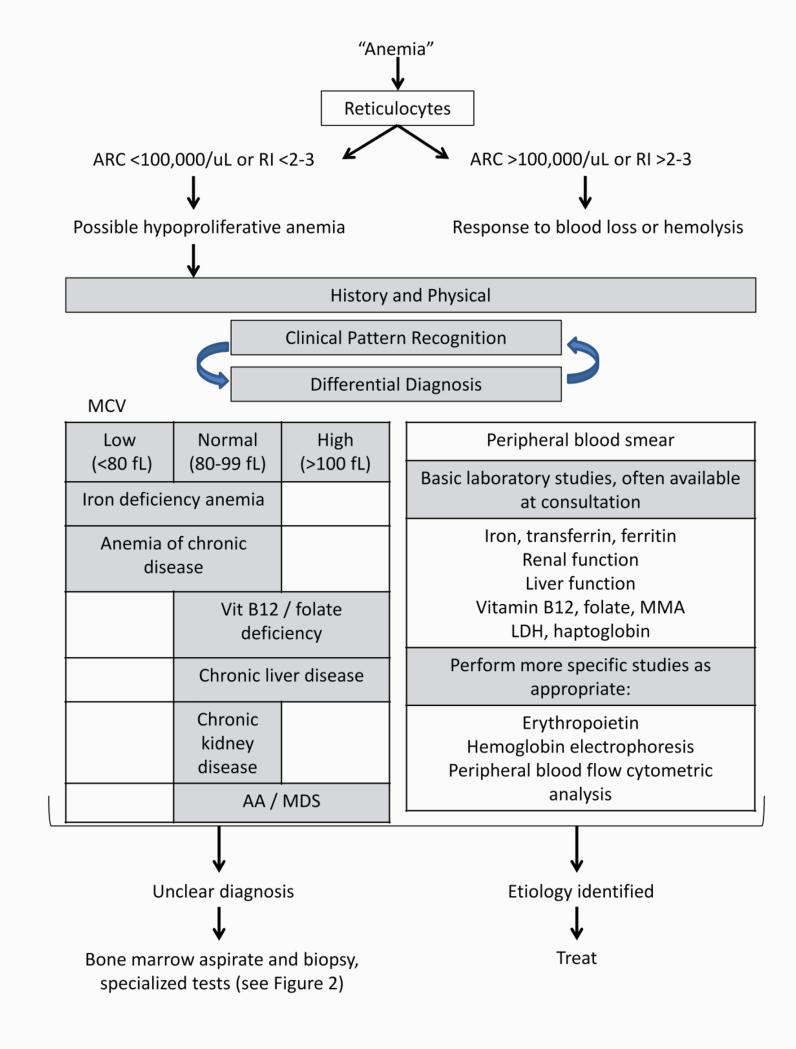
The reticulocyte count, a marker of effective erythropoiesis, is the single blood test most important for distinguishing hypoproliferative anemia from other causes (Figure 1). The reference range for the absolute reticulocyte count (ARC) is typically 20-90 K/uL and may vary according to measurement methods (9). When the reticulocyte count is reported as reticulocyte percentage, the absolute reticulocyte count can be computed by multiplying the reticulocyte percentage by the number of red blood cells. Alternatively, adjustment for the degree of anemia is accomplished as the corrected reticulocyte percentage (= reticulocyte percentage x patient's hematocrit [Hct] / reference Hct). The stressed bone marrow releases reticulocytes prematurely into the peripheral blood, where they remain in circulation, referred to as the reticulocyte maturation time. The reticulocyte maturation time is approximately 1 day for a Hct of 45%, 1.5 days for Hct of 35%, 2 days for Hct of 25%, and 2.5 days for Hct of 15% (10). The reticulocyte index accounts for the maturation time and the degree of anemia, and is calculated as reticulocyte index = corrected reticulocyte percentage / maturation time of reticulocytes in peripheral blood in days. A reticulocyte index higher than 2-3 is not consistent with the diagnosis of hypoproliferative anemia (9).
Figure 1.
Diagnostic approach to hypoproliferative anemia. ARC: absolute reticulocyte count. RI: reticulocyte index. AA: aplastic anemia. CKD: chronic renal disease. MDS: myelodysplastic syndrome. MMA: methylmalonic acid. LDH: lactate dehydrogenase
Interpretation of vitamin B12 and folate levels can be difficult. A fasting serum cobalamin level of around 200 pg/mL is often used as a lower normal limit. However, patients with true vitamin B12 deficiency may present with higher serum cobalamin levels (11), and a sensitivity as low as 0.40 has been reported when a serum cobalamin level of <300 pg/mL is used as the diagnostic threshold (12). Total serum cobalamin levels also poorly reflect metabolically active levels of vitamin B12. Only transcobalamin II-bound cobalamin is bioavailable, and it comprises only approximately 20% of total serum cobalamin, and remaining serum cobalamin is bound to haptocorrin and metabolically unavailable (12). Changes in the level of these cobalamin-binding proteins can affect the measured level of cobalamin, for instance, the haptocorrin level can be falsely low in patients with multiple myeloma, while in patients with myeloproliferative disease, reported serum cobalamin levels may be falsely high (11, 13). Serum folate levels only reflect folate intake over the preceding few days, and a low value (less than approximately 3 ng/mL) does not necessarily indicate more meaningful deficiency at the tissue level (11). Red cell folate levels better reflect folate stores for the 3 months prior to testing (1, 14). Methylmalonic acid (MMA) and homocysteine (Hcy) levels measure vitamin availability at the tissue level, and are more reliable indicators of vitamin deficiencies (11). In general, MMA is elevated only in cobalamin deficiency, while Hcy is less specific and can be elevated in both cobalamin and folate deficiencies as well as in vitamin B6 deficiency, hypothyroidism, methotrexate therapy, phenytoin therapy, and in methylenetetrahydrofolate reductase deficiency and other genetic defects (11, 15, 16). Both MMA and Hcy can be high in renal insufficiency (11). Elevation in plasma MMA and Hcy levels precedes decreased plasma cobalamin and folate levels, respectively. The sensitivity of an elevated plasma MMA is >90% for detecting vitamin B12 deficiency with hematologic abnormalities (17). Although MMA and Hcy tests are more expensive than are standard plasma vitamin levels, a cost-benefit analysis indicated that measurement of MMA is justified in patients with serum cobalamin levels between 80-120 pg/mL and 270-300 pg/mL (12).
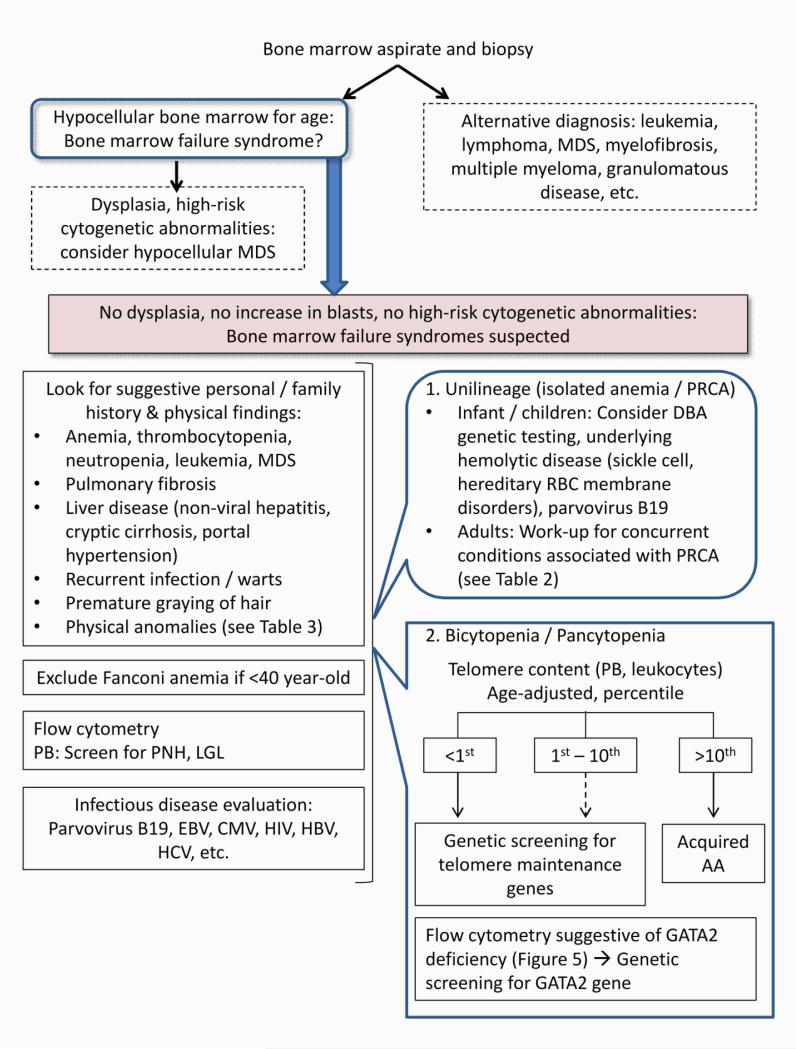
If primary bone marrow abnormalities are suspected or when the diagnosis remains uncertain after initial evaluation, bone marrow examination and further specific hematological tests are indicated to confirm the hypoproliferative nature of bone marrow and to identify other underlying pathologies: leukemia, MDS, myeloma, lymphoma, myelofibrosis, or infiltration by malignancy or granuloma (Figure 1, Figure 2). A core biopsy of adequate size and quality should be obtained because bone marrow cellularity and pathological findings may vary depending on the site (Figure 3-A), and underlying pathology may be distorted by crush artifact or handling error. At National Institutes of Health (NIH), a minimum bone marrow core size of at least 1.5 cm is sought when considering a diagnosis of bone marrow failure. In addition, a good quality bone marrow aspirate should contain macroscopically identifiable “spicules” or particles of bone marrow. An aspirate sample without spicules may indicate contamination by peripheral blood. Further specialized hematological studies, such as flow cytometry, cytogenetics, and molecular studies, will be discussed in the following disease-specific sections.
Figure 2.
Diagnostic approach to bone marrow failure syndromes. MDS: myelodysplastic syndrome. PNH: paroxysmal nocturnal hemoglobinuria. LGL: large granular lymphocytes. PRCA: pure red cell aplasia. RBC: red blood cell. PB: peripheral blood. AA: aplastic anemia
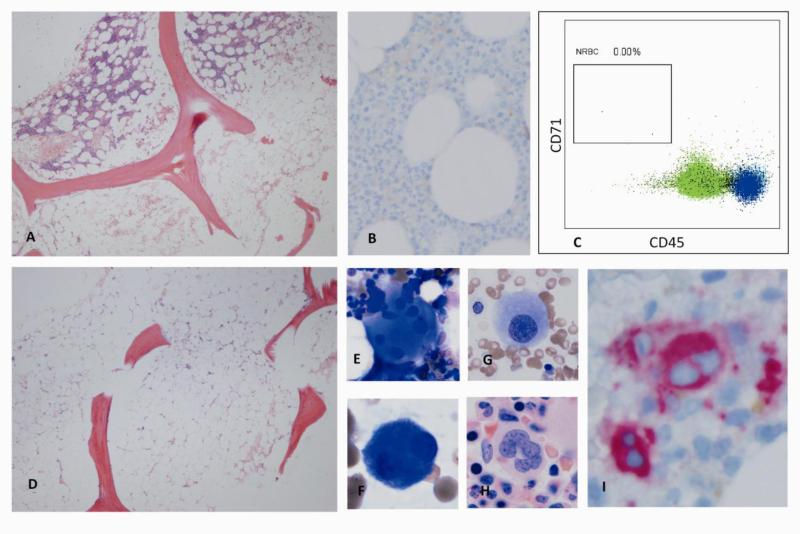
Figure 3.
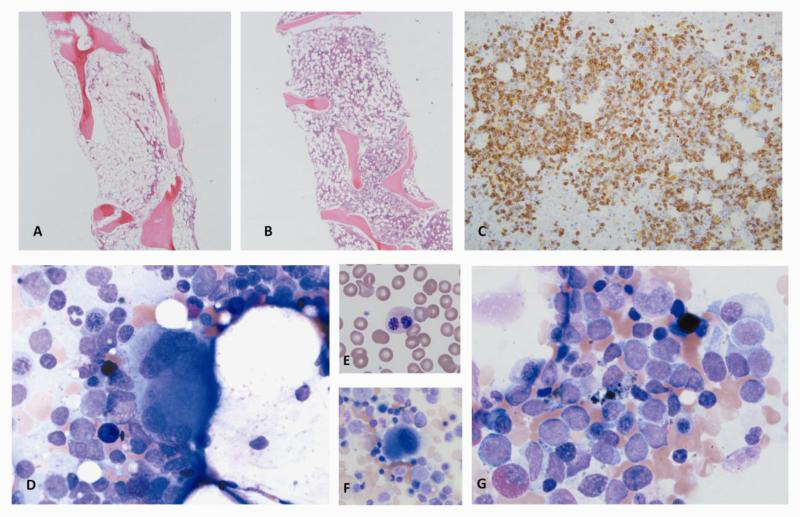
A. Variably hypocellular marrow in aplastic anemia (cellularity <5% to 40%). B. Bone marrow biopsy of a patient with pure red cell aplasia (CD71 stain), revealing lack of erythroid precursors. C. Flow cytomery of bone marrow cells from a patient with pure red cell aplasia. Absence of erythroid lineage is confirmed and quantified. D. Typical bone marrow biopsy in severe aplastic anemia: hypocellular bone marrow replaced with fat. E-G. Examples of atypical megakaryocytes (E: Widely separated lobes without strand. F: small bilobated megakaryoctes. G: small monolobated megakaryocyte). H. Normal megakaryocte for comparison. I. Bone marrow biopsy of a patient with hypocellular MDS. Immunohistochemical staining for CD61 highlights atypical bilobated megakaryoctes. (Figure 3B-C: Courtesy of Dr. Raul Braylan)
HYPOPROLIFERATIVE ANEMIA AS A COMPONENT OF BONE MARROW FAILURE SYNDROMES
The possibility of bone marrow failure syndromes increases once other etiologies of hypoproliferative anemia have been excluded (Table 1, Figure 2). Cytopenia can be limited to a single lineage or can be any combination of bicytopenia or pancytopenia. Bone marrow failure can be constitutional or acquired.
Isolated anemia: PRCA
Pure red cell aplasia (PRCA) is characterized by hypoproliferative anemia in the absence of abnormalities in other hematopoietic lineages. Patients present with symptomatic anemia. Regardless of classification, patients with PRCA share the same characteristic peripheral blood and bone marrow findings of normocytic or macrocytic anemia, profound reticulocytopenia, and severely diminished marrow erythroid precursor cells (Figure 3B-C). Classification of PRCA is shown in Table 2. Age at presentation, family history, and physical anomalies help distinguish constitutional from other forms. Congenital PRCA or Diamond-Blackfan anemia (DBA) is diagnosed at birth or within the first year of life in more than 90% of cases (18), although there are rare reports of presentation in adulthood (19). PRCA in adults are mostly acquired in the setting of underlying infections, autoimmune diseases, or malignancies. Self-limited forms of PRCA include transient aplastic crisis that affects patients with underlying hemolytic anemia following acute parvovirus infection, and transient erythroblastopenia of childhood that occurs in otherwise normal children triggered by yet unidentified viral infections.
Table 2.
Classification of bone marrow failure syndromes
| Acquired | Constitutional |
|---|---|
| Isolated anemia: PRCA | |
| Transient | Diamond-Blackfan anemia |
| • Transient aplastic crisis, parvovirus B19 | Inherited sideroblastic anemia |
| • Transient erythroblastopenia of childhood | Congenital dyserythropoietic anemia |
| Infection | |
| • Persistent parvovirus B19 infection (immunocompromised patients) | |
| • EBV, CMV, HIV, hepatitis viruses | |
| • Other infections | |
| Malignancies | |
| • Thymoma | |
| • LGL leukemia | |
| • CLL | |
| • Other hematologic and solid malignancies | |
| Drugs and chemicals | |
| Pregnancy | |
| Complication of major ABO-mismatch HSCT | |
| Erythropoietin antibody due to usage of recombinant human erythropoietin | |
| Idiopathic | |
| Bicytopenia / Pancytopenia | |
| Radiation | Fanconi anemia |
| Drugs and chemicals | Telomere diseases |
| • Cytotoxic drugs (chemotherapy) | • Dyskeratosis congenita |
| • Benzene | • Telomeropathies |
| Infections | GATA2 deficiency |
| • EBV | Schwachman-Diamond syndrome |
| • Hepatitis viruses | |
| • HIV | |
| • Parvovirus B19 | |
| Immune disease / Malignancies | |
| • LGL leukemia | |
| • Thymoma | |
| • PNH | |
| • Collagen vascular diseases | |
| Pregnancy | |
| Idiopathic | |
Diamond-Blackfan anemia (DBA)
Congenital PRCA or Diamond-Blackfan anemia (DBA) is caused by haploinsufficiency of a ribosomal protein gene. Approximately 60% of DBA cases have identifiable mutations in ribosomal genes (20), among which the RPS19 is most commonly mutated, accounting for about a quarter of DBA cases (21). DBA is inherited in an autosomal dominant pattern, but penetrance and phenotype are variable (20, 22, 23). The severity of DBA varies from in utero complications (preeclampsia, in utero fetal death, in utero growth retardation, hydrop fetalis) (24) to first symptoms of anemia later in life. Thirty to 40% of DBA patients have congenital physical anomalies (21); craniofacial abnormalities are most common and seen in about half of patients, followed by skeletal (commonly malformation of thumbs and upper limb), genitourinary, and cardiac abnormalities (18). Erythrocyte adenosine deaminase (eADA) (25) and hemoglobin F expression are classically increased (26). Genetic sequencing of known ribosomal gene mutations is commercially available, and a positive result supports the diagnosis of DBA (27). Screening for Fanconi anemia with chromosomal breakage analysis and exclusion of other constitutional bone marrow failure syndromes should be considered (discussed later).
Corticosteroids, typically prednisone at the starting dose of 2mg/kg/day (27, 28), are the mainstay of treatment for DBA, with an initial response rate of approximately 80% (29). Once an adequate response is achieved, steroids are slowly tapered (21, 27). However, relapse is frequent and there are insufficient data to support any specific steroid tapering schedule. Since a response is expected within the first few weeks, steroids should be discontinued for non-responders after a maximum of four weeks of administration (21, 27). Hematopoietic stem cell transplantation (HSCT) is the only curative treatment option for DBA, with a 5-year overall survival of approximately 70% for matched sibling donor transplant. The outcome of alternative donor HSCT has substantially improved over the past decade (27). Regardless of prior treatment, one fifth of patients in the DBA registry achieved remission, defined as an adequate hemoglobin level maintained for 6 months or more without any treatment. Overall actuarial survival is approximately 75% at 40 years of age (29).
Transient aplastic crisis and transient erythroblastopenia of childhood
Presentation with acute worsening of anemia in children with underlying hemolytic anemia should raise the concern for transient aplastic crisis (acute B19 parvovirus infection), while sudden onset of severe anemia in previously well children points toward transient erythroblastopenia of childhood (no known infectious etiology). Anemia in children may have different manifestations compared to adults, such as failure to thrive, poor appetite, or apathy. Transient aplastic crisis resolves spontaneously within 1 to 2 weeks of infection, with the appearance of neutralizing antibodies to B19 parvovirus (30, 31). In contrast, it may take a few weeks to months before resolution of transient erythroblastopenia of childhood (32). In addition to reticulocytosis, hemoglobin, white cell, and platelet numbers may temporarily rise to higher than normal values during the process of bone marrow recovery.
Acquired PRCA
Acquired PRCA develops predominantly in adults, and is caused by antibody- and/or cellular-mediated inhibition of erythropoiesis. Evaluation for possible causes and associated concurrent conditions is important, as listed in Table 2. Acquired PRCA is pathophysiologically and clinically associated with autoimmune diseases and malignancies (such as chronic lymphocytic leukemia [CLL], large granular lymphocytic leukemia [LGL leukemia] and thymoma) (33-37). Other causes of acquired PCRA are persistent B19 parvovirus infection in the setting of underlying immunodeficiency (such as acquired immunodeficiency syndrome [AIDS], immunosuppressant recipients) (38-40), antierythropoietin antibodies secondary to admininstration of recombinant human erythropoietin (41-43), pregnancy (44), and major ABO mismatched hematopoietic stem cell transplantation (45). There are numerous drugs and other conditions associated with PRCA, but causation is less well established (1, 43, 46).
Acquired PRCA secondary to persistent B19 parvovirus infection is effectively treated with immunoglobulin infusion (47, 48). For immune-mediated acquired PRCA, various immunosuppressive therapies are employed. Historically, corticosteroids were the first treatment of choice (7), with response rates of approximately 40% (49). However, relapses frequently occurred during steroid tapering, and complications of long-term steroid treatment became problematic. More recently, cyclosporine (CsA) has been advocated as the first treatment choice, and response rate to CsA monotherapy is approximately 70-80% (50, 51). Relapses are also frequent after CsA discontinuation (51), and the reported relapse-free period after discontinuation is 10 months (range 1.5 to 40 months) (51). Cytotoxic drugs are usually reserved for CsA-refractory disease or for patients with contraindications to CsA (7). Cyclophosphamide may offer better response than CsA for LGL leukemia-associated PRCA (52, 53). Several reports have shown that some patients with refractory PRCA with or without underlying lymphoproliferative disease can be successfully treated with alemtuzumab, an anti-CD52 monoclonal antibody (54, 55). Other treatment options include antithymocyte globulin (ATG) (56, 57) and rituximab (58, 59). For thymoma-related PRCA, the hematological response rate after thymectomy is 25-30% at best (60, 61). Thymoma-associated PRCA responds to CsA monotherapy or CsA-containing regimens (62). Matched sibling HSCT has been performed successfully for refractory cases (63-65).
Anemia as a component of bicytopenia or pancytopenia
Acquired aplastic anemia
The definition of aplastic anemia (AA) is reduced blood counts and a hypocellular bone marrow replaced with fat (Figure 3D). Radiation, chemical exposure (benzene), drugs, infection, and pregnancy have all been historically linked to the development of aplastic anemia (46). However, it is often difficult to distinguish association from causation, and the majority of acquired AA cases remain idiopathic (3). Immune-mediated mechanisms appear to be responsible for the severe deficiency of hematopoietic stem and progenitor cells (HSPC) (66), inferred from the success of immunosuppressive therapy (IST) in the majority of patients (67-70). Research laboratory studies also support underlying immune processes, including the demonstration of expansion of cytotoxic T-cells expressing type 1 cytokines and subsequent marrow suppression (71-74), regulatory T-cell deficiency (75, 76), acquired mutations in STAT3 and clonal cytotoxic T-cells in a subset of AA (77), and animal models (78).
Symptoms due to anemia and thrombocytopenia (typically mucocutaneous hemorrhage, petechia, and menorrhagia) are the most common reasons for patients to seek medical care, while infection is an uncommon initial presentation even in the setting of severe aplastic anemia (SAA) (3). SAA has been defined as a hypocellular marrow for age and at least two of the following criteria: absolute neutrophil count <500/uL, absolute reticulocyte count <60,000/uL (or corrected reticulocyte count (CRC) < 1%), and platelet count <20,000/uL (3, 79). Over 40% of patients with non-severe AA present without any symptoms (80), but pancytopenia worsens over time into the severe range in about half of these cases. The bone marrow is hypocellular without overt dysplasia or increase in blasts or other evidence of MDS and leukemia. The initial clinical presentation and bone marrow morphology of hypocellular MDS can be similar to aplastic anemia, and the distinction between these two entities can be difficult. Modest dyserythropoietic and megalobastic changes in red blood cells may be seen in AA, but dysplastic findings in megakaryocytes (especially small and mononuclear megakaryocytes) favor the diagnosis of MDS (Figure 3E-H). Quantification of CD34+ cells may also help distinguish the two conditions: low percentages of CD34+ cells (<0.5%) are associated with AA and higher CD34+ (>= 1%) may be indicative of hypocellular MDS (81). Abnormal cytogenetics results alone do not automatically exclude AA unless the abnormalities are MDS-defining. For some specialists, abnormalities trisomy 8, trisomy 6, and trisomy 15 are accepted in AA cases upon presentation, but the clinical relevance of these abnormalities remains controversial (82-84). Per protocol at NIH, we exclude patients with cytogenetic abnormalities from clinical trials of AA. Patients with AA can gain new cytogenetic abnormalities over time, and specific high-risk abnormalities (especially loss of chromosome 7) are associated with progression to MDS or leukemia (85).
Overlap of paroxysmal nocturnal hemoglobinuria (PNH) with AA is common and identified in approximately 50% of cases (the AA/PNH syndrome) (86, 87). Peripheral blood cell surface flow cytometry for expansion of glycophosphoinositol (GPI) -anchored proteins should be performed to identify and quantify a PNH clone. Whether the presence of PNH clone is a prognostic indicator for a better response to IST is unsettled (86, 88-90). Our retrospective analysis of over 200 AA cases suggested that specific measures to address clinical PNH were rarely required (<5% of the cohort) (86). Patients without a PNH clone at presentation usually do not develop a clone following IST, and clones may disappear over time in some cases. Clinical signs and symptoms of PNH, such as hemolysis or thrombosis, were rarely observed among AA/PNH syndrome patients who underwent IST unless a large clone (>50%) persisted over time (86). Regardless of the PNH clone size at presentation, initial treatment should be immunosuppression or transplant to address underlying marrow failure (3).
Definitive treatment should be initiated promptly for severe aplastic anemia (SAA) to avoid the risk of serious infectious and other complications. Patients with non-severe AA can be monitored, particularly when they do not require transfusion, because it is unknown whether definitive treatment has long-term benefit for milder pancytopenia. Definitive treatment for SAA is either HSCT or IST. HSCT is a curative option, and HSCT is preferred for younger patients (e.g. <40 year-old) with a human leukocyte antigen (HLA)-matched sibling donor (3) (91-93). The risks of graft-versus-host disease (GVHD), related morbidity, and mortality increase with age (94-96). Upfront matched-related donor transplant in children has an excellent outcome, and 3-year overall survival and 3-year event free survival are both around 90% (97). In SAA, bone marrow is the preferred stem cell source because peripheral blood stem cell transplantation (PBSCT) is associated with higher risk of chronic GVHD and mortality (98-100). The outcome of matched unrelated donor (MUD) transplantation is improving, and MUD transplantation is recommended for younger patients who do not have a matched sibling and have failed IST, but generally not as first-line therapy (3, 101).
IST is initial therapy for most patients when HSCT is not a feasible option due to lack of suitable donors, age, or comorbidities. The combination of horse antithymocyte globulin (ATG) and cyclosporine (CsA) is standard (102), with a response rate of 60-70% and overall long-term survival comparable to HSCT (67-70). Baseline ARC and absolute lymphocyte count (ALC) have prognostic implications: patients with a higher baseline ARC (≥ 25K/uL) and ALC (≥ 1000/uL) have better response to IST and better survival compared to lower baseline ARC/ALC group (response rate to IST at 6 months, 83% vs. 41% [p<0.001], and 5-year survival, 92% vs. 53% [p<0.001]) (90).
Recently, an oral thrombopoietin mimetic, eltrombopag was approved by the United States Food and Drug Administration (FDA) for the treatment of SAA. In refractory SAA, eltrombopag monotherapy provided hematological responses in at least one lineage in almost half of study patients at 12 weeks, with many achieving multilineage hematological improvements and transfusion independence (103). Long-term follow-up of the study cohort revealed that improvements were sustained even after discontinuation of eltrombopag (104). Currently, ongoing clinical trials at the NIH are testing the efficacy and safety of eltrombopag in combination with standard IST as upfront therapy. Eltrombopag is generally well tolerated with minimal side effects, but long-term effects of the treatment, including clonal evolution, are yet to be determined. Uncontrolled studies have shown that androgens provide sustained hematological recoveries and survival benefit in some patients (105, 106).
Stereotypical constitutional bone marrow failure syndromes that present in adults
Bone marrow findings of constitutional syndromes are identical to acquired AA (3). Approximately 30% of pediatric bone marrow failures are comprised of constitutional (or inherited) syndromes (46). Some constitutional bone marrow failure syndromes can present in adulthood even without suggestive family history (Table 3). When bone marrow failure syndromes are suspected, it is important to exclude constitutional conditions in appropriate settings because it has important therapeutic implications.
Table 3.
Characteristics of constitutional bone marrow failure syndromes that accompany hypoproliferative anemia
| Disease | Genetics | Median age at diagnosis (range)* | Associated physical anomalies | Diagnostic tests (other than gene sequencing) |
|---|---|---|---|---|
| DBA | Ribosomal protein genes | 3 months (0-64) | Abnormal thumbs, short stature | Elevated erythrocyte adenosine deaminase, elevated Hgb F expression |
| Fanconi anemia | Fanconi anemia genes (DNA repair and genomic stability) | 6.5 (0-49) | Skin pigment changes or café-au-lait spots, short statrure, upper limb anomalites, microcephaly, renal malformations, hypogonadism, ear anomalities and deafness | Increased chromosomal breakage of peripheral blood cells after exposure to the DNA crosslinking agent (DEB, MMC) |
| Telomeropathy | Telomere repair complex or telomere protection complex (TERT, TERC, and others) | 14 (0-75) | Oral leukoplakia, dystrophic nails, skin hypo/hyperpigmentation (lacey reticular rash), pulmonary fibrosis, cryptic cirrhosis, portal hypertension, premature graying of hair | Short telomere contents of peripheral blood leukocytes (flow-FISH, quantitative PCR) |
| GATA2 deficiency | Hematopoietic transcription factor | Insufficient data | Recurrent warts, disseminated non-tuberculous mycobacteria infection, lymphedema, panniculitis, pulmonary diffusion and ventilatory defects, pulmonary alveolar proteinosis | Flow cytometry: peripheral blood - disproportionately reduced monocytes, B cells and NK cells, bone marrow - reduced monocytes, B cells, NK cells, and absent hematogones compared to AA |
Reference 8
Fanconi Anemia
Fanconi anemia (FA), the most common constitutional bone marrow failure syndrome, is inherited in an autosomal recessive or X-linked recessive manner (46). There are at least 16 known FA genes, which encode proteins essential for DNA repair and genomic stability (107). FA is associated with a predisposition for both hematologic and solid malignancies. Bone marrow failure is the most common first hematopoietic presentation of FA (107). Classically, FA is associated with congenital physical anomalies, such as skin hyperpigmentation, short stature, upper limb anomalies, skeletal changes, hypogonadism, renal malformation, and characteristic facial features (8). However, the manifestations of FA are heterogeneous, and up to one third of patients lack these physical features (108). The median age at diagnosis is 6.5 years, but varies widely from 0 to 49 years (8). The cumulative incidence of bone marrow failure, hematologic neoplasms, and non-hematologic neoplasms by 40 years of age is reported to be 90%, 33%, and 28%, respectively (108). Thus, it is reasonable to screen for FA in patients who present with bone marrow failure syndromes up to at least 40 years of age even in the absence of a suggestive family history or physical anomalies.
Increased chromosomal breakage of peripheral blood cells after exposure to the DNA crosslinking agents, diepoxybutane (DEB) or mitomycin C (MMC), is diagnostic of FA. Occasionally when results in peripheral blood cells are normal and there is a high clinical suspicion for FA, chromosome breakage analysis of cultured skin fibroblasts is performed (6). Distinction between acquired AA and FA has important clinical implications: HSCT is the only curative treatment and IST is futile for FA. Because of sensitivity to DNA damages inherent in FA, HSCT regimens require modification and vigilant monitoring for malignancies is important. Moreover, genetic screening of family members is necessary before considering them as potential stem cell donors and for genetic counseling.
Telomere Diseases: Dyskeratosis Congenita in Children and Telomeropathies in Adults
Dyskeratosis congenita is another classical example of constitutional bone marrow failure that usually manifests early in life. Dyskeratosis congenita shares some clinical features with FA, including early development of bone marrow failure, associated physical anomalies, and predisposition to cancers (5). The classic mucocutaneous triad is patchy skin hyperpigmentation, dystrophic nails, and oral leukoplakia. Skin and nail changes typically present during first decade of life (109). Although mucocutaneous manifestations are highly prevalent (110), the complete mucocutaneous triad is seen in less than half of patients (46). Bone marrow failure develops by the third decade of life (median age of onset 8 years old) (110), and is the major cause of mortality. Classical X-linked dyskeratosis congenita is caused by mutations in the DKC1 gene. DKC1 encodes a protein called dyskerin (111), which is a part of the telomere repair complex (or telomerase), and loss of its function destabilizes the complex, leading to an inability of cells to maintain telomeres (4, 5). The telomere repair complex functions to maintain telomeres in cells with high replicative capacity, such as hematopoietic stem cells, preventing chromosome erosion, cell senescence, and genomic instability (5). Mutations of genes that encode components of telomerase result in accelerated telomere attrition and very short telomere length (112-114). Genetic studies in pedigrees with autosomal dominant and recessive patterns have revealed gene mutations in various components of the telomere repair complex or shelterin (the telomere protection complex), including TERC (a RNA template for telomerase) (115, 116), TERT (a reverse transcriptase) (117), and, much less frequently, NOP10 (118), NHP2 (119), and TINF2 (120).
The distinction between constitutional and acquired forms of aplastic anemia is blurred by recent advances in the understanding of telomere biology and its role in hematopoietic stem cell functions. Some acquired aplastic anemia cases among adults without associated physical anomalies or a suggestive family history have TERC and TERT mutations (115, 117). In addition to bone marrow failure, telomeropathies cause pulmonary fibrosis (121) and liver disease such as cryptogenic cirrhosis and portal hypertension (122). Premature graying of hair is a features of telomeropathies and a helpful clue in the clinic (4). TERC and TERT mutations have highly variable penetrance, and they are considered as genetic risk factors that modify the susceptibility of the host to environmental insults rather than genetic determinants of organ failure (5). For example, smoking appears to accelerate progression of pulmonary fibrosis in patients known to have TERT mutations (123).
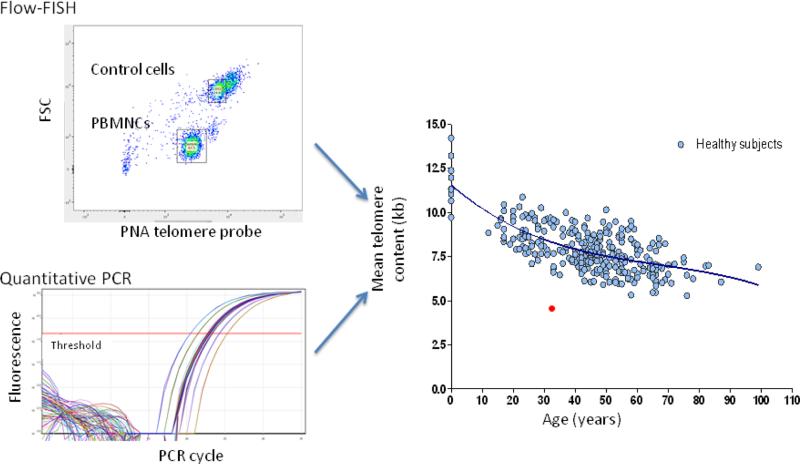
Average chromosomal telomere content in peripheral blood leukocytes can be measured with commercial assays, such as standardized flow-FISH (fluorescent in situ hybridization with labeled probes of telomere repeats measured by flow cytometry) and quantitative polymerase chain reaction (qPCR) amplification for telomere DNA (Figure 4) (124, 125). Results must be adjusted for age, because telomere length normally declines over a lifespan in healthy individuals. Telomere length of total lymphocytes below the first percentile for age strongly supports the diagnosis of telomere disease with high sensitivity and specificity , while telomere content assayed in total leukocytes is less specific (126). However, telomere length above the first percentile does not exclude the diagnosis, as some patients with confirmed mutations in telomerase genes can have normal telomere length (127). Patients with other inherited bone marrow failure syndromes (e.g. FA, DBA, and Shwachman-Diamond syndrome) as well as patients with acquired AA without identifiable mutations can also have short telomeres (114, 128).
Figure 4.
Telomere content of leukocytes measured by standardized flow FISH or quantitative PCR. A patient's result is compared to age-adjusted normalized values. Calculated telomere length of total mononuclear cells below the first percentile for age strongly suggests a diagnosis of telomere disease. (Courtesy of Dr. Bogdan Dumitriu)
The only potentially curable treatment is HSCT, but some patients respond to immunosuppressive therapies as in acquired AA. As in FA, family members must be confirmed to lack mutations before they can serve as donors. Transplant with reduced intensity conditioning regimen has been successfully performed for dyskeratosis congenita patients (129, 130). Androgens increase TERT gene expression and telomerase enzymatic activity (131), and improve hematological findings in about half of the patients (105, 132).
GATA2 deficiency
Haploinsufficiency of the hematopoietic transcription factor GATA2 results in a range of hematologic syndromes (133). GATA2 deficiency can be sporadic or inherited in an autosomal dominant pattern (134, 135). Most mutations occur in the second zinc finger domain or a conserved intronic enhancer element of GATA2, but patients can also have uniallelic expression of GATA2 without an identifiable mutation (133). GATA2 deficiency causes familial MDS / AML (136), monocytopenia and mycobacterial infection (monoMAC syndrome) (135), dendritic cell, myeloid, and NK cell lymphopenia (DCML) (137), Emberger syndrome (lymphedema and MDS) (138), idiopathic bone marrow failure syndromes (139) and aplastic anemia (140). Non-hematological clinical features include susceptibility to nontuberculous mycobacteria (NTM) and human papilloma viruses (HPVs), warts, panniculitis, erythema nodosum, lymphedema, pulmonary complications (including alveolar proteinosis, which is exceedingly rare in general populations), and sensorineural hearing loss (133, 134, 141). The frequency of GATA2 mutations in bone marrow failure syndromes is not well established. In an NIH cohort with a confirmed diagnosis of acquired aplastic anemia, GATA2 mutations were identified in approximately 5% of patients (140). The natural history of bone marrow failure associated with GATA2 mutations can be atypical: Figures 5A-G show peripheral blood and bone marrow findings from a patient who initially presented with pancytopenia and marrow findings consistent with AA, which rapidly evolved into AML with myelodysplastic changes within two years.
Figure 5.
GATA2 deficiency in the clinic: The patient presented as 18 year-old male with pancytopenia and marrow of AA, and within two years, he developed AML with myelodysplastic morphology.
A. Initial bone marrow biopsy at presentation: hypocellular marrow with trilineage hypoplasia compatible with AA. B-E. Bone marrow biopsy two years later: B. AML with myelodysplastic morphology; 30-40% cellularity. C. CD34 immunohistochemistry of biopsy, highlighting increased blasts. D. Dysplastic large osteoclast-like megakaryocyte with separated nuclear lobes, on aspirate smear. E. Pelgeroid PMN, peripheral smear. F. Small mononuclear megakaryocyte, on aspirate smear. G. Increased blasts, 35% on 500 cell differential of aspirate smear. (Courtesy of Dr. Danielle Townsley and Dr. Katherine Calvo).
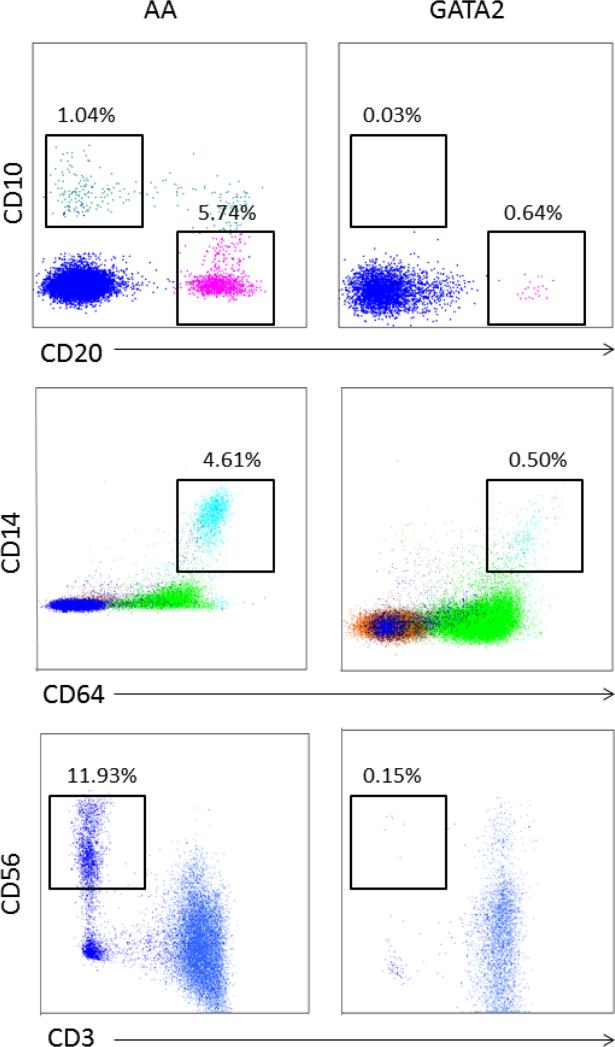
As in other constitutional bone marrow failure syndromes, identification of GATA2 mutations has clinical implications, especially for screening of family donors for the same genetic defects and to recognize increased risks of multi-organ dysfunction associated with the mutations. Flow cytometry of peripheral blood and bone marrow can distinguish GATA2 deficiency from idiopathic AA: GATA2 deficiency is associated with disproportionately reduced numbers of peripheral blood monocytes, B-cells, and NK cells. The bone marrow from patients with GATA2 deficiency also is characterized by markedly reduced monocytes, B-cells, and NK cells, as well as by the absence of hematogones (142) (Figure 6).
Figure 6.
Bone marrow flow cytometry of lymphoid subsets and monocytes in GATA2 patients. Compared to AA patients, GATA2 patients have disproportionately reduced bone marrow mature B cells (CD10−, CD20+), hematogones (CD10+, CD20−), monocytes (CD14+, CD64+), and NK cells (CD3−, CD56+) (Courtesy of Dr. Katherine Calvo).
MYELOPHTHISIC ANEMIA
Myelophthisic anemia is a broad and antique term used to describe hypoproliferative anemia resulting from bone marrow fibrosis and infiltration by abnormal tissues. Myelophthisic anemia may be a manifestation of primary myelofibrosis or fibrosis secondary to other conditions (Table 1). Primary myelofibrosis (PMF) is a clonal myeloproliferative disease, characterized by bone marrow fibrosis, hepatosplenomegaly, extramedullary hematopoiesis, ineffective erythropoiesis, and abnormal cytokine expressions. PMF is classified as one of the myeloproliferative neoplasms (MPNs), along with polycythemia vera (PV) and essential thrombocythemia (ET). Patients commonly present with constitutional symptoms such as fever, night sweats, fatigue, weight loss, pruritis, bone pain, and symptoms related to extrameduallary hematopoiesis (discomfort or pain from splenomegaly, early satiety). Rarely, bleeding or thrombosis can be the presenting symptom. About one-fourth of patients are asymptomatic (143). Janus kinase (JAK) 2 mutations (most commonly V617F) are seen in approximately 60% of patients with PMF or post-ET MF, and are more prevalent among patients with post-PV MF (95%) (144-146).
Secondary processes must be excluded before making the diagnosis of primary myelofibrosis. Other hematological disorders that can be accompanied by bone marrow fibrosis include multiple myeloma, lymphomas, hairy cell leukemia, AML, mastocytosis, and many others (46). Reactive myelofibrosis may occur due to infiltrating metastatic cancers (especially breast, lung, and prostate), disseminated mycobacterial infection or infection with other organisms (1, 46). Myelofibrosis has been associated with autoimmune diseases (147, 148), granulomatous diseases like sarcoidosis (149), and conditions related to bone metabolism, such as renal osteodystrophy (150), hypo- and hyperparathyroidism, vitamin D deficiency, and Paget disease (46).
The peripheral blood smear shows teardrop red blood cells with leukoerythroblastic features, characterized by appearance of immature myeloid cells and nucleated erythrocytes. Bone marrow aspiration is often difficult and a “dry tap” in more than half of cases (151). Treatment and prognosis depends on the etiology of myelofibrosis. Treatable causes must be recognized, because addressing the primary disorder, such as infection or autoimmune disease, may improve marrow fibrosis (147, 148, 152). The choice of treatment for primary myelofibrosis is determined in a risk-adaptive manner. Most experts agree on “wait-and-see” or symptom-guided approach for lower risk disease (153, 154).
TRANSFUSION AND SUPPORTIVE CARE
In all bone marrow failure syndromes, adequate transfusion of blood products is important to correct associated symptoms and prevent organ dysfunction. In general, red blood cell transfusions are provided to maintain a hemoglobin above 7 g/dL (higher than 9 g/dL for patients with ischemic heart disease) (3). Platelet transfusion to maintain a count of 10 K/uL is a routine to avoid spontaneous severe bleeding in stable patients. All red blood cell and platelet products should be leukoreduced to minimize the risk of HLA alloimmunization and resultant transfusion refractoriness, which is problematic for long-term transfusion support. HLA alloimmunization may limit availability of suitable donors and negatively impact transplant outcomes (155, 156). Leukoreduction of blood products also decreases the risk of febrile transfusion reaction and transfusion-related transmission of cytomegalovirus (CMV) (157). Profoundly immunocompromised patients, especially recipients of HSCT, are at risk for the lethal complication of transfusion-associated GVHD (158). The risk of transfusion-associated GVHD can be eliminated by irradiation of blood products (159), although irradiation of blood products is not proven necessary in uncomplicated SAA. The use of G-CSF or erythropoietin is generally ineffective in severe aplastic anemia (160). In patients with severe neutropenia (ANC <500/uL), it is important to promptly evaluate and treat possible infection with empiric broad spectrum antibiotics. Patients who have received substantial amount of red blood cell transfusion develop organ dysfunction secondary to transfusion-associated iron overload. Effective iron chelation regimens are available both parenterally (deferoxamine) and orally (deferasirox) (161-163). Expert consensus has proposed to initiate chelation therapy when the hepatic iron concentration reaches 6–7 mg/g, dry weight, or when the patient has received approximately 100-300 mL/kg of transfusions (21, 27). As a surrogate marker, a serum ferritin level of 1000–1500 ug/L is used as the cutoff to start iron chelation, although serum ferritin levels may unreliably reflect total iron burden (164). Other parameters of iron stores, such as MRI imaging T2 and T2*, may also be applied clinically (164-166).
CONCLUSION
Bone marrow failure syndromes comprise a minority of hypoproliferative anemia in clinical practice, not only for general internists but also for the practicing hematologist. Nevertheless, it is important that clinicians recognize bone marrow failure syndromes and refer patients to a specialist, because timely and optimal treatment may affect the prognosis. As briefly introduced in this review, recent advances in the understanding of pathophysiology have provided us insights into immune-mediated disease mechanisms, hematopoiesis maintenance, and cancer predisposition. Further laboratory and clinical research should improve clinical practice and provide opportunities to address fundamental scientific questions.
Acknowledgment
Intramural Research Program, NHLBI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure / conflict of interest: No conflicts of interest exist.
Contributor Information
Kazusa Ishii, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health.
Neal S. Young, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- 1.Kaushansky K, Williams WJ. Williams hematology. McGraw-Hill Medical; New York: 2010. Available from: http://www.accessmedicine.com/resourceTOC.aspx?resourceID=69 Access for NIH, NIEHS, HSRL via Access Medicine ; 2 concurrent users only. [Google Scholar]
- 2.Cook C. Is clinical gestalt good enough? The Journal of manual & manipulative therapy. 2009;17(1):6–7. doi: 10.1179/106698109790818223. PubMed PMID: 20046560. Pubmed Central PMCID: 2704346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012 Aug 9;120(6):1185–96. doi: 10.1182/blood-2011-12-274019. PubMed PMID: 22517900. Pubmed Central PMCID: 3418715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014 Oct 30;124(18):2775–83. doi: 10.1182/blood-2014-05-526285. PubMed PMID: 25237198. Pubmed Central PMCID: 4215309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calado RT, Young NS. Telomere diseases. The New England journal of medicine. 2009 Dec 10;361(24):2353–65. doi: 10.1056/NEJMra0903373. PubMed PMID: 20007561. Pubmed Central PMCID: 3401586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alter BP. Bone marrow failure: a child is not just a small adult (but an adult can have a childhood disease). Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2005:96–103. doi: 10.1182/asheducation-2005.1.96. PubMed PMID: 16304365. [DOI] [PubMed] [Google Scholar]
- 7.Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. British journal of haematology. 2008 Aug;142(4):505–14. doi: 10.1111/j.1365-2141.2008.07216.x. PubMed PMID: 18510682. Pubmed Central PMCID: 2592349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood reviews. 2010 May;24(3):101–22. doi: 10.1016/j.blre.2010.03.002. PubMed PMID: 20417588. Pubmed Central PMCID: 3733544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piva E, Brugnara C, Chiandetti L, Plebani M. Automated reticulocyte counting: state of the art and clinical applications in the evaluation of erythropoiesis. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2010 Oct;48(10):1369–80. doi: 10.1515/CCLM.2010.292. PubMed PMID: 20666695. [DOI] [PubMed] [Google Scholar]
- 10.Hillman RS, Finch CA. Erythropoiesis: normal and abnormal. Seminars in hematology. 1967 Oct;4(4):327–36. PubMed PMID: 4864985. [PubMed] [Google Scholar]
- 11.Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clinical chemistry. 2000 Aug;46(8 Pt 2):1277–83. PubMed PMID: 10926922. [PubMed] [Google Scholar]
- 12.Holleland G, Schneede J, Ueland PM, Lund PK, Refsum H, Sandberg S. Cobalamin deficiency in general practice. Assessment of the diagnostic utility and cost-benefit analysis of methylmalonic acid determination in relation to current diagnostic strategies. Clinical chemistry. 1999 Feb;45(2):189–98. PubMed PMID: 9931040. [PubMed] [Google Scholar]
- 13.Hansen OP, Drivsholm A, Hippe E. Vitamin B12 metabolism in myelomatosis. Scandinavian journal of haematology. 1977 May;18(5):395–402. doi: 10.1111/j.1600-0609.1977.tb02093.x. PubMed PMID: 877515. [DOI] [PubMed] [Google Scholar]
- 14.Hoffbrand AV, Newcombe FA, Mollin DL. Method of assay of red cell folate activity and the value of the assay as a test for folate deficiency. Journal of clinical pathology. 1966 Jan;19(1):17–28. doi: 10.1136/jcp.19.1.17. PubMed PMID: 5904976. Pubmed Central PMCID: 473152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Archives of internal medicine. 1999 Jun 28;159(12):1289–98. doi: 10.1001/archinte.159.12.1289. PubMed PMID: 10386505. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt DS, Whitehead VM. Cobalamin and folate deficiency: acquired and hereditary disorders in children. Seminars in hematology. 1999 Jan;36(1):19–34. PubMed PMID: 9930566. [PubMed] [Google Scholar]
- 17.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. American journal of hematology. 1990 Jun;34(2):99–107. doi: 10.1002/ajh.2830340205. PubMed PMID: 2339684. [DOI] [PubMed] [Google Scholar]
- 18.Vlachos A, Klein GW, Lipton JM. The Diamond Blackfan Anemia Registry: tool for investigating the epidemiology and biology of Diamond-Blackfan anemia. Journal of pediatric hematology/oncology. 2001 Aug-Sep;23(6):377–82. doi: 10.1097/00043426-200108000-00015. PubMed PMID: 11563775. [DOI] [PubMed] [Google Scholar]
- 19.Balaban EP, Buchanan GR, Graham M, Frenkel EP. Diamond-Blackfan syndrome in adult patients. The American journal of medicine. 1985 Mar;78(3):533–8. doi: 10.1016/0002-9343(85)90352-3. PubMed PMID: 3919581. [DOI] [PubMed] [Google Scholar]
- 20.Ruggero D, Shimamura A. Marrow failure: a window into ribosome biology. Blood. 2014 Oct 30;124(18):2784–92. doi: 10.1182/blood-2014-04-526301. PubMed PMID: 25237201. Pubmed Central PMCID: 4215310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. British journal of haematology. 2008 Sep;142(6):859–76. doi: 10.1111/j.1365-2141.2008.07269.x. PubMed PMID: 18671700. Pubmed Central PMCID: 2654478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrar JE, Dahl N. Untangling the phenotypic heterogeneity of Diamond Blackfan anemia. Seminars in hematology. 2011 Apr;48(2):124–35. doi: 10.1053/j.seminhematol.2011.02.003. PubMed PMID: 21435509. Pubmed Central PMCID: 3078697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khincha PP, Savage SA. Genomic characterization of the inherited bone marrow failure syndromes. Seminars in hematology. 2013 Oct;50(4):333–47. doi: 10.1053/j.seminhematol.2013.09.002. PubMed PMID: 24246701. Pubmed Central PMCID: 3835370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faivre L, Meerpohl J, Da Costa L, Marie I, Nouvel C, Gnekow A, et al. High-risk pregnancies in Diamond-Blackfan anemia: a survey of 64 pregnancies from the French and German registries. Haematologica. 2006 Apr;91(4):530–3. PubMed PMID: 16537118. [PubMed] [Google Scholar]
- 25.Glader BE, Backer K. Elevated red cell adenosine deaminase activity: a marker of disordered erythropoiesis in Diamond-Blackfan anaemia and other haematologic diseases. British journal of haematology. 1988 Feb;68(2):165–8. doi: 10.1111/j.1365-2141.1988.tb06184.x. PubMed PMID: 3348976. [DOI] [PubMed] [Google Scholar]
- 26.Diamond LK, Wang WC, Alter BP. Congenital hypoplastic anemia. Advances in pediatrics. 1976;22:349–78. PubMed PMID: 773132. [PubMed] [Google Scholar]
- 27.Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010 Nov 11;116(19):3715–23. doi: 10.1182/blood-2010-02-251090. PubMed PMID: 20651069. Pubmed Central PMCID: 2981532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willig TN, Gazda H, Sieff CA. Diamond-Blackfan anemia. Current opinion in hematology. 2000 Mar;7(2):85–94. doi: 10.1097/00062752-200003000-00003. PubMed PMID: 10698294. [DOI] [PubMed] [Google Scholar]
- 29.Lipton JM, Atsidaftos E, Zyskind I, Vlachos A. Improving clinical care and elucidating the pathophysiology of Diamond Blackfan anemia: an update from the Diamond Blackfan Anemia Registry. Pediatric blood & cancer. 2006 May 1;46(5):558–64. doi: 10.1002/pbc.20642. PubMed PMID: 16317735. [DOI] [PubMed] [Google Scholar]
- 30.Saarinen UM, Chorba TL, Tattersall P, Young NS, Anderson LJ, Palmer E, et al. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986 May;67(5):1411–7. PubMed PMID: 3008891. [PubMed] [Google Scholar]
- 31.Kurtzman GJ, Cohen BJ, Field AM, Oseas R, Blaese RM, Young NS. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. The Journal of clinical investigation. 1989 Oct;84(4):1114–23. doi: 10.1172/JCI114274. PubMed PMID: 2551923. Pubmed Central PMCID: 329767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glader BE. Diagnosis and management of red cell aplasia in children. Hematology/oncology clinics of North America. 1987 Sep;1(3):431–47. PubMed PMID: 3129394. [PubMed] [Google Scholar]
- 33.Chikkappa G, Zarrabi MH, Tsan MF. Pure red-cell aplasia in patients with chronic lymphocytic leukemia. Medicine. 1986 Sep;65(5):339–51. doi: 10.1097/00005792-198609000-00006. PubMed PMID: 3091991. [DOI] [PubMed] [Google Scholar]
- 34.Visco C, Barcellini W, Maura F, Neri A, Cortelezzi A, Rodeghiero F. Autoimmune cytopenias in chronic lymphocytic leukemia. American journal of hematology. 2014 Nov;89(11):1055–62. doi: 10.1002/ajh.23785. PubMed PMID: 24912821. [DOI] [PubMed] [Google Scholar]
- 35.Go RS, Lust JA, Phyliky RL. Aplastic anemia and pure red cell aplasia associated with large granular lymphocyte leukemia. Seminars in hematology. 2003 Jul;40(3):196–200. doi: 10.1016/s0037-1963(03)00140-9. PubMed PMID: 12876668. [DOI] [PubMed] [Google Scholar]
- 36.Mangan KF, Volkin R, Winkelstein A. Autoreactive erythroid progenitor-T suppressor cells in the pure red cell aplasia associated with thymoma and panhypogammaglobulinemia. American journal of hematology. 1986 Oct;23(2):167–73. doi: 10.1002/ajh.2830230211. PubMed PMID: 2944379. [DOI] [PubMed] [Google Scholar]
- 37.Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cellular & molecular immunology. 2011 May;8(3):199–202. doi: 10.1038/cmi.2010.74. PubMed PMID: 21317916. Pubmed Central PMCID: 4012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young NS, Brown KE. Parvovirus B19. The New England journal of medicine. 2004 Feb 5;350(6):586–97. doi: 10.1056/NEJMra030840. PubMed PMID: 14762186. [DOI] [PubMed] [Google Scholar]
- 39.Geetha D, Zachary JB, Baldado HM, Kronz JD, Kraus ES. Pure red cell aplasia caused by Parvovirus B19 infection in solid organ transplant recipients: a case report and review of literature. Clinical transplantation. 2000 Dec;14(6):586–91. doi: 10.1034/j.1399-0012.2000.140612.x. PubMed PMID: 11127313. [DOI] [PubMed] [Google Scholar]
- 40.Frickhofen N, Abkowitz JL, Safford M, Berry JM, Antunez-de-Mayolo J, Astrow A, et al. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Annals of internal medicine. 1990 Dec 15;113(12):926–33. doi: 10.7326/0003-4819-113-12-926. PubMed PMID: 2173460. [DOI] [PubMed] [Google Scholar]
- 41.Casadevall N, Dupuy E, Molho-Sabatier P, Tobelem G, Varet B, Mayeux P. Autoantibodies against erythropoietin in a patient with pure red-cell aplasia. The New England journal of medicine. 1996 Mar 7;334(10):630–3. doi: 10.1056/NEJM199603073341004. PubMed PMID: 8592526. [DOI] [PubMed] [Google Scholar]
- 42.Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. The New England journal of medicine. 2002 Feb 14;346(7):469–75. doi: 10.1056/NEJMoa011931. PubMed PMID: 11844847. [DOI] [PubMed] [Google Scholar]
- 43.Thompson DF, Gales MA. Drug-induced pure red cell aplasia. Pharmacotherapy. 1996 Nov-Dec;16(6):1002–8. PubMed PMID: 8947971. [PubMed] [Google Scholar]
- 44.Baker RI, Manoharan A, de Luca E, Begley CG. Pure red cell aplasia of pregnancy: a distinct clinical entity. British journal of haematology. 1993 Nov;85(3):619–22. doi: 10.1111/j.1365-2141.1993.tb03359.x. PubMed PMID: 8136286. [DOI] [PubMed] [Google Scholar]
- 45.Bolan CD, Leitman SF, Griffith LM, Wesley RA, Procter JL, Stroncek DF, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. 2001 Sep 15;98(6):1687–94. doi: 10.1182/blood.v98.6.1687. PubMed PMID: 11535498. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman R. Hematology : basic principles and practice. 5th ed. xxvii. Churchill Livingstone/Elsevier; Philadelphia, PA: 2009. p. 2523. [Google Scholar]
- 47.Kurtzman G, Frickhofen N, Kimball J, Jenkins DW, Nienhuis AW, Young NS. Pure red-cell aplasia of 10 years' duration due to persistent parvovirus B19 infection and its cure with immunoglobulin therapy. The New England journal of medicine. 1989 Aug 24;321(8):519–23. doi: 10.1056/NEJM198908243210807. PubMed PMID: 2548098. [DOI] [PubMed] [Google Scholar]
- 48.Crabol Y, Terrier B, Rozenberg F, Pestre V, Legendre C, Hermine O, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Apr;56(7):968–77. doi: 10.1093/cid/cis1046. PubMed PMID: 23243178. [DOI] [PubMed] [Google Scholar]
- 49.Clark DA, Dessypris EN, Krantz SB. Studies on pure red cell aplasia. XI. Results of immunosuppressive treatment of 37 patients. Blood. 1984 Feb;63(2):277–86. PubMed PMID: 6581839. [PubMed] [Google Scholar]
- 50.Mamiya S, Itoh T, Miura AB. Acquired pure red cell aplasia in Japan. European journal of haematology. 1997 Oct;59(4):199–205. doi: 10.1111/j.1600-0609.1997.tb00978.x. PubMed PMID: 9338617. [DOI] [PubMed] [Google Scholar]
- 51.Sawada K, Hirokawa M, Fujishima N, Teramura M, Bessho M, Dan K, et al. Long-term outcome of patients with acquired primary idiopathic pure red cell aplasia receiving cyclosporine A. A nationwide cohort study in Japan for the PRCA Collaborative Study Group. Haematologica. 2007 Aug;92(8):1021–8. doi: 10.3324/haematol.11192. PubMed PMID: 17640861. [DOI] [PubMed] [Google Scholar]
- 52.Go RS, Li CY, Tefferi A, Phyliky RL. Acquired pure red cell aplasia associated with lymphoproliferative disease of granular T lymphocytes. Blood. 2001 Jul 15;98(2):483–5. doi: 10.1182/blood.v98.2.483. PubMed PMID: 11435321. [DOI] [PubMed] [Google Scholar]
- 53.Fujishima N, Sawada K, Hirokawa M, Oshimi K, Sugimoto K, Matsuda A, et al. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a Nationwide Cohort Study in Japan for the PRCA Collaborative Study Group. Haematologica. 2008 Oct;93(10):1555–9. doi: 10.3324/haematol.12871. PubMed PMID: 18641028. [DOI] [PubMed] [Google Scholar]
- 54.Willis F, Marsh JC, Bevan DH, Killick SB, Lucas G, Griffiths R, et al. The effect of treatment with Campath-1H in patients with autoimmune cytopenias. British journal of haematology. 2001 Sep;114(4):891–8. doi: 10.1046/j.1365-2141.2001.03039.x. PubMed PMID: 11564082. [DOI] [PubMed] [Google Scholar]
- 55.Ru X, Liebman HA. Successful treatment of refractory pure red cell aplasia associated with lymphoproliferative disorders with the anti-CD52 monoclonal antibody alemtuzumab (Campath-1H). British journal of haematology. 2003 Oct;123(2):278–81. doi: 10.1046/j.1365-2141.2003.04609.x. PubMed PMID: 14531909. [DOI] [PubMed] [Google Scholar]
- 56.Abkowitz JL, Powell JS, Nakamura JM, Kadin ME, Adamson JW. Pure red cell aplasia: response to therapy with anti-thymocyte globulin. American journal of hematology. 1986 Dec;23(4):363–71. doi: 10.1002/ajh.2830230408. PubMed PMID: 3098093. [DOI] [PubMed] [Google Scholar]
- 57.Mangan KF, Shadduck RK. Successful treatment of chronic refractory pure red cell aplasia with antithymocyte globulin: correlation with in vitro erythroid culture studies. American journal of hematology. 1984;17(4):417–26. doi: 10.1002/ajh.2830170412. PubMed PMID: 6238526. [DOI] [PubMed] [Google Scholar]
- 58.Ghazal H. Successful treatment of pure red cell aplasia with rituximab in patients with chronic lymphocytic leukemia. Blood. 2002 Feb 1;99(3):1092–4. doi: 10.1182/blood.v99.3.1092. PubMed PMID: 11807020. [DOI] [PubMed] [Google Scholar]
- 59.Auner HW, Wolfler A, Beham-Schmid C, Strunk D, Linkesch W, Sill H. Restoration of erythropoiesis by rituximab in an adult patient with primary acquired pure red cell aplasia refractory to conventional treatment. British journal of haematology. 2002 Mar;116(3):727–8. doi: 10.1046/j.1365-2141.2002.3317_3.x. PubMed PMID: 11879264. [DOI] [PubMed] [Google Scholar]
- 60.Thompson CA, Steensma DP. Pure red cell aplasia associated with thymoma: clinical insights from a 50-year single-institution experience. British journal of haematology. 2006 Nov;135(3):405–7. doi: 10.1111/j.1365-2141.2006.06295.x. PubMed PMID: 17032177. [DOI] [PubMed] [Google Scholar]
- 61.Zeok JV, Todd EP, Dillon M, DeSimone P, Utley JR. The role of thymectomy in red cell aplasia. The Annals of thoracic surgery. 1979 Sep;28(3):257–60. doi: 10.1016/s0003-4975(10)63116-5. PubMed PMID: 485627. [DOI] [PubMed] [Google Scholar]
- 62.Hirokawa M, Sawada K, Fujishima N, Nakao S, Urabe A, Dan K, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica. 2008 Jan;93(1):27–33. doi: 10.3324/haematol.11655. PubMed PMID: 18166782. [DOI] [PubMed] [Google Scholar]
- 63.Muller BU, Tichelli A, Passweg JR, Nissen C, Wodnar-Filipowicz A, Gratwohl A. Successful treatment of refractory acquired pure red cell aplasia (PRCA) by allogeneic bone marrow transplantation. Bone marrow transplantation. 1999 Jun;23(11):1205–7. doi: 10.1038/sj.bmt.1701785. PubMed PMID: 10382963. [DOI] [PubMed] [Google Scholar]
- 64.Tseng SB, Lin SF, Chang CS, Liu TC, Hsiao HH, Liu YC, et al. Successful treatment of acquired pure red cell aplasia (PRCA) by allogeneic peripheral blood stem cell transplantation. American journal of hematology. 2003 Dec;74(4):273–5. doi: 10.1002/ajh.10421. PubMed PMID: 14635209. [DOI] [PubMed] [Google Scholar]
- 65.Musso M, Porretto F, Crescimanno A, Polizzi V, Scalone R. Donor lymphocyte infusions for refractory pure red cell aplasia relapsing after both autologous and nonmyeloablative allogeneic peripheral stem cell transplantation. Bone marrow transplantation. 2004 Apr;33(7):769–71. doi: 10.1038/sj.bmt.1704419. PubMed PMID: 14755320. [DOI] [PubMed] [Google Scholar]
- 66.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:76–81. doi: 10.1182/asheducation-2013.1.76. PubMed PMID: 24319166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frickhofen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Herrmann F, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. The German Aplastic Anemia Study Group. The New England journal of medicine. 1991 May 9;324(19):1297–304. doi: 10.1056/NEJM199105093241901. PubMed PMID: 2017225. [DOI] [PubMed] [Google Scholar]
- 68.Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, Mugishima H, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000 Sep 15;96(6):2049–54. PubMed PMID: 10979946. [PubMed] [Google Scholar]
- 69.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995 Jun 1;85(11):3058–65. PubMed PMID: 7756640. [PubMed] [Google Scholar]
- 70.Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, Locatelli F, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO). Blood. 2000 Mar 15;95(6):1931–4. PubMed PMID: 10706857. [PubMed] [Google Scholar]
- 71.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002 Aug 15;100(4):1185–91. doi: 10.1182/blood-2002-01-0035. PubMed PMID: 12149196. [DOI] [PubMed] [Google Scholar]
- 72.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004 Jul 24-30;364(9431):355–64. doi: 10.1016/S0140-6736(04)16724-X. PubMed PMID: 15276395. [DOI] [PubMed] [Google Scholar]
- 73.Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 1995 Jun 1;85(11):3183–90. PubMed PMID: 7538820. [PubMed] [Google Scholar]
- 74.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996 May 15;87(10):4149–57. PubMed PMID: 8639773. [PubMed] [Google Scholar]
- 75.Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, et al. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007 Sep 1;110(5):1603–6. doi: 10.1182/blood-2007-01-066258. PubMed PMID: 17463169. Pubmed Central PMCID: 1975843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, et al. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012 Aug 23;120(8):1624–32. doi: 10.1182/blood-2011-11-390708. PubMed PMID: 22797698. [DOI] [PubMed] [Google Scholar]
- 77.Jerez A, Clemente MJ, Makishima H, Rajala H, Gomez-Segui I, Olson T, et al. STAT3 mutations indicate the presence of subclinical T-cell clones in a subset of aplastic anemia and myelodysplastic syndrome patients. Blood. 2013 Oct 3;122(14):2453–9. doi: 10.1182/blood-2013-04-494930. PubMed PMID: 23926297. Pubmed Central PMCID: 3790512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheinberg P, Chen J. Aplastic anemia: what have we learned from animal models and from the clinic. Seminars in hematology. 2013 Apr;50(2):156–64. doi: 10.1053/j.seminhematol.2013.03.028. PubMed PMID: 24216172. [DOI] [PubMed] [Google Scholar]
- 79.Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976 Jul;48(1):63–70. PubMed PMID: 779871. [PubMed] [Google Scholar]
- 80.Kwon JH, Kim I, Lee YG, Koh Y, Park HC, Song EY, et al. Clinical course of non-severe aplastic anemia in adults. International journal of hematology. 2010 Jun;91(5):770–5. doi: 10.1007/s12185-010-0601-1. PubMed PMID: 20524094. [DOI] [PubMed] [Google Scholar]
- 81.Matsui WH, Brodsky RA, Smith BD, Borowitz MJ, Jones RJ. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006 Mar;20(3):458–62. doi: 10.1038/sj.leu.2404119. PubMed PMID: 16437138. [DOI] [PubMed] [Google Scholar]
- 82.Ishiyama K, Karasawa M, Miyawaki S, Ueda Y, Noda M, Wakita A, et al. Aplastic anaemia with 13q-: a benign subset of bone marrow failure responsive to immunosuppressive therapy. British journal of haematology. 2002 Jun;117(3):747–50. doi: 10.1046/j.1365-2141.2002.03518.x. PubMed PMID: 12028052. [DOI] [PubMed] [Google Scholar]
- 83.Gupta V, Brooker C, Tooze JA, Yi QL, Sage D, Turner D, et al. Clinical relevance of cytogenetic abnormalities at diagnosis of acquired aplastic anaemia in adults. British journal of haematology. 2006 Jul;134(1):95–9. doi: 10.1111/j.1365-2141.2006.06105.x. PubMed PMID: 16803574. [DOI] [PubMed] [Google Scholar]
- 84.Kim SY, Lee JW, Lee SE, Cho BS, Kim M, Eom KS, et al. The characteristics and clinical outcome of adult patients with aplastic anemia and abnormal cytogenetics at diagnosis. Genes, chromosomes & cancer. 2010 Sep;49(9):844–50. doi: 10.1002/gcc.20793. PubMed PMID: 20540166. [DOI] [PubMed] [Google Scholar]
- 85.Dumitriu B, Feng X, Townsley DM, Ueda Y, Yoshizato T, Calado RT, et al. Telomere attrition and candidate gene mutations preceding monosomy 7 in aplastic anemia. Blood. 2015 Jan 22;125(4):706–9. doi: 10.1182/blood-2014-10-607572. PubMed PMID: 25406353. Pubmed Central PMCID: 4304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010 Jul;95(7):1075–80. doi: 10.3324/haematol.2009.017889. PubMed PMID: 20595102. Pubmed Central PMCID: 2895030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young NS. Paroxysmal nocturnal hemoglobinuria and myelodysplastic syndromes: clonal expansion of PIG-A-mutant hematopoietic cells in bone marrow failure. Haematologica. 2009 Jan;94(1):3–7. doi: 10.3324/haematol.2008.001297. PubMed PMID: 19118373. Pubmed Central PMCID: 2625409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kulagin A, Lisukov I, Ivanova M, Golubovskaya I, Kruchkova I, Bondarenko S, et al. Prognostic value of paroxysmal nocturnal haemoglobinuria clone presence in aplastic anaemia patients treated with combined immunosuppression: results of two-centre prospective study. British journal of haematology. 2014 Feb;164(4):546–54. doi: 10.1111/bjh.12661. PubMed PMID: 24261566. [DOI] [PubMed] [Google Scholar]
- 89.Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006 Feb 15;107(4):1308–14. doi: 10.1182/blood-2005-06-2485. PubMed PMID: 16179371. [DOI] [PubMed] [Google Scholar]
- 90.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. British journal of haematology. 2009 Jan;144(2):206–16. doi: 10.1111/j.1365-2141.2008.07450.x. PubMed PMID: 19036108. Pubmed Central PMCID: 4149225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Socie G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:82–6. doi: 10.1182/asheducation-2013.1.82. PubMed PMID: 24319167. [DOI] [PubMed] [Google Scholar]
- 92.Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2010;2010:36–42. doi: 10.1182/asheducation-2010.1.36. PubMed PMID: 21239768. [DOI] [PubMed] [Google Scholar]
- 93.Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy--The European Group for Blood and Marrow Transplantation experience. Seminars in hematology. 2000 Jan;37(1):69–80. doi: 10.1016/s0037-1963(00)90031-3. PubMed PMID: 10676912. [DOI] [PubMed] [Google Scholar]
- 94.Gupta V, Eapen M, Brazauskas R, Carreras J, Aljurf M, Gale RP, et al. Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica. 2010 Dec;95(12):2119–25. doi: 10.3324/haematol.2010.026682. PubMed PMID: 20851870. Pubmed Central PMCID: 2995571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanders JE, Woolfrey AE, Carpenter PA, Storer BE, Hoffmeister PA, Deeg HJ, et al. Late effects among pediatric patients followed for nearly 4 decades after transplantation for severe aplastic anemia. Blood. 2011 Aug 4;118(5):1421–8. doi: 10.1182/blood-2011-02-334953. PubMed PMID: 21653322. Pubmed Central PMCID: 3152503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deeg HJ, Leisenring W, Storb R, Nims J, Flowers ME, Witherspoon RP, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998 May 15;91(10):3637–45. PubMed PMID: 9572999. [PubMed] [Google Scholar]
- 97.Dufour C, Pillon M, Socie G, Rovo A, Carraro E, Bacigalupo A, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. British journal of haematology. 2015 Feb 14; doi: 10.1111/bjh.13297. PubMed PMID: 25683884. [DOI] [PubMed] [Google Scholar]
- 98.Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007 Aug 15;110(4):1397–400. doi: 10.1182/blood-2007-03-081596. PubMed PMID: 17475907. Pubmed Central PMCID: 1939910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu R, Brazauskas R, Kan F, Bashey A, Bredeson C, Camitta B, et al. Comparison of outcomes after transplantation of G-CSF-stimulated bone marrow grafts versus bone marrow or peripheral blood grafts from HLA-matched sibling donors for patients with severe aplastic anemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 Jul;17(7):1018–24. doi: 10.1016/j.bbmt.2010.10.029. PubMed PMID: 21034842. Pubmed Central PMCID: 3114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bacigalupo A, Socie G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M, et al. Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica. 2012 Aug;97(8):1142–8. doi: 10.3324/haematol.2011.054841. PubMed PMID: 22315497. Pubmed Central PMCID: 3409810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. British journal of haematology. 2009 Oct;147(1):43–70. doi: 10.1111/j.1365-2141.2009.07842.x. PubMed PMID: 19673883. [DOI] [PubMed] [Google Scholar]
- 102.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. The New England journal of medicine. 2011 Aug 4;365(5):430–8. doi: 10.1056/NEJMoa1103975. PubMed PMID: 21812672. Pubmed Central PMCID: 3721503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. The New England journal of medicine. 2012 Jul 5;367(1):11–9. doi: 10.1056/NEJMoa1200931. PubMed PMID: 22762314. Pubmed Central PMCID: 3422737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014 Mar 20;123(12):1818–25. doi: 10.1182/blood-2013-10-534743. PubMed PMID: 24345753. Pubmed Central PMCID: 3962161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gardner FH, Juneja HS. Androstane therapy to treat aplastic anaemia in adults: an uncontrolled pilot study. British journal of haematology. 1987 Mar;65(3):295–300. doi: 10.1111/j.1365-2141.1987.tb06856.x. PubMed PMID: 3567083. [DOI] [PubMed] [Google Scholar]
- 106.Androgen therapy in aplastic anaemia: a comparative study of high and low-doses and of 4 different androgens. French Cooperative Group for the Study of Aplastic and Refractory Anemias. Scandinavian journal of haematology. 1986 Apr;36(4):346–52. PubMed PMID: 2872720. [PubMed] [Google Scholar]
- 107.Longerich S, Li J, Xiong Y, Sung P, Kupfer GM. Stress and DNA repair biology of the Fanconi anemia pathway. Blood. 2014 Oct 30;124(18):2812–9. doi: 10.1182/blood-2014-04-526293. PubMed PMID: 25237197. Pubmed Central PMCID: 4314529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003 Feb 15;101(4):1249–56. doi: 10.1182/blood-2002-07-2170. PubMed PMID: 12393516. [DOI] [PubMed] [Google Scholar]
- 109.Vulliamy T, Dokal I. Dyskeratosis congenita. Seminars in hematology. 2006 Jul;43(3):157–66. doi: 10.1053/j.seminhematol.2006.04.001. PubMed PMID: 16822458. [DOI] [PubMed] [Google Scholar]
- 110.Dokal I. Dyskeratosis congenita in all its forms. British journal of haematology. 2000 Sep;110(4):768–79. doi: 10.1046/j.1365-2141.2000.02109.x. PubMed PMID: 11054058. [DOI] [PubMed] [Google Scholar]
- 111.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature genetics. 1998 May;19(1):32–8. doi: 10.1038/ng0598-32. PubMed PMID: 9590285. [DOI] [PubMed] [Google Scholar]
- 112.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood cells, molecules & diseases. 2001 Mar-Apr;27(2):353–7. doi: 10.1006/bcmd.2001.0389. PubMed PMID: 11259155. [DOI] [PubMed] [Google Scholar]
- 113.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998 May 15;91(10):3582–92. PubMed PMID: 9572992. [PubMed] [Google Scholar]
- 114.Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001 Feb 15;97(4):895–900. doi: 10.1182/blood.v97.4.895. PubMed PMID: 11159514. [DOI] [PubMed] [Google Scholar]
- 115.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003 Nov 15;362(9396):1628–30. doi: 10.1016/S0140-6736(03)14797-6. PubMed PMID: 14630445. [DOI] [PubMed] [Google Scholar]
- 116.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003 Aug 1;102(3):916–8. doi: 10.1182/blood-2003-01-0335. PubMed PMID: 12676774. [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. The New England journal of medicine. 2005 Apr 7;352(14):1413–24. doi: 10.1056/NEJMoa042980. PubMed PMID: 15814878. [DOI] [PubMed] [Google Scholar]
- 118.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Human molecular genetics. 2007 Jul 1;16(13):1619–29. doi: 10.1093/hmg/ddm111. PubMed PMID: 17507419. Pubmed Central PMCID: 2882227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 10;105(23):8073–8. doi: 10.1073/pnas.0800042105. PubMed PMID: 18523010. Pubmed Central PMCID: 2430361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. American journal of human genetics. 2008 Feb;82(2):501–9. doi: 10.1016/j.ajhg.2007.10.004. PubMed PMID: 18252230. Pubmed Central PMCID: 2427222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PloS one. 2010;5(5):e10680. doi: 10.1371/journal.pone.0010680. PubMed PMID: 20502709. Pubmed Central PMCID: 2873288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PloS one. 2009;4(11):e7926. doi: 10.1371/journal.pone.0007926. PubMed PMID: 19936245. Pubmed Central PMCID: 2775683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diaz de Leon A, Cronkhite JT, Yilmaz C, Brewington C, Wang R, Xing C, et al. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011 Sep;140(3):753–63. doi: 10.1378/chest.10-2865. PubMed PMID: 21349926. Pubmed Central PMCID: 3168855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nature protocols. 2006;1(5):2365–76. doi: 10.1038/nprot.2006.263. PubMed PMID: 17406480. [DOI] [PubMed] [Google Scholar]
- 125.Martin-Ruiz CM, Baird D, Roger L, Boukamp P, Krunic D, Cawthon R, et al. Reproducibility of telomere length assessment: an international collaborative study. International journal of epidemiology. 2014 Sep 19; doi: 10.1093/ije/dyu191. PubMed PMID: 25239152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007 Sep 1;110(5):1439–47. doi: 10.1182/blood-2007-02-075598. PubMed PMID: 17468339. Pubmed Central PMCID: 1975834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vulliamy TJ, Kirwan MJ, Beswick R, Hossain U, Baqai C, Ratcliffe A, et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PloS one. 2011;6(9):e24383. doi: 10.1371/journal.pone.0024383. PubMed PMID: 21931702. Pubmed Central PMCID: 3172236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alter BP, Giri N, Savage SA, Rosenberg PS. Telomere length in inherited bone marrow failure syndromes. Haematologica. 2015 Jan;100(1):49–54. doi: 10.3324/haematol.2014.114389. PubMed PMID: 25304614. Pubmed Central PMCID: 4281312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ayas M, Nassar A, Hamidieh AA, Kharfan-Dabaja M, Othman TB, Elhaddad A, et al. Reduced intensity conditioning is effective for hematopoietic SCT in dyskeratosis congenita-related BM failure. Bone marrow transplantation. 2013 Sep;48(9):1168–72. doi: 10.1038/bmt.2013.35. PubMed PMID: 23542225. [DOI] [PubMed] [Google Scholar]
- 130.Gadalla SM, Sales-Bonfim C, Carreras J, Alter BP, Antin JH, Ayas M, et al. Outcomes of allogeneic hematopoietic cell transplantation in patients with dyskeratosis congenita. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Aug;19(8):1238–43. doi: 10.1016/j.bbmt.2013.05.021. PubMed PMID: 23751955. Pubmed Central PMCID: 3736557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calado RT, Yewdell WT, Wilkerson KL, Regal JA, Kajigaya S, Stratakis CA, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009 Sep 10;114(11):2236–43. doi: 10.1182/blood-2008-09-178871. PubMed PMID: 19561322. Pubmed Central PMCID: 2745844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Allen DM, Fine MH, Necheles TF, Dameshek W. Oxymetholone therapy in aplastic anemia. Blood. 1968 Jul;32(1):83–9. PubMed PMID: 5658392. [PubMed] [Google Scholar]
- 133.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014 Feb 6;123(6):809–21. doi: 10.1182/blood-2013-07-515528. PubMed PMID: 24227816. Pubmed Central PMCID: 3916876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010 Feb 25;115(8):1519–29. doi: 10.1182/blood-2009-03-208629. PubMed PMID: 20040766. Pubmed Central PMCID: 2830758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011 Sep 8;118(10):2653–5. doi: 10.1182/blood-2011-05-356352. PubMed PMID: 21670465. Pubmed Central PMCID: 3172785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nature genetics. 2011 Oct;43(10):1012–7. doi: 10.1038/ng.913. PubMed PMID: 21892162. Pubmed Central PMCID: 3184204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011 Sep 8;118(10):2656–8. doi: 10.1182/blood-2011-06-360313. PubMed PMID: 21765025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nature genetics. 2011 Oct;43(10):929–31. doi: 10.1038/ng.923. PubMed PMID: 21892158. [DOI] [PubMed] [Google Scholar]
- 139.Zhang MY, Keel SB, Walsh T, Lee MK, Gulsuner S, Watts AC, et al. Genomic analysis of bone marrow failure and myelodysplastic syndromes reveals phenotypic and diagnostic complexity. Haematologica. 2015 Jan;100(1):42–8. doi: 10.3324/haematol.2014.113456. PubMed PMID: 25239263. Pubmed Central PMCID: 4281311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Townsley DM, Hsu A, Dumitriu B, Holland SM, Young NS. Regulatory Mutations in GATA2 Associated with Aplastic Anemia. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):3488. 2012. [Google Scholar]
- 141.Ishida H, Imai K, Honma K, Tamura S, Imamura T, Ito M, et al. GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B- and NK-cell (DCML) deficiency, and myelodysplasia. European journal of pediatrics. 2012 Aug;171(8):1273–6. doi: 10.1007/s00431-012-1715-7. PubMed PMID: 22430350. [DOI] [PubMed] [Google Scholar]
- 142.Ganapathi KA, Townsley DM, Hsu AP, Arthur DC, Zerbe CS, Cuellar-Rodriguez J, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015 Jan 1;125(1):56–70. doi: 10.1182/blood-2014-06-580340. PubMed PMID: 25359990. Pubmed Central PMCID: 4281830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009 Mar 26;113(13):2895–901. doi: 10.1182/blood-2008-07-170449. PubMed PMID: 18988864. [DOI] [PubMed] [Google Scholar]
- 144.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19-25;365(9464):1054–61. doi: 10.1016/S0140-6736(05)71142-9. PubMed PMID: 15781101. [DOI] [PubMed] [Google Scholar]
- 145.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 Apr 28;434(7037):1144–8. doi: 10.1038/nature03546. PubMed PMID: 15793561. [DOI] [PubMed] [Google Scholar]
- 146.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005 Apr 28;352(17):1779–90. doi: 10.1056/NEJMoa051113. PubMed PMID: 15858187. [DOI] [PubMed] [Google Scholar]
- 147.Paquette RL, Meshkinpour A, Rosen PJ. Autoimmune myelofibrosis. A steroid-responsive cause of bone marrow fibrosis associated with systemic lupus erythematosus. Medicine. 1994 May;73(3):145–52. PubMed PMID: 8190037. [PubMed] [Google Scholar]
- 148.Pullarkat V, Bass RD, Gong JZ, Feinstein DI, Brynes RK. Primary autoimmune myelofibrosis: definition of a distinct clinicopathologic syndrome. American journal of hematology. 2003 Jan;72(1):8–12. doi: 10.1002/ajh.10258. PubMed PMID: 12508261. [DOI] [PubMed] [Google Scholar]
- 149.Reich JM. Sarcoidosis and agnogenic myeloid metaplasia. Journal of internal medicine. 1994 Feb;235(2):175–8. doi: 10.1111/j.1365-2796.1994.tb01052.x. PubMed PMID: 8308481. [DOI] [PubMed] [Google Scholar]
- 150.Weinberg SG, Lubin A, Wiener SN, Deoras MP, Ghose MK, Kopelman RC. Myelofibrosis and renal osteodystrophy. The American journal of medicine. 1977 Nov;63(5):755–64. doi: 10.1016/0002-9343(77)90162-0. PubMed PMID: 930950. [DOI] [PubMed] [Google Scholar]
- 151.Varki A, Lottenberg R, Griffith R, Reinhard E. The syndrome of idiopathic myelofibrosis. A clinicopathologic review with emphasis on the prognostic variables predicting survival. Medicine. 1983 Nov;62(6):353–71. PubMed PMID: 6633248. [PubMed] [Google Scholar]
- 152.Viallard JF, Parrens M, Boiron JM, Texier J, Mercie P, Pellegrin JL. Reversible myelofibrosis induced by tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Jun 15;34(12):1641–3. doi: 10.1086/340524. PubMed PMID: 12032901. [DOI] [PubMed] [Google Scholar]
- 153.Cervantes F. How I treat myelofibrosis. Blood. 2014 Oct 23;124(17):2635–42. doi: 10.1182/blood-2014-07-575373. PubMed PMID: 25232060. [DOI] [PubMed] [Google Scholar]
- 154.Tefferi A. Primary myelofibrosis: 2014 update on diagnosis, risk-stratification, and management. American journal of hematology. 2014 Sep;89(9):915–25. doi: 10.1002/ajh.23703. PubMed PMID: 25124313. [DOI] [PubMed] [Google Scholar]
- 155.Kaminski ER, Hows JM, Goldman JM, Batchelor JR. Pretransfused patients with severe aplastic anaemia exhibit high numbers of cytotoxic T lymphocyte precursors probably directed at non-HLA antigens. British journal of haematology. 1990 Nov;76(3):401–5. doi: 10.1111/j.1365-2141.1990.tb06375.x. PubMed PMID: 2261349. [DOI] [PubMed] [Google Scholar]
- 156.Gajewski JL, Johnson VV, Sandler SG, Sayegh A, Klumpp TR. A review of transfusion practice before, during, and after hematopoietic progenitor cell transplantation. Blood. 2008 Oct 15;112(8):3036–47. doi: 10.1182/blood-2007-10-118372. PubMed PMID: 18583566. Pubmed Central PMCID: 2569162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dzik WH. Leukoreduction of blood components. Current opinion in hematology. 2002 Nov;9(6):521–6. doi: 10.1097/00062752-200211000-00010. PubMed PMID: 12394176. [DOI] [PubMed] [Google Scholar]
- 158.Anderson KC, Weinstein HJ. Transfusion-associated graft-versus-host disease. The New England journal of medicine. 1990 Aug 2;323(5):315–21. doi: 10.1056/NEJM199008023230506. PubMed PMID: 2248640. [DOI] [PubMed] [Google Scholar]
- 159.Fast LD. Developments in the prevention of transfusion-associated graft-versus-host disease. British journal of haematology. 2012 Sep;158(5):563–8. doi: 10.1111/j.1365-2141.2012.09197.x. PubMed PMID: 22734439. [DOI] [PubMed] [Google Scholar]
- 160.Marsh JC, Ganser A, Stadler M. Hematopoietic growth factors in the treatment of acquired bone marrow failure states. Seminars in hematology. 2007 Jul;44(3):138–47. doi: 10.1053/j.seminhematol.2007.04.010. PubMed PMID: 17631178. [DOI] [PubMed] [Google Scholar]
- 161.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997 Feb 1;89(3):739–61. PubMed PMID: 9028304. [PubMed] [Google Scholar]
- 162.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000 Feb 15;95(4):1229–36. PubMed PMID: 10666195. [PubMed] [Google Scholar]
- 163.Vichinsky E. Oral iron chelators and the treatment of iron overload in pediatric patients with chronic anemia. Pediatrics. 2008 Jun;121(6):1253–6. doi: 10.1542/peds.2007-1824. PubMed PMID: 18519495. [DOI] [PubMed] [Google Scholar]
- 164.Kolnagou A, Economides C, Eracleous E, Kontoghiorghes GJ. Low serum ferritin levels are misleading for detecting cardiac iron overload and increase the risk of cardiomyopathy in thalassemia patients. The importance of cardiac iron overload monitoring using magnetic resonance imaging T2 and T2*. Hemoglobin. 2006;30(2):219–27. doi: 10.1080/03630260600642542. PubMed PMID: 16798647. [DOI] [PubMed] [Google Scholar]
- 165.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. The New England journal of medicine. 1994 Sep 1;331(9):567–73. doi: 10.1056/NEJM199409013310902. PubMed PMID: 8047080. [DOI] [PubMed] [Google Scholar]
- 166.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005 Aug 15;106(4):1460–5. doi: 10.1182/blood-2004-10-3982. PubMed PMID: 15860670. Pubmed Central PMCID: 1895207. [DOI] [PMC free article] [PubMed] [Google Scholar]