Abstract
Developmentally-tailored diabetes self-care education and support are integral parts of contemporary multidisciplinary T1D care. The patient with T1D must have the support of the family and the diabetes team to maintain the rigors of diabetes management, but the specific roles of patients and families with regard to daily diabetes tasks change considerably throughout the developmental span of early childhood, middle childhood/school-age years, and adolescence. This review provides a framework of key normative developmental issues for each of these developmental stages. Within this context, ideal family diabetes management is reviewed within each developmental stage and anticipated challenges that can arise during these stages and that can adversely impact diabetes management are presented. This paper also summarizes empiric evidence for specific intervention and care strategies to support optimal diabetes management across these stages in order to maximize opportunities for a successful transfer of diabetes management tasks from parents to maturing youth. Finally, the review provides an emphasis on approaches to promote family teamwork and adolescent diabetes self-care adherence as well as opportunities to use novel technology platforms as a means to support optimal diabetes management.
Keywords: Type 1 diabetes, pediatrics, intensive therapy, family teamwork, adolescence, transition
Graphical Abstract
Introduction
The management of type 1 diabetes (T1D) has evolved substantially over the last two and a half decades following publication of the Diabetes Control and Complications Trial (DCCT) (1;2). Since the DCCT, intensive insulin therapy has become the standard of care in T1D with the goal of optimizing blood glucose and hemoglobin A1c (A1c) levels as soon as possible following the diagnosis in order to prevent the development and progression of microvascular and macrovascular complications of diabetes (3–5). In the last two decades, there has been a burgeoning of new therapeutics, such as insulin pumps and continuous glucose monitors (CGMs), to assist in the management of T1D and the implementation of intensive insulin therapy (6;7).
Despite the current era of extraordinary advances in diabetes therapeutics and technologies, childhood management of T1D has remained exceptionally challenging. None of the new therapeutic advances are automated and thus have increased the burden of care associated with treatment of childhood T1D for both patients and families across the age range of childhood and adolescence. For example, studies have shown that it is difficult for pediatric patients to sustain use of CGM, likely due to these additional efforts and burdens to self-care (8–10). Therefore, while these new developments and technologies have a great deal of potential to improve diabetes outcomes, glycemic control remains suboptimal and above the recommended targets for most patients (11) and even in first world countries, only about 1 out of 4 youth with T1D succeeds in reaching the A1c target level of <7.5% (12;13). This is likely due to the ongoing requirement for self-care behaviors related to counting carbohydrates, checking blood glucose levels, delivering insulin in a timely manner, and attending to the effects of exercise, illnesses, and stress.
The roles of the child and family in diabetes management are dynamic; in order to provide optimal care, pediatric diabetes providers must understand the arc of changing patient and family roles over the course of the developmental span. The normal developmental tasks of childhood and adolescence call for the acquisition of slowly increasing levels of independence across many aspects of personal decision making and general self-care (12). However, the premature transfer of diabetes management tasks to the child with T1D is now recognized as a factor that leads to poor glycemic control (14).
The division of diabetes management roles within the family is often directed by the multidisciplinary diabetes care team, which provides ongoing education and support for the youth with T1D and the family. Team members provide anticipatory guidance related to the roles of the patient and the family, especially with respect to the usual developmental transitions and times when the youth is apart from the family, as experienced by all pediatric patients with T1D during childhood and adolescence. Such transitions include entry into preschool or kindergarten for a toddler, or the start of middle school or high school for a young teen. As noted, during these various developmental stages and transitions, there is a need to ensure navigation of diabetes management tasks to avoid non-adherence, uncontrolled diabetes, rising A1c levels, and risk of acute and chronic diabetes complications.
This review will describe the normal developmental stages of early childhood, middle childhood/school-age years, and adolescence, highlighting ideal family diabetes management as well as key developmental challenges that may impact diabetes management. This paper further summarizes empiric evidence for specific intervention and care strategies across the developmental span, with an emphasis on interventions that promote family teamwork and adolescent diabetes self-care adherence as well as novel technology platforms to support optimal diabetes management.
Developmental Issues across Childhood and Adolescence
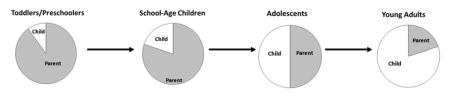
During physical and psychological growth, youth with T1D also experience growth in their ability to manage their diabetes. For example, while older elementary youth are developing skills in various areas and learning to interact within their peer group, they are also learning how to manage their diabetes (with adult help) in various situations, including the occasional situation when they are able to make independent diabetes treatment decisions. Further, managing insulin for the pediatric patient requires particular attention to the ever-changing insulin needs of the toddler and school age child due to ongoing physical growth, which is compounded during adolescence because of pubertal growth and development (15). Over time, management of diabetes moves from being done primarily by parents and caregivers, to a shared responsibility between youth and caregivers, to the older teen holding the majority of the responsibility (see graphical abstract and Table 1 for ideal division of diabetes management roles across developmental stages).
Table 1.
Ideal division of diabetes management roles across developmental stages
| Child age | Patient responsibility | Parent responsibility |
|---|---|---|
| Infant/Toddler (ages 0–2) | Cooperation | Total diabetes management |
| Preschool (ages 3–5) | Interact with parents around checking BG; cooperating with BG checks and treatment of low BGs | Total diabetes management with rare responsibility given to the child with parental supervision (e.g. selecting finger to check blood glucose level) |
| School-age (6–12) | Begin to understand and communicate symptoms of high and low BGs; begin to interpret BGs, start to count carbs, carry supplies | Most responsibilities with parents/adults with more responsibility given to child with parental supervision; child developing more autonomy (e.g. around eating, checking blood glucose for exercise) |
| Early adolescence (13–14) | Perform majority of daily diabetes tasks with supervision; check in with parents around diabetes management; begin to interact with healthcare providers on own | Parents provide more oversight than perform actual tasks; parents overseeing big picture management but share decision making with the teen |
| Late adolescence (15–18) | Ongoing reinforcement of self-care skills; integrating self-care with social and emotional development; routine diabetes foot care, eye exams; understanding need for future care and screening for complications | Supervision of tasks as needed; youth mostly autonomous but should feel able to seek support and help from others, especially parents |
Historically there were no guidelines for family division of diabetes management and what tasks were appropriate at different ages and stages of development. In 1986, Ingersoll et al. (16) found that as youth grew older, parents assumed less responsibility for diabetes tasks, but when parental responsibility decreased, specifically in adjusting insulin, youth did not increase their responsibility within this area and that youth who took more control over insulin adjustments were at “advanced levels of cognitive maturity and had a stronger personal sense of control over diabetes.” This research indicates that parents and providers need to be cognizant of what youth are actually doing in terms of diabetes management. Wysocki et al conducted multicenter surveys of parental and professional estimates of self-care independence of youth with T1D (17). For school-age children, parents reported earlier mastery (compared to professionals’ assessment) for skills involving rote motor action (e.g., fingerstick blood glucose resting) or skills with immediate consequences (e.g., preventing or treating hypoglycemia). In contrast, parents of adolescents reported consistently lower levels of adolescent self-care competence for critical skills involving reliance on planning, anticipation and self-regulation (e.g., preventing hyperglycemia or adjusting insulin doses). Recent research (18;19) indicates that over time, parental involvement in diabetes care declines, but that this varies between families. In addition, shared responsibility for diabetes tasks has been found to be associated with good metabolic control and self-care behavior (20). These results highlight the importance of ongoing, repeated definition of self-care responsibilities by the family and the diabetes care team.
Because treatment adherence is closely associated with better glycemic control (21), and in turn, better glycemic control reduces the risk for chronic diabetes complications (2;22), interventions to support adherence to T1D management during all age ranges are of critical importance.
Early Childhood (Ages 0–5)
Normative developmental issues
This is a period of rapid growth in the brain structures, in language development, and in both fine and gross motor skills. With an increase in gross motor skills, children become more active and lose their baby fat. It is also a time when many children are picky eaters. In early childhood, senses improve, but short attention spans are the norm and youth are beginning to develop their mathematical and literacy skills. During this important developmental period, children begin to develop friendships, which is fostered by their rapid language development (23;24).
Ideal family diabetes management
In early childhood, T1D should be managed mainly by the parents, as young children are not cognitively or physically ready to execute the complicated tasks required for diabetes management (12). Parents are encouraged to take on management completely and to let children help with tasks when they are interested. For example, a six year old child may have the physical dexterity to check her blood sugar and give herself an injection; however, she likely does not have the cognitive ability to understand the math necessary for carbohydrate counting and insulin calculation, nor the emotional maturity to sustain daily diabetes treatment.
Developmental challenges for diabetes management
Care recommendations for this age group focus on parental management of T1D and providing support for parents as they cope with this demanding diagnosis, as parents are responsible for their child’s diabetes care (12;25). In addition, it is important to continue to foster the trusting relationship between youth and caregiver around T1D management, as young children need to feel like they are safe and secure within the context of their diabetes care. With young children, there is focus on identifying, preventing, and treating hypoglycemia, as young children often have difficulty identifying hypoglycemia and avoiding extreme swings in blood glucose. As very young children tend to be variable eaters and have unpredictable physical activity, it is important for care providers and medical providers and take this into account with diabetes treatment plans and to focus on consistency within the home environment (26;27).
Childhood is also fraught with the common occurrence of acute pediatric illnesses that make diabetes management additionally challenging, whether from a sick day that brings risk of hyperglycemia and ketosis or from a gastrointestinal illness with potential for hypoglycemia. Indeed, it is not unusual for the healthy child with (or without) T1D to experience 7–10 acute illnesses a year, thereby playing havoc with routine insulin dosing and adding stress to already burdened families around diabetes management.
Intervention and care strategies
Treatment adherence in young children is an area of concern and in this age group interventions are often focused on the parents, as they are responsible for diabetes management. One example of this is a pilot study implemented by Monaghan et al. (28). The authors created and tested a telephone-based support intervention for parents of young children, ages 2–5, with T1D. The intervention included cognitive behavioral and problem-solving strategies for coping with parenting stress, managing children’s behavior, and seeking social support. Parents in the intervention reported decreased stress and increased social support and rated the program as favorable. Other interventions focus on mealtimes. Patton et al. (29) created an intervention to decrease stress at mealtimes in families of children ages 2–6 with T1D. In a pilot study, they found that a six session group intervention comprised of discussion of topics to make eating less stressful such as behavioral management, healthy eating, and insulin management, was associated with decreased mean daily glucose. In this population of young children with T1D, parent-based interventions that provide support and concrete problem solving strategies appear to be beneficial to address the unique developmental challenges of this age range.
Middle childhood/school age (ages 6–12)
Normative developmental issues
Children continue to grow at a regular pace during middle childhood, but development during this period is less than that of preschool and adolescence. Fine and gross motor skills continue to develop, with fine motor skills at adult levels by the end of this developmental period. In addition, rules become more important in this age group and children become more independent. Understanding and use of language improves during this period, which aids in improving conversations among peers. Children also learn to read with the continued growth in cognitive development; however children remain concrete thinkers. During this time period, peer relationships become more important and youth start organizing into peer groups (23;24).
Ideal family diabetes management
While it is important for children to learn the basics of diabetes management and for them to be able to treat hypoglycemia and talk about carbohydrate counting with their caregivers, it is equally important for parents to remain involved in diabetes management as much as possible, as studies have shown that greater parental involvement is associated with better outcomes (20;30). Care strategies for this age group include a continued focus on preventing hypoglycemia and focusing on unpredictable food intake and physical activity. It is also imperative to provide youth with positive reinforcement for caring for their diabetes and educating other caregivers. Because autonomy grows during this developmental period, making sure diabetes treatment plans are flexible and appropriate for an individual child’s life is key, as is maintaining optimal quality of life (12).
Developmental challenges for diabetes management
During this developmental stage, children start developing more autonomy in all areas of their life. With this increasing independence comes more time away from parents and often more desire for autonomy in diabetes management. In 1990, Anderson et al. (31) developed the first Diabetes Family Responsibility Questionnaire (DFRQ), to assess sharing of diabetes-related management tasks, as well as discrepancies between youth and parent report. They found that disagreement between youth and mothers was associated with higher A1c. Following this study, Wysocki et al. (32) found that youth who were caring for diabetes with more autonomy displayed less treatment adherence and worse glycemic control than those youth who had more parental involvement in diabetes care. In 1997, Anderson et al. (30) found that families with greater parental involvement in blood glucose monitoring showed greater adherence to checking blood glucose and, in turn, found that frequency of blood glucose monitoring was significantly associated with glycemic control.
Quality of parental support around diabetes management is important with respect to glycemic control, as parental warmth has been associated with greater diabetes adherence (33;34). In terms of perceptions of diabetes support at school, children with T1D cite improved flexibility by nurse and teachers, care plans that are individualized, and help managing hypoglycemia in school as factors that would help them feel more supported at school and better manage their diabetes (35).
Intervention and care strategies
Although emerging knowledge and skills as youth grow up may allow for youth to assume a greater role in diabetes management, there is ample evidence supporting a family teamwork approach in the pre-adolescent/school age years, suggesting that this might be an ideal time to lay the foundation for parents to continue to play a significant role in diabetes management and establish good self-care habits in preparation for adolescence.
Anderson et al. conducted the first study evaluating an office-based intervention for family teamwork in diabetes management (36). Families were randomized to either the teamwork intervention or one of two comparison groups. The teamwork intervention was comprised of four behavioral sessions that were family-based and consisted of material focusing on the importance of responsibility sharing for diabetes management and reducing conflict and was conducted over 12 months. Those in the teamwork intervention showed no deterioration in parental involvement in BG monitoring and insulin administration at 12 months, compared to deterioration in the comparison groups. In addition, families in the teamwork intervention reported less diabetes-specific conflict and youth in the teamwork group were significantly more likely to improve their glycemic control than the comparison groups.
Since this original study, there have been many others examining ways to leverage family involvement in diabetes management with the goal of increasing treatment adherence and positive outcomes. Studies have found family involvement in diabetes care to be associated with improvements in treatment adherence and glycemic control (30;37;38), quality of life (30;39), coping skills(39;40), and family communication (41;42).
The concept of family teamwork in T1D can also be extended to teamwork in general, including all care providers in the youth’s life. Interventions are also being developed for these relationships and often utilize new technology. For example, Izquierdo et al. (43) developed an intervention that consisted of video conferencing between clinics and school nurses consisting of quarterly medical visits and contact between clinic providers and school nurses as needed. The intervention included monthly virtual meetings between the patient, school nurse, and clinic providers, in addition to usual care. After six months, the intervention group showed a significant decrease in A1c relative to the usual care group and demonstrated significant improvement in several pediatric diabetes quality of life subscales, as well as fewer hospitalizations and emergency department visits.
Adolescence (ages 13–18)
Normative developmental issues
Adolescence is the transitional period between the start of puberty and adulthood in human development and marks a time of great change in both physical and psychosocial realms. Adolescents experience rapid physical growth and sexual maturation. An increasing awareness of sexuality and greater preoccupation with body image are psychosocial hallmarks of the adolescent stage. Identity formation is a central developmental challenge for adolescents. As adolescence progresses, individuals acquire skills needed to carry out adult roles and develop expanded capacity for abstract reasoning. Adolescence is also a period of risk during which social contexts and peer relationships exert powerful influences (23;44;45).
Ideal family diabetes management
For adolescents with T1D, family support and involvement and decreased diabetes-related family conflict are associated with improved adherence to adolescent diabetes self-care (30;46;47). While diabetes care responsibility is transferred to adolescent patients, parents should remain involved in positive, supportive roles. Parent and adolescent roles in diabetes management may need to be renegotiated in order for roles to be acceptable to both parties (12).
Self-efficacy, or the belief that one can carry out specific behaviors in specified situations, is a critically important factor for optimal diabetes management as adolescents acquire new self-care roles in the face of developmental and social challenges (48). Adolescents with strong self-efficacy should be better equipped to overcome barriers to diabetes self-care (48), as evidenced by data demonstrating correlations with improved outcomes such as increased self-care and glycemic control (48–50). In a recent study (19), it was found that youth who reported a decrease in parental responsibility and also an increase in self-efficacy were able to maintain treatment adherence over time, compared to those who did not report increases in self-efficacy. In addition, for those youth with lower self-efficacy, better glycemic control is associated with greater parental responsibility for carel (51). Therefore, self-efficacy is an area that is important for medical providers to consider when working with adolescents with T1D.
Developmental challenges for diabetes management
Glycemic control deteriorates during adolescence in association with the insulin resistance of pubertal growth and development and as teens naturally become preoccupied with academic, athletic, social, and other natural distractions (15;47;52–54). In an analysis of 7,303 adolescents with T1D (ages 13–19 years) in the T1D Exchange Clinic Registry, only 21% had A1c values in the target range of less than 7.5% (11;53). This deterioration in glycemic control is in part related to the physiological changes that lead to greater insulin resistance in puberty (15).
The shift in diabetes self-care responsibility from parent to child is a major developmental challenge in adolescent diabetes care. In addition to physiological changes and shifting self-care responsibilities, the adolescent period poses additional challenges for diabetes care adherence. A recent study by Hilliard et al. analyzed predictors of deterioration in diabetes management and control in 150 adolescents with T1D over an 18–24 month period (55). Approximately two-thirds of subjects did not meet American Diabetes Association targets for blood glucose self-monitoring (≥4 times daily) or A1c (<7.5%). A number of non-modifiable and modifiable factors predicted poor diabetes management and control. Non-modifiable factors, which may alert providers to increased risk and allow for preventative efforts included older youth age, ethnic minority status, injection-based insulin regimens, and unmarried caregiver status. The modifiable psychological factors included general and diabetes-specific distress and diabetes-specific family conflict; these results reaffirm the importance of incorporating mental health assessment and treatment into adolescent diabetes care.
Increasing concerns about peer relationships and social context are hallmarks of adolescence. Palladino et al. (56) completed a review of the literature on peer influence in youth with T1D and found mixed evidence regarding peer influences. For example, some data show a positive impact of peer relationships on diabetes care behaviors (57) and other data describe little impact (58).
A recent study by Borus et al. highlights the importance of considering social context and its potential effect on adherence behaviors in adolescents with T1D. Over a 14-day period, adolescents with T1D, age 14–18 years, carried handheld devices that prompted them to report social context variables associated with self-monitoring of blood glucose throughout the day. Interestingly, the odds of checking blood glucose were higher when participants expressed a strong desire to blend in with peers, which may be related to desire for avoidance of embarrassing situations (e.g., hypoglycemia). In contrast, a strong desire to impress others was associated with decreased likelihood of checking blood glucose. Such results suggest areas in which providers might help adolescents anticipate and problem-solve relationships between social situations and diabetes self-care adherence (59).
Intervention and care strategies
Different versions of systems-based family therapy have been used in families of adolescents with diabetes with the goal of improving glycemic control. Wysocki et al. (60) conducted a 6-month behavioral intervention using Behavioral Family Systems Therapy for Diabetes (BFST-D) compared to two comparison groups (educational support; standard care). Those in the BFST-D group showed greater treatment adherence at follow-up compared to the comparison groups. Ellis et al. (61) conducted a 6-month behavioral intervention using multisystemic family therapy (MST) in youth with poor glycemic control, compared to a group that received telephone support. Adolescents in the MST group showed a significant reduction in A1c at the post-treatment time point and also after an additional 12 months. These interventions highlight the importance of including the family and other systems in which children are embedded in interventions aimed at improving diabetes outcomes.
Related lines of research have utilized cost-effective interventions to improve follow-up, including a non-medically trained “Care Ambassador” who provides support to families between medical visits. Studies have shown that Care Ambassadors compared to standard care result in improved results including consistent outpatient follow-up, significant reduction in hospitalizations and emergency department visits, decrease in A1c, and maintained or increased parental involvement (62–64).
In addition to family-based interventions, motivational interviewing has also shown potential for the support of adherence to self-care in adolescents with T1D. Motivational interviewing is a collaborative communication style that is designed to strengthen personal motivation for change and commitment to specific goals. In the United Kingdom, Channon et al. (65) conducted a randomized controlled trial examining the impact of motivational interviewing during clinic visits in 66 adolescents with T1D, ages 14–17 years, over 12 months. Compared to controls, patients receiving the motivational interviewing had lower mean A1c, and this improvement was still maintained a year after the intervention ended. In addition, patients in the motivational interviewing group demonstrated improved satisfaction, well-being, and belief that diabetes self-care mattered, relative to controls.
Mobile health (mHealth) technologies hold promise to support youth adherence to T1D management and glycemic control while maintaining caregiver involvement in T1D care. As of January 2014, 90% of American adults owned a cellphone, and 58% owned a smartphone (66). Many mobile users are adolescents, and text messaging is the principal mode of communication for this population. Given these trends, text messaging interventions may serve as a valuable method to amplify the benefits of clinic-based diabetes care without significantly increasing health care provider resources.
Text messaging for T1D management has been studied in the pediatric population and has been shown to improve diabetes self-efficacy and treatment adherence (67). In Scotland, Franklin et al. (68) conducted a randomized controlled trial of Sweet Talk, a text-messaging support system designed to enhance self-efficacy and improve glycemic control in youth with T1D. Sweet Talk consisted of automatically delivered personalized daily text messages, which reinforced goals set in the clinic. While the study did not demonstrate a consistent improvement in A1c, Sweet Talk was associated with significant improvements in diabetes self-efficacy. Moreover, >80% of patients felt that Sweet Talk had improved their self-management adherence, and 90% wanted to continue receiving text messages. Along similar lines, Markowitz et al. (69) studied an mHealth intervention using text messaging incorporating general healthy lifestyle messages, with the goal of enhancing goal-setting among adolescents and young adults with T1D. Over a one-month pilot intervention period, self-efficacy and glycemic control measures did not change, but the text messaging intervention was acceptable to patients and was rated highly, again suggesting the potential of text messaging interventions to increase motivation for change in these age groups.
Regarding internet interventions, Grey et al. (70) evaluated the impact of internet psycho-educational programs on glycemic control and quality of life in 320 youth with T1D, ages 11–14 years. The Coping Skills Training internet program (TeenCope) was comprised of content aimed at increasing skills such as communication, social problem solving, stress management, and conflict resolution and the internet diabetes health education program (Managing Diabetes) was comprised of content aimed toward diabetes education and problem solving. Each was delivered weekly over 5 weeks; patients were then invited to cross over to the other internet program after 12 months. After 18 months, youth who completed both programs had lower A1c, higher quality of life, self-efficacy, and social acceptance, and lower family conflict. These results suggest that youth require diabetes management education as well as behavioral coping interventions (rather than one or the other), and that the internet is an effective mode of intervention delivery.
In summary, review of successful adherence-promoting interventions in adolescents with T1D reveals a number of unifying themes, including optimizing family functioning around diabetes care and increasing family teamwork, as well as fostering adolescent motivation for change, evaluating social context, and harnessing the power of internet and mobile technology in reaching this population. Drawing on the available evidence, a recent position statement of the American Diabetes Association (12) outlined key priorities in the clinical management of adolescents with T1D. These include supporting the development of teen self-management, preventing and addressing family conflict related to T1D. In addition, given the increased risk of poor glycemic control in adolescents with depression and disordered eating behaviors (71–73), the guidelines also underscore the need to routinely monitor adolescents with T1D for signs of comorbid mental illness (12) and provide appropriate treatment or referrals.
Emerging Adulthood
The young adult developmental stage from the late teens through the twenties has been defined as “emerging adulthood,” a period characterized by competing educational, social, occupational, and economic demands (74). This emerging adult period presents special challenges for patients with T1D, as described in detail in Monaghan et al. (this issue).
Conclusion
Management of T1D in childhood and adolescence is demanding for the patient and the family, and impacts the entire community that interacts with the growing and developing youth. The roles of the child and family members are constantly changing and evolving throughout the child’s development, as the child traverses from childhood, through the stage of pubertal growth and development, and onto older adolescence and emerging adulthood. Throughout childhood and the period of rapid physical and emotional changes of adolescence, there are natural transitions related to who manages and who performs fundamental tasks like bathing, homework, household chores, etc. For the child with T1D, there are parallel needs to assess and shift the division of roles and responsibilities for youths’ diabetes management across developmental stages.
Throughout all the developmental stages, there are numerous opportunities to capitalize on the modern diabetes advances of the 21st century to enhance diabetes management and self-care in order to optimize glycemic control. A recent publication from the DCCT and Epidemiology of Diabetes Complications (EDIC) highlights the importance of intensive insulin therapy and optimal glycemic control to reduce the threat to premature mortality for patients with T1D (75). This requires the multidisciplinary team working with the child and family as well as with the school and community to help in the care of the child with diabetes. The team not only provides education and guidance in the proper use of diabetes technologies but is fundamental to ensuring the timely delivery of diabetes education and support to the growing youth across developmental stages as roles change for both the patient with T1D and the family members. There is need for patience and renegotiation in the changing roles of youth with T1D. Together, it is important to strive to achieve the A1c goals to prevent complications, preserve health, and protect the futures of pediatric patients with T1D across childhood. With ongoing efforts focused on family teamwork, goal setting, and leveraging emerging technologies, it will be increasingly possible for many youth and families to achieve these goals and maintain good quality of life.
Acknowledgments
JM, KG, and LL researched and wrote this paper.
Footnotes
Conflict of Interest:
Dr. Markowitz and Dr. Garvey have no have no conflicts of interest to disclose. Dr. Laffel has no conflicts of interest associated with this report. Dr. Laffel reports that she serves as a consultant for Johnson & Johnson, Eli Lilly, Sanofi, Bristol-Myers Squibb, Astra-Zeneca, Menarini, Lifescan/Animas, Roche, Oshadi, Bayer Healthcare, NovoNordisk, DexCom, and Boehringer Ingelheim. This work was partially supported by NIH/NIDDK 1K23DK092335; 1K23DK102655; K12DK094721 and P30DK036836
Contributor Information
Jessica T. Markowitz, Email: jessica.markowitz@joslin.harvard.edu, Pediatric, Adolescent, & Youth Adult Section, Joslin Diabetes Center, Boston, MA, 617-309-4723 (p); 617-309-2451 (f)
Katharine C. Garvey, Division of Endocrinology, Children’s Hospital Boston.
Lori M.B. Laffel, Pediatric, Adolescent, & Youth Adult Section, Genetics and Epidemiology Section, Joslin Diabetes Center, Boston, MA.
Reference List
- 1.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993 Jul 29;329(5):304–9. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994 Aug;125(2):177–88. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 3.The Writing Team for the Diabetes Control and Complications Trial-Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002 May 15;287(19):2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003 Jun 5;348(23):2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood JR, Laffel LMB. Technology and intensive management in youth with type 1 diabetes: State of the art. Curr Diab Rep. 2007 Apr;7(2):104–13. doi: 10.1007/s11892-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SN, Wolfsdorf JI. Contemporary management of patients with type 1 diabetes. Endocrinol Metab Clin North Am. 2010 Sep;39(3):573–93. doi: 10.1016/j.ecl.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Lane JE, Shivers JP, Zisser H. Continuous glucose monitors: current status and future developments. Curr Opin Endocrinol Diabetes Obes. 2013 Apr;20(2):106–11. doi: 10.1097/MED.0b013e32835edb9d. [DOI] [PubMed] [Google Scholar]
- 9.Chase HP, Beck RW, Xing D, Tamborlane WV, Coffey J, Fox LA, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010 Jul;12(7):507–15. doi: 10.1089/dia.2010.0021. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz JT, Pratt K, Aggarwal J, Volkening LK, Laffel LM. Psychosocial correlates of continuous glucose monitoring use in youth and adults with type 1 diabetes and parents of youth. Diabetes Technol Ther. 2012 Jun;14(6):523–6. doi: 10.1089/dia.2011.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, Dubose SN, Hall CA. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012 Dec;97(12):4383–9. doi: 10.1210/jc.2012-1561. jc.2012-1561 [pii]; [DOI] [PubMed] [Google Scholar]
- 12.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014 Jul;37(7):2034–54. doi: 10.2337/dc14-1140. dc14-1140 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rewers MJ, Pillay K, de BC, Craig ME, Hanas R, Acerini CL, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014 Sep;15(Suppl 20):102–14. doi: 10.1111/pedi.12190. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BJ. Behavioral research in pediatric diabetes: putting the evidence to work for advocacy and education. Pediatr Diabetes. 2012 Feb;13(1):77–80. doi: 10.1111/j.1399-5448.2011.00778.x. [DOI] [PubMed] [Google Scholar]
- 15.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986 Jul 24;315(4):215–9. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 16.Ingersoll GM, Orr DP, Herrold AJ, Golden MP. Cognitive maturity and self-management among adolescents with insulin- dependent diabetes mellitus. J Pediatr. 1986 Apr;108(4):620–3. doi: 10.1016/s0022-3476(86)80852-6. [DOI] [PubMed] [Google Scholar]
- 17.Wysocki T, Meinhold PA, Abrams KC, Barnard MU, Clarke WL, Bellando BJ, et al. Parental and professional estimates of self-care independence of children and adolescents with IDDM. Diabetes Care. 1992 Jan;15(1):43–52. doi: 10.2337/diacare.15.1.43. [DOI] [PubMed] [Google Scholar]
- 18.King PS, Berg CA, Butner J, Butler JM, Wiebe DJ. Longitudinal trajectories of parental involvement in Type 1 diabetes and adolescents’ adherence. Health Psychol. 2014 May;33(5):424–32. doi: 10.1037/a0032804. 2013-21805-001 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiebe DJ, Chow CM, Palmer DL, Butner J, Butler JM, Osborn P, et al. Developmental processes associated with longitudinal declines in parental responsibility and adherence to type 1 diabetes management across adolescence. J Pediatr Psychol. 2014 Jun;39(5):532–41. doi: 10.1093/jpepsy/jsu006. jsu006 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: links to health outcomes. J Pediatr Psychol. 2008 Jun;33(5):497–508. doi: 10.1093/jpepsy/jsm081. jsm081 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009 Dec;124(6):e1171–e1179. doi: 10.1542/peds.2009-0207. [DOI] [PubMed] [Google Scholar]
- 22.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 23.Berk LE. Development Through the Lifespan. 2. 2001. [Google Scholar]
- 24.Human Development in Multicultural Contexts. Upper Saddle River, NJ: Prentic Hall; 2002. [Google Scholar]
- 25.Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep. 2014;14(9):520. doi: 10.1007/s11892-014-0520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton SR, Dolan LM, Powers SW. Parent report of mealtime behaviors in young children with type 1 diabetes mellitus: implications for better assessment of dietary adherence problems in the clinic. J Dev Behav Pediatr. 2006 Jun;27(3):202–8. doi: 10.1097/00004703-200606000-00004. 00004703-200606000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Monaghan M, Herbert LJ, Wang J, Holmes C, Cogen FR, Streisand R. Mealtime behavior and diabetes-specific parent functioning in young children with type 1 diabetes. Health Psychology. 2015 doi: 10.1037/hea0000204. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaghan M, Hilliard ME, Cogen FR, Streisand R. Supporting parents of very young children with type 1 diabetes: results from a pilot study. Patient Educ Couns. 2011 Feb;82(2):271–4. doi: 10.1016/j.pec.2010.04.007. S0738-3991(10)00177-1 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patton SR, Odar C, Midyett LK, Clements MA. Pilot study results for a novel behavior plus nutrition intervention for caregivers of young children with type 1 diabetes. J Nutr Educ Behav. 2014 Sep;46(5):429–33. doi: 10.1016/j.jneb.2013.11.007. S1499-4046(13)00715-X [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1997 Feb;130(2):257–65. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 31.Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. 1990 Aug;15(4):477–92. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- 32.Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy. Association with diabetes outcomes. Diabetes Care. 1996 Feb;19(2):119–25. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]
- 33.Faulkner MS, Chang LI. Family influence on self-care, quality of life, and metabolic control in school-age children and adolescents with type 1 diabetes. J Pediatr Nurs. 2007 Feb;22(1):59–68. doi: 10.1016/j.pedn.2006.02.008. S0882-5963(06)00136-9 [pii]; [DOI] [PubMed] [Google Scholar]
- 34.Davis CL, Delamater AM, Shaw KH, La Greca AM, Eidson MS, Perez-Rodriguez JE, et al. Parenting styles, regimen adherence, and glycemic control in 4- to 10-year-old children with diabetes. J Pediatr Psychol. 2001 Mar;26(2):123–9. doi: 10.1093/jpepsy/26.2.123. [DOI] [PubMed] [Google Scholar]
- 35.Nabors L, Lehmkuhl H, Christos N, Andreone TL. Children with diabetes: perceptions of supports for self-management at school. Journal of School Health. 2003;73(6):216–21. doi: 10.1111/j.1746-1561.2003.tb06563.x. [DOI] [PubMed] [Google Scholar]
- 36.Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999 May;22(5):713–21. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 37.Delamater AM, Bubb J, Davis SG, Smith JA, Schmidt L, White NH, et al. Randomized prospective study of self-management training with newly diagnosed diabetic children. Diabetes Care. 1990 May;13(5):492–8. doi: 10.2337/diacare.13.5.492. [DOI] [PubMed] [Google Scholar]
- 38.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003 Apr;142(4):409–16. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 39.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr. 2000 Jul;137(1):107–13. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 40.Greco P, Shroff Pendley J, McDonell K, Reeves G. A peer group intervention for adolescents with type 1 diabetes and their best friends. J Pediatr Psychol. 2001;26:485–90. doi: 10.1093/jpepsy/26.8.485. [DOI] [PubMed] [Google Scholar]
- 41.Wysocki T, Miller KM, Harvey LM, Taylor A, Elder-Danda C, McDonell K, et al. Behavior therapy for families of adolescents with diabetes: Effects on directly observed family interactions. Behav Ther. 2000;30:507–25. [Google Scholar]
- 42.Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, et al. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. J Pediatr Psychol. 2000 Jan;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- 43.Izquierdo R, Morin PC, Bratt K, Moreau Z, Meyer S, Ploutz-Snyder R, et al. School-centered telemedicine for children with type 1 diabetes mellitus. J Pediatr. 2009 Sep;155(3):374–9. doi: 10.1016/j.jpeds.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Adolescent Development. 2015. Feb 4, Ref Type: Online Source. [Google Scholar]
- 45.Fisher M, Elizabeth A, Kreipe R, Rosenfeld W, editors. Textbook of Adolescent Health Care. 2011. [Google Scholar]
- 46.Wysocki T, Nansel TR, Holmbeck GN, Chen R, Laffel L, Anderson BJ, et al. Collaborative involvement of primary and secondary caregivers: associations with youths’ diabetes outcomes. J Pediatr Psychol. 2009 Sep;34(8):869–81. doi: 10.1093/jpepsy/jsn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilliard ME, Holmes CS, Chen R, Maher K, Robinson E, Streisand R. Disentangling the roles of parental monitoring and family conflict in adolescents’ management of type 1 diabetes. Health Psychol. 2013 Apr;32(4):388–96. doi: 10.1037/a0027811. 2012-10739-001 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iannotti RJ, Schneider S, Nansel TR, Haynie DL, Plotnick LP, Clark LM, et al. Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. J Dev Behav Pediatr. 2006 Apr;27(2):98–105. doi: 10.1097/00004703-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Herge WM, Streisand R, Chen R, Holmes C, Kumar A, Mackey ER. Family and youth factors associated with health beliefs and health outcomes in youth with type 1 diabetes. J Pediatr Psychol. 2012 Oct;37(9):980–9. doi: 10.1093/jpepsy/jss067. jss067 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rasbach L, Jenkins C, Laffel L. An Integrative Review of Self-efficacy Measurement Instruments in Youth With Type 1 Diabetes. Diabetes Educ. 2014 Sep 12; doi: 10.1177/0145721714550254. 0145721714550254 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer DL, Berg CA, Butler J, Fortenberry K, Murray M, Lindsay R, et al. Mothers’, fathers’, and children’s perceptions of parental diabetes responsibility in adolescence: examining the roles of age, pubertal status, and efficacy. J Pediatr Psychol. 2009 Mar;34(2):195–204. doi: 10.1093/jpepsy/jsn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010 Aug;22(4):405–11. doi: 10.1097/MOP.0b013e32833a46a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013 Jul;36(7):2035–7. doi: 10.2337/dc12-1959. dc12-1959 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hood KK, Beavers DP, Yi-Frazier J, Bell R, Dabelea D, McKeown RE, et al. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health. 2014 Oct;55(4):498–504. doi: 10.1016/j.jadohealth.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hilliard ME, Wu YP, Rausch J, Dolan LM, Hood KK. Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. J Adolesc Health. 2013 Jan;52(1):28–34. doi: 10.1016/j.jadohealth.2012.05.009. S1054-139X(12)00210-8 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palladino DK, Helgeson VS. Friends or foes? A review of peer influence on self-care and glycemic control in adolescents with type 1 diabetes. J Pediatr Psychol. 2012 Jun;37(5):591–603. doi: 10.1093/jpepsy/jss009. jss009 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skinner TC, John M, Hampson SE. Social support and personal models of diabetes as predictors of self-care and well-being: a longitudinal study of adolescents with diabetes. J Pediatr Psychol. 2000 Jun;25(4):257–67. doi: 10.1093/jpepsy/25.4.257. [DOI] [PubMed] [Google Scholar]
- 58.Helgeson VS, Reynolds KA, Escobar O, Siminerio L, Becker D. The role of friendship in the lives of male and female adolescents: does diabetes make a difference? J Adolesc Health. 2007 Jan;40(1):36–43. doi: 10.1016/j.jadohealth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Borus JS, Blood E, Volkening LK, Laffel L, Shrier LA. Momentary assessment of social context and glucose monitoring adherence in adolescents with type 1 diabetes. J Adolesc Health. 2013 May;52(5):578–83. doi: 10.1016/j.jadohealth.2012.10.003. S1054-139X(12)00401-6 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006 Oct;31(9):928–38. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 61.Ellis DA, Naar-King S, Chen X, Moltz K, Cunningham PB, Idalski-Carcone A. Multisystemic therapy compared to telephone support for youth with poorly controlled diabetes: findings from a randomized controlled trial. Ann Behav Med. 2012 Oct;44(2):207–15. doi: 10.1007/s12160-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laffel LM, Brackett J, Ho J, Anderson BJ. Changing the process of diabetes care improves metabolic outcomes and reduces hospitalizations. Qual Manag Health Care. 1998 Sep;6(4):53–62. doi: 10.1097/00019514-199806040-00006. [DOI] [PubMed] [Google Scholar]
- 63.Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LMB. Reducing acute adverse outcomes in youths with type 1 diabetes: a randomized, controlled trial. Pediatrics. 2003;112(4):914–22. doi: 10.1542/peds.112.4.914. [DOI] [PubMed] [Google Scholar]
- 64.Katz ML, Volkening LK, Butler DA, Anderson BJ, Laffel LM. Family-based psychoeducation and Care Ambassador intervention to improve glycemic control in youth with type 1 diabetes: a randomized trial. Pediatr Diabetes. 2014 Mar;15(2):142–50. doi: 10.1111/pedi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care. 2007 Jun;30(6):1390–5. doi: 10.2337/dc06-2260. [DOI] [PubMed] [Google Scholar]
- 66.Pew Research Internet Project. Cell Phone and Smartphone Ownership Demographics. 2014. Dec 23, Ref Type: Online Source. [Google Scholar]
- 67.Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS call phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009 Feb;11(2):99–106. doi: 10.1089/dia.2008.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006 Dec;23(12):1332–8. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 69.Markowitz JT, Cousineau T, Franko DL, Schultz AT, Trant M, Rodgers R, et al. Text messaging intervention for teens and young adults with diabetes. J Diabetes Sci Technol. 2014 Sep;8(5):1029–34. doi: 10.1177/1932296814540130. 1932296814540130 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grey M, Whittemore R, Jeon S, Murphy K, Faulkner MS, Delamater A. Internet psycho-education programs improve outcomes in youth with type 1 diabetes. Diabetes Care. 2013 Sep;36(9):2475–82. doi: 10.2337/dc12-2199. dc12-2199 [pii]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rydall AC, Rodin GM, Olmsted MP, Devenyi RG, Daneman D. Disordered eating behavior and microvascular complications in young women with insulin-dependent diabetes mellitus. N Engl J Med. 1997 Jun 26;336(26):1849–54. doi: 10.1056/NEJM199706263362601. [DOI] [PubMed] [Google Scholar]
- 72.Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2007 Dec;8(6):408–18. doi: 10.1111/j.1399-5448.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 73.McGrady ME, Laffel L, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with type 1 diabetes: mediational role of blood glucose monitoring. Diabetes Care. 2009 May;32(5):804–6. doi: 10.2337/dc08-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000 May;55(5):469–80. [PubMed] [Google Scholar]
- 75.Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JYC, et al. Association between seven years of intensive treatment of type 1 diabetes and long term mortality. JAMA. 2015 Jan; doi: 10.1001/jama.2014.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]


