Abstract
Interstrand cross-links in cellular DNA are highly deleterious lesions that block transcription and replication. We recently characterized two new structural types of interstrand cross-links derived from the reaction of abasic (Ap) sites with either guanine or adenine residues in duplex DNA. Interestingly, these Ap-derived cross-links are forged by chemically-reversible processes, in which the two strands of the duplex are joined by hemiaminal, imine, or aminoglycoside linkages. Therefore, understanding the stability of Ap-derived cross-links may be critical in defining the potential biological consequences of this lesion. Here we employed bacteriophage ϕ29 DNA polymerase, which can couple DNA synthesis and strand displacement, as a model system to examine whether dA-Ap cross-links can withstand DNA-processing enzymes. We first demonstrated that a chemically stable interstrand cross-link generated by hydride reduction of the dG-Ap cross-link completely blocked primer extension by ϕ29 DNA polymerase at the last unmodified nucleobase preceding cross-link. We then showed that the nominally reversible dA-Ap cross-link behaved, for all practical purposes, like an irreversible, covalent DNA-DNA cross-link. The dA-Ap cross-link completely blocked progress the ϕ29 DNA polymerase at the last unmodified base before the cross-link. This suggests that Ap-derived cross-links have the power to block various DNA-processing enzymes in the cell. In addition, our results reveal ϕ29 DNA polymerase as a tool for detecting the presence and mapping the location of interstrand cross-links (and possibly other lesions) embedded within regions of duplex DNA.
Graphical Abstract
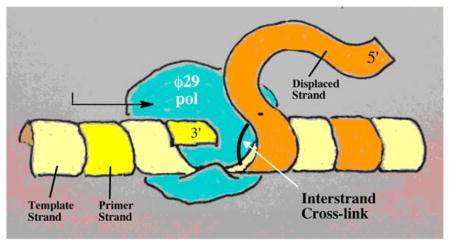
INTRODUCTION
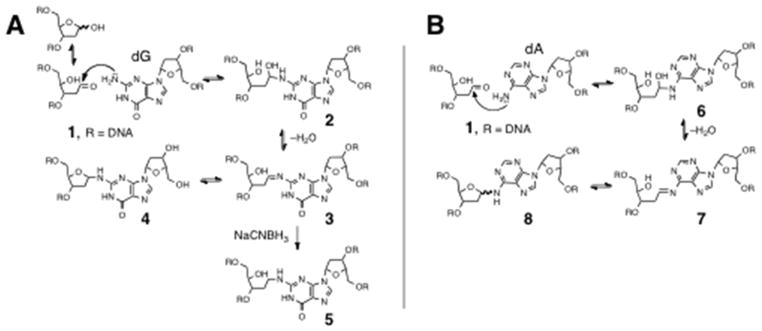
Interstrand cross-links in cellular DNA are highly deleterious because they prevent the strand separation that is necessary for cellular machinery to extract genetic information from the double helix during transcription and replication.1–3 We recently characterized two new structural types of interstrand cross-links derived from the reaction of abasic (Ap) sites with either guanine or adenine residues in duplex DNA (Scheme 1).4–8 Ap sites are abundant in cellular DNA9–12 and cross-links derived from these lesions could play an important role in spontaneous mutagenesis, neurodegeneration, and aging.13–19 Interestingly, these Ap-derived cross-links are forged by chemically-reversible processes, in which the two strands of the duplex are joined by hemiaminal, imine, or aminoglycoside linkages (Scheme 1). Therefore, understanding the stability of Ap-derived cross-links may be critical in defining the potential biological consequences of this lesion. Early work showed that these cross-links are stable to gel electrophoretic analysis.4,6–8 Furthermore, recent work has shown that, once formed in duplex DNA, the cross-links are stable for days at pH 7 and 37 °C.4,7 Nonetheless, with regard to the potential biological activity of these lesions, it may be most important to consider whether the Ap-derived cross-links can block DNA-processing enzymes that induce strand separation.
Scheme 1.
In the studies described here, we employed bacteriophage ϕ29 DNA polymerase as a model system to examine whether dA-Ap cross-links can withstand DNA-processing enzymes. ϕ29 DNA polymerase displays remarkably high processivity and strand-displacement activity.20 In fact, this polymerase is able to separate the strands of a DNA duplex and carry out DNA synthesis without assistance from helicases, clamp proteins, or other accessory factors.21 Accordingly, ϕ29 DNA polymerase has been described as a hybrid polymerase-helicase.22 We anticipated that these properties might make ϕ29 DNA polymerase a useful tool for examining whether Ap-derived cross-links can withstand enzyme-induced melting of the DNA duplex. As a test of this methodology, we first examined the action of ϕ29 DNA polymerase on substrates containing the chemically stable interstrand cross-link 5 (Scheme 1A) generated by hydride reduction of the dG-Ap cross-link.4–6 This chemically stable cross-link blocked primer extension by ϕ29 DNA polymerase at the last unmodified nucleobase preceding the cross-link. We then characterized ϕ29 DNA polymerase-mediated primer extension on substrates containing the chemically-reversible dA-Ap cross-link (8, Scheme 1B) embedded in various locations and orientations within a region of duplex DNA. We found that the dA-Ap cross-link behaves like an irreversible, covalent DNA-DNA cross-link that completely blocks the progress of the ϕ29 DNA polymerase. The results suggest that, once formed in genomic DNA, Ap-derived cross-links may have the power to block various DNA-processing enzymes and exert significant biological effects. In addition, our results establish ϕ29 DNA polymerase as a tool for determining the presence and location of interstrand cross-links (and possibly other lesions) embedded within regions of duplex DNA.
EXPERIMENTAL
Reagents
Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Uracil DNA glycosylase (UDG), ϕ29 DNA polymerase, and T7 DNA polymerase were from New England Biolabs (Ipswich, MA). A mixture of the four 2′-deoxynucleotide triphosphates (dNTP) was purchased from Promega (Madison, WI). [γ-32P]-ATP (6000 Ci/mmol) was purchased from Perkin-Elmer. C-18 Sep-Pak cartridges were purchased from Waters (Milford, MA), and BS Poly-prep columns were obtained from BioRad (Hercules, CA). Acrylamide/bis-acrylamide 19:1 (40% Solution/Electrophoresis) was purchased from Fisher Scientific (Waltham, MA), and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Representative Procedure for Preparation of Cross-linked Templates Involving a Reduced dG-Ap Cross-link or dA-Ap Cross-link
A single-stranded, uracil-containing 2′-deoxy-oligonucleotide was annealed with its complimentary strand and treated with the enzyme UDG (50 units/mL, final concentration) to generate duplexes containing an Ap sites at a defined location.23,24 The UDG enzyme was removed by phenol-chloroform extraction. The DNA was then ethanol precipitated25 and the duplexes were redissolved in either sodium acetate buffer (750 mM, pH 5.2) containing NaCNBH3 (250 mM) and incubated at 37 °C for 24 h to form the reduced dG-Ap cross-link or redissolved in HEPES buffer (50 mM, pH 7.0) containing NaCl (100 mM) and incubated at 37 °C for 120 h to form the dA-Ap cross-link. The DNA was then ethanol precipitated, resuspended in formamide loading buffer,25 loaded onto a 2 mm thick 20% denaturing polyacrylamide gel, and the gel electrophoresed for 10 h at 200 V. The DNA bands in the gel were visualized by UV shadowing, and the slow-moving band corresponding to cross-linked duplex6,8 was cut from the gel with a razor blade. The gel slice was crushed and then agitated in an elution buffer composed of aqueous NaCl (200 mM) and 1 mM EDTA (1 mM, pH 8) at 24 °C for 1 h. The gel fragments were removed by filtering through a Poly-Prep column, and the filtrate was desalted using a C18 Sep-Pak (100 mg size). The resulting solution was dried using a Speed-Vac concentrator, and the residue redissolved in HEPES buffer (50 mM, pH 7.0) containing NaCl (100 mM) and stored frozen at −20 °C prior to use.
Representative Procedure for ϕ29 DNA Polymerase Primer Extension Reactions
The primer was α-32P 5′-end-labeled using standard procedures.25 The labeled primer (100,000 cpm) was annealed to the template DNA (200 pmoles) in MOPS buffer (50 mM, pH 7.0) containing NaCl (100 mM) by incubation at 24 °C for 12 h to generate substrates for the polymerase assay. The DNA duplex was then ethanol precipitated, redissolved in an appropriate amount of concentrated (1.1x) solution of the polymerase assay buffer (see below) to give approximately 2,222 cpm/μL and aliquotted into the assays. For each primer extension assay, the polymerization reaction was started by addition of ϕ29 DNA polymerase (1 μL, 10 units) to 9 μL of the concentrated (1.1x) stock solution mentioned above to give an assay with a final volume of 10 μL that was contained Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), the four canonical dNTPs (1 mM in each), bovine serum albumin (0.1 mg/ml) and 20,000 cpm [α-32P]-labeled DNA substrate. After incubation at 24 °C for 30 min (for the un-cross-linked templates or templates containing a reduced dG-Ap cross-link) or 60 min (for the templates containing the dA-Ap cross-link), the reaction was stopped by addition of EDTA (1 μL of a 110 mM, pH 8 solution) to give a final concentration of 10 mM EDTA. Protein was removed from the sample by phenol-chloroform extraction,25 the primer extension products ethanol precipitated, resuspended in formamide loading buffer, loaded onto a 20% denaturing polyacrylamide gel, and the gel electrophoresed for 9 h at 1000 V. Marker lanes were generated by hydroxyl radical cleavage, Maxam-Gilbert G-reactions, and Maxam-Gilbert A+G-reactions on a 5′-32P-labeled single-stranded authentic synthetic standard of the full-length extension product using literature protocols.26,27 The radiolabeled DNA fragments in the gel were visualized and the amount of radioactivity in each band measured by phosphorimager analysis using a Personal Molecular Imager (BioRad) with Quantity One software (v.4.6.5).
Representative Procedure for T7 DNA Polymerase Primer Extension Reaction
Substrates consisting of a template hybridized to a α-32P 5′-end-labeled primer were prepared as described above and 20,000 cpm of labeled DNA substrate was aliquotted into each reaction mixture. Primer extension reactions were initiated by addition of T7 DNA polymerase (10 units) to 10 μL of a solution containing Tris-HCl (20 mM, pH 7.5), MgCl2 (10 mM), DTT (1 mM), bovine serum albumin (0.1 mg/mL), the four dNTPs (0.5 mM in each) and 20,000 cpm of the labeled substrate. The mixture was incubated at 24 °C for 8 min, followed by quenching by addition of formamide loading buffer (80 μL, composed of 95% deionized formamide, 25 mM EDTA (pH 7.5), 0.1% bromophenol and xylene cyanol). The DNA was loaded onto a 20% denaturing polyacrylamide gel and the gel electrophoresed for 9 h at 1000 V and the radiolabeled DNA fragments in the gel visualized by phosphorimager analysis.
Melting Temperature Measurement
The melting temperature of duplex B (no primer annealed) was determined by monitoring the increase in absorbance at 260 nm as a function of temperature. The temperature was increased from 20 °C to 70 °C at a rate of 0.5 °C/min. The melting temperature was determined using Cary WinUV-Thermal software.
RESULTS AND DISCUSSION
Preparation and Characterization of Polymerase Substrates Containing Ap-Derived Cross-links
The polymerase templates used here were 42 nucleotide (nt), mixed-sequence 2′-deoxyoligomers (Figures 1 and 2). At the 5′-end of most templates was located a 21 base pair (bp) duplex region. The duplex region of some substrates contained Ap-derived cross-links, indicated by the blue lines joining the complementary strands in Figures 1 and 2. These cross-linked substrates were prepared using previously described methods.6,8 Briefly, Ap sites were generated at defined locations by treatment of the corresponding 2′-deoxyuridine-containing duplexes with the enzyme uracil DNA glycosylase (UDG).23,24 Substrates C–G and L, containing the chemically stable, reduced dG-Ap cross-link (5, Scheme 1A) in the duplex region were generated by incubation of the Ap-containing duplex in sodium acetate buffer (750 mM, pH 5.2) containing NaCNBH3 (250 mM) at 37 °C for 24 h. Templates O–S and X, containing a dA-Ap cross-link at various locations in the duplex region, were prepared by incubation of the requisite Ap-containing duplex in HEPES buffer (50 mM, pH 7) containing NaCl (100 mM) at 37 °C for 120 h. The cross-linked DNA duplexes were separated from un-cross-linked material by electrophoresis on a 20% denaturing polyacrylamide gel. The cross-linked DNA was excised from the gel and desalted prior to use in the polymerase experiments. The location of interstrand cross-linking (shown in Figures 1 and 2) in the sequences used here has been established previously.4–8
Figure 1.
Polymerase substrates containing the chemically-stable reduced dG-Ap cross-link (5).
Figure 2.
Polymerase substrates containing dA-Ap cross-link (8).
We first set out to demonstrate that the 21 bp duplex region of the un-cross-linked polymerase substrates used here remains annealed to the template strand under the conditions used for the primer extension experiments. A UV-vis thermal melting experiment on the uncross-linked duplex B (freshly prepared Ap site, without the primer bound) showed a Tm of 51.3 ± 0.9 °C in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)SO4 (10 mM), DTT (4 mM), containing 0.1 mg/mL bovine serum albumin at a duplex concentration of approximately 1 μM (Figure S1). In addition, we examined duplex B as a substrate for T7 DNA polymerase. T7 DNA polymerase is not capable of efficient strand displacement synthesis and was expected to stall at, or near, the single strand-duplex junction of this substrate.28 A 15-nt 32P-labeled primer was hybridized to the 3′-end of the template strand and T7 DNA polymerase allowed to extend the primer in the presence of all four dNTPs. As expected, we observed that the primer extension by T7 DNA polymerase stalled near the beginning of the double helical region of substrate B (Figure S2). A control experiment showed that T7 DNA polymerase was able to fully extend a primer annealed to the single-stranded template A (Figure S2). Together, these results provided evidence that the duplex region of the polymerase substrates used in these studies remains hybridized under conditions identical to (Tm experiments) or similar to (T7 experiments) those used for the ϕ29 DNA polymerase experiments described below.
Chemically Stable, Reduced dG-Ap Cross-links (5) Block Primer Extension by ϕ29 DNA Polymerase
To establish ϕ29 DNA polymerase as a useful tool for probing the properties of Ap-derived cross-links, we first examined the ability of this enzyme to carry out primer extension on five different substrates (duplexes C–G) containing the chemically-stable, reduced dG-Ap cross-link (5, Scheme 1A) in various locations. A 32P-labeled primer was hybridized to the template and incubated with ϕ29 DNA polymerase in the presence of all four dNTPs (1 mM in each) for 30 min at 24 °C. The resulting primer extension products were analyzed by electrophoresis on a 20% denaturing polyacrylamide gel. In each case, the longest extension product observed corresponded to stalling at the −1 position immediately preceding the cross-linked guanine residue on the template strand (Figures 1 and 3). The −1 product was generated alongside a collection of shorter products corresponding to extension of the primer to locations within the duplex region of the substrate (the position of the arrow in the Figures corresponds to extension of the primer to the last base in the single-stranded region of the template). In the case of duplex G, bands in the −2 to −8 region were relatively weak. Control experiments showed that ϕ29 DNA polymerase fully extended a labeled primer that was annealed to the single-stranded substrate A and the non-cross-linked, duplex-containing substrate B (Figure S3).
Figure 3.
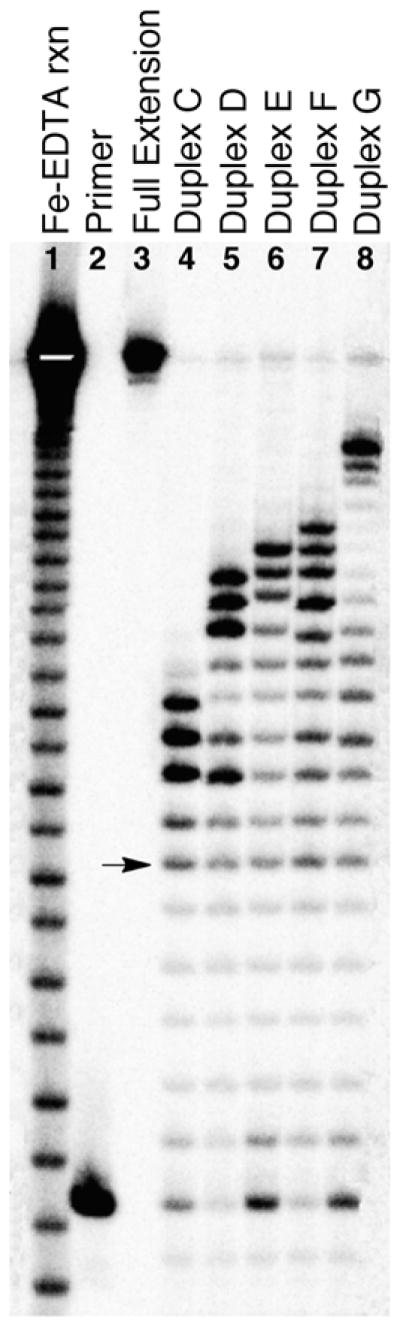
The chemically-stable, reduced dG-Ap cross-link 5 blocks primer extension by ϕ29 DNA polymerase. The 32P-labeled primers were extended by incubation of the DNA substrates with ϕ29 DNA polymerase (10 units) and the four dNTPs (1 mM in each) in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), and bovine serum albumin (0.1 mg/mL) for 30 min at 24 °C. After reaction work-up, the primer extension products were analyzed by electrophoresis on a 20% denaturing polyacrylamide gel. Lane 1 is an iron-EDTA cleavage reaction on a synthetic standard of the full-length extension product (5′-32P-GAT CAC AGT GAG TAC AAT AGA ATA GAT GAA CTA AGA CAT ATA), lane 2 is the 15 nt, 5′-32P-labeled primer, lane 3 is the 5′-32P-labeled full-length extension product, and lanes 4–8 depict the results of primer extension on templates C–G. The arrow corresponds to extension of the primer to the last base in the single-stranded region of the template. The slight difference in gel mobility observed for the iron-EDTA cleavage products and the primer extension products reflects the fact that the cleavage products generated by iron-EDTA possess 3′-phosphate termini,26 while the primer extension products possess 3′-hydroxyl termini.
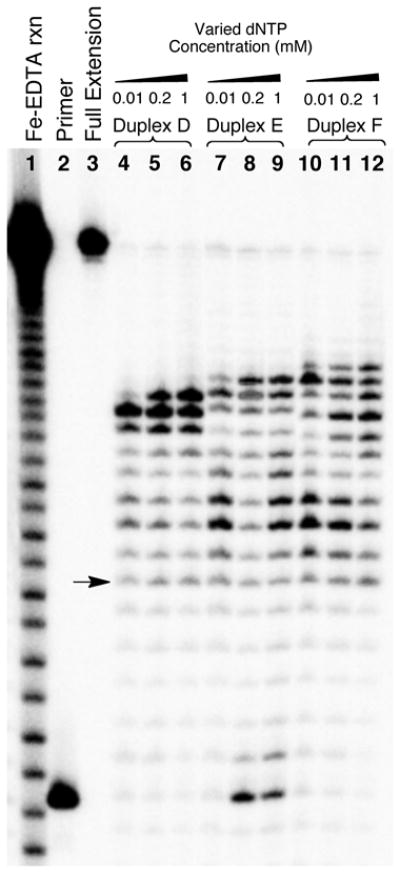
We investigated the effect of dNTP concentration on these primer extension reactions. At higher dNTP concentrations larger fractions of the −1 extension product were observed (Figure 4). Conversely, at the lower dNTP concentrations, larger fractions of the −2 and −3 products as well as products resulting from stalling approximately 2–3 bases past the single strand-duplex junction were observed. Control experiments showed that all of the dNTP concentrations used here supported extension of the primer to full length on the un-cross-linked substrate B (Figure S4). These experiments showed that the chemically-stable reduced dG-Ap cross-link completely blocks primer extension by ϕ29 DNA polymerase. For the purposes of detecting the presence and mapping the location of a cross-link within a region of duplex DNA, higher dNTP concentrations were most effective.
Figure 4. Higher dNTP concentrations favor extension of primers to the −1 position immediately preceding the reduced dG-Ap cross-link 5.
The 32P-labeled primers were extended by incubation of the DNA substrates with ϕ29 DNA polymerase (10 units) and the four dNTPs (0.01–1 mM in each) in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), and bovine serum albumin (0.1 mg/mL) for 30 min at 24 °C. After reaction work-up, the primer extension products were subjected to electrophoretic analysis on a 20% denaturing polyacrylamide gel. Lane 1 is an iron-EDTA cleavage reaction on a synthetic standard of the full-length extension product (5′-32P-GAT CAC AGT GAG TAC AAT AGA ATA GAT GAA CTA AGA CAT ATA), lane 2 is the 15 nt, 5′-32P-labeled primer, lane 3 is the 5′-32P-labeled full-length extension product, and lanes 4–12 depict the results of primer extension on templates D–F in the presence of the indicated dNTP concentrations. The arrow corresponds to extension of the primer to the last base in the single-stranded region of the template.
dA-Ap Cross-links In Duplex DNA Block Primer Extension by ϕ29 DNA Polymerase
We next examined the ability of ϕ29 DNA polymerase to carry out primer extension on five different substrates O–S containing the chemically-reversible dA-Ap cross-link 8 at different locations within the duplex region. The 5′-32P-labeled primer was hybridized to the templates and incubated with ϕ29 DNA polymerase in the presence of all four dNTPs (1 mM in each) for 60 min at 24 °C. In each case, we observed that primer extension was blocked primarily at the −1 position, the last unmodified base preceding the cross-link (Figure 5). The intensity of other bands corresponding to stalling at other sites preceding the cross-link was relatively weak. Importantly, no significant extension of the primer past the cross-link was observed. Analogous results were observed when primer extension reactions were carried out at 37 °C (Figures S5 and S6).
Figure 5. The dA-Ap (8) cross-link blocks primer extension by ϕ29 DNA polymerase.
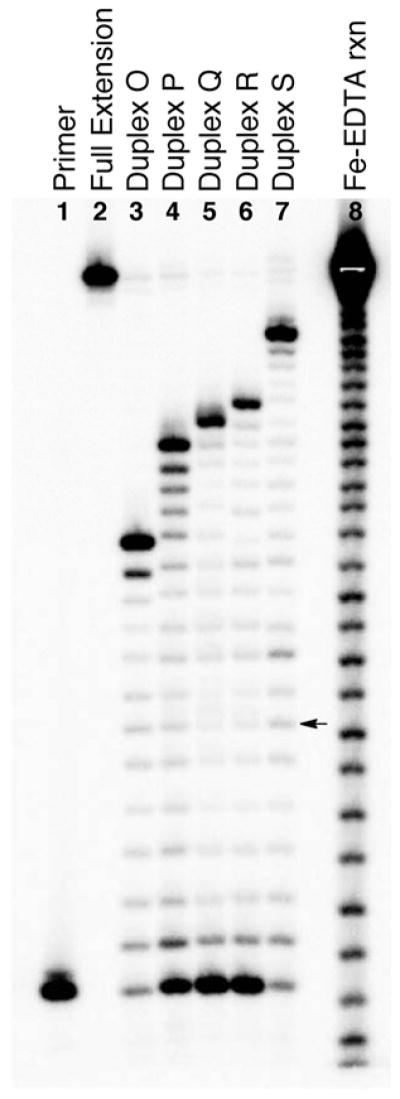
The 32P-labeled primers were extended by incubation of the DNA substrates with ϕ29 DNA polymerase (10 units) and the four dNTPs (1 mM in each) in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), and bovine serum albumin (0.1 mg/mL) for 60 min at 24 °C. After reaction work-up, the primer extension products were subjected to electrophoretic analysis on a 20% denaturing polyacrylamide gel. Lane 1 is the 15 nt, 5′-32P-labeled primer, lane 2 is the 5′-32P-labeled full-length extension product, lanes 3–7 depict the results of primer extension reactions on substrates O–S, and lane 8 is an iron-EDTA cleavage reaction on a synthetic standard of the full-length extension product (5′-32P-GAT CAC AGT GAG TAC AAT AGA ATA GAT GAA CTA AGA CAT ATA). The arrow corresponds to extension of the primer to the last base in the single-stranded region of the template.
Time Courses For Primer Extension By ϕ29 DNA Polymerase On Various Substrates
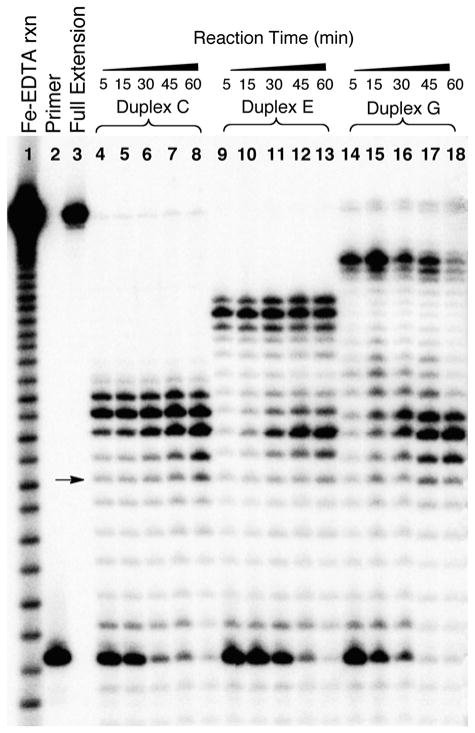
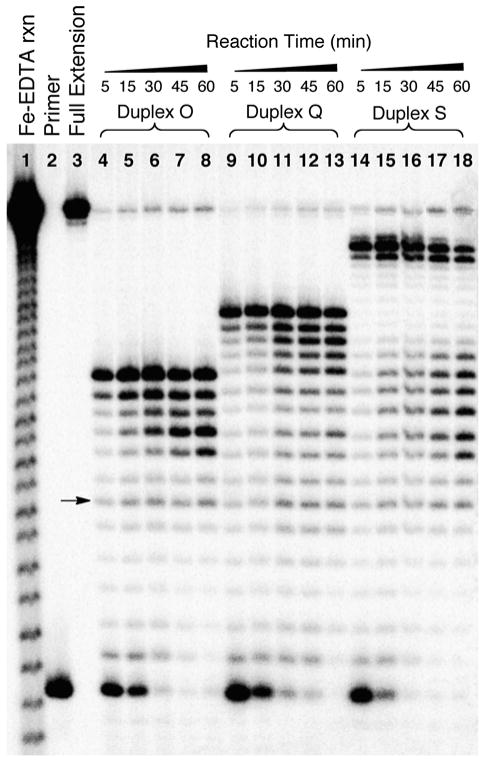
We examined time courses for primer extension on six different substrates containing the reduced dG-Ap cross-link and the dA-Ap cross-link at different locations within the duplex region (Figures 6 and 7). Early in the reactions, the major products correspond to unextended primer and primer extended to the −1, −2, and −3 positions preceding the cross-links. This is consistent with processive extension of the primer to stall sites near the cross-link. No significant amounts of full-length extension products resulting from bypass of the cross-link were generated at any time point. For both the reduced dG-Ap cross-link (5) and the dA-Ap cross-link (8), longer incubation times led to increases in the intensity of several shorter extension products. For the most part, these shorter products corresponded to primers extended to locations near the single strand-duplex junction of the template (Figures 6 and 7). The size distribution of these shorter products, presumably generated by the exonuclease function of ϕ29 DNA polymerase,29–33 differ somewhat for the two types of cross-link examined here. The intensity of these truncated products was greater in the case of the substrates containing the reduced dG-Ap cross-link. With respect to the use of ϕ29 DNA polymerase as a tool for detecting the presence and location of cross-links in duplex DNA, it is clear that, under the conditions of our experiments, shorter incubation times yield a sharper picture of cross-link location.
Figure 6. The effects of incubation time on the collection of products generated by ϕ29 DNA polymerase-mediated primer extension on substrates containing the reduced dG-Ap cross-link 5.
The 32P-labeled primers were extended by incubation of the DNA substrates with ϕ29 DNA polymerase (10 units) and the four dNTPs (1 mM in each) in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), and bovine serum albumin (0.1 mg/mL) for 5–60 min at 24 °C. After reaction work-up, the primer extension products were subjected to electrophoretic analysis on a 20% denaturing polyacrylamide gel. Lane 1 is an iron-EDTA cleavage reaction on a synthetic standard of the full-length extension product (5′-32P-GAT CAC AGT GAG TAC AAT AGA ATA GAT GAA CTA AGA CAT ATA), lane 2 is the 15 nt, 5′-32P-labeled primer, lane 3 is the 5′-32P-labeled full-length extension product, and lanes 4–18 depict primer extension reactions on templates C, E, and G for the indicated times. The arrow corresponds to extension of the primer to the last base in the single-stranded region of the template.
Figure 7. The effects of incubation time on the collection of products generated by ϕ29 DNA polymerase-mediated primer extension on substrates containing the dA-Ap cross-link 8.
The 32P-labeled primers were extended by incubation of the DNA substrates with ϕ29 DNA polymerase (10 units) and the four dNTPs (1 mM in each) in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), and bovine serum albumin (0.1 mg/mL) for 5–60 min at 24 °C. After reaction work-up, the primer extension products were subjected to electrophoretic analysis on a 20% denaturing polyacrylamide gel. Lane 1 is an iron-EDTA cleavage reaction on a synthetic standard of the full-length extension product (5′-32P-GAT CAC AGT GAG TAC AAT AGA ATA GAT GAA CTA AGA CAT ATA), lane 2 is the 15 nt, 5′-32P-labeled primer, lane 3 is the 5′-32P-labeled full-length extension product, and lanes 4–18 depict primer extension reactions on duplexes O, Q, and S for the indicated times. The arrow corresponds to extension of the primer to the last base in the single-stranded region of the template.
Cross-linked Substrates Containing the Abasic Site in the Template Strand
We examined primer extension on substrates in which the Ap site of the cross-link was located in the template strand rather than the displaced strand. We first examined primer extension on the 2′-deoxyuridine-containing duplexes (H and J) that served as precursors to the abasic duplexes (I and K). As expected, these substrates were fully extended by ϕ29 DNA polymerase (Figure 8, lanes 6 and 8). On the other hand, the Ap site in the template strand of substrates I and K acted as a partial block to primer extension under the conditions used here (Figure 8, lanes 7 and 9). Substrates L and X, containing the reduced dG-Ap (5) and dA-Ap cross-link (8), respectively, blocked primer extension by ϕ29 DNA polymerase. This shows that the dA-Ap cross-link blocks primer extension by ϕ29 DNA polymerase regardless of whether the Ap component of the cross-link is located in the template or displaced strand.
Figure 8. Cross-links containing the abasic site in the template strand block primer extension by ϕ29 DNA polymerase extension, while an un-cross-linked abasic site in the template strand is a partial block.
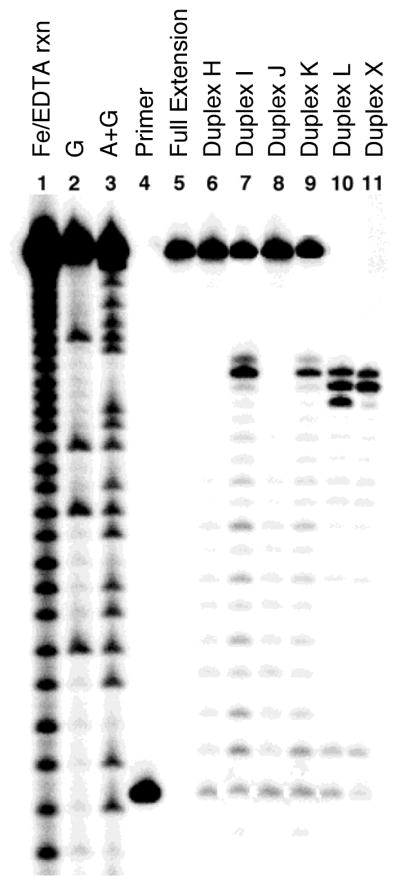
The 32P-labeled primers were extended by incubation of the DNA substrates with ϕ29 DNA polymerase (10 units) and the four dNTPs (1 mM in each) in Tris-HCl (50 mM, pH 7.5), MgCl2 (10 mM), (NH4)2SO4 (10 mM), DTT (4 mM), and bovine serum albumin (0.1 mg/mL) for 60 min at 24 °C. After reaction work-up, the primer extension products were subjected to electrophoretic analysis on a 20% denaturing polyacrylamide gel. Lane 1 is an iron-EDTA cleavage reaction on a synthetic standard of the full-length extension product (5′-32P-GAT CAC AGT GAG TAC AAT AGA ATA GAT GAA CTA AGA CAT ATA); lanes 2 and 3 are Maxam-Gilbert G- and A+G-reactions carried out on the 5′-32P-labeled full-length extension product; lane 4 is the 15 nt, 5′-32P-labeled primer; lane 5 is the 5′-32P-labeled full-length extension product; lane 6, primer extension on the single-strand substrate H containing dU in the template; lane 7, single-strand substrate I containing Ap in the template; lane 8, duplex substrate J containing dU in the template; lane 9, duplex substrate K containing an un-cross-linked Ap site in the template strand; lane 10, duplex substrate L containing reduced dG-Ap cross-link 5 with the Ap residue in the template strand; lane 11, duplex substrate X containing the dA-Ap cross-link in which the Ap residue is in the template strand.
Conclusions
Our results show that the dA-Ap cross-link blocks primer extension by the bacteriophage ϕ29 helicase-polymerase. On all substrates examined here, primer extension was blocked at the last unmodified base prior to the cross-link. The dA-Ap cross-linkage is nominally reversible but functioned, for all practical purposes, as an irreversible covalent cross-link in these experiments. Previous measurements using optical tweezers showed that the fork junction destabilization energy of ϕ29 is greater than that of other polymerases and helicases examined.22 This suggests that ϕ29 polymerase serves as a stringent test of the ability of the dA-Ap cross-link to withstand enzyme-driven strand separation.
DNA in the cells of normal mammalian tissue carry a substantial burden of Ap sites.9–12 Certainly, not all of the Ap sites generated in cellular DNA go forward to forge interstrand cross-links. Cross-link formation exists in competition with other processes including repair and strand cleavage.9,34–42 However, even small numbers of cross-links in cellular DNA can lead to profound consequences including mutagenesis, cell death, or cell senescence.43 Our work suggests that, to the extent that Ap-derived cross-links are generated in cells, these lesions have the potential to exert significant biological effects via blockage of helicases, DNA polymerases, and RNA polymerases.
Analysis of ϕ29-DNA co-crystal structures suggested that the template strand of the DNA duplex is fed into the polymerase active site via a tunnel that extends 5–6 nt downstream.21,44 This tunnel, composed of the TPR2 and exo domains of the protein, plays an important role in the remarkable processivity and strand-displacement activity of ϕ29.21 Our data support the idea44 that the active site entry tunnel observed in the crystal structures of ϕ29 is created by a dynamic flap that can open to allow substrate access. If the tunnel were a rigid structure, the interstrand cross-links in our substrates likely would have stalled primer extension several nucleotides prior to the cross-link lesion, rather than at the last unmodified nucleotide as observed.
Finally, these studies introduce ϕ29 DNA polymerase as a tool for detecting the presence and mapping the location of interstrand DNA cross-links in duplex DNA. Our results showing that an un-cross-linked Ap site serves as a partial block to primer extension further suggest that ϕ29 polymerase may also be useful for the detection of non-cross-linked lesions on the template strand. This method could provide a useful alternative to T7 RNA polymerase as a tool for mapping the location of DNA adducts generated by natural products such as anthramycin, ecteinascidin, saframycin, or tomaymycin that are stable in duplex DNA but potentially unstable in single-stranded DNA.45–50
Supplementary Material
Acknowledgments
Funding
We thank the National Institute of Environmental Health Science of the National Institutes of Health for support of this work (ES 021007).
We thank Professor Margarita Salas for helpful comments on the manuscript and we thank Professor Carmelo Rizzo for an insightful discussion that inspired this work.
ABBREVIATIONS
- Ap
DNA abasic site
- dA
2′-deoxyadenosine
- dG
2′-deoxyguanosine
- UDG
uracil DNA glycosylase
- dNTP
2′-deoxynucleoside triphosphate
- HEPES
4-(2-hydroxyethyl-1-piperazineethanesulfonic acid
- MOPS
3-(N-morpholino)propanesulfonic acid
- EDTA
ethylenediamine tetraacetic acid
- Tris
tris(hydroxymethyl)aminomethane
- DTT
1,4-dithio-D-threitol
- nt
nucleotide
- bp
base pair
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
UV-vis thermal melting analysis of substrate B, T7 DNA polymerase-mediated primer extension on substrate B, control reactions involving ϕ29-mediated primer extension on substrate B, and primer extension reactions at 37 °C. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Schärer OD. DNA interstrand crosslinks: natural and drug-induced DNA adducts that induce unique cellular responses. ChemBioChem. 2005;6:27–32. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 2.Rajski SR, Williams RM. DNA cross-linking agents as antitumor drugs. Chem Rev. 1998;98:2723–2795. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]
- 3.Muniandy PA, Liu J, Majumdar A, Liu ST, Seidman MM. DNA interstrand crosslink repair in mammalian cells: step by step. Crit Rev Biochem Mol Biol. 2010;45:23–49. doi: 10.3109/10409230903501819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano MJ, Liu S, Andersen N, Yang Z, Johnson KM, Price NA, Wang Y, Gates KS. Chemical structure and properties of the interstrand cross-link formed by the reaction of guanine residues with abasic sites in duplex DNA. J Am Chem Soc. 2015;137 doi: 10.1021/jacs.5b00669. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta S, Chowdhury G, Gates KS. Interstrand crosslinks generated by abasic sites in duplex DNA. J Am Chem Soc. 2007;129:1852–1853. doi: 10.1021/ja067294u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KM, Price NE, Wang J, Fekry MI, Dutta S, Seiner DR, Wang Y, Gates KS. On the Formation and Properties of Interstrand DNA-DNA Cross-links Forged by Reaction of an Abasic Site With the Opposing Guanine Residue of 5′-CAp Sequences in Duplex DNA. J Am Chem Soc. 2013;135:1015–1025. doi: 10.1021/ja308119q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price NE, Catalano MJ, Liu S, Wang Y, Gates KS. Chemical and structural characterization of interstrand cross-links formed between abasic sites and adenine residue in duplex DNA. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv174. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price NE, Johnson KM, Wang J, Fekry MI, Wang Y, Gates KS. Interstrand DNA–DNA Cross-Link Formation Between Adenine Residues and Abasic Sites in Duplex DNA. J Am Chem Soc. 2014;136:3483–3490. doi: 10.1021/ja410969x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gates KS. An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chem Res Toxicol. 2009;22:1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillet M, Bioteux S. Origin of endogenous DNA abasic sites in Saccaromyces cerevisiae. Mol Cell Biol. 2003;23:8386–8394. doi: 10.1128/MCB.23.22.8386-8394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura J, Swenberg JA. Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res. 1999;59:2522–2526. [PubMed] [Google Scholar]
- 12.Nakamura J, Walker VE, Upton PB, Chiang SY, Kow YW, Swenberg JA. Highly sensitive apurinic/apyrimidinic site assay can detect spontaneous and chemically induced depurination under physiological conditions. Cancer Res. 1998;58:222–225. [PubMed] [Google Scholar]
- 13.Zheng H, Wang X, Warren AJ, Legerski RJ, Nairn RS, Hamilton JW, Li L. Nucleotide excision repair- and polymerase eta-mediated error-prone removal of mitomycin C interstrand cross-links. Mol Cell Biol. 2003;23:754–761. doi: 10.1128/MCB.23.2.754-761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen X, Li L. Mutagenic repair of interstrand crosslinks. Env Mol Mutagenesis. 2010;51:493–499. doi: 10.1002/em.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grillari J, Katinger H, Voglauer R. Contributions of DNA interstrand cross-links to aging of cells and organisms. Nucleic Acids Res. 2007;35:7566–7576. doi: 10.1093/nar/gkm1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergstrahl DT, Sekelsky J. Interstrand crosslink repair: can XPF-ERCC1 be let off the hook? Trends in Genetics. 2007;24:70–76. doi: 10.1016/j.tig.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Brooks PJ. The case for 8,5′-cyclopurine-2′-deoxynucleosides as endogenous DNA lesions that cause neurodegeneration in xeroderma pigmentosum. Neuroscience. 2007;145:1407–1417. doi: 10.1016/j.neuroscience.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedernhofer LJ, Lalai AS, Hoeijmakers JHJ. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Garinis GA, van der Horst GTJ, Vijg J, Hoeijmakers JHJ. DNA damage and aging: new-age ideas for an age-old problem. Nat Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco L, Bernad A, Lázaro JM, Martín G, Garmendia C, Salas M. Highly efficient DNA synthesis by the phage ϕ29 DNA polymerase. Symmetrical mode of DNA replication. J Biol Chem. 1989;264:8935–8940. [PubMed] [Google Scholar]
- 21.Rodríguez I, Lázaro JM, Blanco L, Kamtekar S, Berman AJ, Wang J, Steitz TA, Salas M, de Vega M. A specific subdomain in ϕ29 DNA polymerase confers both processivity and strand-displacement capacity. Proc Nat Acad Sci USA. 2005;102:6407–6412. [Google Scholar]
- 22.Morin JA, Cao FJ, Lázaro JM, Arias-Gonzalaz R, Valpuesta JM, Carrascosa JL, Salas M, Ibarra B. Active DNA unwinding dynamics during processive DNA replication. Proc Nat Acad Sci USA. 2012;109:8115–8120. doi: 10.1073/pnas.1204759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindahl T, Ljunquist S, Siegert W, Nyberg B, Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977;252:3286–3294. [PubMed] [Google Scholar]
- 24.Varshney U, van de Sande JH. Specificities and kinetics of uracil excision from uracil-containing DNA oligomers by Escherichia coli uracil DNA glycosylase. Biochemistry. 1991;30:4055–4061. doi: 10.1021/bi00230a033. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Lab Manual. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 26.Pogozelski WK, McNeese TJ, Tullius TD. What species is responsible for strand scission in the reaction of [Fe(II)EDTA]2− and H2O2 with DNA? J Am Chem Soc. 1995;117:6428–6433. [Google Scholar]
- 27.Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 28.Manosas M, Spiering MM, Ding F, Bensimon D, Allemand JF, Benkovic SJ, Croquette V. Mechanism of strand displacement synthesis by DNA replicative polymerases. Nucleic Acids Res. 2012;40:6174–6186. doi: 10.1093/nar/gks253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco L, Salas M. Characterization of a 3′-5′ exonuclease activity in the phage ϕ29-encoded DNA polymerase. Nucleic Acids Res. 1985;13:1239–1249. doi: 10.1093/nar/13.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esteban JA, Soengas MS, Salas M, Blanco L. 3′-5′ exonuclease active site of ϕ29 DNA polymerase. Evidence favoring a metal ion-assisted reaction mechanism. J Biol Chem. 1994;269:31946–31954. [PubMed] [Google Scholar]
- 31.Garmendia C, Bernad A, Esteban JA, Blanco L, Salas M. The bacteriophage ϕ29 DNA polymerase, a proofreading enzyme. J Biol Chem. 1992;267:2594–2599. [PubMed] [Google Scholar]
- 32.de Vega M, Lázaro JM, Salas M, Blanco L. Primer-terminus stabilization at the 3′-5′ exonuclease active site of ϕ29 DNA polymerase. Involvement of two amino acid residues highly conserved in proofreading DNA polymerases. EMBO J. 1996;15:1182–1192. [PMC free article] [PubMed] [Google Scholar]
- 33.de Vega M, Lázaro JM, Salas M, Blanco L. Mutational analysis of ϕ29 DNA polymerase residues acting as ssDNA ligands for 3′-5′ exonucleolysis. J Mol Biol. 1998;279:807–822. doi: 10.1006/jmbi.1998.1805. [DOI] [PubMed] [Google Scholar]
- 34.Bailly V, Verly WG. Possible roles of β-elimination and δ-elimination reactions in the repair of DNA containing AP (apurinic/apyrimidinic) sites in mammalian cells. Biochem J. 1988;253:553–559. doi: 10.1042/bj2530553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayley CR, Brammer KW, Jones AS. The nucleotide sequence in deoxyribonucleic acids. Part V. The alkaline degradation of apurinic sites. J Chem Soc. 1961:1903–1907. [Google Scholar]
- 36.Boiteux S, Guillet M. Abasic sites in DNA: repair and biological consequences in Saccaromyces cerevisiae. DNA Repair. 2004;3:1–12. doi: 10.1016/j.dnarep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl T, Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972;11:3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- 38.Admiraal SJ, O’Brien PJ. Base Excision Repair Enzymes Protect Abasic Sites in Duplex DNA from Interstrand Cross-Links. Biochemistry. 2015;54:1849–1857. doi: 10.1021/bi501491z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luke AM, Chastain PD, Pachkowski BF, Afonin V, Takeda S, Kaufman DG, Swenberg JA, Nakamura J. Accumulation of true single strand breaks and AP sites in base excision repair deficient cells. Mutation Res. 2010;694:65–71. doi: 10.1016/j.mrfmmm.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sczepanski JT, Wong RS, McKnight JN, Bowman GD, Greenberg MM. Rapid DNA-protein cross-linking and strand scission by an abasic site in a nucleosome core particle. Proc Nat Acad Sci USA. 2010;107:22475–22480. doi: 10.1073/pnas.1012860108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou C, Sczepanski JT, Greenberg MM. Mechanistic studies on histone catalyzed cleavage of apyrimidinic/apurinic sites in nucleosome core particles. J Am Chem Soc. 2012;134:16734–16741. doi: 10.1021/ja306858m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sczepanski JT, Zhou C, Greenberg MM. Nucleosome core particle-catalyzed strand scission at abasic sites. Biochemistry. 2013;52:2157–2164. doi: 10.1021/bi3010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clauson C, Schärer OD, Niedernhofer LJ. Advances in understanding the complex mechanisms of DNA interstrand cross-link repair. Cold Spring Harbor Perspectives in Biology. 2013;5:a012732/012731–a012732/012725. doi: 10.1101/cshperspect.a012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamtekar S, Berman AJ, Wang J, Lázaro JM, de Vega M, Blanco L, Salas M, Steitz TA. The phi29 DNA polymerase:protein-primer structure suggests a model for the initiation to elongation transition. EMBO J. 2006;25:1335–1343. doi: 10.1038/sj.emboj.7601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lown WJ, Joshua AV, Lee JS. Molecular mechanisms of binding and single strand scission of DNA by the antitumor antibiotics saframycin A and C. Biochemistry. 1982;21:419–428. doi: 10.1021/bi00532a001. [DOI] [PubMed] [Google Scholar]
- 46.Hurley LH, Allen CS, Feola JM, Lubaway WC. In vitro and in vivo stability of anthramycin-DNA conjugate and its potential application as an anthramycin prodrug. Cancer Res. 1979;39:3134–3140. [PubMed] [Google Scholar]
- 47.Rahman KM, James CH, Thurston DE. Observation of the reversibility of a covalent pyrrolobenzodiazepine (PBD) DNA adduct by HPLC/MS and CD spectroscopy. Org Biomol Chem. 2011;9:1632–1641. doi: 10.1039/c0ob00762e. [DOI] [PubMed] [Google Scholar]
- 48.Gates KS. Covalent Modification of DNA by Natural Products. In: Kool ET, editor. Comprehensive Natural Products Chemistry. Pergamon; New York: 1999. pp. 491–552. [Google Scholar]
- 49.Puvvada MS, Forrow SA, Hartley JA, Stephenson P, Gibson I, Jenkins TC, Thurston DE. Inhibition of Bacteriophage T7 RNA Polymerase in Vitro Transcription by DNA-Binding Pyrrolo[2,1-c][1,4]benzodiazepines. Biochemistry. 1997;36:2478–2484. doi: 10.1021/bi952490r. [DOI] [PubMed] [Google Scholar]
- 50.Zewail-Foote M, Hurley LH. Differential rates of reversibility of ecteinascidin 743-DNA covalent adducts from different sequences lead to migration to favored bonding sites. J Am Chem Soc. 2001;123:6485–6495. doi: 10.1021/ja004023p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.