Abstract
Cocaine-induced locomotion is mediated by dopamine in the nucleus accumbens (NAc). Recent evidence indicates that the medial preoptic area (mPOA), a region in the rostral hypothalamus, modulates cocaine-induced dopamine in the NAc. Specifically, rats with lesions of the mPOA experienced a greater increase in dopamine following cocaine administration than rats with sham lesions. Whether the mPOA similarly influences cocaine-induced locomotion is not known. Here we examined whether radiofrequency or neurotoxic lesions of the mPOA in male rats influence changes in locomotion that follow cocaine administration. Locomotion was measured following cocaine administration in male rats with neurotoxic, radiofrequency, or sham lesions of the mPOA. Results indicate that bilateral lesions of the mPOA facilitated cocaine-induced locomotion. This facilitation was independent of lesion type, as increased locomotion was observed with either approach. These findings support a role for the mPOA as an integral region in the processing of cocaine-induced behavioral response, in this case locomotor activity.
Keywords: Cocaine, Locomotion, Dopamine, Medial preoptic area, Nucleus accumbens, Lesions
In 1976, Kelly and Iversen [1] introduced the idea that dopamine in the nucleus accumbens (NAc) stimulates locomotion after discovering that selective lesions of dopamine fibers in the NAc significantly reduced cocaine-induced locomotor activity. Extensive analyses by Costall and colleagues followed, showing that dopamine administration directly into the NAc increases locomotion [2, 3]. This effect is specific to dopamine, as increased primary hyperactivity occurs following microinjections of dopamine into the NAc, but not norepinephrine, serotonin, GABA, or acetylcholine [2]. Cocaine increases locomotion through activation of this system.
Cocaine-induced behavioral changes are dependent on dopamine in the NAc [4]. A host of studies, employing microinjections or microdialysis, support this connection. For example, freely moving rats experience a five-fold increase in levels of extracellular dopamine in the NAc following cocaine administration [5]. Cocaine-induced increases in dopamine follow either experimenter-administered or self-administered drug [6]. Microinjection experiments similarly support the connection between cocaine, dopamine in the NAc, and increased locomotion, by showing that cocaine, but not other anesthetics, directly administered into the NAc causes motor activation in rats [7].
While the preponderance of evidence justifiably points to the mesolimbic dopamine system as the necessary neurobiological endpoint when describing cocaine’s behavioral effects, this system does not operate in isolation, and input from other structures may play an equally critical role in cocaine-induced behaviors. We recently provided evidence describing a novel estradiol-dependent input into the mesolimbic system that modulates cocaine-induced neural, neurochemical, and behavioral activity in female rats [8, 9]. Our findings demonstrated that neurotoxic lesions of the medial preoptic area (mPOA) increased cocaine-induced dopamine release in the NAc, that many of the neurons projecting from the mPOA to the ventral tegmental area (VTA) are sensitive to estradiol, and that estradiol microinjections into the mPOA 24-hours prior to cocaine administration increased cocaine-induced dopamine release in the NAc [9]. These results are in line with additional work from our laboratory revealing that lesions of the mPOA enhanced cocaine-induced conditioned place preference (CPP) and cocaine-induced cellular activity in the NAc [8]. Together, these results point to the mPOA as an integral region in the processing of cocaine-induced response. However, whether the mPOA similarly affects cocaine-induced behaviors like locomotion in male rats was hitherto unknown. To help answer this question, we examined whether radiofrequency or neurotoxic lesions in the mPOA of male rats influenced cocaine-induced locomotion.
All experimental procedures were in accordance with the National Institutes of Health Guidelines for the Use of Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Male Sprague Dawley rats (Harlan, Indianapolis), weighing 300–400 g, were double housed in large plastic cages on a 12/12 h light-dark cycle, lights off at 10am, with free access to food and water. Subjects were randomly assigned to surgical condition (sham or lesion) as well as drug administration order (saline first or cocaine first), and were then housed such that cage mates received all the same treatments (surgery and injections).
Stereotaxic surgeries were performed three weeks before behavioral testing. All animals received isofluorane (2–5%) anesthesia. For both radiofrequency and neurotoxic lesions, bilateral boreholes were drilled in the skull above the central region of the mPOA (AP, −0.4 mm; ML, ±0.5 mm; DV, −8.2 mm; according to coordinates from [10]). To produce radiofrequency lesions, a TCZ thermo-coupled electrode (0.25 mm exposed tip) was lowered into the mPOA and a Radionics radiofrequency lesion generator was used to heat the electrode to 80°C (±3°C) for 20 seconds. Sham radiofrequency animals underwent the same procedure without the electrode being heated. For neurotoxic lesions, injections of N-methyl-D-aspartate (NMDA; 25 ug/ul) in 0.1M PBS into each hemisphere of the mPOA were used. NMDA was injected into the mPOA with a 1ul Hamilton syringe at 0.1ul/min for 2 minutes for each hemisphere. The syringe was then left in the mPOA for 5 minutes before being removed. The same procedure was followed for sham NMDA lesions except vehicle was microinjected in place of NMDA. Lesion placement was verified histologically after behavioral testing was complete. For verification, methyl green was used for subjects with radiofrequency lesions whereas an antibody against NeuN was used to evaluate NMDA lesions.
Of the animals receiving radiofrequency lesions, 11 were removed because they had lesions outside the mPOA, presented with tumors, or experienced seizures. Of the animals receiving sham-radiofrequency lesions, 4 were removed for similar reasons. Of the animals receiving neurotoxic lesions, 12 were removed with failed lesions. Of the remaining animals with neurotoxic lesions, 13 had unilateral and 7 had bilateral lesions; thus, bilateral and unilateral lesions of the mPOA were treated as separate levels of surgery during analysis. After exclusions, the four groups contained the following number of animals: radiofrequency-sham lesions (n=15), radiofrequency lesions (n=13); neurotoxic-sham lesions (n=18), neurotoxic-unilateral lesions (n=13), neurotoxic-bilateral lesions (n=7).
Med Associates open field boxes (44cm × 44cm × 30cm) equipped with photodiodes (2.5cm apart on each side of the box; 16 per side) were used to measure locomotor activity. Locomotion was operationalized as beam breaks, with a beam break defined as breaking every 5th photodiode. Locomotor activity was recorded in 5-minute bins using Activity Monitor 5 software (Med Associates Inc.).
All locomotion tests were carried out during the dark cycle. Subjects were removed from the colony in their home cage and brought to the behavioral testing room; during transportation, a dark sheet was draped over the home cage. On the first day of testing, all subjects were allowed 25 minutes to habituate to the locomotion test chamber before being returned to the colony. The following day, subjects were placed in the chamber for 5 (radiofrequency experiment) or 10 (neurotoxic experiment) minutes to establish baseline locomotion and then given an intraperitoneal (IP) injection of either saline (0.9% NaCl) 0.5 ml/kg or cocaine 15 mg/kg/0.5mL. After 20 minutes, subjects were removed from the test chamber and returned to the colony. Six days later, all subjects underwent a second 25-minute habituation session, and received a counterbalanced test session; the following day, subjects that received cocaine first received saline and vice-versa (see Figure 1 for summary).
Fig 1.
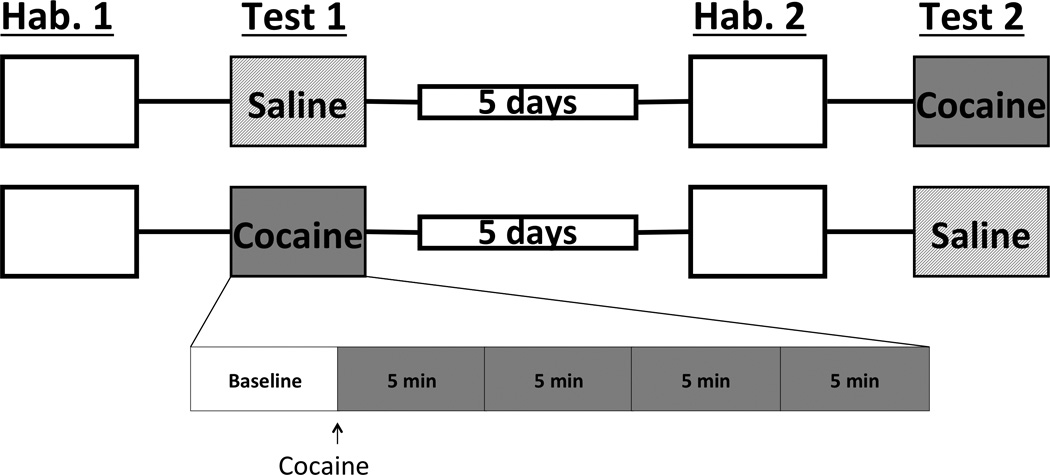
Timeline of events for locomotion experiments. Being placed, on day 1, in the locomotion chamber for 25 minutes habituated all animals to the testing apparatus (blank box). The next day, animals received either saline or cocaine. Five days later, subjects were allowed to explore the chamber once again to insure prior drug exposure had no lasting effects and then received the opposite compound the following day. (Hab., habituation).
All subjects received an overdose of Euthasol (130mg/kg), were tested for deep anesthesia, and were then transcardially perfused with 50 mL of PBS and 250 mL of 4% paraformaldehyde. Brains were removed and stored in 4% paraformaldehyde for 1-hour before being transferred to 30% sucrose and stored at 4° C overnight; after 24-hours brains were transferred to another 30% sucrose solution and stored at 4° C until sectioning. Brains were sliced at 40 μm and stored in cryoprotectant (30% ethylene glycol, 30% sucrose, and 0.0002% sodium azide in 0.1 Μ phosphate buffer (PB)) at −20° C.
In order to determine neurotoxic lesion placement, immunohistochemistry was performed to visualize NeuN, a neuronal marker. Brain slices containing the mPOA were washed in 0.1M PB and incubated in a monoclonal mouse anti-NeuN antibody (1:15000, Millipore, MAB337) overnight. Tissues were further incubated in an anti-mouse biotinylated antibody (1:1000, Vector) for 1-hour and enhanced with avidin/biotin (1:1000, ABC kit, Vector). NeuN immunoreactivity was visualized by incubating tissues in a 3,3’Diaminobenzidine (DAB) for 10 minutes. Between all incubations tissue was washed in 0.1 M PB 4x5 min. Slices were then mounted, dehydrated, and cover slipped in preparation for bright-field microscopy.
Radiofrequency lesion placement was determined by staining tissue with methyl green. Brain slices containing the mPOA were mounted on charged slides and soaked in methyl green solution for 5 minutes. Slides where then dehydrated and cover-slipped in preparation for bright field microscopy.
All statistics were performed using R, version 3.2.2. In order to account for within subject variability, all locomotion data was analyzed as a percent change from baseline (time point prior to the administration of saline/cocaine). For subjects with radiofrequency lesions, a 4x2x2 mixed factorial ANOVA was performed with surgery (sham/lesion) as a between subject factor, and drug (saline/cocaine) and time (four 5-minute intervals after drug administration) as within-subject factors. A two-way interaction between surgery and drug was then decomposed using Welch’s two sample t-tests (holding drug constant) and paired t-tests (holding surgery constant). For subjects with neurotoxic lesions, a 4x3x2 mixed factorial ANOVA was performed with surgery (sham, bilateral, or unilateral lesion) as a between-subjects factor and drug (saline/cocaine) and time (four 5-minute intervals after drug administration) as within-subject factors. A two-way interaction between surgery and drug was then decomposed using one-way ANOVAs (holding drug constant) followed by Tukey’s HSD and paired t-tests (holding surgery constant). For subjects with either radiofrequency or neurotoxic lesions, a two way interaction between time and drug was decomposed using a repeated measures ANOVA (holding drug constant) followed by Tukey’s HSD and a series of paired t-tests (holding time constant).
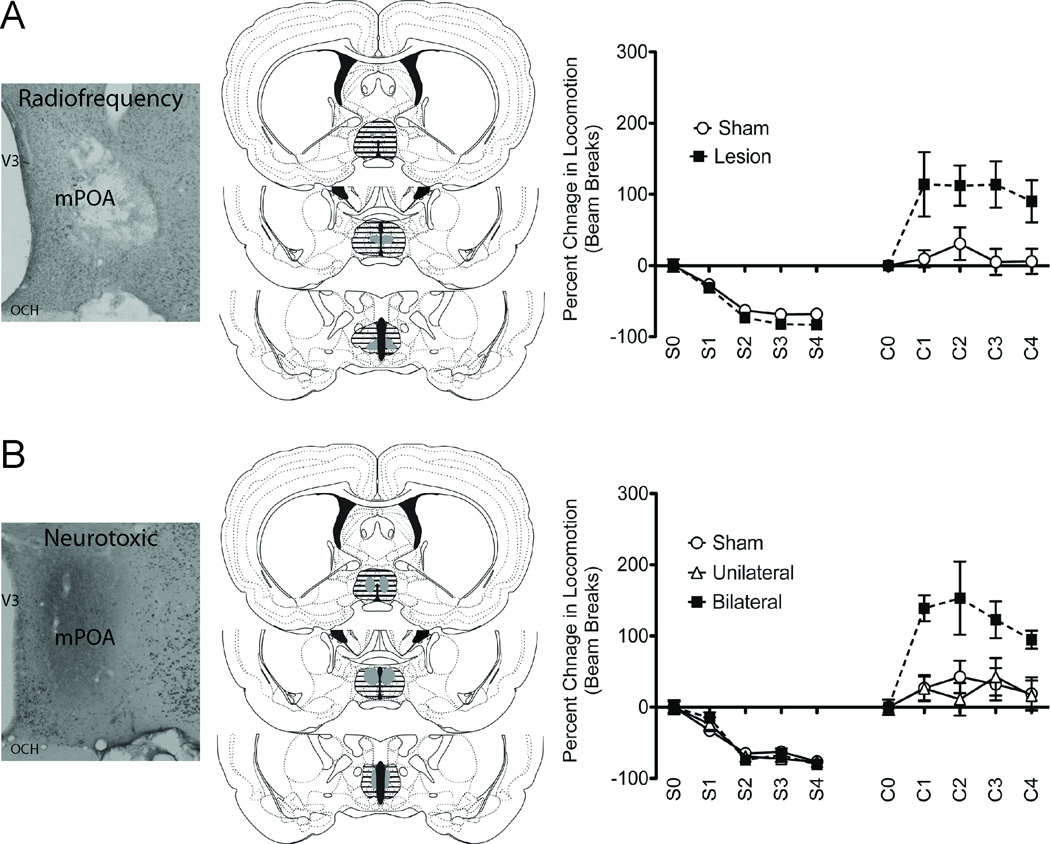
Results indicate that lesions of the mPOA facilitated cocaine-induced locomotor activity in male rats. Specifically, a 4x2x2 mixed factorial ANOVA performed on subjects receiving radiofrequency or sham lesions revealed an interaction between surgery and drug (F(1,185) = 40.958, p < 0.05); there was no three-way interaction (F(3,182) = 0.159, p > 0.05) nor was there a surgery by time interaction (F(3,185) = 0.228, p > 0.05) or time and drug (F(3,185) = 1.809, p < 0.05) (Figure 2A/B). Post hoc decomposition of the surgery by drug interaction revealed that subjects with radiofrequency lesions exhibited greater cocaine-induced locomotion (t(21.368) = 3.12, p < 0.05), but less locomotion when exposed to saline (t(23.383) = −2.45, p < 0.05) compared to sham operated subjects.
Fig 2.
Lesions of the mPOA enhanced cocaine-induced locomotion in male rats. Description of radiofrequency-lesion experiment is on A, the top row; description of neurotoxic-lesion experiment is on B, the bottom row. Representative micrographs are to the left; radiofrequency lesions were confirmed following staining tissue with methyl green, whereas neurotoxic lesions were confirmed following immunohistochemistry staining for NeuN. The middle column contains representative perimeters of smallest (solid gray) and largest (dashed black lines) radiofrequency or neurotoxic lesions of the mPOA; drawn from [10]. To the right are graphical representations of percent change in locomotion in response to an IP injection of either saline or cocaine in animals receiving (A) radiofrequency or (B) neurotoxic lesions, compared to sham lesions, of the mPOA. Saline and cocaine administration occurred one week apart. S0 and C0 are baseline locomotion measurement prior to injection whereas S1–4 and C1–4 are 5-min locomotion bins after injection. Subjects with lesions had a greater overall increase in cocaine-induced locomotion compared to sham-treated subjects (p < 0.05). All subjects exhibited a cocaine-induced increase in locomotion compared to saline treatment (p < 0.05). (C, cocaine; S, saline).
Results obtained following neurotoxic lesions support findings obtained with radiofrequency lesions. Specifically, a 4x3x2 mixed factorial ANOVA performed on subjects receiving neurotoxic or sham lesions, and using the average of the 2 five-minute locomotion intervals as a baseline, revealed an interaction between surgery and drug (F(2,251) = 20.541, p < 0.05), as well as an interaction between time and drug (F(3,251) = 3.329, p < 0.05); there were no three-way interactions (F(6,245) = 0.519, p > 0.05), nor a surgery by time interaction (F(6,251) = 0.589, p > 0.05) (Figure 2C/D). Post hoc decomposition of the time by drug interaction revealed percent change in locomotion decreased over time in response to saline (F(3,111) = 61.59, p < 0.05) but did not change over time in response to cocaine (F(3,111) = 1.47, p > 0.05). Specifically, Tukey corrected contrasts revealed percent change in locomotion was significantly lower at the last three time points compared to the first for saline treatment. When compared to saline, cocaine-induced locomotion was higher across all time points. Post hoc decomposition of the surgery by drug interaction revealed that lesion type modulated cocaine (F(2,35) = 5.048, p < 0.05) induced locomotion but not the response to saline (F(2,35) = 0.041, p > 0.05). Specifically, Tukey corrected post hoc contrasts revealed subjects with bilateral neurotoxic lesions exhibited a greater increase in cocaine-induced locomotion than subjects with unilateral or sham lesions. All three groups exhibited greater locomotor response to cocaine than saline.
Here, we demonstrated that the mPOA modulates cocaine-induced changes in locomotion. The facilitation of cocaine-induced locomotion following lesions of the mPOA was observed with either radiofrequency or neurotoxic lesions, suggesting that cells in the mPOA, and not only passing fibers, are modulating the locomotion-promoting properties of cocaine. This effect requires input from both hemispheres of the mPOA, as cocaine-induced locomotion in animals with unilateral lesions did not differ from sham-operated animals. We also observed a decrease in locomotion over time in response to saline. This is likely due to reduced exploration as the behavioral sessions proceeded.
The idea that lesions of the mPOA facilitate cocaine-induced locomotion is consistent with our hypothesis that the mPOA sends inhibitory input into the VTA; its removal should disinhibit cellular activity in the VTA, facilitating cocaine-induced increase of dopamine in the NAc. Similarly, as the mPOA is essential for sexual [11] and maternal behaviors [12], contains one of the highest concentrations of cells expressing sex-steroid hormone receptors [13], and contains robust connections with the mesolimbic system [14], it is not surprising that it should also modulate cocaine-induced behavioral activity. Dopamine in the NAc increases locomotion [2, 3], removing a source of inhibition into the mesolimbic dopamine system, namely the mPOA [8, 9], should then facilitate locomotion, as is evidenced in our present findings. However, how the mPOA achieves this modulation is not entirely clear.
Maternal care and male sexual behavior, two behaviors heavily regulated by the mPOA [11, 12], also induce dopamine release in the NAc [15, 16], suggesting a functional link between the mPOA and the NAc. However, anatomical studies suggest few neurons project directly from the mPOA to the NAc [14]. The VTA, which sends dopaminergic projections to the NAc [17], and modulates both CPP [18] and locomotion [19], is densely innervated by the mPOA [14], particularly the rostral end [8]. This suggests that the mPOA may modulate dopamine activity in the NAc via direct interactions with the VTA. Similarly, manipulations of the VTA modulate mPOA-dependent behaviors. Electrical stimulation [20], as well as pharmacological manipulation [21], of the VTA facilitates male sexual behavior. Pharmacological depression of the VTA impairs CPP for pups in female rats as well as pup directed maternal care [22]. Additionally, unilateral lesions of the mPOA and contralateral VTA lesions abolish pup retrieval but not nursing behavior [23].
In the present study, removal of the mPOA increased cocaine-induced locomotion, suggesting that the mPOA inhibits dopamine activity in the NAc of male rats, as it does in females [8, 9], through its GABAergic output. As evidence of this, a substantial number of neurons within the mPOA are GABAergic [24]. Additionally, pharmacological inactivation of the mPOA with muscimol increases locomotion, as well as dopamine levels in the NAc [25]. Furthermore, a substantial number of neurons in the mPOA that project to the VTA exhibit GABA immunoreactivity [8]. Thus, it is likely that the mPOA sends GABAergic projections to the VTA that inhibit dopamine neurons, which in turn reduces dopamine release in the NAc. Alternatively, it is important to note that the mPOA also projects to 62 other regions, in addition to the VTA [14]; a multitude of these other regions also interact with the mesolimbic system. Therefore, in addition to direct interactions with the VTA, removal of the mPOA might impact cocaine-induced mesolimbic activity and locomotion as a result of changes in other regions interacting with the mesolimbic system. We should also like to note that while our previous study [9] examined a role for the mPOA in cocaine-induced response of only females and the present study examined only males, it is still probable that mPOA-mesolimbic connections mediate gender differences resulting from cocaine administration, this is the focus of future and ongoing studies in our lab.
In conclusion, we observed an increase in cocaine-induced locomotion in male rats following neurotoxic or radiofrequency lesions of the mPOA. This potentiation required lesions of both hemispheres of the mPOA, as this effect was not observed with unilateral lesions alone. The mPOA may modulate cocaine-induced behavioral response through interactions with the VTA, as the mPOA projects directly to the VTA. While the neurochemical profile of the mPOA-VTA afferents remains to be elucidated, GABA is a good candidate transmitter, as the majority of cells in the mPOA are GABAergic. If true, then lesions of the mPOA decrease GABAergic input to the VTA, allowing for greater dopaminergic output to the NAc, facilitating sensitivity to the locomotor stimulant effects of cocaine, as evidenced by our findings (see Figure 3 for summary).
Fig. 3.
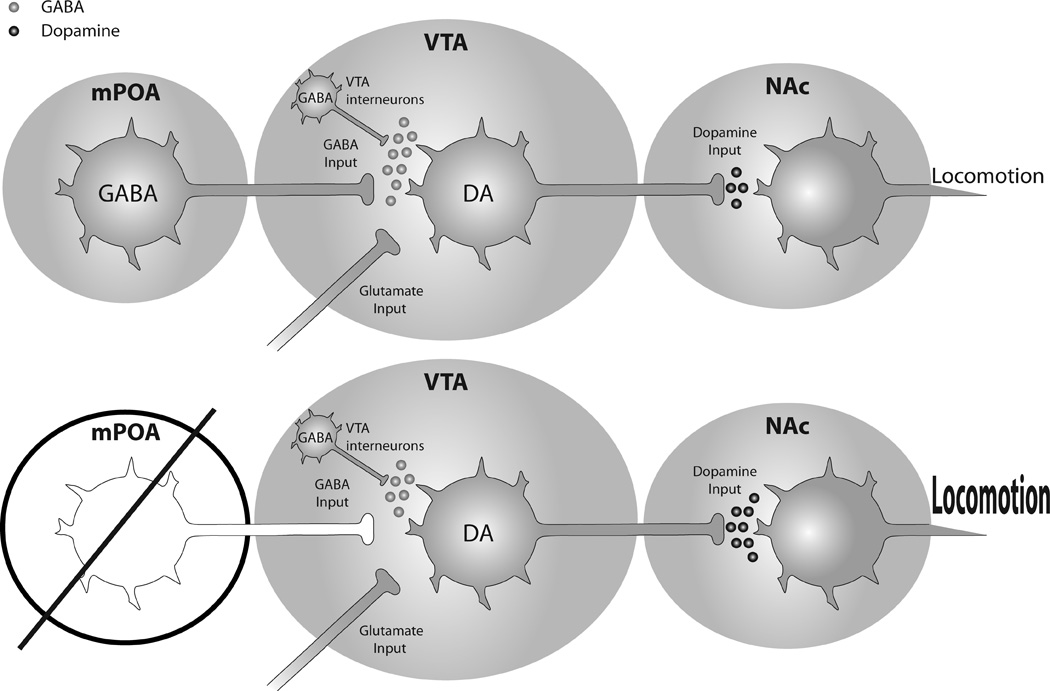
A summary of the proposed mechanism that results in enhanced cocaine-induced locomotion following lesions of the mPOA, adapted from [9]. (Top row) The mPOA is a source of GABA input to the VTA [8, 9]; dopamine-containing efferents in the VTA are a major source of dopamine to the NAc. (Bottom row) In the absence of mPOA-GABAergic input, dopamine efferents in the VTA experience disinhibition, enhancing cocaine-induced dopamine in the NAc, and subsequent locomotion, as evidenced by present findings.
Acknowledgments
This project was funded by NIDA grant R01-DA032789 to JMD. RGW is on NIAAA training grant T32-AA007471. JRM was awarded an Undergraduate Research Fellowship from the Office of the Vice President for Research, The University of Texas at Austin. Parts of this study were performed as fulfillment of JRM’s Honors Thesis. We wish to thank Dr. Chris Robison for comments on an earlier version of this manuscript.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. European Journal of Pharmacology. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- 2.Costall B, Domeney AM, Naylor RJ. Locomotor hyperactivity caused by dopamine infusion into the nucleus accumbens of rat brain: specificity of action. Psychopharmacology. 1984;82:174–180. doi: 10.1007/BF00427768. [DOI] [PubMed] [Google Scholar]
- 3.Costall B, Hui SC, Naylor RJ. Hyperactivity induced by injection of dopamine into the accumbens nucleus: actions and interactions of neuroleptic, cholinomimetic and cholinolytic agents. Neuropharmacology. 1979;18:661–665. doi: 10.1016/0028-3908(79)90032-7. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sciences. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 6.Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacology, Biochemistry, and Behavior. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- 7.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. The Journal of Neuroscience. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobiansky DJ, Roma PG, Hattori T RGW, Nutsch VL, Dominguez JM. The medial preoptic area modulates cocaine-induced activity in female rats. Behavioral Neuroscience. 2013;127:293–302. doi: 10.1037/a0031949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, et al. Estradiol in the Preoptic Area Regulates the Dopaminergic Response to Cocaine in the Nucleus Accumbens. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson LW. Brain Maps III: Structure of the Rat Brain. 3rd. San Diego: California Academic Press; 2004. [Google Scholar]
- 11.Hull EM, Dominguez J. Sexual Behavior. In: Nelson RJ, Mizumori S, editors. Handbook of Psychology: Wiley&Sons. 2012. pp. 331–364. [Google Scholar]
- 12.Lonstein JS, Levy F, Fleming AS. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and Behavior. 2015;73:156–185. doi: 10.1016/j.yhbeh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauber AH, Romano GJ, Pfaff DW. Gene expression for estrogen and progesterone receptor mRNAs in rat brain and possible relations to sexually dimorphic functions. The Journal of Steroid Biochemistry and Molecular Biology. 1991;40:53–62. doi: 10.1016/0960-0760(91)90167-4. [DOI] [PubMed] [Google Scholar]
- 14.Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. The Journal of Comparative Neurology. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- 15.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. The Journal of Neuroscience. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behavioral Neuroscience. 1992;106:181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- 17.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- 19.Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. The Journal of Pharmacology and Experimental Therapeutics. 2000;292:406–414. [PubMed] [Google Scholar]
- 20.Eibergen RD, Caggiula AR. Ventral midbrain involvement in copulatory behavior of the male rat. Physiology & Behavior. 1973;10:435–441. doi: 10.1016/0031-9384(73)90202-3. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat associated with intra-VTA injections of opiates. Pharmacology, Biochemistry, and Behavior. 1990;35:643–650. doi: 10.1016/0091-3057(90)90302-x. [DOI] [PubMed] [Google Scholar]
- 22.Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behavioral Neuroscience. 2009;123:1325–1338. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behavioral Neuroscience. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 24.Herbison AE, Augood SJ, McGowan EM. Expression of glutamic acid decarboxylase messenger RNA in rat medial preoptic area neurones during the oestrous cycle and after ovariectomy. Brain Research, Molecular Brain Research. 1992;14:310–316. doi: 10.1016/0169-328x(92)90098-v. [DOI] [PubMed] [Google Scholar]
- 25.Osborne PG, Mataga N, Onoe H, Watanabe Y. Behavioral activation by stimulation of a GABAergic mechanism in the preoptic area of rat. Neuroscience Letters. 1993;158:201–204. doi: 10.1016/0304-3940(93)90264-l. [DOI] [PubMed] [Google Scholar]