Abstract
The GABAA receptor mediates fast, inhibitory signaling, and cortical expression of the α1 subunit increases during postnatal development. Certain pathological stimuli such as stressors or prenatal cocaine exposure can interfere with this process, but causal relationships between GABAAα1 deficiency and complex behavioral outcomes remain unconfirmed. We chronically reduced GABAAα1 expression selectively in the medial prefrontal cortex (mPFC; prelimbic subregion) of mice using viral-mediated gene silencing of Gabra1. Adolescent-onset Gabra1 knockdown delayed the acquisition of a cocaine-reinforced instrumental response but spared cocaine seeking in extinction and in a cue-induced reinstatement procedure. To determine whether response acquisition deficits could be associated with impairments in action-outcome associative learning and memory, we next assessed behavioral sensitivity to instrumental contingency degradation. In this case, the predictive relationship between familiar actions and their outcomes is violated. Adolescent-onset knockdown, though not adult-onset knockdown, delayed the expression of goal-directed response strategies in this task, resulting instead in inflexible habit-like modes of response. Thus, the maturation of mPFC GABAAα1 systems during adolescence appears necessary for goal-directed reward-related decision making in adulthood. These findings are discussed in light of evidence that prolonged Gabra1 deficiency may impair synaptic plasticity.
Keywords: response-outcome, addiction, habit, GABA(a), mouse
Introduction
The gamma-aminobutyric acid A (GABAA) receptor family is made up of ligand-gated chloride ion channels, which mediate fast inhibitory neurotransmission. These pentameric receptors may be composed of any combination of 19 known subunits. The most common, α1, is found in approximately half of GABAA receptors in the central nervous system, including throughout the cerebral cortex (Olsen and Sieghart, 2009).
During postnatal development, prefrontal cortical GABAAα1 expression levels progressively increase (Fritschy et al., 1994; Hashimoto et al., 2009; Duncan et al., 2010; Datta et al., 2014), and exposure to stressors and drugs of abuse can impinge upon this process. For example, prenatal cocaine exposure decreases postnatal GABAAα1 expression, with impairments first detectable in close proximity to the onset of adolescence (Lu et al., 2009; Huang et al., 2011). As adults, rodents with a history of prenatal cocaine exposure develop anxiety-like behavior and blunted behavioral sensitization to psychostimulants (Crozatier et al., 2003; Lu et al., 2009; Salas-Ramirez et al., 2010; Huang et al., 2011; Wang et al., 2013). A key question, however, is whether adolescent-emergent GABAAα1 deficiency (due to cocaine or other insults) causally regulates behavioral outcomes pertinent to human psychopathology. Alternatively, we may ask: What is the role of typical GABAAα1 expression during adolescent development?
Here we reduced GABAAα1 expression in the medial prefrontal cortex (mPFC), prelimbic subregion, using viral-mediated gene silencing of Gabra1. Viral vectors were initially delivered on postnatal day (P) 31, corresponding to early adolescence in rodents (Spear, 2000; Green and McCormick, 2013). Adolescent-onset Gabra1 knockdown delayed the acquisition of a cocaine-reinforced instrumental response in adulthood, but spared response extinction and cue-induced reinstatement of cocaine seeking, suggesting that healthy mPFC GABAAα1 tone may be essential to developing goal-directed reward-seeking behaviors. To test this perspective, we assessed whether adolescent-onset Gabra1 knockdown impairs the ability of mice to select responses according to action-outcome contingencies – that is, to select behaviors based on the likelihood that they will be reinforced with a particular outcome. To do so, we used a modified form of classical instrumental contingency degradation in which mice were trained to generate two unique instrumental responses, then the likelihood that one response would be reinforced was greatly reduced. Typical mice preferentially engaged the other response, the one most likely to result in a food reinforcer. Adolescent-onset knockdown, however, biased response patterns towards inflexible habit-like strategies that were insensitive to action-outcome contingencies. Interestingly, mice with adult-onset knockdown were largely unaffected in the same task, providing evidence that GABAAα1-dependent maturation of mPFC systems during adolescence optimizes the performance of goal-directed action selection in adulthood.
Methods
Subjects
Transgenic Gabra1-tm1Geh mice back-crossed onto a C57BL/6 background were used (see Vicini et al., 2001; Heldt and Ressler, 2010; Jackson Labs). loxP sites flank the α1 exon encoding an essential transmembrane domain, and Cre recombinase (Cre) deletes this domain. Mice were fed ad libitum except during food-reinforced instrumental conditioning when mice were food-restricted and maintained at ~93% of their original body weight to motivate responding. Males were used throughout. All procedures were Emory University IACUC-approved.
Intracranial surgery
Cre-expressing lentiviruses were generated as described; intracranial delivery results in reduced GABAAα1 expression within at least 2 weeks (Swanson et al., 2015). Mice were anaesthetized with ketamine/xylazine and placed in a digitized stereotaxic frame (Stoelting Co., Wood Dale, IL, USA) at P31 or 56, corresponding to early adolescence and early adulthood, respectively (Spear, 2000; Green and McCormick, 2013). The scalp was incised, skin retracted, bregma and lambda identified, the head leveled, and coordinates located. Viral vectors expressing Cre or Green Fluorescent Protein (GFP) under the cytomegalovirus (CMV) promoter were infused over 5 min in a volume of 0.5 μl at AP+2.0, DV−2.5, ML+/−0.1 (Gourley et al., 2010). Needles were left in place for 5 min prior to withdrawal and suturing.
Intravenous catheterization
Mice were anaesthetized with ketamine/xylazine, and the dorsal and ventral sides were shaved and disinfected. The right jugular vein was exposed by blunt dissection, and a sterile Silastic catheter was placed as described (Thomsen and Caine, 2005) and then exteriorized posterior to the scapulae. The entrance and exit wounds were sutured, and mice were housed individually. During the 5-7 day recovery period, catheter patency was ensured by infusing mice daily with 0.05 ml heparinized saline. Subsequently, catheter patency was tested immediately prior to cocaine self-administration and then again prior to extinction training using a 0.03 ml ketamine challenge (15 mg/ml). If mice were insensitive to ketamine at any point, defined by a failure to lose muscle tone within 10 seconds of infusion, they were excluded.
Behavioral testing
Mice were tested using illuminated Med-Associates conditioning chambers (Georgia, VT, USA) with 2 or 3 nose poke recesses and a separate magazine.
i. Food-reinforced responding
25 days after intracranial surgery, all mice were trained to nose poke for food reinforcers (20 mg Bioserv precision pellets; Flemington, NJ, USA) using a fixed ratio 1 (FR1) schedule of reinforcement; 30 reinforcers were available for responding on each of 2 distinct nose poke recesses, resulting in 60 reinforcers/session. 5-7 daily response acquisition sessions were conducted, as shown, and response rates represent responses on both recesses. We identified no side preferences throughout. Next, mice were used in either cocaine self-administration or instrumental contingency degradation studies.
ii. Cocaine self-administration
Following food-reinforced nose poke training (described immediately above), mice were implanted with in-dwelling jugular catheters. Then, following the 5-7 day recovery period, cocaine self-administration was tested in contextually distinct conditioning chambers. Mice were tested daily, during which a single nose poke response on the center of 3 nose poke recesses was reinforced with an infusion of cocaine (20 μl; 1.25 mg/ml; Sigma) delivered through a catheter connected to a swivel holding armored polyethylene tubing. Delivery culminated in extinction of the house light and a 20-sec timeout. Sessions ended when mice self-administered 30 infusions or at 120 min. Responding was considered stable when mice self-administered ≥20 mg/kg/day, with ≥70% responding on the active nose poke, and ≤20% variability in response rate for 2 sequential days (adapted from Thomsen and Caine, 2005).
After mice reached these criteria, responding was extinguished by placing mice in the conditioning chambers and attaching the catheter tubing, but responding was non-reinforced. Mice were considered to have extinguished when response rates on the previously-active aperture were <25% of the last day of training (Kruzich, 2007). All mice were trained for at least 5 days; sessions 1–5 for all mice are shown in the associated figure.
Finally, cue-induced reinstatement of cocaine seeking was assessed by presenting mice with a single non-contingent cue (house light off, pump on), followed by contingent cue presentations, but no drug infusion, for 60 min (Grimm et al., 2002).
This experiment included 5 intact mice, i.e., mice that did not undergo intracranial surgery. Throughout, GFP-expressing control mice and intact mice did not differ, and their data are combined for statistical and graphical purposes. A total of 18 mice was tested, however 4 control mice died unexpectedly between the initial training and extinction phases.
iii. Instrumental contingency degradation
Following food-reinforced nose poke training, separate groups of mice were tested using a modified form of classical instrumental contingency degradation to assess whether they were sensitive to the predictive relationship between a response and the associated outcome. As previously described (Gourley et al., 2012,2013; Swanson et al., 2013,2015; Hinton et al., 2014), on one day, one of the two nose poke apertures was occluded and pellet delivery was contingent upon responses on the available aperture. Specifically, responding on this aperture was reinforced using a variable ratio 2 (VR2) schedule of reinforcement for 25 min. During a separate session, only the opposite aperture was made available. Here, pellets were delivered independent of animals' interactions with this remaining aperture for 25 min, thus violating, or “degrading,” the predictive relationship between nose poking on that particular aperture and pellet delivery. The pellet delivery rate was matched to each animal's mean reinforcement rate from the previous session (also as in the method of Barker et al., 2013). Thus, the response-outcome relationship associated with each response changed relative to the initial nose poke training period, and responding on one aperture became significantly less predictive of pellet delivery than the other. The order of these sessions and location of the “degraded aperture” were counter-balanced.
The following day, both apertures were available for 10 min during a probe test; responding was not reinforced. Preferential engagement of the highly-reinforced response during this probe test is considered “goal-directed,” while non-selective responding reflects a failure in action-outcome conditioning (Balleine and O'Doherty, 2010).
Mice that had had surgery during adolescence then received 2 additional nose poke training sessions, then the contingency degradation procedure was repeated.
Histology
After behavioral testing, mice were euthanized and brains were fixed by submersion in chilled 4% paraformaldehyde for 48 hours, then chilled 30% w/v sucrose. Brains were sectioned into 40 μm sections at −15°C. Infusion sites in knockdown mice were verified by immunostaining for Cre as described (DePoy et al., 2013). Alternatively, GFP was imaged.
Immunoblotting
Separate mice infused with lenti-Cre or lenti-GFP as adults were euthanized approximately 3 months after infusion. Brains were frozen at −80°C to verify that GABAAα1 protein expression was reduced following lenti-Cre infusion. Standard tissue-punch dissection and electrophoresis techniques were used (as per Swanson et al., 2015). Primary antibodies were anti-GABAAα1 (Millipore, Billerica, MA, USA; Rb; 1:500) and anti-HSP-70 (Santa Cruz, Dallas, TX, USA; Ms; 1:5000), and samples were analyzed in duplicate.
Statistics
Data were analyzed by SPSS. Response rates were compared by ANOVA with repeated measures when appropriate. Following interactions, post-hoc Tukey's t-tests were applied, and results are indicated graphically. For cocaine self-administration experiments, additional metrics (sessions required to ingest 20 mg/kg/day, sessions required to develop stable response rates, and total cocaine infusions) were compared by unpaired t-test. For western blotting experiments, densitometry values were normalized to the corresponding loading control (HSP-70) and compared by unpaired t-test. Throughout, values lying 2 standard deviations outside of the mean were considered outliers and excluded.
Results
Adolescent-onset Gabra1 knockdown delays the acquisition of a cocaine-reinforced response
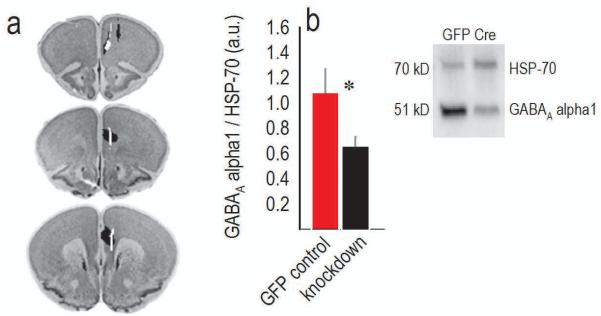
To decrease GABAAα1 expression, we delivered Cre-expressing viral vectors to the mPFC of adolescent `floxed' Gabra1 mice. Histological analyses indicated that viral vectors infected primarily the prelimbic cortex with some spread to the cingulate cortex (fig.1a). Caudally, limited spread into the dorsal infralimbic cortex was detected in a small number of animals, but throughout, the lateral PFC was spared. In 2 mice, Cre was detected in M2, but the majority remained contained within the dorsal mPFC. Two infusions were mis-targeted, and the affected mice were excluded. Using this protocol, GABAAα1 expression was reduced to ~61% of baseline in homogenized tissue punches [t(23)=−2.4, p=0.03] (fig.1b), indicating that targeted knockdown reduced regional protein expression as expected.
Figure 1. GABAAα1 expression is reduced following viral-mediated Gabra1 knockdown.
(a) Histological representations of adolescent infusion sites are transposed onto images from the Mouse Brain Library (Rosen et al., 2000). Black indicates the largest regions affected, and white the smallest. Infusions were bilateral. (b) GABAAα1 receptor expression was reduced in tissue punches collected from the infusion site. Signal was normalized to an HSP-70 loading control, and representative blots are adjacent. n=12–13. Means+SEMs,*p<0.05.
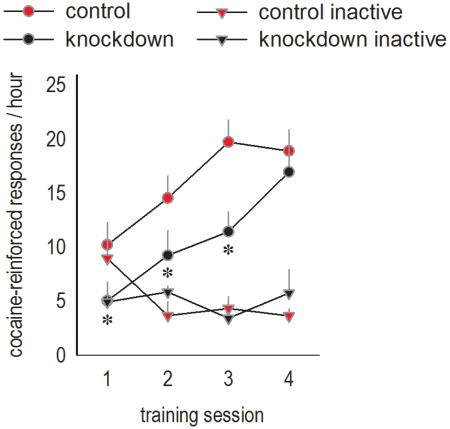
Viral vectors were infused in early adolescence, at P31 (timing based on Spear, 2000). As adults, mice were implanted with indwelling jugular catheters for intravenous cocaine self-administration studies (timeline in fig.2a). During the initial training of the cocaine-reinforced response, response rates in knockdown mice lagged, with lower rates on the active, but not inactive, aperture [interaction F(1,48)=10.4, p=0.005] (fig.2b). [A main effect of session was also detected as expected [F(3,48)=2.9, p=0.05], with no overall effect of group [F(1,16)=2.4, p=0.14].]
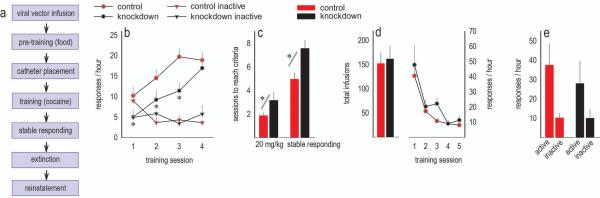
Figure 2. Adolescent-onset GABAAα1 silencing retards the acquisition of a cocaine-reinforced response; responding in extinction and cue-induced reinstatement are intact.
(a) Experimental timeline. (b) The initiation of cocaine-reinforced responding lagged following Gabra1 knockdown. Cocaine-reinforced response rates are represented, as are response rates on inactive nose poke recesses. (c) Accordingly, knockdown mice required more sessions to obtain 20 mg/kg/day (left) and to develop stable response rates (right). (d) Nonetheless, knockdown mice ultimately ingested as much cocaine as GFP-expressing counterparts (left), and responding in extinction was intact (right). (e) Additionally, all mice developed cue-induced reinstatement of cocaine seeking. Means+SEMs,*p<0.05. Control n=13, knockdown n=5.
As would be expected by the response acquisition curves, control mice ingested 20 mg/kg more rapidly than knockdown mice [t(15)=−2.4, p=0.03] (fig.2c, left). Similarly, mice with Gabra1 knockdown required more training sessions to meet response stability criteria [t(14)=−2.2, p<0.05] (fig.2c, right).
Mice were trained until they met stability criteria, ensuring that groups ultimately did not differ in the total cocaine infusions self-administered [t(14)=−0.26, p>0.8] (fig.2d, left). With drug exposure thus matched, we next extinguished the cocaine-reinforced response, and here, response rates did not differ [interaction F<1; effect of group F(1,12)=1.5, p>0.2; effect of session F(4,48)=16.9, p<0.001] (fig.2d, right). Additionally, mice did not differ in the degree of response reinstatement when presented with cocaine-associated stimuli [effect of knockdown F<1] (fig.2e). Thus, Gabra1 knockdown impaired response initiation, but spared cocaine seeking in extinction, as well as relapse-like behavior.
Adolescent-onset Gabra1 knockdown impairs goal-directed action selection
Based on the hypothesis that the initial delay in the development of a cocaine-reinforced instrumental response might be associated with impaired action-outcome associative learning and memory, we generated another group of mice with Gabra1 knockdown in the prelimbic prefrontal cortex and assessed action-outcome decision-making strategies (fig.3a–b). We used a task in which mice were trained to generate 2 operant responses equally, then the likelihood that one response would be reinforced was greatly reduced. Meanwhile, the other response remained reinforced. Preferential engagement of the highly-reinforced response during a subsequent probe test is prelimbic cortical-dependent and considered “goal-directed,” while non-selective responding reflects a failure in action-outcome conditioning (Balleine and O'Doherty, 2010).
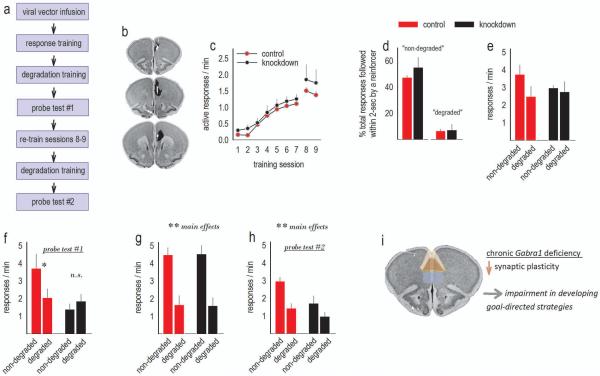
Figure 3. Adolescent-onset GABAAα1 silencing impairs goal-directed action selection in adulthood.
(a) Experimental timeline. (b) Histological representations of infusion sites are transposed onto images from the Mouse Brain Library (Rosen et al., 2000). Black indicates the largest regions affected, and white the smallest. Infusions were bilateral. (c) Mice were trained to respond for food reinforcers; breaks in the acquisition curves annotate tests for sensitivity to action-outcome contingency degradation. Response rates represent both trained responses/min. (d) Following training, the schedules of reinforcement were modified such that roughly half of responses directed towards one nose poke recess were reinforced, while pellet delivery followed only ~7% of responses directed towards a different nose poke recess. These contingencies are referred to as “non-degraded” and “degraded.” (e) Response rates during these training sessions are shown. (f) During a subsequent probe test, control mice preferentially generated the response most likely to be reinforced in a goal-directed manner (“non-degraded”). Mice with Gabra1 knockdown instead generated both responses at equivalent rates, a failure in action-outcome conditioning. (g) With additional task experience, both groups generated higher response rates during training sessions when the response was likely to be reinforced. (h) Correspondingly, mice were also ultimately able to select actions that were more, vs. less, likely to be reinforced in a probe test. (i) Our model: Chronic Gabra1 deficiency has been associated with decreased synaptic marker expression and decreased expression of mushroom-shaped spines in the cortex (see Discussion). We suggest that these abnormalities in the prelimbic cortex (highlighted at center) delay the development of goal-directed response strategies. The anterior cingulate (dorsal) and the medial orbitofrontal cortex (ventral) are also highlighted on this image from the Mouse Brain Library (Rosen et al., 2000). Means+SEMs,*p<0.05,**p≤0.01. n=5/group.
Response acquisition curves represent both responses/min, and we detected no differences in response rates between groups [interaction F<1; effect of group F(1,8)=2.1, p=0.18; effect of session F(8,64)=17.6, p<0.001] (fig.3c). Next, one nose poke recess was occluded, and responding on the remaining available nose poke aperture was reinforced using a VR2 schedule of reinforcement. In this case, roughly 50% of responses were reinforced, and this percentage did not differ between groups [t(8)=0.98, p=0.4] (fig.3d, left). In another session, the opposite nose poke recess was occluded, and food pellets associated with the other response were delivered non-contingently at a rate yoked to each animal's own reinforcement rate from the previous session. In this case, only ~7% of pellets were delivered (by chance) within 2 seconds of a response. This percentage did not differ between groups [t(8)=−0.14, p=0.9] (fig.3d, right). Response rates generated during these 2 training sessions are shown (fig.3e). Control mice appeared to generate the highly reinforced response with greater frequency than the response that was unlikely to be reinforced, while response rates generated by the knockdown mice did not appear to differ between conditions. During this training period, however, neither an interaction effect (which would support this perspective), nor a main effect of response selection (which could refute it), was detected [F(1,8)=2.5, p=0.15; F(1,8)=1.8, p=0.22, respectively]. We also detected no effect of group [F<1].
During the subsequent probe test, response patterns crystalized, and control mice clearly preferentially generated the response more likely to be reinforced, evidence of goal-directed action selection (fig.3f). Meanwhile, mice with adolescent-onset knockdown failed to differentiate between the responses [interaction F(1,8)=10.6, p=0.02] (fig.3f). Significant main effects were not detected [of response selection, F(1,8)=3.4, p=0.1; of group, F(1,8)=3.5, p=0.1].
To determine whether adolescent-onset knockdown delays the acquisition of goal-directed action selection strategies or prevents them entirely, we retrained the nose poke responses (see fig.3c, sessions 8,9), and then again “degraded” one action-outcome contingency. In reaction, both groups preferentially engaged the response most likely to be reinforced during training and during the probe test [main effects, F(1,8)=40.3, p<0.001; F(1,8)=11.4, p=0.01 respectively] (fig.3g–h). No significant interaction effects were detected during these periods [F<1; F(1,8)=1.4, p=0.27, respectively]. Notably, however, as in cases of prelimbic cortical lesions (Corbit and Balleine, 2003), knockdown mice responded less overall during the probe test [main effect F(1,8)=17.2, p=0.003] (fig.3h), though not during training [F<1] (fig.3g).
Thus, adolescent-onset Gabra1 knockdown in the prelimbic cortex delayed, but did not block, the expression of goal-directed decision-making strategies. As will be addressed in the Discussion, we propose a model in which prolonged Gabra1 silencing impairs synaptic plasticity in the prelimbic cortex, which then delays the development of goal-directed response selection strategies (fig.3i).
The effects of mPFC Gabra1 knockdown in adolescence and adulthood are dissociable
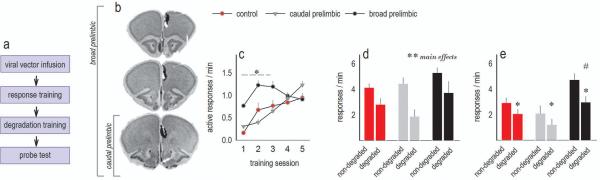
To assess whether knockdown had age-specific effects, we next delayed knockdown until adulthood. In this case, histological analyses revealed 2 populations of mice – those with infusions contained within the caudal prelimbic cortex, and mice that were more similar to the adolescent-onset knockdown group, with broader infection including the rostral prelimbic and a portion of the anterior cingulate cortex (fig.4a–b). These groups are referred to as “caudal prelimbic” and “broad prelimbic” and are represented separately. Behaviorally, lenti-GFP control groups did not differ and have been combined.
Figure 4. Site-selective Gabra1 knockdown in adulthood does not obviously impair goal-directed action selection.
(a) Experimental timeline. (b) Histological representation of viral vector expression imposed onto images from the Mouse Brain Library (Rosen et al., 2000). Infusions were bilateral. Some infections were contained within the caudal prelimbic cortex, and are referred to as “caudal prelimbic.” Meanwhile, others were more broadly distributed within the mPFC and are referred to as “broad prelimbic.” (c) Acquisition of food-reinforced responses was augmented by broad prelimbic Gabra1 knockdown, but all mice ultimately responded at equivalent rates. Response rates represent both trained responses/min. (d) Unlike with adolescent-onset knockdown, knockdown mice here inhibited responses that were unlikely to be reinforced during training. (e) Also, knockdown mice could differentiate between responses that were more, or less, likely to be reinforced during a probe test. Mice with broad mPFC knockdown responded more overall. Means+SEMs,*p<0.05,**p<0.001. # signifies higher rates of responding overall in the “broad prelimbic” group, p<0.05. Total control n=14, broad prelimbic knockdown n=8, caudal prelimbic knockdown n=8.
Mice were food-restricted and trained to generate 2 distinct food-reinforced responses. Again, response acquisition curves represent both responses/minute. Broad knockdown acutely enhanced response rates during training, while caudal prelimbic-selective knockdown had no effects [interaction F(8,108)=4.8, p<0.001; main effect of group F(2,27)=4.5, p=0.02] (fig.4c). A main effect of session was also detected as expected [F(4,108)=13.5, p<0.001].
Next, pellets associated with one response were delivered non-contingently. All mice inhibited responding during this period of action-outcome contingency degradation [main effect of response choice F(1,27)=23.2, p<0.001; of group F(2,27)=2.7, p=0.08; interaction F<1] (fig.4d). Subsequently, all mice showed evidence of knowledge of action-outcome associative contingencies, in that they preferentially engaged the response that was most likely to be reinforced; additionally, broad prelimbic infection resulted in higher response rates overall [interaction F(2,27)=4.7, p=0.02; main effect of response F(1,27)=52.7, p<0.001; main effect of group F(2,27)=6.1, p=0.007] (fig.4e).
Discussion
The mPFC develops considerably during adolescence (Spear, 2000; Green and McCormick, 2013). Across mammalian species, this process includes the up-regulation of GABAAα1 expression (Fritschy et al., 1994; Hashimoto et al., 2009; Duncan et al., 2010; Datta et al., 2014). Certain pathological stimuli such as prenatal cocaine exposure or early-life stress, by contrast, decrease cortical GABAAα1 or Gabra1 expression when measured immediately prior to adolescence, in adolescence, or in adulthood (Hsu et al., 2003; Lu et al., 2009; Huang et al., 2011). Conversely, stressor resilience is associated with an enhancement in mPFC GABAAα1 (Caldji et al., 2000). Nevertheless, the causal effects of adolescent-onset GABAAα1 deficiency on complex decision making pertinent to human psychopathology remain unclear. We report that prelimbic cortical adolescent-onset Gabra1 knockdown retards the acquisition of a cocaine-reinforced instrumental response. Stimulus-elicited relapse-like behavior is intact in a reinstatement test however, suggesting that healthy mPFC GABAAα1 tone may be essential in particular to developing goal-directed decision-making strategies – that is, selecting actions based on the likelihood of reinforcement, rather than in response to reward-related cues. To test this perspective, we used a modified form of classical instrumental contingency degradation and found that adolescent-onset, but not adult-onset, knockdown indeed delays the development of goal-directed response strategies in adulthood.
mPFC GABAAα1 regulates cocaine self-administration and goal-directed action selection
In our first series of experiments, we delivered viral vectors selectively to the prelimbic cortex during early adolescence, causing a site-selective knockdown of Gabra1. We then implanted in-dwelling jugular catheters in adulthood for cocaine self-administration studies. Adolescent-onset Gabra1 knockdown impaired the initiation of cocaine self-administration. Why might this be? First, it is possible that mPFC-targeted Gabra1 knockdown decreased initial, though not long-term, sensitivity to the incentive motivational properties of cocaine, which could account for delayed response acquisition, but intact responding during a reinstatement test. This perspective is based on evidence that both rats and rabbits exposed to prenatal cocaine – which reduces mPFC GABAAα1 expression to roughly 70% of control levels (Hsu et al., 2003; Lu et al., 2009; Huang et al., 2011) – are initially resistant to the locomotor-activating effects of amphetamine, but locomotor activation after a challenge injection is intact (Lu et al., 2009; Huang et al., 2011; Simansky and Kachelries, 1996; Wang et al., 2013).
Additionally of note, the prelimbic cortex regulates cocaine-seeking behaviors. For example, lesions or inactivation of the prelimbic cortex decrease cocaine-reinforced responding in rodents and interfere with cocaine-conditioned place preference (for excellent review, see Moorman et al., 2014). This is particularly relevant because viral-mediated prefrontal cortical Gabra1 knockdown, as used here, causes synaptic marker elimination as early as 2 weeks following viral vector infusion (Swanson et al., 2015). Further, Gabra1+/− mice have fewer mature, mushroom-shaped dendritic spines – those likely to contain synapses – in the cerebral cortex relative to genetically-intact control mice (Heinen et al., 2003). Lastly, experiments using in vivo multiphoton imaging indicate that repeatedly inhibiting GABAA receptor activity accelerates typical dendritic spine pruning, resulting in a net reduction in dendritic spines in the cerebral cortex (Chen, 2014). All together, these findings suggest that Gabra1 knockdown here may have weakened cocaine-seeking behaviors by destabilizing synaptic plasticity in the prelimbic cortex.
We hypothesized that an additional possibility, which is not mutually exclusive, is that prelimbic cortical Gabra1 knockdown impairs action-outcome conditioning – that is, associating a specific behavior or behaviors with reinforcement. This could contribute to the delayed development of a cocaine-reinforced response following Gabra1 knockdown. We tested the possibility that Gabra1 knockdown weakens action-outcome associative learning and memory using an instrumental contingency degradation task. Mice were first trained to generate two distinct food-reinforced instrumental responses that were equally likely to be reinforced. Then, the likelihood of reinforcement associated with one of the responses was greatly decreased. In reaction, control mice preferentially generated the remaining response, that likely to be reinforced, providing evidence of knowledge of the action-outcome relationship (Balleine and O'Doherty, 2010; Dickinson, 1980). Mice with adolescent-onset knockdown, however, failed to differentiate between the responses and generated both equally. A failure to modify response strategies following the degradation of an instrumental contingency is thought to reflect a failure in goal-directed decision making; instead, responding is interpreted as stimulus-dependent and reflexive, or “habitual.”
As with chronic Gabra1 knockdown here, lesions of the prelimbic cortex block sensitivity to instrumental contingency degradation, inducing stimulus-elicited habits (Balleine and Dickinson, 1998; Corbit and Balleine, 2003; Killcross and Coutureau, 2003; Dutech et al., 2011). These deficits are also similar to those resulting from Gabra1 knockdown in the ventrolateral orbital prefrontal cortex (VLO; Swanson et al., 2015). The VLO is interconnected with medial wall structures such as the prelimbic cortex in primates, rats, and mice (Sesack et al., 1989; Carmichael and Price, 1996; Ongur and Price, 2000; Gremel and Costa, 2013). Also, both the prelimbic cortex and VLO innervate the dorsomedial striatum, and the VLO additionally has terminals in the central/lateral striatum (Sesack et al., 1989; Schilman et al., 2009; Gremel and Costa, 2013). Instrumental conditioning induces immediate-early gene expression in these dorsomedial and central/lateral striatal compartments (Maroteaux et al., 2014), and studies using lesions or temporary inactivation approaches indicate that, unlike the dorsolateral compartment, the dorsomedial and central/lateral striatum are necessary for selecting actions based on expected outcomes (Yin et al., 2008; Gourley et al., 2013). We suggest that chronic Gabra1 knockdown interferes with the integrity of these cortico-striatal circuits and thereby biases response strategies towards reflexive habits at the expense of goal-directed action selection.
Notably, Gabra1 knockdown mice were ultimately able to develop goal-directed response patterns with additional training, indicating that knockdown delayed, but did not block, action-outcome conditioning. These findings are in agreement with evidence that failures in goal-directed decision making following permanent or temporary inactivation of the prelimbic cortex are attributable to impairments in the development, as opposed to expression, of outcome-based response strategies (Ostlund and Balleine, 2005; Tran-tu-Yen et al., 2009). Considering our finding that Gabra1 knockdown also delayed the acquisition of a cocaine-reinforced response, we suggest that intact prelimbic cortical neurocircuits may energize the development of goal-directed behaviors including in the context of drugs of abuse. This would allow for flexible drug seeking as behavioral strategies evolve and become ingrained.
The lack of effect of Gabra1 knockdown on response extinction may seem to contradict evidence that acute microinfusion of the GABAA agonist muscimol into the mPFC occludes the extinction of cocaine-reinforced responding (LaLumiere et al., 2010). However, LaLumiere and colleagues (2010) found that infusions targeted to the infralimbic cortex, and not prelimbic cortex, regulate response extinction. Our infusions overwhelmingly targeted the prelimbic cortex and spared response extinction, in agreement with LaLumiere et al. (2010).
The effects of mPFC Gabra1 knockdown in adolescence and adulthood are dissociable
Remarkably, when we delayed knocking down mPFC Gabra1 until adulthood, a different behavioral response pattern emerged: First, behavioral sensitivity to instrumental contingency degradation was intact, even in mice with infusions selective to the prelimbic compartment. Given that the mature prelimbic cortex is essential to goal-directed action selection (Balleine and Dickinson, 1998; Corbit and Balleine, 2003; Killcross and Coutureau, 2003), this outcome was unexpected. Nonetheless, it importantly indicates: 1) that deficiencies following adolescent-onset knockdown are unlikely to reflect the acute effects of knockdown at test in adulthood, but rather the progressive effects of knockdown during a critical period of neocortical development. Additionally: 2) adolescence is a critical period during which even subtle disruptions in mPFC GABA-ergic systems may have long-term maladaptive consequences, and mature cortical systems are by contrast resilient. Finally: 3) given that adult-onset Gabra1 knockdown in the VLO impairs action-outcome conditioning (Swanson et al., 2015), these findings together raise the possibility that mPFC GABAergic systems are essential to goal-directed action selection during adolescence and early adulthood, but that VLO GABAergic systems “come on-board” or otherwise play a more predominant role later in adulthood.
Response rates during the initial phases of food-reinforced response training were elevated in mice with knockdown broadly distributed throughout the anterior cingulate and rostral and caudal prelimbic cortices. By contrast, mice with knockdown restricted to the caudal prelimbic cortex were unaffected, suggesting that Gabra1 silencing in the rostral mPFC accounts for increased response rates. Chronic Gabra1 knockdown decreases synaptic marker expression in the PFC (Swanson et al., 2015), and certain forms of learning and memory are associated with dendritic spine elimination (Lai et al., 2012; Sanders et al., 2012). Thus, Gabra1 knockdown-mediated plasticity or possibly synapse clearance in the rostral prelimbic and/or anterior cingulate cortex may account for augmented response acquisition, but future investigations would be needed to explicitly investigate this possibility.
Conclusions
The double dissociation between the effects of adolescent- vs. adult-onset Gabra1 knockdown on outcome-based decision making reported here highlights a challenge in basic research – that neurobehavioral studies using genetic or environmental manipulations in adult rodents may be ineffective in informing psychopathologies with neurodevelopmental etiology. As another example, cocaine exposure results in a hypo-metabolic state in the prefrontal cortex of mature rats, while the same cocaine exposure protocol applied in adolescence has the opposite effects (Cass et al., 2013). Despite challenges (e.g., associated with intracranial surgery in adolescent mice and intravenous catheterization of the same subjects later in life, as was performed to complete the studies reported here), experimental approaches applied in developmentally-sensitive ways may more optimally illuminate ontogenic factors in complex behavior.
Acknowledgements
We thank Dr. Kerry Ressler for generously providing the transgenic mice used here and Dr. Ressler and Ms. Elizabeth Hinton for valuable feedback. This work was supported by the Emory University Research Council, DA015040 (PI: Kuhar), and DA034808 (PI: Gourley). The Yerkes National Primate Research Center is supported by the Office of Research Infrastructure Programs/OD P51OD11132. The Emory Viral Vector Core is supported by an NINDS Core Facilities grant, P30NS055077.
Footnotes
The authors have no conflicts to disclose.
References
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR. Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Front. Neurosci. 2013;7:126. doi: 10.3389/fnins.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol. Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. GABA-A receptor-dependent mechanisms prevent excessive spine elimination during postnatal maturation of the mouse cortex in vivo. FEBS Lett. 2014;588:4551–4560. doi: 10.1016/j.febslet.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav. Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Crozatier C, et al. Altered cocaine-induced behavioral sensitization in adult mice exposed to cocaine in utero. Brain Res. Dev. Brain Res. 2003;147:97–105. doi: 10.1016/j.devbrainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Datta D, Arion D, Lewis DA. Developmental expression patterns of GABAA receptor subunits in layer 3 and 5 pyramidal cells of monkey prefrontal cortex. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu040. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Contemporary Animal Learning Theory. Cambridge University Press; Cambridge: 1980. [Google Scholar]
- Duncan CE, et al. Prefrontal GABAA receptor α-subunit expression in normal postnatal human development and schizophrenia. J. Psych. Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Dutech A, Coutureau E, Marchand AR. A reinforcement learning approach to instrumental contingency degradation in rats. J. Physiol. Paris. 2011;105:36–44. doi: 10.1016/j.jphysparis.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: An immunohistochemical study. J. Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of goal-directed action within mouse prefrontal cortex. Eur. J. Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc. Natl. Acad. Sci. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, et al. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur. J. Neurosci. 2013;38:2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, McCormick CM. Effects of stressors in adolescence on learning and memory in rodent models. Hormones Behav. 2013;64:364–379. doi: 10.1016/j.yhbeh.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual action. Nat. Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm J, Shaham Y, Hope B. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav. Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, et al. Protracted developmental trajectories of GABAA receptor α1 and α2 subunit expression in the primate prefrontal cortex. Biol. Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Baker RE, Spijker S, Rosahl T, van Pelt J, Brussaard AB. Impaired dendritic spine maturation in GABAA receptor alpha1 subunit knock out mice. Neuroscience. 2004;122:699–705. doi: 10.1016/s0306-4522(03)00477-9. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J. Neurosci. 2010;30:7139–7151. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton EA, Wheeler MG, Gourley SL. Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn. Mem. 2014;21:253–257. doi: 10.1101/lm.033290.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, et al. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc. Natl. Acad. Sci. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. Prenatal cocaine exposure enhances long-term potentiation induction in rat medial prefrontal cortex. Int. J. Neuropsychopharmacol. 2011;14:431–443. doi: 10.1017/S1461145710000258. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex. Cereb. Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ. Does response-contingent access to cocaine reinstate previously extinguished cocaine-seeking behavior in C57BL/6J mice? Brain Research. 2007;1149:165–171. doi: 10.1016/j.brainres.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodeling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn. Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Lim B, Poo MM. Cocaine exposure in utero alters synaptic plasticity in the medial prefrontal cortex of postnatal rats. J. Neurosci. 2009;29:12664–12674. doi: 10.1523/JNEUROSCI.1984-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux M, Valjent E, Longueville S, Topilko P, Girault JA, Hervé D. Role of the plasticity-associated transcription factor zif268 in the early phase of instrumental learning. PLoS One. 2014;9:e81868. doi: 10.1371/journal.pone.0081868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.024. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisitionbut not the expression of goal-directed learning. J. Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G, et al. The mouse brain library; International Mouse Genome Conference; 2000. p. 166. at www.Mbl.Org. [Google Scholar]
- Salas-Ramirez KY, Frankfurt M, Alexander A, Luine VN, Friedman E. Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience. 2010;169:1287–1295. doi: 10.1016/j.neuroscience.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Cowansage K, Baumgartel K, Mayford M. Elimination of dendritic spines with long-term memory is specific to active circuits. J. Neurosci. 2012;32:12570–12578. doi: 10.1523/JNEUROSCI.1131-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simansky KJ, Kachelries WJ. Prenatal exposure to cocaine selectively disrupts motor responding to D-amphetamine in young and mature rabbits. Neuropharmacology. 1996;35:71–78. doi: 10.1016/0028-3908(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Swanson AM, Shapiro LP, Whyte AJ, Gourley SL. Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Comm. Int. Biol. 2013:e26068. doi: 10.4161/cib.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson AM, Allen AG, Shapiro LP, Gourley SL. GABAAα1-mediated plasticity in the orbitofrontal cortex regulates context-dependent action selection. Neuropsychopharmacology. 2015;40:1027–1036. doi: 10.1038/npp.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr. Protoc. Neurosci. 2005 doi: 10.1002/0471142301.ns0920s32. Unit 9.20. [DOI] [PubMed] [Google Scholar]
- Tran-tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur. J. Neurosci. 2009;30:464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J. Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur. J. Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, et al. Overinhibition of corticostriatal activity following prenatal cocaine exposure. Ann. Neurol. 2013;73:355–369. doi: 10.1002/ana.23805. [DOI] [PMC free article] [PubMed] [Google Scholar]