Abstract
Purpose
To test whether palisade endings are a general feature of mammalian extraocular muscles (EOMs).
Methods
Thirteen species, some frontal-eyed (human, monkey, cat, and ferret), and others lateral-eyed (pig, sheep, calf, horse, rabbit, rat, mouse, gerbil, and guinea pig) were analyzed. Palisade endings were labeled by using different combinations of immunofluorescence techniques. Three-dimensional reconstructions of immunolabeled palisade endings were done.
Results
In all frontal-eyed species, palisade endings were a consistent feature in the rectus EOMs. Their total number was high and they exhibited an EOM-specific distribution. In particular, the number of palisade endings in the medial recti was significantly higher than in the other rectus muscles. In the lateral-eyed animals, palisade endings were infrequent and, when present, their total number was rather low. They were only found in ungulates (sheep, calf, pig, and horse) and in rabbit. In rodents (rat, guinea pig, mouse, and gerbil) palisade endings were found infrequently (e.g., rat) or were completely absent. Palisade endings in frontal-eyed species and in some lateral-eyed species (pig, sheep, calf, and horse) had a uniform morphology. They generally lacked α-bungarotoxin staining, with a few exceptions in primates. Palisade endings in other lateral-eyed species (rabbit and rat) exhibited a simplified morphology and bound α-bungarotoxin.
Conclusions
Palisade endings are not a universal feature of mammalian EOMs. So, if they are proprioceptors, not all species require them. Because in frontal-eyed species, the medial rectus muscle has the highest number of palisade endings, they likely play a special role in convergence.
Keywords: oculomotor system, proprioception, frontal-eyed species, lateral-eyed species, palisade endings, convergence
The eyes are the most mobile organs in the body and are moved by contraction of the extraocular muscles (EOMs). It has been supposed that EOMs are richly endowed with proprioceptors providing feedback about the direction of gaze, that in turn allows individuals to determine where foveated objects are located in space. But surprisingly, classical proprioceptors are only found in the EOMs of even-toed ungulates (i.e., pigs), where muscle spindles and Golgi tendon organs are present in very high numbers.1 In the eye muscles of primates, muscle spindles are only sometimes present, whereas Golgi tendon organs are entirely absent. Muscle spindles are reported to be frequent in humans, but are rare in monkeys, and in both species they display a simplified morphology.2–4 Unlike even-toed ungulates, the phylogenetically closely related odd-toed ungulates (horses) do not possess muscle spindles or Golgi tendon organs.1 In other species, like cat, dog, rabbit, rat, and mouse, they are likewise absent.1 In conclusion, classical proprioceptors are only present in few species, but the reason for these interspecies variations is still unclear and there is no evolutionary and/or functional explanation for their pattern of existence.5
Because classical proprioceptors are an exception in mammalian eye muscles, scientists searched for an alternative proprioceptive structure. A nervous end organ, termed the palisade ending or innervated myotendinous cylinder, was first described by Dogiel6 and has been found in every species so far investigated, including human, monkey, cat, dog, sheep, rabbit, and rat.7–13 Palisade endings are a peculiar structure, solely found in EOMs and located in myotendinous junctions at the tip of individual muscle fibers. Palisade endings consist of a dense ramification of axonal branches and boutons. The muscle fibers associated with palisade endings are the multiply innervated muscle fibers of the EOM global layer, which possess several en grappe terminals along their length.7,13
For a century, palisade endings have been considered as sensory structures substituting for muscle spindles and Golgi tendon organs in EOMs. Interest in palisade endings was reignited when molecular analysis and neuronal tracing experiments determined that palisade endings are cholinergic and originate from the EOM motor nuclei.14–18
Today, the prevailing opinion is that palisade endings are a universal feature of mammalian EOMs. Here, we have analyzed palisade endings in 13 mammalian species, including frontal-eyed (human, monkey, cat, and ferret) and lateral-eyed (pig, sheep calf, horse, rabbit, rat, guinea pig, mouse, and gerbil). In those species endowed with palisade endings, we quantified these EOM-specific organs and analyzed their structure and molecular pattern. Our findings do not support the theory that palisade endings are ubiquitous in mammals and instead indicate a relationship between the presence of palisade endings and the oculomotor repertoire.
Materials and Methods
The following animal species were analyzed: primates (human and monkey), carnivores (cat and ferret), even-toed ungulates (pig, sheep, and cow), odd-toed ungulates (horse), lagomorphs (rabbit), and rodents (rat, guinea pig, mouse, and gerbil). Two individuals in each case were analyzed from human, monkey, cow, and horse; three from ferret, pig, sheep, guinea pig, mouse, and gerbil; four from cat, rabbit, and rat. Material used in this study was obtained from a variety of sources. Specifically, human bulbi were obtained from bodies donated to the Department of Anatomy of the Innsbruck Medical University by people who had given informed consent for their use for scientific and educational purposes before death. Monkey tissue was received from the University of Mississippi Medical Center; material from pig, rat, mouse, and guinea pig from the Medical University Vienna; material from cat and rabbit from the University of Seville; ferret material from the Lund University; gerbil tissue from the Ludwig-Maxmilians University Munich; horse tissue from the University of Veterinary Medicine in Vienna; and sheep and calf tissue from a local abattoir in Seville. Surgical and handling procedures for experiments followed the guidelines of the National Institutes of Health (NIH; http:/oacu.od.nih.gov, in the public domain), specific recommendations for maintenance of higher mammals during neuroscience experiments (NIH publication 94-3207, 1994), and were in accordance with national legislation for the use and care of laboratory animals (R.D. 53/2013, BOE 34/11370-421, 2013). Human tissue was collected 12 hours after death; sheep, calf, and horse tissue within the same day; and all other tissue immediately after death.
Eye muscles from humans, horses, pigs, sheep, and calves were excised and fixed by immersion in 4% paraformaldehyde containing 0.1 M sodium phosphate buffer, pH 7.4. All other animals (monkey, cat, ferrets, rabbit, rat, guinea pig, mouse, and gerbil) were deeply anesthetized with a terminal dose of sodium pentobarbital (50 mg/kg, intraperitoneally) and intracardially perfused with physiological saline followed by the above-mentioned fixative. Then, bulbi, including the EOMs, were dissected and postfixed for 2 hours. Thereafter, all tissue was stored for up to 4 weeks in buffer containing 0.05% sodium azide at 4°C before further processing.
For analysis, EOM whole-mount preparations were used. In ferret, rat, guinea pig, mouse, and gerbil, the muscles were small and the complete EOMs, including the distal tendon, were used as whole-mounts. In human, monkey, cat, pig, sheep, calf, horse, and rabbit, the EOMs were large and were therefore cut transversally into two pieces: one piece containing the muscle belly and the second containing the distal part of the muscle with the attached tendon. Exclusively, the distal EOM myotendinous pieces were used as whole-mount preparations.
Immunofluorescence Labeling
Extraocular muscle whole-mounts were labeled using either double-fluorescent staining or two combinations of triple-fluorescent labeling. For double labeling, we used an antibody against neurofilaments, as well as phalloidin staining. For triple fluorescent labeling, we used either antibodies against neurofilaments, synaptophysin, and phalloidin staining, or antibody against neurofilaments along with α-bungarotoxin and phalloidin stainings. The antibody against neurofilaments was used to stain axons and the antibody against synaptophysin, a synaptic vesicle membrane protein, was used to label nerve terminals; α-bungarotoxin, which binds to nicotinic acetylcholine receptors in the muscle fiber membrane, was used to detect motor terminals at the postsynaptic level. Phalloidin, which binds to actin filaments, labels muscle fibers. Primary antibodies were from Merck Millipore (Darmstadt, Germany); secondary antibodies, phalloidin, and α-bungarotoxin from were Molecular Probes (Waltham, MA, USA).
Before applying antibodies, the tissue was blocked for 2 hours with 10% normal goat serum in sodium PBS containing 1% Triton (PBS-T). In double fluorescent labeling, tissue was incubated for 48 hours with the primary antibody, rinsed thoroughly with PBS-T, and incubated for 24 hours with the secondary antibody and phalloidin. In triple fluorescent labeling, the tissue was incubated for 48 hours with the primary antibodies, washed with PBS-T, and incubated for 24 hours with one of the secondary antibodies. After another washing step, tissue was incubated overnight with the other secondary antibody and phalloidin (staining combination 1), or with α-bungarotoxin and phalloidin (staining combination 2). Finally, the tissue was rinsed again and mounted in vol/vol 60% glycerin + 40% PBS. Primary antibodies (anti-neurofilament 1:2500 and anti-synaptophysin 1:300) were applied at room temperature, secondary antibodies (goat anti-chicken Alexa Fluor 568 and goat anti-mouse Alexa Fluor 488, both 1:500), phalloidin (1:100), and α-bungarotoxin (1:500) at 37°C.
For negative control experiments, primary antibodies were omitted and secondary antibodies were used alone. In all cases, the omission of the primary antibodies resulted in a complete lack of immunostaining.
Fluorescently-labeled whole-mounts were analyzed with a confocal laser scanning microscope (CLSM; LSM 510 and LSM 700; Carl Zeiss Meditec, Oberkochen, Germany). A series of virtual CLSM sections of 0.5 to 1.0 μm thickness were cut through the structures of interest. Each section was photo-documented and 3D projections were formulated on a computer using LSM ZEN software (Carl Zeiss Meditec). Images were generated in three different fluorescence channels: excitation wavelengths of 488, 568, and 633 nm.
Three-Dimensional Reconstructions
Three-dimensional (3D) reconstructions were made from single palisade endings of human, monkey, cat, ferret, rabbit, and rat. For 3D reconstruction, we used z-stacks from the CLSM images, which were transferred to a PC workstation equipped with the software package AMIRA 5.4 (Mercury Computer Systems, Chelmsford, MA, USA). The image stacks were loaded into the AMIRA software and converted to volume data. The voxel size of the preparation was as follows: human, 0.17 × 0.17 × 0.93 μm3; monkey, 0.4 × 0.4 × 1 μm3; cat, 0.4 × 0.4 × 0.5 μm3; ferret, 0.2 × 0.2 × 0.89 μm3; rabbit, 0.4 × 0.4 × 1 μm3; and rat, 0.12 × 0.12 × 0.51 μm3. Using the volume rendering and segmentation tools of the AMIRA software, we created detailed, 3D computer models of the muscle fibers, nerves, and nerve terminals for each specimen.
Quantification and Statistics
The number of palisade endings was counted in the two vertical (superior and inferior) and two horizontal (medial and lateral) EOMs from humans, monkeys, cats, ferrets, pigs, sheep, rabbits, and rats. Data were expressed as mean and SEM. For comparison between groups, we used the 2-way ANOVA test. The two factors analyzed were the type of EOM muscle and the species. The ANOVA test was followed by the Holm-Sidak method for the post hoc analysis. Statistics were carried out using the platform SigmaPlot (Systat Software, Inc., San Jose, CA, USA).
Results
For our analyses, we used EOMs labeled with fluorescent markers in whole-mount preparations. In contrast to sectioning, where tissue is fragmented, whole-mount preparations remain in toto and the overall morphology of structures is accessible to microscopic observation. When palisade endings were identified, the following analyses were done: First, palisade endings were quantified in each rectus EOM. This was done for each species endowed with palisade endings with the exception of calf (only the horizontal eye muscles were analyzed) and horse (only muscle fragments were available). Several species lacked palisade endings (mice, gerbil, and guinea pig) and so these were also excluded from the analysis. Second, 3D reconstructions were done to give a spatial impression of this organ. Third, the molecular features of palisade endings were determined using immunofluorescence and α-bungarotoxin labeling. We start this section with the findings in frontal-eyed animals followed by lateral-eyed animals.
Frontal-Eyed Animals
Findings in frontal-eyed animals were obtained from primates (human and monkey) and carnivores (cat and ferret).
Palisade Endings Are a Constant Feature in the EOMs of Frontal-Eyed Animals With the Highest Density in the Medial Rectus.
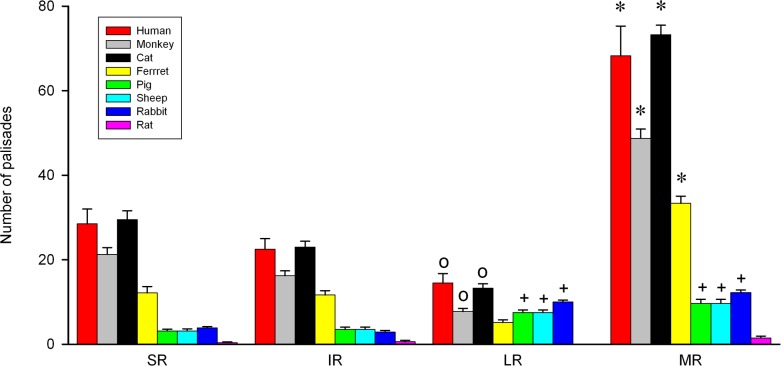
In immunolabeled EOM whole-mounts of frontal-eyed animals, we observed many axons coming from the muscle and penetrating the tendon. Within the tendon, the axons made U-turns and approached individual muscle fiber tips. There, the axons branched and finally terminated. According to Dogiel,6 such structures are termed palisade endings. We found palisade endings in each rectus EOM of each of the frontal-eyed species. They were counted in several specimens for each rectus muscle. The results of this analysis are illustrated in Figure 1 in the form of a bar chart showing the mean and SEM for every muscle and species. The 2-way ANOVA test was used to reveal significant differences between the four recti for the eight species analyzed. This analysis revealed that the total number of palisade endings was higher for all the examined frontal-eyed species compared with lateral-eyed species. Notably, the number of palisade endings varied among the EOMs in a specific pattern. In particular, the number of palisade endings in the medial recti was significantly higher than in the other three rectus muscles, and the lateral recti always contained the lowest number of palisade endings. The superior and inferior rectus muscles had intermediate values (F3,162 = 362.32; P < 0.001; Figs. 1–3).
Figure 1.
Bar chart showing the total number and the muscle-specific distribution of palisade endings. The total number of palisade endings was higher in frontal-eyed than lateral-eyed species. In frontal-eyed species, the medial rectus muscle (MR) always contained the highest number of palisade endings and the lateral rectus (LR) the lowest. The values of the vertical eye muscles (superior rectus, SR, and inferior rectus, IR) were similar to each other. In lateral-eyed species, the MR and LR had more palisade endings than the SR and IR, except in the rat where the number of palisade endings was extremely low. Data represent mean and SEM. Eight muscles for each EOM were analyzed in cat, rabbit, and rat; six muscles in ferret, pig, and sheep; four in monkey and human LR and MR; and two for human SR and IR. *Significantly higher number of palisade endings in MR with respect to the other muscles. +, significant differences of MR and LR with SR and IR; O, significantly lower number of palisade endings in LR than in the other three muscles (2-way ANOVA test followed by Holm-Sidak method for post hoc multiple comparisons at a significance level of 0.05).
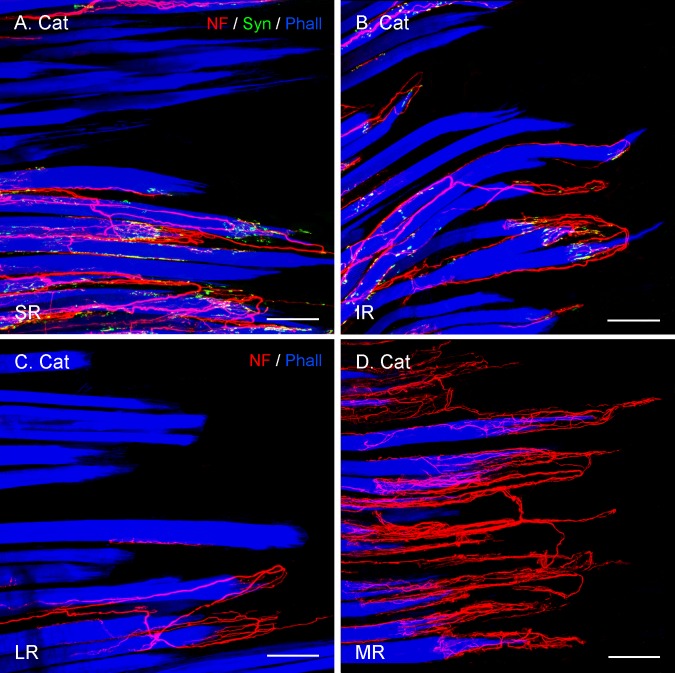
Figure 3.
Projection of CLSM z-stacks showing segments of the distal muscle–tendon junction of the four rectus muscles (SR, IR, LR, MR) in cat. (A, B) Axons were labeled with anti-neurofilament (NF, red), anti-synaptophysin (Syn, green), and muscle fibers with phalloidin (Phall, blue). (C, D) Staining with anti-neurofilament and phalloidin, but lacking anti-synaptophysin labeling. (A–D) The relative EOM-specific abundance of palisade endings with the medial rectus (D) highest and the lateral rectus (C) lowest. The values of the vertical eye muscles (A, B) are in-between. Scale bars: 100 μm.
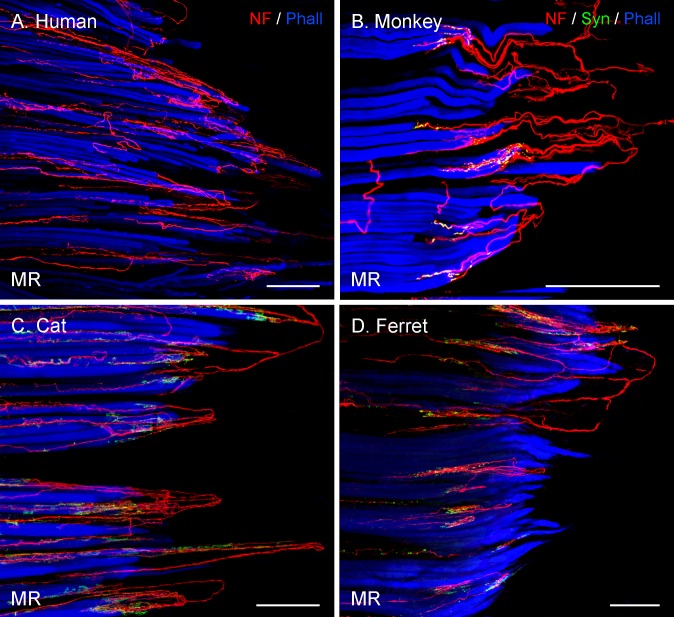
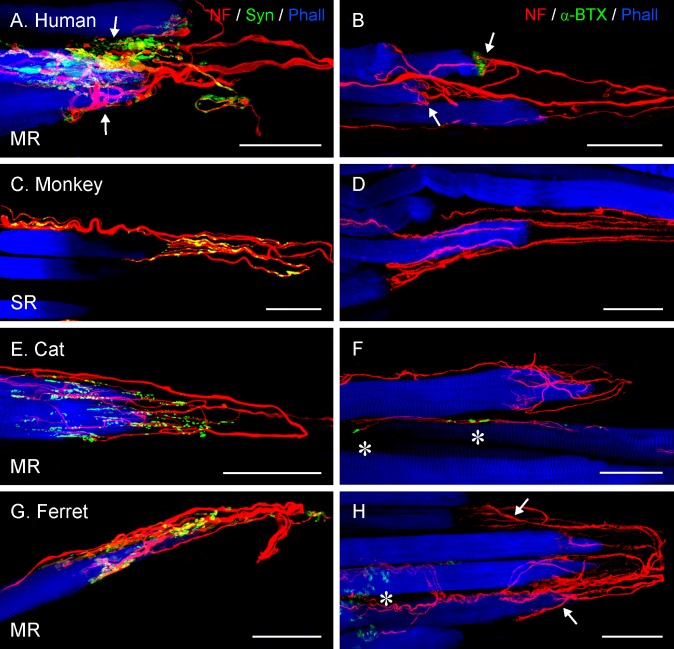
Figure 2.
Projection of CLSM z-stacks showing the distal muscle–tendon junction of MR muscles in frontal-eyed species. Only segments of the muscle–tendon junction are shown due to the size of the muscle. (A) Axons were labeled with anti-neurofilament (NF, red) and muscle fibers with phalloidin (Phall, blue). (B, D) Additional labeling of nerve terminals with anti-synaptophysin (Syn, green). In these and other photomicrographs, the tendon extends from the muscles to the right of the image and is not visible. (A–D) A high number of palisade endings were detected in the MR. (B–D) Terminal branches exhibited synaptophysin-positive varicosities. Scale bars: 200 μm.
Palisade Endings in Frontal-Eyed Animals Have a Comparable Morphology.
Three-dimensional reconstruction showed that palisade endings in frontal-eyed animals conformed to a common pattern in their morphology. In most cases, a palisade ending was supplied by a single axon, which sometimes branched to give rise to up to three palisade endings (Fig. 4A). Alternatively, up to four axons supplied palisade endings at the same muscle fiber. The palisade ending itself had a complex appearance: with arbor branches either coursing in parallel or forming a network to produce terminal varicosities (including en passant terminals) at the attachment of the muscle fiber with the tendon (Figs. 4A, 4C, 4E, 4G). Some terminal varicosities of palisade endings lay at the tendon level, far from the muscle fiber tip (Figs. 4B, 4D, 4F, 4H; arrows), whereas others were located around the muscle fiber tip (Figs. 4B, 4D, 4F, 4H; asterisks) or between the fingerlike protrusions of the muscle fiber at its tendon attachment. Most terminals at the muscle level did not directly contact the muscle fiber surface, but were separated from it by a small gap (Fig. 4F; arrowheads).
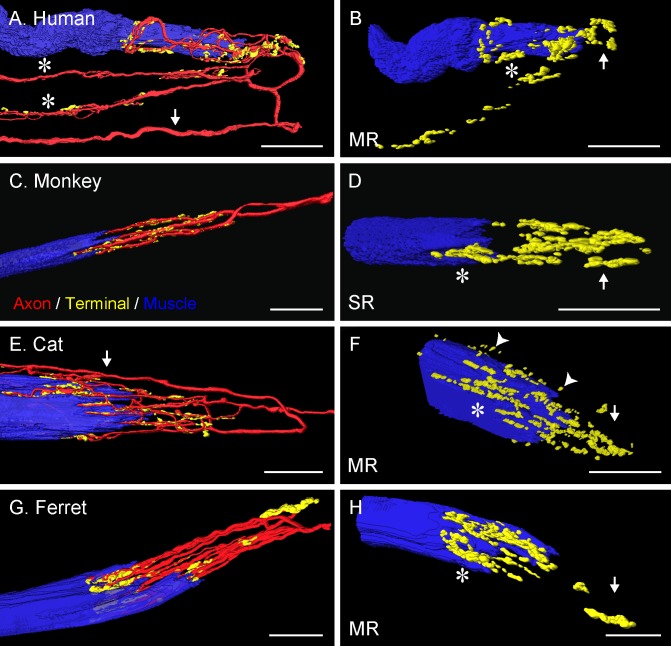
Figure 4.
Three-dimensional reconstruction of palisade endings in frontal-eyed species. In monkey (C), the palisade ending is from an SR muscle; in the other species they are from MR muscles. Nerve fibers were labeled with anti-neurofilament (red), terminal varicosities with anti-synaptophysin (green), and muscle fibers with phalloidin (blue). The yellow color represents the merging of green (synaptophysin) and red (neurofilament) signals. (A, E) Showing the whole formation of the palisade ending with the axon (arrows) indicated. (A) The axon supplies three palisade endings, of which only the upper one is completely shown. Asterisks indicate the collateral supplying the other palisade endings. (C, G) From the axon supplying the palisade endings, only the recurrent part coming from the tendon is shown. (B, D, F, H) Showing the same palisade endings as in (A, C, E, G), respectively, but rotated and the nerve fibers removed. Terminal varicosities are found far away from the muscle fiber (arrows), at the tendon level, and around the muscle fiber tip (asterisks). Many nerve terminals around the muscle fiber tip are separated from the muscle fiber surface by a small gap (arrowheads [F]). Scale bars: 30 μm.
Palisade Endings in Frontal-Eyed Animals Are Positive for Synaptophysin, Whereas α-Bungarotoxin Staining Is an Exceptional Feature.
Staining with anti-neurofilament/anti-synaptophysin/phalloidin showed that terminal varicosities of palisade endings always exhibited synaptophysin immunoreactivity (Figs. 5A, 5C, 5E, 5G). However, when staining with anti-neurofilament/α-bungarotoxin/phalloidin, all palisade endings in carnivores and most palisade endings in primates exhibited no α-bungarotoxin signal at all. In other primate palisade endings, a small number of terminals exhibited α-bungarotoxin signal. When present, the α-bungarotoxin–positive nerve terminals were never found within the tendon. They were exclusively located around the muscle fiber tip and presumably represented motor neuromuscular contacts (Figs. 5B, 5D, 5F, 5H).
Figure 5.
Projection of z-stacks from CLSM images showing palisade endings in frontal-eyed species. All palisade endings are from MR muscles with the exception of (C), which is from an SR muscle. (A, C, E, G) Staining was done with anti-neurofilament (NF, red), anti-synaptophysin (Syn, green), and phalloidin (Phall, blue). (B, D, F, H) Staining with anti-neurofilament, α-bungarotoxin (α-BTX, green), and phalloidin. (A, B, H) Two palisade endings (arrows) in each panel are shown supplying neighboring muscle fibers. In the rest of the images, only a single palisade ending is demonstrated. (A, C, E, G) Terminal varicosities of palisade endings exhibit synaptophysin immunoreactivity. (D, F, H) Absence of α-bungarotoxin signal associated with palisade endings. (F, H) α-Bungarotoxin-positive neuromuscular contacts (asterisks) are on neighboring muscle fibers. (B) Showing two human palisade endings (arrows); the upper one is associated with α-bungarotoxin staining, but not the lower one. Scale bars: 50 μm.
Lateral-Eyed Animals
Findings in lateral-eyed species were obtained from even-toed ungulates (pig, sheep, and calf), odd-toed ungulates (horse), lagomorphs (rabbit), and rodents (rat, guinea pig, mouse, and gerbil).
Palisade Endings Are Not Always Present in the EOMs of Lateral-Eyed Animals.
Lateral-eyed species exhibited a nonuniform pattern with respect to the occurrence of palisade endings. Specifically, in EOMs of even-toed ungulates, odd-toed ungulates and in lagomorphs, relatively few axons formed palisade endings in each rectus EOM (Figs. 6A, 6B). Axons forming palisade endings sometimes established neuromuscular contacts alongside the muscle fiber (Figs. 7A, 7C). In rat EOMs, the occurrence of palisade endings was irregular. In some cases, one to three palisade endings were found in the medial, superior, and inferior rectus muscles. More commonly, all the horizontal and vertical EOMs lacked palisade endings (Fig. 6C). In the EOMs of all other rodents (guinea pig, mouse, and gerbil), all axons terminated before reaching the muscle–tendon junction and no palisade endings were present at all (Figs. 6D, 6E). Axons terminating before the muscle–tendon junction established several neuromuscular contacts alongside the muscle fibers and they obviously belonged to motoneurons that supply these multiply innervated muscle fibers. Among those species presenting palisade endings (pig, sheep, calf, horse, rabbit, and rat) their number was determined in each rectus muscle, with the exception of calf and horse, where staining intensity varied within the muscles and only muscle parts could be evaluated. Quantitative analyses in pig, sheep, rabbit, and rat revealed that the average number of palisade endings (4.9 ± 0.4) was rather low when compared with frontal-eyed species (25.1 ± 2.0; t192 = 10.8, P < 0.001). Except in rat (where the average number of palisade endings in the four rectus muscles was extremely low), the remaining lateral-eyed species contained a similar number of palisade endings in the two vertical EOMs (superior and inferior rectus), as well as in the two horizontal EOMs (medial and lateral rectus). However, there were significantly fewer palisade endings in the vertical than in the horizontal EOMs (Fig. 1; 2-way ANOVA, F3,162 = 362.32; P < 0.001 with Holm-Sidak post hoc test). In calf and horse, our less complete evaluation of muscle parts also indicated that the number of palisade endings was likewise lower than that of frontal-eyes species, and comparable to that in the other lateral-eyed species with palisade endings.
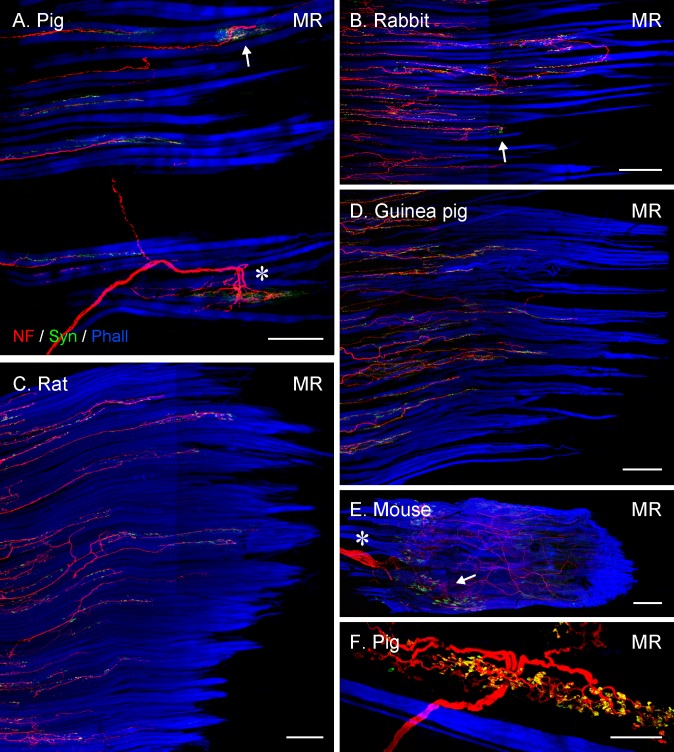
Figure 6.
Projection of CLSM z-stacks showing the distal muscle–tendon junction of MR muscles (A–D), a whole MR muscle (E) and a Golgi tendon organ (F) in lateral-eyed species. (A) and (B) illustrating only segments of the muscle–tendon junctions, whereas (C) and (D) the entire muscle-tendon junctions. Axons were labeled with anti-neurofilament (NF, red), nerve terminals with anti-synaptophysin (Syn, green), and muscle fibers with phalloidin (Phall, blue). Only in pig (A) and rabbit (B), are individual palisade endings (arrow) seen, and most axons stop at variable distances away from the muscle–tendon junction. Such axons establish numerous synaptophysin-positive contacts, indicating they supply multiply innervated muscle fibers. (A) A Golgi tendon organ (asterisk) is visible as well. In (C–E), all axons stop before reaching the muscle–tendon junction and no palisade endings are present. (E) Showing the nerve entry site (asterisk) and the motor endplate zone (arrow) in the proximal part of the muscle. (I) High magnification image of a Golgi tendon organ with synaptophysin-positive nerve terminals. Scale bars: (A, C) 200 μm; (B, D, E) 300 μm; (F) 50 μm.
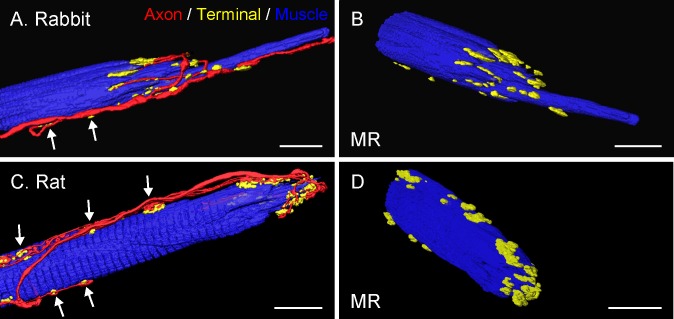
Figure 7.
Three-dimensional reconstruction of palisade endings in lateral-eyed species. All examples are from MR muscles. Nerve fibers were labeled with anti-neurofilament (red), nerve terminals with anti-synaptophysin (green), and muscle fibers with phalloidin (blue). (A, C) The whole extent of the palisade ending is shown with nerve fibers, nerve terminals, and muscle fibers. Axons forming palisade endings establish nerve terminals (arrows) alongside the muscle fibers. The palisade complex itself appears rather simple, without extensive axonal branching and with terminal varicosities around the muscle fiber tip. (B, D) Showing the same palisade endings in different views with the nerve fibers removed. Scale bars: (A, B) 25 μm; (C, D) 15 μm.
Consistent with other studies, Golgi tendon organs were also a regular constituent at the muscle–tendon junction in even-toed ungulates.19,20 Unlike palisade endings, Golgi tendon organs have a capsule formed by perineural cells.20 Nerve fibers supplying Golgi tendon organs also came from the muscle and penetrated the tendon, but they had a larger diameter than those forming palisade endings. Axons of Golgi tendon organs entered the organ in its middle section and divided into branches that extended in opposite directions. After further arborization, nerve branches established synaptophysin-positive nerve terminals in the tendon (Figs. 6A, 6F).
Palisade Endings in Lateral-Eyed Animals Exhibit Morphologic Differences From the Canonical Palisade Ending.
In lateral-eyed animals, palisade endings were usually supplied by a single axon, which, in the case of even-toed ungulates, sometimes branched to supply two palisade endings on neighboring muscle fibers. Analogous to frontal-eyed animals, palisade endings in even-toed and odd-toed ungulates exhibited a complex morphology consisting of dense network of axonal arbors and terminal varicosities at the tendon level and around the muscle fiber tip. Palisade endings in lagomorphs and, when present, in rat exhibited a much simpler morphology. In those species, the arborization of palisade endings displayed fewer branches and boutons. Terminal varicosities at the tendon level were entirely lacking. Instead, they exclusively invested the muscle fiber tip (Figs. 7A–D).
Palisade Endings in Lateral-Eyed Animals Are Positive for Synaptophysin and Those in Rabbit and Rat for α-Bungarotoxin as Well.
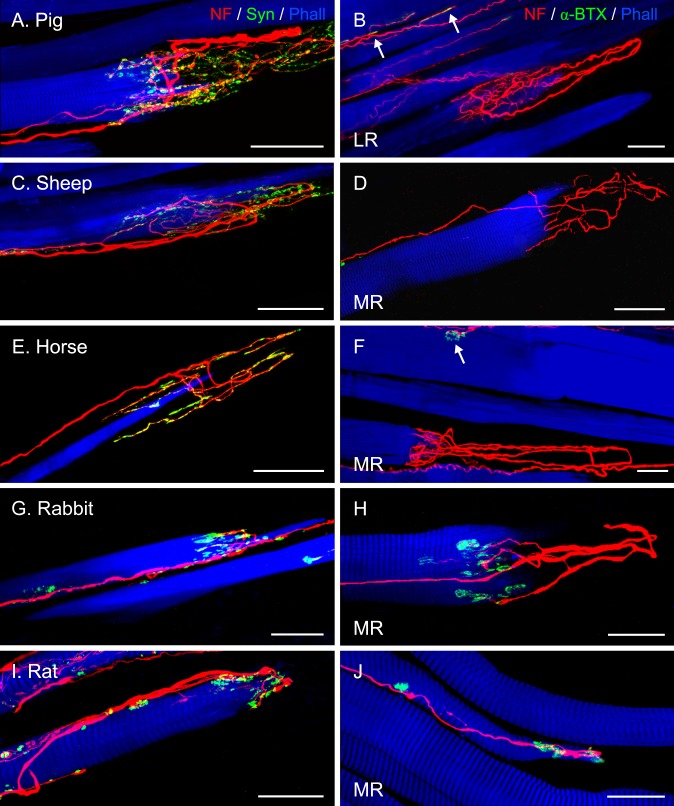
Staining with anti-neurofilament/anti-synaptophysin/phalloidin demonstrated that terminal varicosities in palisade endings of lateral-eyed animals were synaptophysin-positive. (Figs. 8A, 8C, 8E, 8G, 8I). However, after labeling with anti-neurofilament/α-bungarotoxin/phalloidin, no α-bungarotoxin signal was detected in palisade endings of even-toed and odd-toed ungulates (Figs. 8B, 8D, 8F). Only in the palisade endings of rabbits and rats was α-bungarotoxin binding detected in the terminal varicosities that invested the muscle fiber tip, suggesting the presence of acetylcholine receptors (Figs. 8H, 8J).
Figure 8.
Projection of z-stacks from CLSM images showing palisade endings in lateral-eyed species. Palisade endings are from LR muscles in (A, B), and the rest from MR muscles. (A, C, E, G, I) Staining with anti-neurofilament (NF, red), anti-synaptophysin (Syn, green), and phalloidin (Phall, blue), and (B, D, F, H, J) with anti-neurofilament, α-bungarotoxin (α-BTX), and phalloidin. (A, C, E, G, I) Terminal varicosities in palisade endings exhibit synaptophysin immunoreactivity. In pig (A), sheep (C), and horse (E), terminal varicosities of palisade endings are found at the tendon level and also around the muscle fiber tip, whereas in rabbit (G) and rat (I), varicosities of palisade endings are only at the muscle fiber tip. (B, D, F) Alpha-bungarotoxin labeling is generally absent in palisade endings, but is found on neighboring muscle fibers ([B, F] arrows). However, in (H) and (J), α-bungarotoxin is present in association with palisade endings. Scale bars: (A–G) 50 μm; (H–J) 25 μm.
Discussion
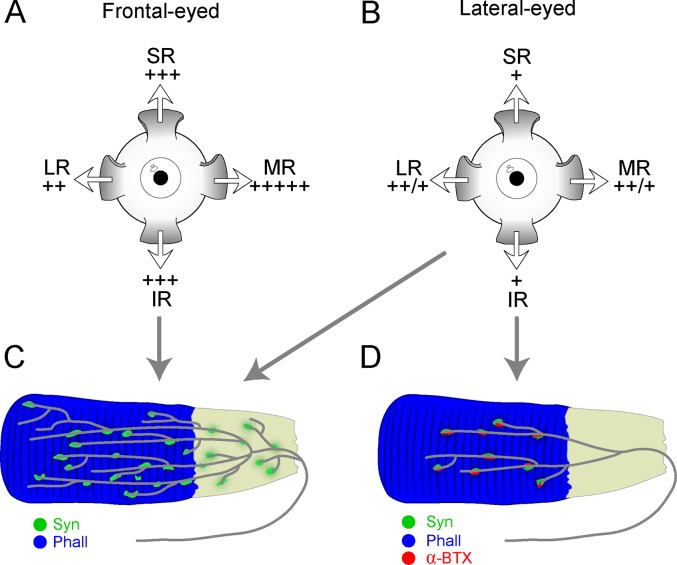
We analyzed 13 mammals, including both frontal-eyed and lateral-eyed species, and extended previous findings on palisade endings. We found that palisade endings were not a general feature of the EOMs of all species; their number was higher in frontal-eyed than lateral-eyed species; they were less elaborate in some lateral-eyed species (rabbit and rat), but could bind α-bungarotoxin; and their number varied among the EOMs. Figure 9 summarizes our results.
Figure 9.
Schematic drawing summarizing the distribution pattern and molecular properties of palisade endings in frontal-eyed and lateral-eyed species. In (A) and (B), relative density of palisades is indicated by + in the SR, IR, LR, and MR (differences between rats and nonrodent lateral-eyed species indicated by a ++/+). Frontal-eyed species (A) showed a different specific distribution of palisade endings in the rectus muscles from lateral-eyed species (B). (C) Palisade endings in frontal-eyed species had terminal varicosities at the tendon level and around the muscle fiber tip. Terminal varicosities displayed synaptophysin immunoreactivity (Syn), whereas α-bungarotoxin labeling (α-BTX) was exceptional. (D) In rabbit and rat, palisades exhibited a more simplified morphology and lacked terminal varicosities at the tendon level, but they displayed synaptophysin and α-bungarotoxin staining. However, ungulates exhibited palisade endings whose structure and molecular properties came closer to those found in frontal-eyed animals (C). The tendon in (C) and (D) is illustrated in gray in continuity with the phalloidin (Phall)-stained muscle fiber in blue.
Palisade Endings Are a Constant Feature in Frontal-Eyed, But Not in Lateral-Eyed Species
Because palisade endings had been found in all species investigated to date, it was assumed that they are a regular constituent of mammalian EOMs.21 We tested this hypothesis. Consistent with prior observations, palisade endings were found in frontal-eyed species like humans,11 monkeys,13 and cats.7 To this list, we have added the ferret. Palisade endings have also been demonstrated in the dog, another frontal-eyed carnivore.12 Among lateral-eyed species, we confirmed palisade endings in EOMs of sheep,8 rabbits,22 and rats,9 and have added their occurrence in even-toed ungulates (pig and calf) and in odd-toed ungulates (horse). To date, rats were the only rodent investigated. In those we examined (guinea pig, mouse, and gerbil), no palisade endings were found at all. Consequently, our survey does not support the assumption that palisades are ubiquitous in mammalian EOMs and irrespective of whether their function is sensory or motor, it is clear that not all species require them.
Comparison With Previous Quantitative Studies
This is the first exhaustive cross-species quantitative analysis of palisade endings. Clearly, the total number of palisade endings in those lateral-eyed species that possess them was lower than in frontal-eyed species. Their muscle distribution also differed. Among frontal-eyed species, palisade endings were most frequent in medial recti and least frequent in lateral recti muscle (Fig. 1). In lateral-eyed species, both horizontal eye muscles outnumbered the vertical eye muscles. Prior studies focused mostly on individual EOMs. Curiously, with the exception of humans, the older counts exceed ours. Briefly, counts in frontal-eyed species revealed: 50 palisade endings in cat inferior rectus, 350 in monkey medial rectus, and between 20 and 30 per muscle in human.7,10,13 Counts in lateral-eyed-species revealed 30 palisade endings in rabbit medial rectus and only 27 among 17 rat EOMs.9,22 Most of the discrepancies between older and present values might be explained by the different methods used for quantification, with the exception of the amazingly high previous count in the monkey. Although values vary, there is consensus among studies that palisade endings are more frequent in frontal-eyed than in those lateral-eyed species that possess them. We therefore conclude that palisade endings are more relevant for frontal-eyed than lateral-eyed animals.
Variations With Respect to Structure and α-Bungarotoxin Expression
In all frontal-eyed species and in four lateral-eyed species (pig, sheep, calf, and horse), palisade endings were uniform, with terminal expansions at the tendon level and around the muscle fiber tip, whereas in two other lateral-eyed animals (rabbit and rat) they exhibited a simpler morphology, with terminals exclusively located around the muscle fiber tip. The findings in ferret, pig, calf, and horse are new and those in the other species consistent with prior studies.7–9,13,22,23
When α-bungarotoxin, a snake venom that binds to nicotinic acetylcholine receptors, was used to detect motor terminals in palisade endings, it was only observed in few primate palisade endings at the muscle fiber tip, but generally in the palisade varicosities of rabbits and rats. Our findings confirm prior studies in primates, cat, sheep, and rabbit.10,15,18,22 However, in a previous rat study,9 α-bungarotoxin binding was not detected, perhaps due to the different processing approach used. In conclusion, palisade endings in rabbit and rat depart from the canonical palisade ending, in that they are much less elaborate and contain acetylcholine receptors.
The Number of Palisade Endings Correlates With the Oculomotor Repertoire
Both frontal- and lateral-eyed species have reflexive eye movements, whereas more complex eye movements, like smooth pursuit and convergence, are restricted to, or much more prevalent, in frontal-eyed species.24,25 The high number of palisade endings in frontal-eyed species may correlate with a more complex oculomotor repertoire. Moreover, as the medial rectus in frontal-eyed species has the highest density of palisade endings, they may play a specific role in convergence. Support for this interpretation comes from prior studies. Specifically, the cell bodies supplying medial and inferior rectus palisades in monkeys lie in a distinct group of neurons (C group) close to the preganglionic Edinger-Westphal nucleus.26 Moreover, dendrites of C group neurons extend into the Edinger-Westphal nucleus and increase their synaptic density there.27,28 These findings indicate that the cells supplying palisade endings likely share synaptic inputs related to convergence with preganglionic neurons, which generate pupil constriction and lens accommodation during convergence. Frontal-eyed animals have primarily bilateral visual fields, as well as retinal specializations such as the fovea or area centralis. Thus, the medial rectus muscle they use to make accurate convergence movements for stereopsis is characterized by elevated palisade numbers that may play a crucial role in their visual perception.
Based on their cholinergic phenotype and their origin in the EOM motor nuclei, palisade endings indicate they are effector organs.13,16,18,29 Following excitation, they may generate contraction of terminal portion of the muscle fiber and the large number of medial rectus palisades in frontal-eyed species may be related to the need keeping the eye position stable, while accurately pointing retinal specializations at the target. It is, however, important to note that although they contain cholinergic vesicles, postsynaptic acetylcholine receptors for cholinergic transmission are not generally present in palisade endings, with the exception of the rabbit and rat. Thus, the function of palisade endings remains speculative and, clearly, molecular and physiological studies aimed at identifying the signals in palisade neurons are imperative.
Acknowledgments
The authors thank Regina Mayer, Marietta Lipowec, and Marlene Rodler for their valuable technical assistance. We further thank Germund Hesslow (Department of Experimental Medicine, Lund University, Lund, Sweden) and Barbara Braus (Department of Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria) for the kind provision of ferret and horse material.
Supported by Grant P20881-B09 from the Fonds zur Foerderung der Wissenschaftlichen Forschung, an Austria–Spain concerted action ES04/2010 and AT2009-0039 (AMP, RB), Grant BFU2009-07121 from MEC-FEDER, and CVI-6053 from Junta de Andalucía-FEDER (AMP).
Disclosure: R. Blumer, None; B. Maurer-Gesek, None; B. Gesslbauer, None; M. Blumer, None; E. Pechriggl, None; M.A. Davis-López de Carrizosa, None; A.K. Horn, None; P.J. May, None; J. Streicher, None; R.R. de la Cruz, None; Á.M. Pastor, None
References
- 1. Maier A,, DeSantis M,, Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J Morphol. 1974; 143: 397–408. [DOI] [PubMed] [Google Scholar]
- 2. Ruskell GL. The fine structure of human extraocular muscle spindles and their potential proprioceptive capacity. J Anat. 1989; 167: 199–214. [PMC free article] [PubMed] [Google Scholar]
- 3. Blumer R,, Lukas JR,, Aigner M,, Bittner R,, Baumgartner I,, Mayr R. Fine structural analysis of extraocular muscle spindles of a two-year-old human infant. Invest Ophthalmol Vis Sci. 1999; 40: 55–64. [PubMed] [Google Scholar]
- 4. Greene T,, Jampel R. Muscle spindles in the extraocular muscles of the macaque. J Comp Neurol. 1966; 126: 547–549. [PubMed] [Google Scholar]
- 5. Büttner-Ennever JA,, Konakci KZ,, Blumer R. Sensory control of extraocular muscles. Prog Brain Res. 2006; 151: 81–93. [DOI] [PubMed] [Google Scholar]
- 6. Dogiel AS. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Saeugetieren. Arch Mikroskop Anal. 1906; 68: 501–506. [Google Scholar]
- 7. Alvarado Mallart RM Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979; 11: 567–584. [DOI] [PubMed] [Google Scholar]
- 8. Blumer R,, Lukas JR,, Wasicky R,, Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci Lett. 1998; 248: 49–52. [DOI] [PubMed] [Google Scholar]
- 9. Eberhorn AC,, Horn AK,, Eberhorn N,, Fischer P,, Boergen KP, Büttner Ennever JA. Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity. J Anat. 2005; 206: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lukas JR,, Blumer R,, Denk M,, Baumgartner I,, Neuhuber W,, Mayr R. Innervated myotendinous cylinders in human extraocular muscles. Invest Ophthalmol Vis Sci. 2000; 41: 2422–2431. [PubMed] [Google Scholar]
- 11. Richmond FJ,, Johnston WS,, Baker RS,, Steinbach MJ. Palisade endings in human extraocular muscles. Invest Ophthalmol Vis Sci. 1984; 25: 471–476. [PubMed] [Google Scholar]
- 12. Rungaldier S,, Pomikal C,, Streicher J,, Blumer R. Palisade endings are present in canine extraocular muscles and have a cholinergic phenotype. Neurosci Lett. 2009; 465: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruskell GL. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J Neurocytol. 1978; 7: 693–708. [DOI] [PubMed] [Google Scholar]
- 14. Lienbacher K,, Mustari M,, Hess B,, Büttner-Ennever J,, Horn AK. Is there any sense in the Palisade endings of eye muscles? Ann N Y Acad Sci. 2011; 1233: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blumer R,, Konakci KZ,, Pomikal C,, Wieczorek G,, Lukas JR,, Streicher J. Palisade endings: cholinergic sensory organs or effector organs? Invest Ophthalmol Vis Sci. 2009; 50: 1176–1186. [DOI] [PubMed] [Google Scholar]
- 16. Konakci KZ,, Streicher J,, Hoetzenecker W,, Blumer MJ,, Lukas JR,, Blumer R. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest Ophthalmol Vis Sci. 2005; 46: 155–165. [DOI] [PubMed] [Google Scholar]
- 17. Zimmermann L,, May PJ,, Pastor AM,, Streicher J,, Blumer R. Evidence that the extraocular motor nuclei innervate monkey palisade endings. Neurosci Lett. 2011; 489: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmermann L,, Morado-Diaz CJ,, Davis-Lopez de Carrizosa MA,, et al. Axons giving rise to the palisade endings of feline extraocular muscles display motor features. J Neurosci. 2013; 33: 2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blumer R,, Konakci KZ,, Brugger PC,, et al. Muscle spindles and Golgi tendon organs in bovine calf extraocular muscle studied by means of double fluorescent labelling, electron microscopy and three-dimensional reconstruction. Exp Eye Res. 2003; 77: 447–462. [DOI] [PubMed] [Google Scholar]
- 20. Ruskell GL. Golgi tendon organs in the proximal tendon of sheep extraocular muscles. Anat Rec. 1990; 227: 25–31. [DOI] [PubMed] [Google Scholar]
- 21. Spencer RF,, Porter JD. Structural organization of the extraocular muscles. Rev Oculomot Res. 1988; 2: 33–79. [PubMed] [Google Scholar]
- 22. Blumer R,, Wasicky R,, Hotzenecker W,, Lukas JR. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp Eye Res. 2001; 73: 787–796. [DOI] [PubMed] [Google Scholar]
- 23. Sodi A,, Corsi M. Faussone Pellegrini MS, Salvi G. Fine structure of the receptors at the myotendinous junction of human extraocular muscles. Histol Histopathol. 1988; 3: 103–113. [PubMed] [Google Scholar]
- 24. Land MF. Eye movements of vertebrates and their relation to eye form and function. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015; 201: 195–214. [DOI] [PubMed] [Google Scholar]
- 25. Missal M,, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol. 2001; 86: 2413–2425. [DOI] [PubMed] [Google Scholar]
- 26. Büttner Ennever JA, Horn AK, Scherberger H, D'Ascanio P. Motoneurons of twitch and nontwitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001; 438: 318–335. [DOI] [PubMed] [Google Scholar]
- 27. Tang X,, Buttner-Ennever JA,, Mustari MJ,, Horn AK. Internal organization of medial rectus and inferior rectus muscle neurons in the C group of the oculomotor nucleus in monkey. J Comp Neurol. 2015; 523: 1809–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erichsen JT,, Wright NF,, May PJ. Morphology and ultrastructure of medial rectus subgroup motoneurons in the macaque monkey. J Comp Neurol. 2014; 522: 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lienbacher K,, Mustari M,, Ying HS,, Büttner-Ennever JA,, Horn AK. Do palisade endings in extraocular muscles arise from neurons in the motor nuclei? Invest Ophthalmol Vis Sci. 2011; 52: 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]