Abstract
Background/Aims
In cerebral arteries, nitric oxide (NO) release plays a key role in suppressing vasomotion. Our aim was to establish the pathways affected by NO in rat middle cerebral arteries.
Methods
In isolated segments of artery, isometric tension and simultaneous measurements of either smooth muscle membrane potential or intracellular [Ca2+] ([Ca2+]SMC) changes were recorded.
Results
In the absence of l-NAME, asynchronous propagating Ca2+ waves were recorded that were sensitive to block with ryanodine, but not nifedipine. l-NAME stimulated pronounced vasomotion and synchronous Ca2+ oscillations with close temporal coupling between membrane potential, tone and [Ca2+]SMC. If nifedipine was applied together with l-NAME, [Ca2+]SMC decreased and synchronous Ca2+ oscillations were lost, but asynchronous propagating Ca2+ waves persisted. Vasomotion was similarly evoked by either iberiotoxin, or by ryanodine, and to a lesser extent by ODQ. Exogenous application of NONOate stimulated endothelium-independent hyperpolarization and relaxation of either l-NAME-induced or spontaneous arterial tone. NO-evoked hyperpolarization involved activation of BKCa channels via ryanodine receptors (RYRs), with little involvement of sGC. Further, in whole cell mode, NO inhibited current through L-type voltage-gated Ca2+ channels (VGCC), which was independent of both voltage and sGC.
Conclusion
NO exerts sGC-independent actions at RYRs and at VGCC, both of which normally suppress cerebral artery myogenic tone.
Keywords: Nitric oxide, Membrane potential, Calcium signalling, Vascular smooth muscle, Cerebral arteries, Vasomotion
Introduction
Cerebral arteries typically display spontaneous, sub-maximal constriction that is dependent on the level of intraluminal pressure or isometric stretch, termed myogenic tone. This myogenic tone is an essential mechanism in the local control of blood flow and tissue perfusion in the cerebral vasculature both in vivo and in vitro, and in many other vascular beds [1, 2]. The development of myogenic tone is generally characterized by vascular smooth muscle cell depolarization, leading to an increase in intracellular [Ca2+] ([Ca2+]SMC) and associated constriction of the artery [1, 3]. Myogenic responses, by definition, can occur without a functional endothelial cell layer; however, the endothelium can considerably modulate the degree of myogenic tone by releasing a number of factors including nitric oxide (NO), prostacyclin and endothelium-derived hyperpolarizing factor.
In addition to suppressing myogenic tone, endothelium-derived factors also modulate the vasomotion that often occurs in tandem with the development of myogenic constriction. Vasomotion describes rhythmic oscillations in tension or diameter that are normally synchronous with oscillations in Ca2+ and membrane potential (Em). In the brain, oscillations in middle cerebral artery blood flow velocity (as a result of vasomotion) have been observed in many species, including humans [4] and rats [5]. The role of the endothelium in the control of vasomotion is unclear; in some vascular beds the NO/cGMP pathway has been shown to augment vasomotion [6]. However, in other beds, including the cerebral vasculature [5, 7], NO/cGMP attenuates this response as NO synthase (NOS) inhibitors stimulate vasomotion. This vasomotion manifests as a reduction in capillary blood flow, which tends to oscillate in synchrony within the bed [8]. Therefore, any disruption of the ability to synthesize NO can potentially lead to vasomotion and/or spasm, as observed under pathophysiological conditions such as subarachnoid haemorrhage [9, 10].
In arteries isolated from both coronary [11, 12] and cerebral [13–20] beds, a continual, basal release of NO suppresses myogenic tone, with inhibition of NOS leading to depolarization and constriction in the absence of vasoconstrictor agents. NO can either stimulate hyperpolarization and closure of voltage-gated Ca2+ channels (VGCC), or directly close VGCC, both of which suppress myogenic tone. In terms of hyperpolarization, NO can activate smooth muscle cell BKCa channels either directly [21–23] or via PKG-dependent mechanisms [24, 25]. NO can also stimulate ryanodine-sensitive calcium stores (by opening the ryanodine receptor, RYR) in the sarcoplasmic reticulum, evoking discrete calcium events termed ‘sparks’ that activate adjacent clusters of BKCa channels. This mechanism has been suggested to underpin NO-dependent relaxation in the rat posterior cerebral artery [26] where the presence of NO is reported to be a prerequisite to activate the RYRs. Stimulation of RYRs by NO could be either direct or indirect, such as nitrosylation of thiol groups [27], or via cGMP-mediated phosphorylation of the channel and the sarcoplasmic reticulum calcium ATPase [28], respectively. In addition, NO can close VGCC in a membrane potential-independent manner, which can occur either via sGC/PKG [29–31] and/or by nitrosylation [32–34].
Therefore, we investigated further the mechanisms underlying the modulation of myogenic tone and the development of vasomotion associated with the basal release of NO in the rat (middle) cerebral arteries. Although our data support the suggestion that NO does stimulate RYR channels to release calcium that drives BKCa channel-mediated hyperpolarization, they also suggest 2 further important aspects of NO activity. First, that inhibition of NOS masks (rather than inhibits) spontaneous oscillations in smooth muscle cell calcium due to activation of VGCCs and the appearance of vasomotion, which is consistent with activation of RYRs via NO-independent pathways. Second, a direct inhibitory action of NO on VGCCs can suppress cerebral artery myogenic tone.
Materials and Methods
Male Wistar rats (200–300 g) were euthanized using procedures defined by the Animals (Scientific Procedures) Act 1986, UK (schedule 1 procedure) and the brain was rapidly removed and stored immediately in ice-cold physiological salt solution for a maximum of 30 min.
Simultaneous Measurement of Tension and Membrane Potential
A 2-mm segment of the middle cerebral artery (internal diameter of approx. 175 µm) was mounted in a Mulvany-Halpern myograph (model 410A; Danish Myotechnology) in Krebs solution containing (in mm): NaCl, 118.0; NaCO3, 25; KCl, 3.6; MgSO4·7H2O, 1.2; KH2PO4, 1.2; glucose, 11.0; CaCl2, 2.5; and gassed with 95% O2 and 5% CO2 at 37°C. The vessels were allowed to equilibrate for 20 min and were then tensioned to 1–1.5 mN (approximates wall tension at 60 mm Hg). Vessel viability was assessed by the addition of exogenous K+ (15–55 mm), only vessels developing tension ≥3 mN were used. Endothelial cell viability was assessed by the ability of SLIGRL (20 µm; a protease-activated receptor 2 ligand) to relax U46619-induced tone by >70% and to hyperpolarize the smooth muscle cell membrane by >15 mV. All blocking drugs were allowed to equilibrate for 20 min before study except nifedipine and ryanodine which produced immediate responses or whose effects were studied over a 20-min period. In some experiments, endothelial cells were removed by gently rubbing the luminal surface with a human hair; subsequent relaxation of <15% to SLIGRL (20 µm) was considered as successful removal. Smooth muscle cell tension and Em were measured simultaneously as previously described [35] and were recorded with the use of Powerlab system (AD Instruments). Briefly, individual smooth cells were impaled with a glass electrode (filled with 2 M KCl, tip resistance 60–100 MΩ) held perpendicular to the cells.
Simultaneous Measurement of Changes in [Ca2+]SMC and Tension
A segment of middle cerebral artery was mounted as described above except in a Mulvany-Halpern myograph designed for use on a confocal microscope (model 120CW; Danish Myotechnology) and in MOPS buffer containing (in mm): NaCl, 145; KCl, 4.7; CaCl2, 2.0; MgSO4, 1.17; MOPS, 2.0; NaH2PO4, 1.2; glucose, 5.0; pyruvate, 2.0; EDTA, 0.02; NaOH, 2.75 (the pH of the solution was adjusted to 7.39–7.41 at 37°C using NaOH or HCl, as appropriate). The arteries were loaded with the calcium-sensitive fluorescent dye Oregon Green 488 BAPTA-1 AM [10 µm; dissolved in DMSO and 0.02% (w/v) Pluronic F-127] for 1 h. After excitation at 488 nm, the fluorescence emission intensity at 515 nm was recorded using a spinning disc confocal microscope (Yokogawa CSU22) fitted with an Andor iXON DV887ECS-BV camera mounted on an Olympus IX70 inverted microscope using a water immersion objective (×40, aperture 0.8, working distance 3.3 mm; Olympus) and images (512 × 512 pixels, 20 Hz) were stored for offline analysis (iQ; Andor). Following background subtraction, average relative changes in [Ca2+]SMC were calculated as changes in intensity of fluorescence divided by fluorescence at time 0 s (F/F0), within selected cell regions (5 × 5 pixels).
Isolated Smooth Muscle Cell Patch Clamp Experiments
Freshly dissected middle cerebral arteries were placed in ice-cold Ca2+-free isolation solution containing (in mm): NaCl, 140; KCl, 4.7; MgCl2, 1.2; glucose, 10; HEPES, 10 (pH 7.4). After incubation on ice for 20 min, the arteries were transferred to Ca2+-free isolation solution, containing 1 mg/ml albumin, 1 mg/ml papain (Sigma) and 1 mg/ml dithiothreitol, and allowed to digest for 20 min at 37°C. The tissue was then transferred into a solution containing 0.1 mm CaCl2 and 1 mg/ml collagenase type H (Roche) plus 1 mg/ml collagenase type F (Sigma). Following digestion for 10 min at 37°C, the tissue was washed in isolation solution containing 1 mg/ml albumin and 0.1 mm CaCl2. After gentle trituration, cells were centrifuged for 5 min at 1,000 rpm, the supernantant removed, and resuspended in fresh isolation solution. The concentration of extracellular calcium was increased over the next 30 min to 750 µm. Freshly isolated cells were maintained on ice for use on the same day.
Cells were placed in a heated recording chamber (RC-25F; Warner Instruments) and left for approximately 10 min to adhere to the cover glass. Cells were then continually superfused (approx. 1 ml/min) with heated solution (SH-27B Inline Heater; Warner Instruments) via a multi-barrel gravity-fed perfusion system. Experiments were performed using an agar bridge (2% agar filled with 3 m KCl). During seal formation, cells were superfused with physiological saline solution containing (in mm): NaCl, 140; KCl, 4; CaCl2, 1.5; MgCl2, 1.2; HEPES, 10; glucose, 10; pH 7.4. To record membrane potential, the pipette solution contained (in mm): KCl, 130; NaCl, 10; HEPES, 10; MgCl2, 0.5; CaCl2, 0.5; Amphotericin B (200 µg/ml). To record L-type calcium current (ICaL), the whole cell mode was used and Ba2+ was used as the charge carrier. Cells were perfused with solution containing (in mm): NaCl, 120; CsCl, 4; TEA-Cl, 10; BaCl2, 10; MgCl2, 1.2; HEPES, 10; glucose; pH 7.4. The pipette solution contained (in mm): CsCl, 130; MgCl2, 0.4; HEPES, 10; EGTA 2; CaCl2, 0.4; GTP, 0.5; MgATP, 5; pH 7.3. The osmolarity of all solutions was measured and corrected to 300 ± 5 mOsm using mannitol. All electrophysiological recordings were performed at 37°C.
ICaL was recorded using a 1-second ramp protocol, from –100 to +80 mV from a holding potential of –80 mV at a frequency of 0.05 Hz. Nifedipine (1 µm) was applied at the end of the protocol and subtracted from the current records obtained in barium-containing solution, and the data presented as nifedipine-sensitive current. Cell membrane capacitance was measured using a 10-mV hyperpolarizing step and used to correct ICaL currents for cell size. Currents were expressed as current density (pA/pF). Any cell exhibiting current rundown in control conditions was excluded from the analysis. NONOate was freshly diluted with physiological saline solution, and infused via an injection port in the superfusion line directly upstream from the recording chamber. In experiments with the sGC inhibitor 1H-(1,2,4)oxadiazolo(4,3-a)-quinoxalin-1-one (ODQ), cells were incubated in 10 µm ODQ for 15 min, and it was also included in the perfusion solutions.
Data were analyzed and leak subtracted offline using pClamp 8 (Axon Instruments). Values are expressed as means ± SEM of n cells (from at least 3 animals). The paired two-tail t test was used to compare parameters obtained in control and test conditions in the same cell. A non-paired t test was used to compare the differences between groups of data.
Solutions and Drugs
Exogenous K+ was added as an isotonic solution, and expressed as the final bath concentration. Caffeine, NG-nitro-L-arginine methyl ester (l-NAME), nifedipine, ryanodine, BayK 8644 and papaverine were all obtained from Sigma. Iberiotoxin (IbTx) was obtained from Latoxan; DEA-NONOate from Alexis; ODQ from Tocris; SLIGRL from Auspep; Oregon Green 488 BAPTA-AM from Molecular Probes; TRAM-34 was a gift from Dr. H. Wulff (University of California, Davis, Calif., USA); U46619 was obtained from Calbiochem. All drugs were made in 0.9% NaCl except ryanodine, nifedipine, ODQ, U46619 and Amphotericin B in DMSO; NONOate in 0.01 m NaOH (and stored at –80°C), and (–) BayK 8644 in EtOH. All subsequent dilutions of all drugs were made in 0.9% NaCl and vehicle had no effect. NONOate dilutions were kept on ice in the dark and were discarded after 20 min.
Statistical Analysis
Results are expressed as the mean ± SEM of n animals. Relaxation is expressed as the peak percentage reduction of the total vascular tone (from the myogenic tone to the tension/diameter following addition of papaverine, 150 µm) or as mN, as appropriate. Constriction is expressed in mN or as a percentage of maximal constriction induced by exogenous K+ (55 mm), as appropriate; all values were the peak values. When oscillations in membrane potential or tension were observed, values are the average of 10 s. Graphs were drawn and statistical comparisons made using either Student’s t test or one-way ANOVA with Tukey’s or Dunnett’s post hoc test using Prism software (Graphpad).
Results
Effect of Inhibiting NOS, sGC, RYRs and BKCa Channels on Myogenic Tone
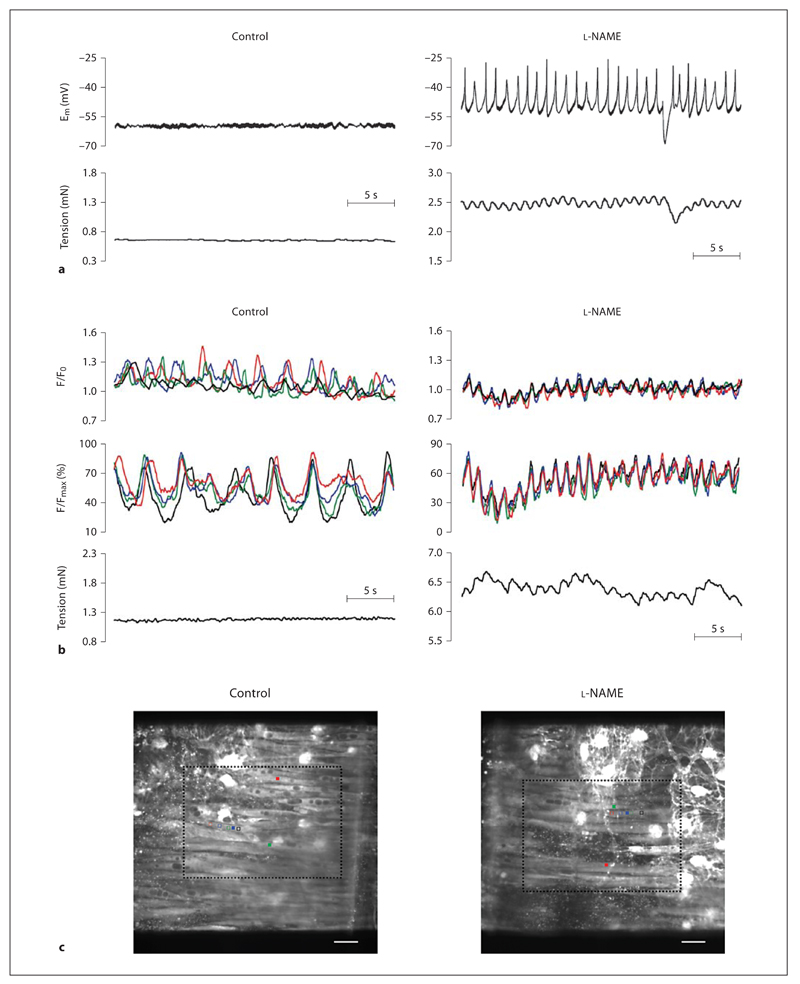
Rat middle cerebral arteries exhibit myogenic tone in a wire myograph equivalent to approximately 15% of the maximum tension the vessel can develop [18], and associated with a resting membrane potential (Em) of –50 ± 0.2 mV (n = 9). Addition of the NOS inhibitor l-NAME (100µm) evoked depolarization (to Em –43.7 ± 1.9 mV, n = 6) and constriction (increase in tension of 3.7 ± 0.5 mN, n = 7; fig. 1). In addition, oscillations in Em developed, temporally linked to oscillations in tension (fig. 1; table 1). Fluorescence imaging revealed that in unstimulated control arteries, smooth muscle cells displayed spontaneous and asynchronous propagating Ca2+ waves (172 of 210 cells; fig. 1; online supplementary video 1, www.karger.com/doi/10.1159/000235964). The oscillations occurred with a frequency of 0.27 ± 0.02 Hz (n = 21; table 1) and were not associated with any change in tension (fig. 1). Addition of l-NAME increased the global [Ca2+]SMC (data not shown) associated with the development of synchronous Ca2+ oscillations between smooth muscle cells that clearly linked temporally to changes in tension (fig. 1; online supplementary video 2; table 1).
Fig. 1.
Spontaneous NO release prevents vasomotion. Original traces showing simultaneous recordings of membrane potential (Em) and tension (a) or simultaneous recordings of [Ca2+]SMC (2 upper panels) and tension (b) under control resting conditions or in the presence of the NOS inhibitor l-NAME (100 µm) in rat middle cerebral arteries. Under control conditions, membrane potential and tension are relatively stable, and at the same time, [Ca2+]SMC is constantly oscillating, but these oscillations are asynchronous between smooth muscle cells and can be observed as waves passing along cells (asynchronous propagating Ca2+ waves). In the presence of l-NAME, the smooth muscle cells depolarized and developed regular depolarizing oscillations, which were associated with increased tension and oscillations in tension; the peaks in Em immediately preceded peaks in tension. In the presence of l-NAME, oscillations in [Ca2+]SMC were now synchronized and regular (synchronous Ca2+ oscillations) and were temporally linked to oscillations in tension. c The top coloured traces correspond to the average F/F0 in 3 cells indicated by filled coloured squares on the images of the preparations, and the black traces are the average change in fluorescence from 10 equivalent regions in separate cells (see online version for colour). The lower coloured traces correspond to the percentage maximum change in fluorescence in single cells indicated by the open coloured squares (see online version for colour). Scale bar = 20 µm. Video files corresponding to the cropped regions (dashed lines) shown in control and l-NAME are available online (online supplementary videos). Summary data are shown in table 1.
Table 1.
Amplitude and frequency of smooth muscle cell Em, tension and synchronous Ca2+ oscillations
| Smooth muscle cell Em |
[Ca2+]SMC oscillation frequency |
|||||
|---|---|---|---|---|---|---|
| oscillation amplitude |
oscillation frequency |
|||||
| Em, mV | tension, mN | Em, Hz | tension, Hz | Ca2+, Hz | tension, Hz | |
| l-NAME | 16.4±1.8 | 0.14±0.2 | 0.85±0.06 | 0.84±0.05 | 0.75±0.05 | 0.76±0.04 |
| IbTx | 19.7±2.3 | 0.11±0.02 | 0.97±0.05 | 0.96±0.06 | ND | ND |
| ODQ | 8.1±0.7* | 0.13±0.02 | 0.57±0.06* | 0.56±0.07* | ND | ND |
| Ryanodine | 19.5±3.4 | 0.06±0.01* | 1.08±0.06 | 1.06±0.06 | 1.04±0.04* | 1.01±0.05* |
Data are expressed as means ± SEM (n = 4−11). Time-matched, paired values were obtained from simultaneous records of either Em and tension or Ca2+ and tension. ND = Not determined. * p < 0.05 significant difference from l-NAME.
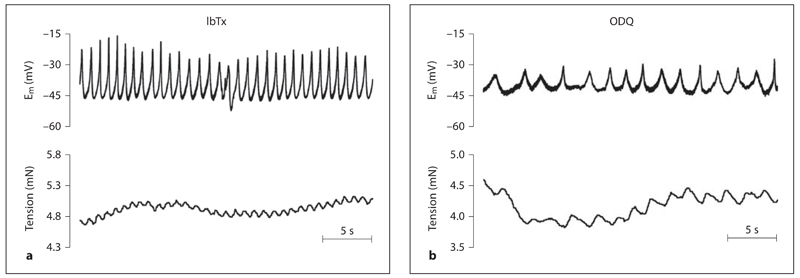
The BKCa channel inhibitor IbTx (100 nm) and the sGC inhibitor ODQ (10 µm) both mimicked this effect of l-NAME. Each caused depolarization (to Em –40.3 ± 1.8 and –35.5 ± 6.0 mV, n = 5 and n = 3, respectively; fig. 2) and vasoconstriction (increases in tension of 3.7 ± 0.8 and 3.8 ± 0.7 mN, n = 6 and n = 3, respectively; fig. 2) associated with the development of oscillations in Em temporally linked to oscillations in tone (table 1). Note that the vasomotion induced by ODQ was at a significantly lower frequency than that with either IbTx or l-NAME (table 1).
Fig. 2.
Spontaneous activation of BKCa channels and sGC prevent vasomotion. Original traces showing the effect of either the BKCa channel inhibitor IbTx (100 nm, a) or the sGC inhibitor ODQ (10 μM; b) on simultaneous recordings of membrane potential (Em) and tension. Both IbTx and ODQ caused depolarization and increased tension and development of vasomotion.
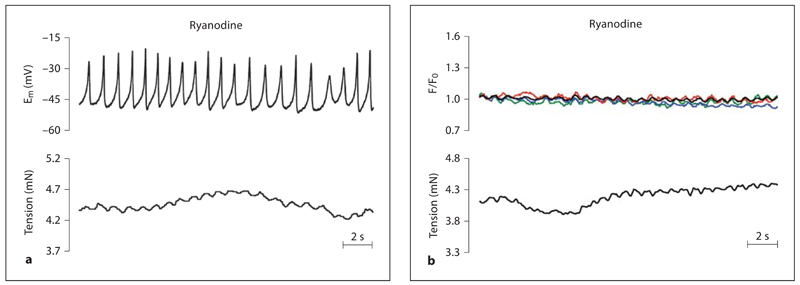
Inhibition of RYRs with ryanodine (10 µm) also mimicked the effect of l-NAME causing depolarization (13.5 ± 3.6 mV) and tension increases (2.3 ± 0.2 mN, n = 5), and associated development of synchronous Em oscillations temporally linked with tension oscillations (fig. 3; table 1). Similarly, ryanodine stimulated synchronous Ca2+ oscillations in phase with tension changes (fig. 3; table 1).
Fig. 3.
Spontaneous activation of RYRs prevents vasomotion. Original traces of simultaneous recordings of membrane potential and tension (a) or simultaneous recordings of [Ca2+]SMC and tension (b). The traces show recordings obtained in the presence of the inhibitor of RYRs (ryanodine, 10 μm ). Ryanodine caused depolarization and increased tension of the middle cerebral artery associated with development of depolarizing oscillations in Em that were temporally coupled to changes in tension. Ryanodine also caused development of synchronous Ca2+ oscillations that were temporally linked to oscillations in tension. [Ca2+]SMC responses from 3 randomly selected cells are displayed (see online version for colour) as well as the 10-cell average (black).
Effect of l-NAME and Ryanodine on Oscillations in [Ca2+]SMC in the Presence of Nifedipine
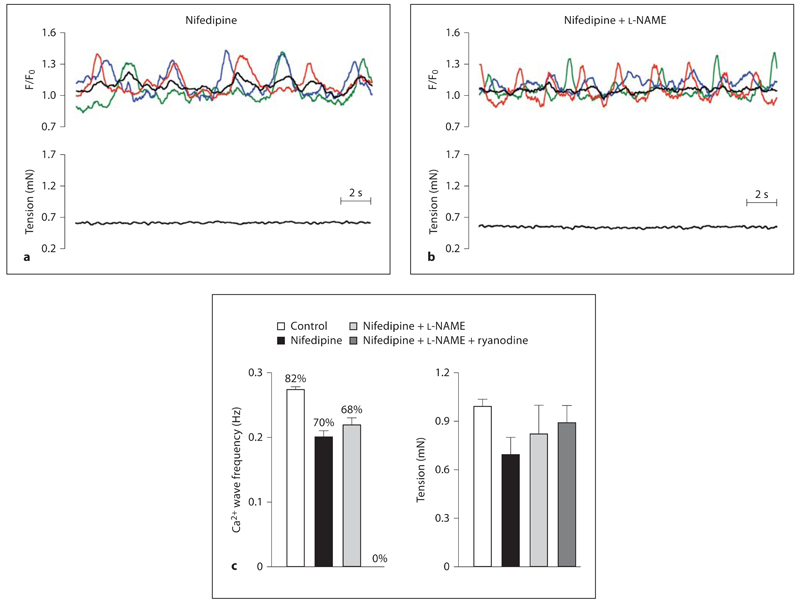
As ryanodine and l-NAME each evoke constrictor responses associated with depolarization (and consequent calcium entry via VGCC) the effects of these drugs were assessed in the presence of the L-type VGCC inhibitor, nifedipine (1 µm). Under control conditions, nifedipine alone hyperpolarized (6.4 ± 2.4 mV) and relaxed (0.76 ± 0.03 mN) myogenic tone (n = 4), associated with a slight but significant reduction in both the frequency (0.20 ± 0.02 Hz, n = 4, p < 0.01) and the number of cells exhibiting asynchronous propagating Ca2+ waves (to 70%, 28 of 40 cells; fig. 4). Subsequent addition of l-NAME repolarized the smooth muscle Em (depolarization of 7.5 ± 3.8 mV, n = 4) and caused a small increase in tension (0.8 ± 0.01 mN, n = 4), returning Em and tension values close to values recorded in quiescent vessels. In the presence of nifedipine, l-NAME had no significant effect on the number of cells exhibiting asynchronous propagating Ca2+ waves (68%, 27 of 40 cells) or the wave frequency (0.22 ± 0.02 Hz, n = 4; fig. 4). In contrast, ryanodine completely abolished these Ca2+ waves (to 0 in 60 cells; fig. 4).
Fig. 4.
Spontaneous NO release does not inhibit control, asynchronous propagating Ca2+ waves. Original traces showing the basal, asynchronous propagating Ca2+ waves from 3 representative cells (see online version for colour) and the 10-cell average (black; upper traces) and associated tension records (lower traces) in rat middle cerebral arteries in the presence of the L-type VGCC inhibitor nifedipine (1 µm; a) and the combination of nifedipine and the NOS inhibitor l-NAME (100 µm; b). Under control conditions, changes in [Ca2+]SMC were not synchronized between individual cells and were not coupled to changes in tension (as in fig. 1a). Nifedipine had no effect on the size of the asynchronous propagating Ca2+ waves. Subsequent addition of l-NAME also had no effect on these Ca2+ waves. c Average data showing the frequency of Ca2+ waves and the percentage of cells exhibiting this behaviour and the associated tension in control vessels and in the presence of nifedipine, nifedipine + l-NAME and nifedipine + ryanodine (10 µm). Ryanodine completely abolished the Ca2+ waves in all cells of all vessels tested (60 cells). Data are expressed as means ± SEM.
Effect of Blocking VGCC and Application of Exogenous NO or Caffeine on l-NAME-Induced Tone
In vessels pre-constricted with l-NAME, nifedipine (1 μM ) abolished oscillations in Em and caused a repolarization (hyperpolarization of 11.7 ± 1.9 mV, n = 3) to circa −50 mV, the resting membrane potential in the absence of NOS inhibition. This was associated with complete reversal of l-NAME-induced tone (93.1 ± 1.6%, n = 3). Furthermore, nifedipine caused a large decrease in [Ca2+] SMC (data not shown) and abolished the synchronous Ca2+ oscillations between SMC, unmasking the asynchronous propagating Ca2+ waves (compare fig. 4b to fig. 1b).
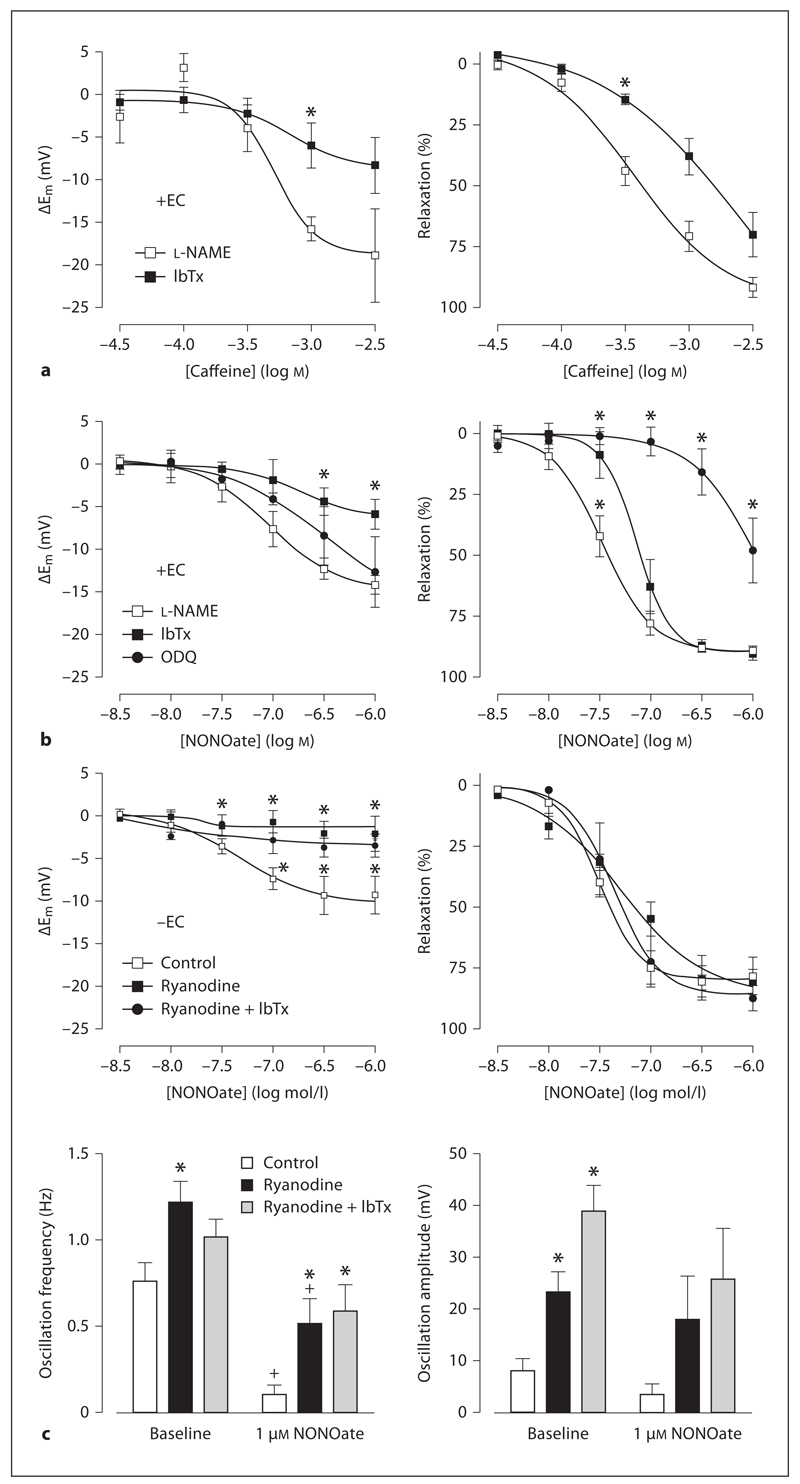
In vessels pre-constricted with l-NAME, application of caffeine (30 µm to 3 mm) induced concentration-dependent hyperpolarization (log EC50: –3.29 ± 0.16, n = 4–9; 3 mm: 18.9 ± 5.5 mV, n = 4) and relaxation (log EC50: –3.43 ± 0.10, n = 7–8; 3 mm: 91.8 ± 4.2% relaxation, n = 8; fig. 5a). Hyperpolarization and relaxation to caffeine (1 mm: 15.8 ± 1.4 mV, n = 9 and 70.7 ± 6.3%, n = 8, respectively) were attenuated by IbTx (1 mm: 6.0 ± 2.7 mV, n = 4 and 38.0 ± 7.5%, n = 4, respectively; fig. 5a) and by ryanodine (1 mm: 4.4 ± 1.9 mV and 40.0 ± 30.9%, respectively, n = 6).
Fig. 5.
Caffeine and NONOate stimulate hyperpolarization and relaxation. Concentration response curves showing hyperpolarization (left panels) and relaxation (right panels) produced by caffeine (a) or the NO donor DEA-NONOate (b) in endothelium-intact (+EC) or endothelium-damaged arteries (–EC; c). Vessels were pre-incubated with l-NAME (100µm), IbTx (100 nm), ODQ (10 µm) and/or ryanodine (10 µm). Data are expressed as means ± SEM (n = 4–9). * p < 0.05, significant difference from l-NAME (+EC) or control (–EC); + p < 0.05, significant difference from baseline.
Application of the NO donor NONOate (3 nm to 1 µm) stimulated concentration-dependent hyperpolarization (log EC50: –7.05 ± 0.18, n = 11–13; 1 µm: 14.1 ± 1.1 mV, n = 11) and relaxation (log EC50: –7.47 ± 0.05, n = 13–14; 1 µm: 89.0 ± 1.8% relaxation, n = 13; fig. 5b). The sGC inhibitor ODQ (1 µm) did not significantly affect hyperpolarization to NONOate (n = 6–7; 1 µm: 12.6 ± 4.1 mV, n = 6) but significantly attenuated the relaxation (n = 6–7; 1 µm: 47.8 ± 13.3% relaxation, n = 7; fig. 5b). Blockade of BKCa-channels with IbTx (100 nm) significantly inhibited both NONOate-induced hyperpolarization and relaxation (n = 3–4; fig. 5b).
Effect of Removing the Endothelium on Myogenic Tone and the Response to Application of Exogenous NO
Following removal of the endothelium, cerebral artery smooth muscle cells were depolarized (Em –45.9 ± 2.2 mV, n = 12) and spontaneously developed tension (1.5 ± 0.2 mN, n = 12) sometimes (9 of 12 records) associated with oscillations in both Em (amplitude: 4.5 ± 1.2 mV; frequency: 0.84 ± 0.20 Hz, n = 12) and tension. In these denuded cerebral arteries, l-NAME did not further increase tension (data not shown).
The NO donor, NONOate (3 nm to 1 µm) evoked concentration-dependent hyperpolarization and relaxation in denuded arteries (logEC50: –7.50 ± 0.06; 1 µm: 9.2 ± 2.2 mV and 78.0 ± 7.9% relaxation, n = 6; fig. 5c). Ryanodine (10 µm) caused a small increase in tone, which was associated with slight depolarization (Em –41.7 ± 1.0 mV, n = 12) and a significant increase in the amplitude of oscillations in Em (23.3 ± 2.7 mV; frequency: 1.24 ± 0.09 Hz, n = 12; fig. 5c). These oscillations were not coupled to a detectable tension change. Ryanodine markedly reduced the hyperpolarization produced by NONOate, but did not significantly affect the relaxation. Interestingly, NONOate reduced the amplitude and frequency of ryanodine-mediated oscillations in Em (1 µm; fig. 5c). The addition of IbTx did not modify the effects of ryanodine, apart from further increasing the amplitude of oscillations by around 10 mV (amplitude significantly increased to 34.9 ± 3.4 mV, frequency 1.29 ± 0.09 Hz, n = 8; fig. 5c).
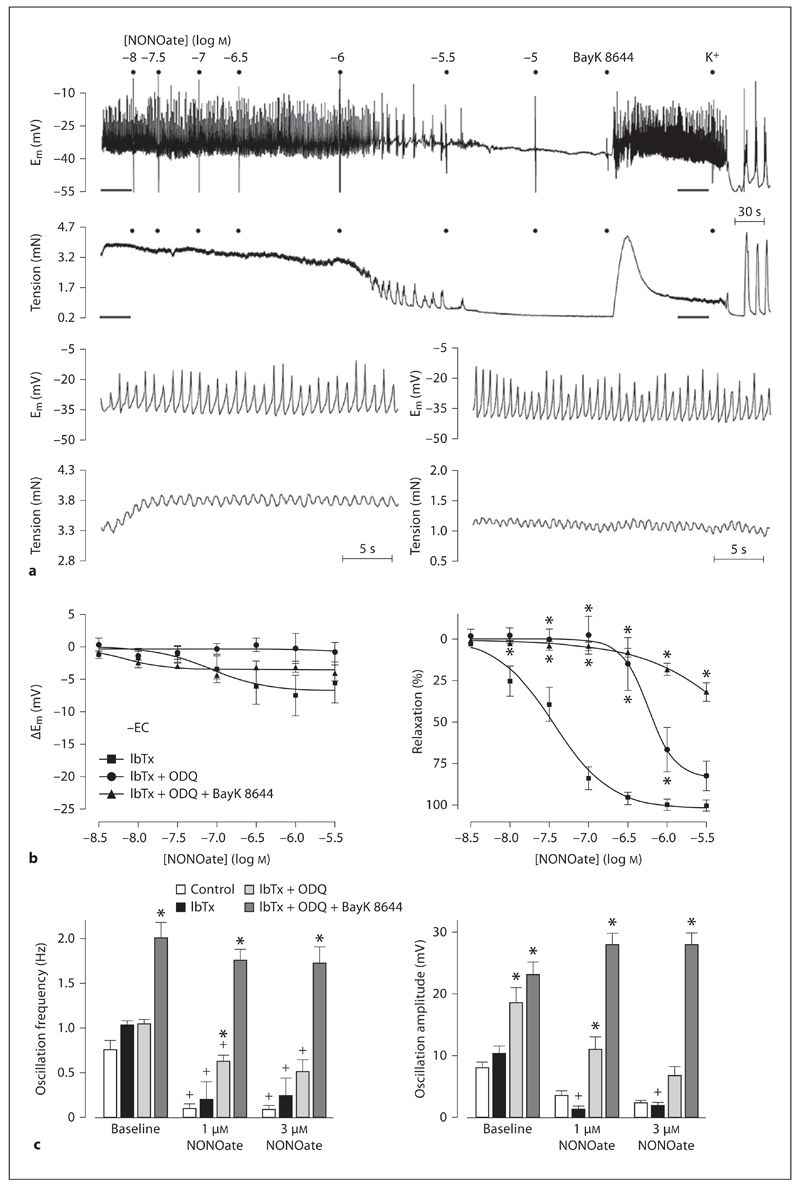
To further characterize the action of NO, experiments were performed to assess an action at VGCC. In endothelium-denuded arteries, IbTx had no significant effect on hyperpolarization and relaxation responses to NONOate (1 µm: 7.4 ± 3.1 mV and 99.6 ± 3.4%, n = 6; compare fig. 5c to fig. 6b). However, in the additional presence of ODQ, the hyperpolarization and relaxation to NONOate were reduced (fig. 6a, b). Subsequent addition of the L-type VGCC opener BayK 8644 did not significantly alter membrane potential (hyperpolarization of 3.7 ± 3.8 mV) but contracted arteries (1.2 ± 0.2 mN, n = 5), and significantly increased both the frequency and amplitude of oscillations in Em (fig. 6c). In the presence of this combination of inhibitors, the hyperpolarization to NONOate was effectively abolished, and the relaxation to NONOate markedly reduced.
Fig. 6.
Application of exogenous NO (NONOate 3 nm to 3 µm) appears to directly inactivate VGCCs in endothelium-denuded middle cerebral arteries. a Original trace showing that in the combined inhibition of BKCa channels (IbTx, 100 nm) and sGC (ODQ, 10 µm), NONOate induces a reduction in membrane potential oscillation frequency and amplitude (upper trace) that is associated with relaxation (lower trace). The effects of NONOate were fully reversed by an opener of L-type VGCCs (BayK 8644, 1 µm). Highlighted regions (gray lines) are reproduced in an extended time base to demonstrate that BayK 8644 fully reverses the effects of NONOate. Also shown are concentration response curves (b) showing the effect of NONOate on membrane potential and tension as well as histograms (c) showing the effect of NONOate on oscillation frequency and amplitude in the presence of IbTx, the combined presence of IbTx and ODQ and in the additional presence of BayK 8644. Note that following inhibition of BKCa and sGC, NONOate-mediated relaxation does not seem to involve a true hyperpolarization but results from a reduction in both frequency and amplitude of the oscillations in membrane potential. Data are expressed as means ± SEM. * p < 0.05, significant difference from control; + p < 0.05, significant difference from baseline.
Direct Action of NO on VGCC
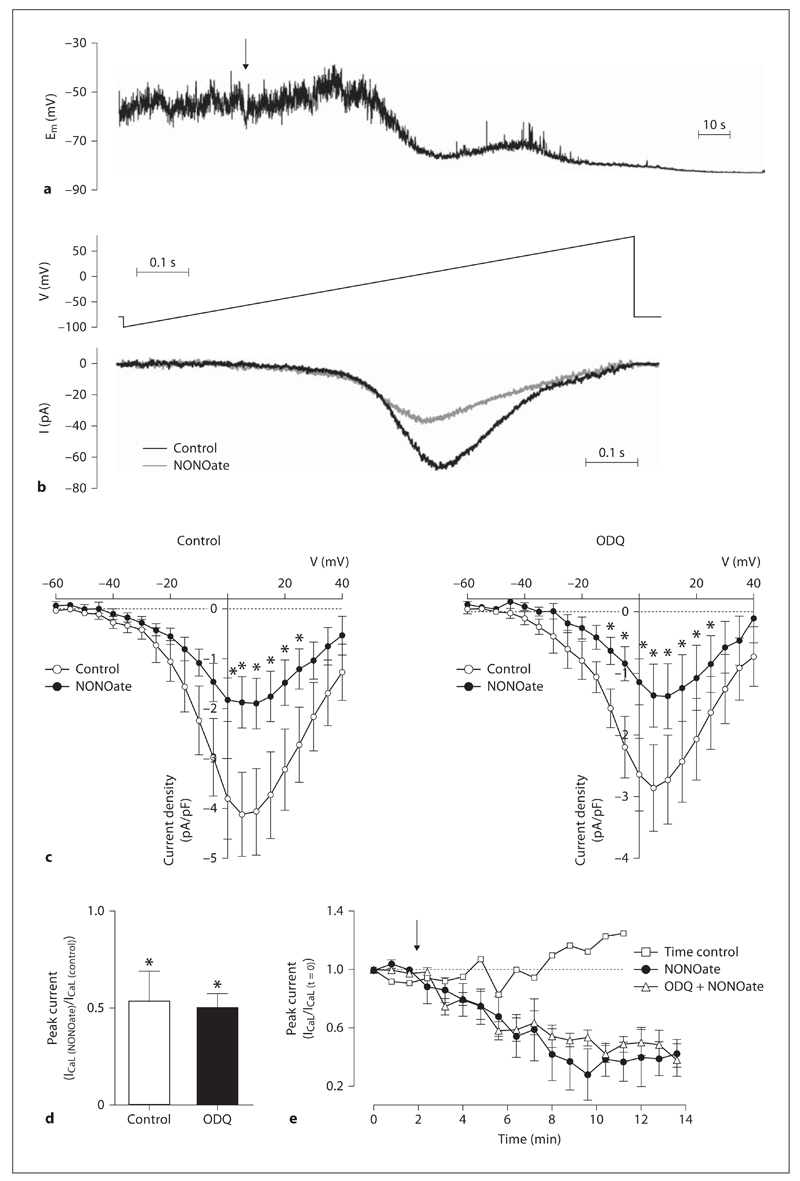
The average resting Em of isolated smooth muscle cells was –51.1 ± 2.0 mV (n = 12). In these unstretched and unstimulated cells, Em tended to oscillate (amplitude of 15.4 ± 2.8 mV, n = 12), but a clear pattern was not observed (fig. 7a). In contrast, under similar conditions at 37°C, the resting Em of smooth muscle cells isolated from mesenteric arteries tended to remain stable at –54.5 ± 0.6 mV (with less frequent and lower amplitude oscillations of 5.2 ± 0.5 mV, n = 11). Addition of 1 µm NONOate to the superfusion solution stimulated hyperpolarization and abolished the oscillations in Em (fig. 7a). In whole cell mode, steady-state ICaL was recorded for 1 min using the ramp protocol. Application of NONOate (1 µm) to the bath induced a significant reduction in ICaL that was not inhibited by ODQ (fig. 7c, d; n = 6–7). The effect of NONOate on ICaL was time dependent (fig. 7d), so values were taken at 10 min following application of NONOate.
Fig. 7.
NONOate inhibits VGCC via a sGC-independent mechanism. a In isolated smooth muscle cells at 37°C under current-clamp conditions the resting Em oscillated. Addition of 1 µm NONOate (indicated by arrow) hyperpolarized the cell and abolished the oscillations in Em. b The voltage protocol for detecting ICaL (top) resulted in inward current that was reduced by 1 µm NONOate (bottom). c Mean current voltage relationships under both control conditions (n = 6, left) and after pretreatment with ODQ (n = 7, right) show that the inhibition of ICaL by 1 µm NONOate was not sensitive to ODQ. d The peak current was reduced by approximately 50% under both conditions. Data are expressed as means ± SEM. * p < 0.05, significant difference from control. e Effect of 1 µm NONOate (added at arrow) on peak ICaL amplitude over time, for data shown in c and d. NONOate-induced ICaL inhibition took minutes to occur, and was not due to current rundown (time control).
Discussion
These data from the rat middle cerebral artery indicate that myogenic tone and vasomotion are normally suppressed by basal release of endothelium-derived NO that inhibits VGCC largely via sGC-independent pathways. This can occur either through an effect at RYR and activation of smooth muscle cell BKCa channels, or a direct action independent of voltage. The activation of BKCa channels appears to involve in part an indirect action of NO due to stimulation of Ca2+ release from ryanodine-sensitive Ca2+ stores, but also in part a direct action of NO on the KCa channel. Therefore, upon inhibition of NOS, smooth muscle cell depolarization due to closure of BKCa channels and the removal of an inhibitory influence, both lead to opening of VGCCs which is followed by a rise in [Ca2+]SMC and tension leading to arterial vasomotion.
The finding that myogenic tone is normally suppressed by basal release of NO in rat middle cerebral arteries is consistent with previous studies using cerebral arteries [14–19] and a variety of other vessels that exhibit myogenic tone including small coronary arteries [11, 12]. By suppressing myogenic tone, NO also suppresses vasomotion in the middle cerebral artery. Nifedipine fully reversed the effects of l-NAME, reversing tension and abolishing synchronized oscillations in both Em and [Ca2+]SMC. Therefore, it is apparent that opening VGCCs is essential for vasomotion to develop, consistent with many other vessels [1, 36]. Despite this, we cannot rule out the involvement of other ion channels. Once the intracellular Ca2+ levels rise and the membrane depolarizes, other channels would be stimulated to open, including voltage-gated Na+ channels, Ca2+-activated Cl– channels and KCa channels. Furthermore, as both the endothelial and smooth muscle cells are coupled by homocellular and heterocellular gap junctions in this artery [37], it remains possible that the endothelium influences membrane potential through NO or other mediators. For example, changes in endothelial cell Ca2+ are responsible for the release of NO, so endothelial cell KCa channels may also play a role in the observed changes in membrane potential.
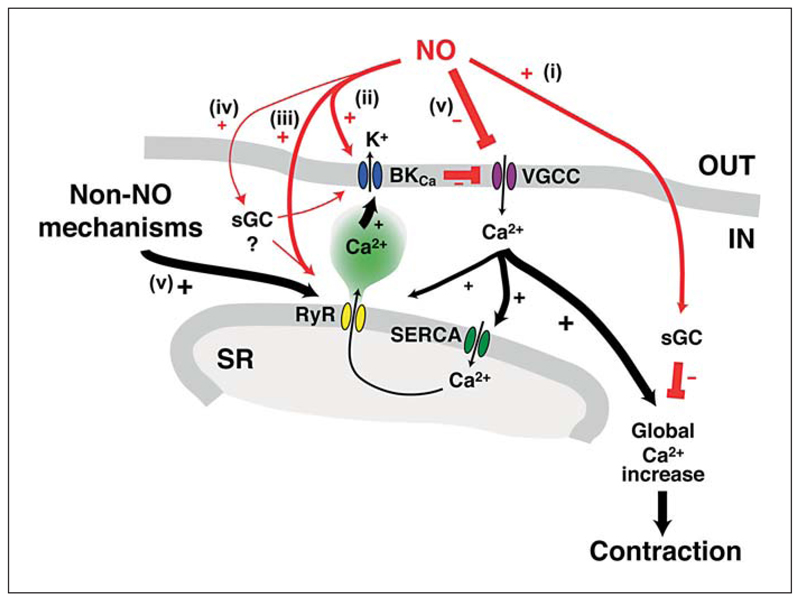
While basal release of NO is known to suppress myogenic tone (and vasomotion), the precise mechanisms are unclear. However, it is likely that NO acts via multiple mechanisms, a few of which are shown in figure 8. NO can suppress the contractile apparatus of the smooth muscle cells via the cGMP pathway. Indeed, ODQ produced increases in tension and depolarization (similar to l-NAME, albeit with a lower frequency of vasomotion), suggesting that sGC somehow stimulates hyperpolarization, perhaps via an action at BKCa channels through PKG-dependent mechanisms [24, 25] or by an action on RYRs [27, 28]. In addition, our data are consistent with a cGMP-independent action of NO at BKCa channels, because in the presence of ODQ the BKCa channel-mediated hyperpolarization induced by the NO donor was reduced, but not significantly, leaving an ODQ-insensitive hyperpolarization. This suggests that endogenous NO activates BKCa channels either directly [21, 22] or via stimulation of Ca2+ release (for example sparks) from ryanodine-sensitive stores (by opening RYRs). Evidence for the latter comes from the ability of ryanodine to block NONOate-induced hyperpolarization in endothelium-damaged vessels. Despite this block, NONOate was still able to reduce the frequency and amplitude of the depolarizing spikes (oscillations in Em) linked with the vasomotion generated by ryanodine. This suggests that NO acts to prevent the opening of the ion channel responsible for the depolarization. Further evidence consistent with a cGMP-independent action of NO on RYRs was the ability of ryanodine to (1) stimulate vasomotion, mimicking the effect of NOS inhibition, and (2) inhibit the IbTx-sensitive hyperpolarization to caffeine.
Fig. 8.
Schematic depicting actions of NO in cerebral artery smooth muscle cells. Release of NO from endothelial cells can suppress vasomotion via multiple mechanisms. (i) Stimulation of sGC can relax smooth muscle cells via voltage-independent pathways. (ii) NO can directly activate BKCa channels, leading to hyperpolarization, closure of VGCC and relaxation. The action of NO on BKCa channel activity can be indirect, via a direct action of NO at RYRs, or (iv) via an intermediate (such as sGC/PKG). RyRs are also activated by NO-independent mechanisms (v) including those related to store filling via the Ca2+ ATPase (SERCA). This depiction is based on the close association of RyRs to BKCa-channels, which are spatially separated from the Ca2+ release and influx mechanisms associated with contraction [43, 44].
Although it is likely that a major component of NO-induced suppression of myogenic tone involves a stimulation of Ca2+ release events, our data argue against an essential role for NO in the activation of RYRs. In the presence of nifedipine, l-NAME did not markedly prevent the basal asynchronous propagating Ca2+ waves, whereas ryanodine did. This is in contrast to previous observations in cerebral arteries by Mandala et al. [26], who suggested that NO was absolutely essential for RYR activation (and thus for activation of BKCa channels) because spontaneous Ca2+ sparks were reduced by around 50% with NOS inhibitors or endothelium removal. However, following on from our observations, it is likely that asynchronous propagating Ca2+ waves were masked by the Ca2+ influx through the L-type VGCCs and development of synchronous Ca2+ oscillations, as observed in the present study. Therefore, while the activation of BKCa channels by NO likely involves direct stimulation of RYR-controlled Ca2+ stores, this action of NO is not an essential step in the activation of RYR. It follows that as RYR stimulation is not necessarily associated with NO, an as yet unidentified process may also modulate vasomotion. In support of this conclusion, inhibition of RYR in the absence of a functional endothelium (and therefore NO synthesis) resulted in a small increase in tension as well as development of large, regular depolarizing oscillations in Em.
Further experiments in the absence of functional endothelium showed that NONOate appears to directly inhibit VGCCs. In the presence of both IbTx and ODQ, NONOate responses mimicked those of nifedipine under control conditions and in the presence of l-NAME, that is, complete block of the oscillations in Em associated with a small hyperpolarization, and relaxation. This direct effect of NONOate on VGCC was confirmed in isolated smooth muscle cells, where ICaL was markedly reduced. The effect of NONOate on ICaL in the isolated cells appears to be, at least in part, via a direct action on the channel protein or associated proteins, rather than via a cGMP-dependent mechanism. This is consistent with previous findings in the carotid body, where Summers et al. [33] showed that NO-mediated inhibition of ICaL occurs via S-nitrosylation of the channel protein, and that S-alkylation of the free cysteine residues by NEM prevented the modulation by the NO donor sodium nitroprusside, rather than via the activation of sGC.
Further evidence for the action of NO on ICaL in our studies to be induced by nitrosylation rather than via the cGMP/PKG pathway may come from the time course of NONOate-induced inhibition, which took minutes to tens of minutes to occur. Previous studies of neuronal BKCa channels suggest that not only does nitrosylation require a higher concentration of NO than the PKG pathway, but it develops with a much slower time course [38, 39].
The action of NO at VGCC was fully reversed by adding the direct opener of L-type VGCC, suggesting that the sites of action are independent. Although there is evidence that both NO and BayK 8644 each evoke their effects on the L-type VGCC via the pore-forming α1c-subunit, BayK 8644, which competitively competes with nifedipine, binds from the extracellular surface to access the dihydopyridine receptor site within the channel [40, 41]. The site of NO-induced VGCC modulation by nitrosylation still remains to be elucidated, although there is evidence that VGCC function can be impaired by nitrosylation of an intracellular tyrosine residue (Y2134) situated in the src kinase protein-binding domain of the carboxy terminal of the α1c-subunit [42].
In summary, in rat middle cerebral arteries a basal release of NO from the endothelium suppresses myogenic tone. This suppression of myogenic tone is due, at least in part, to the ability of NO to stimulate BKCa channels by activating ryanodine-sensitive Ca2+ stores. Following inhibition of NOS, the BKCa channels close, leading to depolarization, with an associated increase in tension and the development of vasomotion. Therefore, our data indicate that basal NO release represents an important controlling mechanism on myogenic tone in cerebral arteries. In disease states where NO synthesis is compromised, disruption of this constitutive suppression of myogenic tone would be predicted to increase significantly the risk of brain ischaemia.
Supplementary Material
Acknowledgments
This work was supported by the British Heart Foundation (PG/04/069) and the Wellcome Trust (079677 and 07543/04).
References
- 1.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 2.Fujii K, Heistad DD, Faraci FM. Ionic mechanisms in spontaneous vasomotion of the rat basilar artery in vivo. J Physiol. 1990;430:389–398. doi: 10.1113/jphysiol.1990.sp018297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 4.Diehl RR, Diehl B, Sitzer M, Hennerici M. Spontaneous oscillations in cerebral blood flow velocity in normal humans and in patients with carotid artery disease. Neurosci Lett. 1991;127:5–8. doi: 10.1016/0304-3940(91)90880-3. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Lindauer U, Villringer A. Nitric oxide synthase blockade enhances vasomotion in the cerebral microcirculation of anesthetized rats. Microvasc Res. 1993;45:318–323. doi: 10.1006/mvre.1993.1028. [DOI] [PubMed] [Google Scholar]
- 6.Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- 7.Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545:615–627. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswal BB, Hudetz AG. Synchronous oscillations in cerebrocortical capillary red blood cell velocity after nitric oxide synthase inhibition. Microvasc Res. 1996;52:1–12. doi: 10.1006/mvre.1996.0039. [DOI] [PubMed] [Google Scholar]
- 9.Jung CS, Oldfield EH, Harvey-White J, Espey MG, Zimmermann M, Seifert V, Pluta RM. Association of an endogenous inhibitor of nitric oxide synthase with cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:945–950. doi: 10.3171/JNS-07/11/0945. [DOI] [PubMed] [Google Scholar]
- 10.Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol Ther. 2005;105:23–56. doi: 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Garcia SR, Bund SJ. Nitric oxide modulation of coronary artery myogenic tone in spontaneously hypertensive and Wistar-Kyoto rats. Clin Sci (Lond) 1998;94:225–229. doi: 10.1042/cs0940225. [DOI] [PubMed] [Google Scholar]
- 12.Graves JE, Greenwood IA, Large WA. Tonic regulation of vascular tone by nitric oxide and chloride ions in rat isolated small coronary arteries. Am J Physiol Heart Circ Physiol. 2000;279:H2604–H2611. doi: 10.1152/ajpheart.2000.279.6.H2604. [DOI] [PubMed] [Google Scholar]
- 13.Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 14.Golding EM, Steenberg ML, Johnson TD, Bryan RM. Nitric oxide in the potassium-induced response of the rat middle cerebral artery: a possible permissive role. Brain Res. 2001;889:98–104. doi: 10.1016/s0006-8993(00)03121-8. [DOI] [PubMed] [Google Scholar]
- 15.Peng HL, Jensen PE, Nilsson H, Aalkjar C. Effect of acidosis on tension and [Ca2+]i in rat cerebral arteries: is there a role for membrane potential? Am J Physiol Heart Circ Physiol. 1998;274:H655–H662. doi: 10.1152/ajpheart.1998.274.2.H655. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 17.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeish AJ, Dora KA, Garland CJ. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke. 2005;36:1526–1532. doi: 10.1161/01.STR.0000169929.66497.73. [DOI] [PubMed] [Google Scholar]
- 19.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 20.Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvasc Res. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- 21.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 22.Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homer KL, Wanstall JC. Cyclic GMP-independent relaxation of rat pulmonary artery by spermine NONOate, a diazeniumdiolate nitric oxide donor. Br J Pharmacol. 2000;131:673–682. doi: 10.1038/sj.bjp.0703613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 25.Archer SL, Huang JMC, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandala M, Heppner TJ, Bonev AD, Nelson MT. Effect of endogenous and exogenous nitric oxide on calcium sparks as targets for vasodilation in rat cerebral artery. Nitric Oxide. 2007;16:104–109. doi: 10.1016/j.niox.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Eu JP, Xu L, Stamler JS, Meissner G. Regulation of ryanodine receptors by reactive nitrogen species. Biochem Pharmacol. 1999;57:1079–1084. doi: 10.1016/s0006-2952(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 28.Suko J, Maurer-Fogy I, Plank B, Bertel O, Wyskovsky W, Hohenegger M, Hellmann G. Phosphorylation of serine 2843 in ryanodine receptor-calcium release channel of skeletal muscle by cAMP-, cGMP- and CaM-dependent protein kinase. Biochim Biophys Acta. 1993;1175:193–206. doi: 10.1016/0167-4889(93)90023-i. [DOI] [PubMed] [Google Scholar]
- 29.Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca2+ channels in cerebral arterioles. Circ Res. 2001;88:359–365. doi: 10.1161/01.res.88.3.359. [DOI] [PubMed] [Google Scholar]
- 30.Quignard JF, Frapier JM, Harricane MC, Albat B, Nargeot J, Richard S. Voltage-gated calcium channel currents in human coronary myocytes. Regulation by cyclic GMP and nitric oxide. J Clin Invest. 1997;99:185–193. doi: 10.1172/JCI119146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tewari K, Simard JM. Sodium nitroprusside and cGMP decrease Ca2+ channel availability in basilar artery smooth muscle cells. Pflugers Arch. 1997;433:304–311. doi: 10.1007/s004240050281. [DOI] [PubMed] [Google Scholar]
- 32.Almanza A, Navarrete F, Vega R, Soto E. Modulation of voltage-gated Ca2+ current in vestibular hair cells by nitric oxide. J Neurophysiol. 2007;97:1188–1195. doi: 10.1152/jn.00849.2006. [DOI] [PubMed] [Google Scholar]
- 33.Summers BA, Overholt JL, Prabhakar NR. Nitric oxide inhibits L-type Ca2+ current in glomus cells of the rabbit carotid body via a cGMP-independent mechanism. Journal of neurophysiology. 1999;81:1449–1457. doi: 10.1152/jn.1999.81.4.1449. [DOI] [PubMed] [Google Scholar]
- 34.Jian K, Chen M, Cao X, Zhu XH, Fung ML, Gao TM. Nitric oxide modulation of voltage-gated calcium current by S-nitrosylation and cGMP pathway in cultured rat hippocampal neurons. Biochem Biophys Res Commun. 2007;359:481–485. doi: 10.1016/j.bbrc.2007.05.113. [DOI] [PubMed] [Google Scholar]
- 35.Garland CJ, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566:645–656. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 38.Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- 39.Klyachko VA, Ahern GP, Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike afterhyperpolarization. Neuron. 2001;31:1015–1025. doi: 10.1016/s0896-6273(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 40.Alborch E, Salom JB, Torregrosa G. Calcium channels in cerebral arteries. Pharmacol Ther. 1995;68:1–34. doi: 10.1016/0163-7258(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 41.Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H. Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol Sci. 1998;19:108–115. doi: 10.1016/s0165-6147(98)01171-7. [DOI] [PubMed] [Google Scholar]
- 42.Kang M, Ross GR, Akbarali HI. COOH-terminal association of human smooth muscle calcium channel CaV1.2b with Src kinase protein binding domains: effect of nitrotyrosylation. Am J Physiol Cell Physiol. 2007;293:C1983–C1990. doi: 10.1152/ajpcell.00308.2007. [DOI] [PubMed] [Google Scholar]
- 43.Sanders KM. Invited review: mechanisms of calcium handling in smooth muscles. J Appl Physiol. 2001;91:1438–1449. doi: 10.1152/jappl.2001.91.3.1438. [DOI] [PubMed] [Google Scholar]
- 44.Lee CH, Poburko D, Kuo KH, Seow CY, van Breemen C. Ca2+ oscillations, gradients, and homeostasis in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1571–H1583. doi: 10.1152/ajpheart.01035.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.