Abstract
Objective
Low body mass index (BMI) is a risk factor for poor longterm outcomes in rheumatoid arthritis (RA). The purpose of this study was to identify factors associated with longterm changes in BMI.
Methods
Subjects with RA from the Veterans Affairs (VA) Rheumatoid Arthritis (VARA) Registry (n = 1474) were studied. Information on inflammatory markers, presence of erosions, and smoking status were extracted from the VARA database. BMI was extracted from VA electronic medical records within 14 days of each visit date. VA pharmacy records were queried to identify prescriptions for specific RA therapies within 1 month of the visit date. We used robust generalized estimating equations marginal regression models to calculate independent associations between clinical variables and BMI over time. Similar models determined predictors of change in weight and risk of weight loss over the subsequent study observation period.
Results
Increasing age, active smoking, and the presence of erosions at baseline were associated with lower BMI. Weight decreased over time among older adults. Factors associated with greater reductions in BMI over time and a greater risk of weight loss were higher inflammatory markers, smoking, older age, higher BMI, and less subsequent improvement in inflammation. Methotrexate use was associated with a lower risk of weight loss. The use of prednisone or anti-tumor necrosis factor therapies was not associated with change in BMI or the risk of weight loss independent of other factors.
Conclusion
Greater age, greater inflammatory activity, and active smoking are associated with greater weight loss in RA over time.
Key Indexing Terms: RHEUMATOID ARTHRITIS, BODY MASS INDEX, CACHEXIA, INFLAMMATION
Low body mass index (BMI) is a strong predictor of poor longterm outcomes in rheumatoid arthritis (RA). Subjects with low BMI have greater deficits in muscle mass, greater joint destruction, and greater longterm mortality1,2,3,4,5. Low BMI in RA therefore might be evidence of more aggressive disease manifesting as weight loss over time as a result of greater resting energy expenditure.
Rheumatoid cachexia, or wasting associated with uncontrolled RA, has been described6,7,8,9. However, in the current era of RA management, this phenomenon has been considered rare. In fact, more recent data have suggested that BMI is normal or greater overall among patients with RA compared to controls8,10. There are limited data evaluating factors associated with change in BMI over time among subjects with RA. One clinical trial of 236 subjects demonstrated that a lower Disease Activity Score at 28 joints was associated with greater increases in BMI at 6 months11. Other studies have also suggested that RA subjects with low baseline weight tend to gain weight after the initiation of anti-tumor necrosis factor (TNF) therapy12. Improved disease control resulting in greater weight gain (and a lower risk of weight loss) potentially explains why obese RA subjects experience better longterm outcomes.
Change in weight represents an important factor because it predicts longterm outcomes in other populations. For instance, greater weight loss among the elderly independently carries an increased risk of premature death13. In our study, we determined the factors that independently predict change in BMI (in particular weight loss) for a cohort of patients with RA. To achieve this goal, we examined several time-varying and time-invariant candidate predictors of change in body weight, including markers of systemic inflammation, improvements in inflammation, erosive disease at baseline, autoantibody seropositivity, active smoking at baseline, prednisone use, and disease-modifying antirheumatic drug use [specifically methotrexate (MTX) and anti-TNF use]. We hypothesized that greater systemic inflammation and more severe disease would place subjects at a greater risk of weight loss.
MATERIALS AND METHODS
Study sample
Our sample consisted of subjects from the US Veterans Affairs (VA) Rheumatoid Arthritis (VARA) Registry1,14,15,16,17,18,19,20. The VARA national repository and multicenter chronic disease registry began enrollment in January 2003 and has operated for more than 10 years. At the time of our study, 12 VA sites participated (Salt Lake City, Utah; Washington, DC; Jackson, Michigan; Philadelphia, Pennsylvania; Brooklyn, New York; Omaha, Nebraska; Dallas, Texas; Iowa City, Iowa; Denver, Colorado; Little Rock, Arkansas; Portland, Oregon; Birmingham, Alabama). Veterans with RA were identified by the treating physician at the individual sites. Veterans who fulfilled the 1987 American College of Rheumatology criteria for RA and were over 18 years of age were eligible for VARA enrollment. The physician-investigators at each site recorded clinical data at enrollment and at followup visits, including the Multidimensional Health Assessment Questionnaire (MD-HAQ), swollen and tender joint counts (0 to 28), inflammatory markers, pain score, and patient’s and physician’s disease assessment scores. Each individual site had Institutional Review Board approval and all study patients provided informed written consent at enrollment.
Key study measures
Demographics and disease-specific characteristics at baseline and in followup were extracted from the VARA registry database. Results of testing of inflammatory markers [erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)], clinical joint counts, global disease assessments [patient and physician visual analog scale (0–100)], physician-reported presence of erosions at baseline, and smoking status at baseline were extracted from the VARA database.
Other data used in our study included the VA Corporate Data Warehouse and the Decisions Support System accessed by the VA Informatics and Computing Infrastructure. Weight measurements recorded for clinical care 14 days before or after each VARA visit date were extracted from the VA electronic medical records. Weight in kilograms was converted to BMI by dividing by the square of the modal height (in meters). If weight was not measured during the 14 days before or after the clinic visit, the observation was dropped from analyses.
The electronic prescription of specific RA therapies within 1 month prior to or following the visit date was determined by querying the VA pharmacy records using previously validated algorithms17. Anticyclic citrullinated peptide antibodies (anti-CCP) were measured using a second-generation anti-CCP (anti-CCP2) ELISA (Diastat, Axis-Shield; positive test ≥ 5 U/ml). A subset of participants (over 90% of the study cohort) was genotyped for HLA-DRB shared epitope containing alleles as previously described18.
Baseline comorbid conditions that were considered a priori to be potential confounders were defined based on the International Classification of Diseases-9-Clinical Modification (ICD-9-CM) codes, and Current Procedural Terminology codes identified prior to or within 1 year of enrollment in the registry. Coding of comorbidities was based on previously validated algorithms21,22, or algorithms appearing in peer-reviewed publications14,23,24,25. Specific comorbidities assessed included a history of cardiovascular disease (including heart failure), any malignancy, diabetes, chronic kidney disease, and depression.
Statistical analysis
Data were analyzed using Stata 12 software (StataCorp LP). Baseline associations between demographic and clinical variables and BMI were assessed using Student t tests and Wilcoxon rank-sum tests for data not fitting a normal distribution. Skewed data were log-transformed in linear regression models (ESR and CRP). The greatest number of missing values in the registry was observed for the physician-reported presence of erosions at enrollment (468/1950, 24%). These subjects exhibited disease characteristics similar to those of subjects with nonmissing data. Therefore, multiple imputation was performed based on covariables from regression models to replace missing values for this variable. For all other variables, observations with missing values were dropped from analyses. The statistical models for weight change were approached in the following 3 ways:
1. Predictors of BMI and interactions with time
We used sequential robust generalized estimating equations (GEE) in linear regression models (exchangeable correlation structure) to determine independent associations between clinical variables and BMI as the dependent variable. The robust “sandwich” estimator typically gives more consistent estimates of the standard errors when the correlation matrix is specified incorrectly. We confirmed the results of the GEE models with mixed-effects models with random intercept and random slope (unstructured and exchangeable correlation matrix). Results of mixed-effects models were comparable and are not reported.
We examined time-invariant exposures (baseline erosive disease, CCP seropositivity, baseline smoking, comorbid conditions) and time-varying exposures such as natural logarithm of CRP [ln(CRP)], treatments, and subsequent change in CRP. Based on a previous study demonstrating increases in weight up to age 50 and subsequent decline, we evaluated the association between age and BMI using splines by placing a knot at age 50 years in regression models26. To test for associations between clinical variables and the change in BMI over time, multiplicative interaction terms were used within these models. Significant interactions with age were illustrated by graphing the predicted mean BMI over the first 10 visits among subjects younger than 50 years or at least 50 years. The actual mean BMI over time was also graphed over the first 10 visits among a subset of subjects with persistently elevated CRP levels above the normal range (≥ 1.1 mg/dl, n = 162) over the first 3 visits compared with subjects with persistently normal CRP levels (< 1.1 mg/dl, n = 330) over the first 3 visits.
2. Factors associated with change in BMI over the subsequent followup interval
Fewer subjects and observations were included in these analyses because subjects were necessarily dropped if they only had 1 BMI measurement available (thus change could not be calculated). Where the change in BMI was the outcome in regression models, we used the absolute change in BMI adjusting for the baseline BMI based on previous work27. Differences in the change in BMI over the followup interval were evaluated using robust GEE models with exchangeable correlation structures. A time lag of 1 visit between the independent variable and the change in BMI was used. For significant predictors of weight loss, we also evaluated for effect modification by sex. Mixed-effect models (using random intercept, random slope, unstructured correlation structure) were used to confirm the findings of GEE models (results not shown).
3. Predictors of weight loss over the subsequent followup interval
Based on previous publications, we defined weight loss as a negative change in BMI of > 1 kg/m2 from the preceding visit13,28,29. A loss of 1 kg/m2 of BMI has been associated with death in other populations13,28. For an individual 67 inches tall, this loss in BMI would translate to a loss of weight of 2.9 kg or 6.4 lbs. Population-averaged robust GEE models with exchangeable correlation structures were used to evaluate associations between hypothesized variables and the risk of a loss in BMI of > 1 kg/m2 over the subsequent followup interval.
RESULTS
Baseline characteristics of study subjects
Out of 1950 total VARA registry subjects, 1474 had sufficient data to contribute to analyses with a median followup time of 5.9 years (4.1–8.3). The primary reasons for excluding subjects from analyses were a lack of available data for CCP seropositivity in 233 subjects, lack of BMI measurements in 269, lack of CRP measurements in 253, and lack of ESR measurements in 220. Baseline characteristics of study subjects are presented in Table 1. More than 90% of subjects were male with an average age of 63.4 years, consistent with the demographics of the VA.
Table 1.
Baseline clinical and demographic characteristics of study subjects from the VARA registry. Values are mean (SD) or median (interquartile range) unless otherwise specified.
| Variable | VARA Participants |
|---|---|
| Total subjects, n | 1474 |
| Age, yrs | 63.5 (11.0) |
| Male, % (n) | 91 (1344) |
| White, % (n) | 76 (1127) |
| Black, % (n) | 16 (241) |
| BMI, kg/m2 | 28.5 (5.4) |
| RF-positive, % (n) | 80 (283/1383) |
| CCP-positive, % (n) | 78 (1156) |
| Active smoker, % (n) | 27 (396) |
| Disease duration, yrs | 7.4 (1.7–17.0) |
| Presence of erosions, % (n) | 51 (615/1198) |
| Erosions, imputed, % (n) | 52 (764) |
| DAS28-ESR | 4.07 (1.6) |
| CRP, mg/dl | 0.8 (0.4–2) |
| ESR, mm/h | 22 (10–38) |
| MD-HAQ score | 0.94 (0.60) |
| Prednisone, % (n) | 39 (572) |
| Methotrexate, % (n) | 50 (744) |
| Sulfasalazine, % (n) | 13 (185) |
| Hydroxychloroquine, % (n) | 28 (409) |
| Leflunomide, % (n) | 11 (167) |
| Any TNF inhibitor use, % (n) | 20 (299) |
| Shared epitope positive, % (n) | 72 (960/1342) |
VARA: Veterans Affairs Rheumatoid Arthritis; BMI: body mass index; RF: rheumatoid factor; CCP: cyclic citrullinated peptide antibodies; DAS28: Disease Activity Score at 28 joints; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; MD-HAQ: Multidimensional Health Assessment Questionnaire; TNF: tumor necrosis factor.
Associations with BMI and interactions with time
In 164 subjects under 50 years of age, BMI was higher among older subjects. However, among subjects older than 50, older age was associated with lower BMI (Table 2, Model 1). BMI was also lower among those with erosive disease and who were actively smoking at baseline (Table 2, Model 1). Subjects treated with MTX had a higher weight at the time of the observation (p = 0.02). There were no associations between BMI and sex, race, or the use of prednisone or anti-TNF therapies at the time of the observation. There were no associations between MD-HAQ score, joint counts, global evaluator scores, or shared epitope positivity and BMI (not shown). On average, there was a decrease in BMI over time [per 100 days, β −0.015 (−0.026 – −0.0032), p = 0.01]. This equates to an average decrease in weight of about 0.19 kg or 0.41 lb per year.
Table 2.
Sequential robust GEE regression models assessing predictors of BMI over all study visits and interactions with time in subjects with all necessary data available.
| Variable | Model 1, n = 1474, Obs = 15,318 | Model 2, n = 1474, Obs = 15,318 | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Time, per 100 days | −0.015 (−0.026 – −0.0032) | 0.01 | 0.22 (0.15–0.29) | < 0.001 |
| Baseline age, per yr, < 50 | 0.14 (0.047–0.23) | 0.003 | 0.17 (0.079–0.26) | < 0.001 |
| Baseline age, per yr, ≥ 50 | −0.18 (−0.21 – −0.15) | < 0.001 | −0.15 (−0.18 – −0.12) | |
| Female | 0.082 (−1.06–1.23) | 0.7 | 0.13 (−1.01–1.28) | 0.8 |
| White | −0.41 (−1.09–0.22) | 0.3 | −0.40 (−1.05–0.25) | 0.2 |
| ln(CRP), mg/dl | −0.066 (−0.11 – −0.017) | 0.008 | 0.019 (−0.061−0.097) | 0.6 |
| Prednisone use | −0.028 (−0.15–0.094) | 0.7 | 0.015 (−0.13–0.10) | 0.8 |
| Methotrexate use | 0.15 (0.021–0.28) | 0.02 | 0.11 (−0.0073–0.23) | 0.07 |
| Anti-TNF use | 0.15 (−0.034–0.33) | 0.1 | 0.16 (−0.017–0.33) | 0.08 |
| Baseline CCP-positive | 0.22 (−0.40–0.83) | 0.5 | 0.19 (−0.43–0.81) | 0.5 |
| Baseline erosions | −1.82 (−2.36 – −1.29) | < 0.001 | −1.80 (−2.34 – −1.26) | < 0.001 |
| Baseline smoking | −2.96 (−3.58 – −2.34) | < 0.001 | −2.99 (−3.61 – −2.38) | < 0.001 |
| Interactions with observation time* | ||||
| Age × time, 1 yr of age × 100 days | — | — | −0.0038 (−0.0047 – −0.0029) | < 0.001 |
| ln(CRP) × time, 1 unit × 100 days | — | — | −0.0079 (−0.014 – −0.0014) | 0.02 |
Interactions tested, but not significant: Prednisone Use × Time; Methotrexate Use × Time; Anti-TNF Use × Time; Baseline Erosions × Time. GEE: generalized estimating equations; BMI: body mass index; Obs: observations; CRP: C-reactive protein; TNF: tumor necrosis factor; CCP: cyclic citrullinated peptide antibodies.
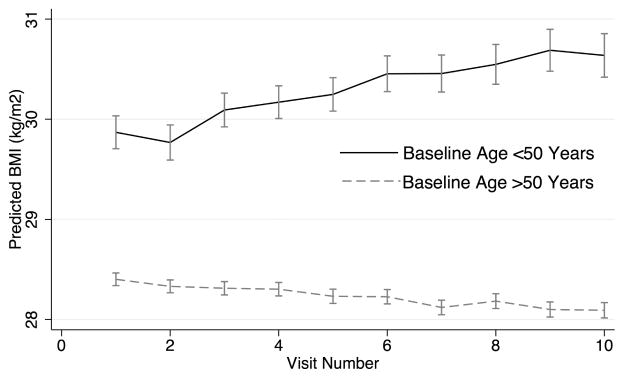
There was a significant interaction between baseline age and time from enrollment that suggested a greater decrease in BMI among older subjects (Table 2, Model 2). The interaction with age confirmed weight gain up until about the age of 57, with subsequent weight loss. Figure 1 illustrates the overall lower BMI and the greater decline in mean BMI over time among subjects greater than 50 years old at enrollment over the first 10 visits.
Figure 1.
Predicted mean BMI (SE) over time in patients at least 50 years old (n = 1312) compared to those younger than 50 (n = 162). BMI: body mass index.
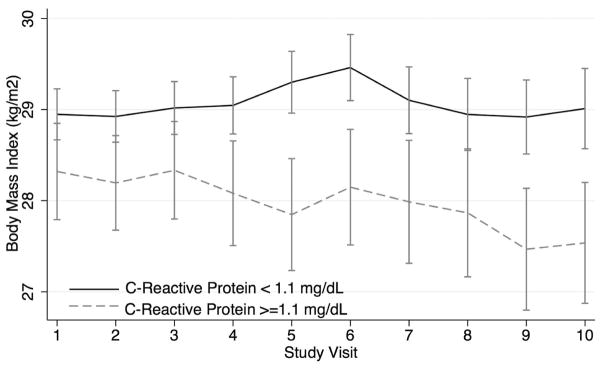
There was also a significant interaction between ln(CRP) and time from enrollment that suggested a greater decrease in weight among those with higher CRP levels (Table 2, Model 2). Figure 2 illustrates the greater decline in mean BMI over time among 154 subjects who had persistently elevated CRP levels (> 1.1 mg/dl) over the first 3 visits compared to 162 subjects who had persistently low CRP levels (< 0.5 mg/dl).
Figure 2.
Mean BMI (SE) over the first 10 visits in groups of subjects with CRP levels that remained above or equal to 1.1 mg/dl (n = 154) or below 1.1 mg/dl (n = 330) over the first 3 visits. BMI: body mass index; CRP: C-reactive protein.
There was no interaction between time from enrollment and baseline erosive disease, CCP seropositivity, active smoking at baseline, or the use of MTX, anti-TNF therapies, or prednisone (not shown). There was no interaction between any of the baseline comorbidities and time from enrollment.
Factors associated with change in BMI over the subsequent followup interval
A total of 1395 subjects contributed to 10,681 observations that included a current BMI and the change in BMI over the subsequent followup interval. The length of followup was not associated with change in weight (p = 0.9) and was not included as a covariable. Table 3 demonstrates that older age, higher baseline BMI, active smoking at baseline, and higher CRP were significant and independent predictors of a greater decrease in BMI over the subsequent observation period. A high CRP (> 1.1 mg/dl) was associated with greater weight loss in similar models [β −0.056 (−0.10 – −0.011), p = 0.02]. When ln(ESR) replaced ln(CRP) in this model, it also tended to be associated with a greater decrease in BMI [β −0.017 (−0.036–0.0015), p = 0.07, n = 1449; full model not shown]. In a subset of subjects who had sufficient data to calculate the change in CRP over the subsequent observation period (n = 1272, Obs = 9493), the change in CRP (per 1 mg/dl) was inversely associated with weight change [β −0.018 (−0.033 – −0.0018), p = 0.03].
Table 3.
GEE in linear regression models evaluating independent predictors of the change in BMI at the subsequent visit.
| Variable | Change in BMI at Subsequent Visit, n = 1395, Obs = 10,681 | |
|---|---|---|
| β (95% CI) | p | |
| Age, per 1 yr | −0.0069 (−0.0089 – −0.0049) | < 0.001 |
| Female | 0.042 (−0.026–0.11) | 0.2 |
| White | −0.012 (−0.054–0.031) | 0.6 |
| BMI, per 1 kg/m2 | −0.0065 (−0.011 – −0.0020) | 0.005 |
| ln(CRP), mg/dl | −0.024 (−0.040 – −0.0071) | 0.005 |
| Prednisone | 0.020 (−0.027–0.068) | 0.4 |
| Anti-TNF therapy | 0.032 (−0.013–0.078) | 0.2 |
| Methotrexate | 0.016 (−0.025–0.057) | 0.5 |
| Baseline CCP-positive | 0.013 (−0.033–0.059) | 0.6 |
| Baseline erosive disease | −0.029 (−0.067–0.0076) | 0.1 |
| Baseline smoker | −0.057 (−0.10 – −0.017) | 0.009 |
GEE: generalized estimating equations; BMI: body mass index; Obs: observations; CRP: C-reactive protein; TNF: tumor necrosis factor; CCP: cyclic citrullinated peptide antibodies.
The effect of CRP, smoking, and BMI was similar in men and women (i.e., no significant interaction). However, the effect of age (per 1 yr older) was more pronounced among men [β −0.0081 (−0.010 – −0.0059), p < 0.001] compared with the 126 women [β −0.0022 (−0.0067–0.0023), p = 0.3; p for interaction = 0.02].
There was no difference in the change in weight observed in subjects receiving MTX, anti-TNF therapies, or prednisone. Further, the initiation of prednisone was not associated with change in weight over the subsequent followup in final models [β 0.045 (−0.056–0.15), p = 0.4]. CCP seropositivity and the presence of erosions at baseline were not associated with change in BMI. There was no association between MD-HAQ score, joint counts, shared epitope positivity, global evaluator scores, or baseline comorbidities and the change in BMI at subsequent visits (not shown). There was no association between the interval duration and weight change, and the adjustment for interval duration did not alter the estimates of association.
Predictors of weight loss over the subsequent followup period
Weight loss > 1 kg/m2 of BMI occurred in 1917 out of 11,775 observation periods (14.0%). Weight loss over the subsequent observation period was more likely among older subjects, whites, baseline smokers, those with greater BMI, and those with greater CRP levels (Table 4). A high CRP (> 1.1 mg/dl) was associated with higher odds of weight loss in similar models [OR 1.32 (1.18–1.48), p < 0.001]. Greater ln(ESR) was also associated with a greater risk of weight loss when it replaced ln(CRP) in these models [OR 1.12 (1.06–1.18), p < 0.001, n = 1449]. In the subset of subjects with available data (n = 1272, Obs = 9493), less improvement in CRP over the following observation period was associated with a greater risk of weight loss [OR 1.07 (1.04–1.10), p < 0.001].
Table 4.
Final GEE population averaged marginal model examining predictors of weight loss at the subsequent visit (> 1 kg/m2 loss in BMI).
| Variable | Risk of Weight Loss, n = 1395, Obs = 10,681 | |
|---|---|---|
| OR (95% CI) | p | |
| Age, per 1 yr | 1.01 (1.00–1.02) | 0.002 |
| Female | 0.99 (0.80–1.21) | 0.9 |
| White | 1.19 (1.04–1.37) | 0.01 |
| BMI, per kg/m2 | 1.07 (1.06–1.08) | < 0.001 |
| ln(CRP), mg/dl | 1.13 (1.08–1.19) | < 0.001 |
| Current prednisone | 1.04 (0.92–1.18) | 0.5 |
| Current methotrexate | 0.84 (0.74–0.94) | 0.002 |
| Current anti-TNF | 0.99 (0.88–1.11) | 0.9 |
| CCP-positive | 1.07 (0.93–1.23) | 0.3 |
| Baseline erosive disease | 1.11 (0.98–1.25) | 0.1 |
| Baseline smoker | 1.26 (1.10–1.45) | 0.001 |
GEE: generalized estimating equations; BMI: body mass index; Obs: observations; CRP: C-reactive protein; TNF: tumor necrosis factor; CCP: cyclic citrullinated peptide antibodies.
MTX use, but not anti-TNF or prednisone use, was independently associated with a lower risk of weight loss. There was no association between MD-HAQ score, joint counts, shared epitope positivity, global evaluator scores, or baseline comorbidities, and the risk of weight loss at subsequent visits (data not shown).
DISCUSSION
To our knowledge, this is the first study to comprehensively evaluate clinical and demographic risk factors associated with change in BMI in RA subjects using a large, real-world, registry database. These data consistently demonstrated greater weight loss (and less weight gain) among older patients, those who smoked at enrollment, and those with greater systemic inflammation.
The observation that greater systemic inflammatory activity (higher CRP or ESR) is associated with a greater risk of weight loss (and less weight gain) supports the hypothesis that a history of more severe disease and greater systemic inflammation in RA is an important factor in determining an individual’s current BMI. A CRP above the normal range (> 1.1 mg/dl) was associated with about a 32% increased risk of significant weight loss. These observations are important because they suggest that BMI is affected by the longterm activity of the underlying inflammatory disease even in the current era of more aggressive RA management and after the advent of biologic therapies. Previous studies have demonstrated that low BMI is associated with poor outcomes in RA, including greater radiographic progression and greater mortality9,30. These observations are likely to be explained, at least in part, by weight loss or lack of weight gain seen in more severe and resistant disease over time.
The weight loss (and lack of weight gain) among subjects with high inflammatory activity may be because of the greater resting energy expenditure and greater catabolism seen with the active inflammatory process. Cachexia in other populations has been linked to cytokine excess, as well as deficiency in testosterone and insulin-like growth factor-131,32. Similar pathways are likely to be affected in RA. The data presented here suggest that even subtle changes in weight may be reflective of a greater inflammatory burden leading to upregulation of pathways associated with the cachectic phenotype. Improvements in CRP over the same period were also associated with a lower risk of weight loss (and greater weight gain). Therefore, when considering the effect of RA therapies on weight, it is important to consider that effective reversal of the systemic inflammatory process is likely to directly affect changes in weight.
These data also demonstrate that active smoking at baseline is predictive of a greater decline in BMI and greater risk of weight loss between clinical visits. Previous studies demonstrated greater weight gain among nonsmokers and greater weight gain associated with cessation of smoking33,34. Nicotine may act as an appetite suppressant and smoking is a behavioral alternative to eating35. Smoking may also increase metabolic rate and blunt the expected increase in food intake in response35. Active smoking has been linked to the severity of RA36,37,38, and therefore may help to explain why patients with RA with low BMI have more severe disease and poorer longterm outcomes.
These data suggest that advancing age is a risk factor for greater weight loss over time among subjects with RA, particularly among men. These data both confirm previous studies suggesting increases in weight up to age 50 and subsequent declines while also demonstrating increasing rates of weight loss at older ages26. We are not aware of previous data demonstrating greater weight loss among older subjects with RA. However, weight loss is more common among the elderly in the general population, possibly because of an increase in systemic cytokines and circulating hormones associated with early satiety39. Weight loss in the elderly is meaningful and predictive of premature mortality40,41. Thus, the increase in the rate of weight loss with advancing age among RA subjects is likely to be important and might conceivably be comparable with that observed in the general population.
Greater BMI was associated with a greater risk of weight loss. With all other variables the same, a subject with a BMI of < 20 kg/m2 in this cohort would be expected to gain weight while an obese subject would be expected to lose weight. Subjects with an obese BMI have the greatest opportunity to lose weight while subjects who are very thin may have the most to gain back by the initiation of effective therapies and reversal of the systemic inflammatory disease. Greater weight loss among more obese subjects has been described previously in other populations42,43. Similarly, weight gain has been observed among thin subjects with RA who initiate biologic therapies for RA12.
There was no clear effect of prednisone use on change in BMI after adjusting for measures of systemic inflammation. This observation might be unexpected given studies describing weight gain with glucocorticoid use44. However, the observations in the current study support a previous study showing that weight gain among those treated with prednisone was explained by a lower disease activity in that group11. The lack of association between prednisone use and weight gain might also be explained by the relatively low doses of prednisone that are typically used in this disease. We were not able to assess whether higher doses of prednisone might be associated with weight gain in this cohort.
We also did not see a more positive change in weight among anti-TNF users after adjustment for disease severity measures. Previous data have suggested weight gain among subjects initiating anti-TNF therapy12. However, these associations may have been confounded by greater disease control among patients treated with anti-TNF in these studies.
In our study, MTX use was not associated with change in overall BMI, but was associated with a lower risk of weight loss compared with subjects who did not use MTX. To our knowledge, this is the first study to describe a lower risk of weight loss among subjects receiving MTX and this is hypothesis-generating only. While models evaluating the effect of medication use on change in weight were adjusted for a number of potential confounders, including measures of disease severity and disease activity, studies of the effect of therapies within observational cohort studies are always limited by the potential for residual confounding by indication.
Strengths of our study are the large sample size, well-characterized clinical data, the multisite study design, and the longterm followup available. There are several limitations to consider in interpreting the results. While the VA setting was ideal for many aspects of the study, the proportion of men was higher than in other RA cohorts. Therefore, these observations may not be entirely generalizable and should be confirmed in other study populations. The use of BMI as the primary outcome does not consider that muscle and fat composition changes over time and therefore may not be an ideal measure of obesity and cachexia in chronic disease states. While the use of the VA data warehouse provided a unique opportunity to comprehensively study weight change in a registry setting, some clinical data were missing because of the dependence on the use of data routinely collected at regular clinic visits. Thus, some observations were imputed and some dropped because of missing data. Because missing data appear to be missing at random with regard to body mass, it seems unlikely that this would have introduced systematic bias. We were not able to assess compliance with medical therapies, explore dosing of therapies, or specifically assess other biologic therapies used with less frequency in this cohort. Finally, because most patients were enrolled years after they were diagnosed, we were not able to evaluate changes in weight immediately following diagnosis with RA.
Greater age, greater inflammatory activity, and active smoking are associated with greater weight loss among subjects with RA.
Acknowledgments
The Veterans Affairs Rheumatoid Arthritis (VARA) registry is supported by the Nebraska Arthritis Outcomes Research Center at the University of Nebraska Medical Center and by the Veterans Affairs Health Services Research and Development Program of the Veterans Health Administration. Dr. Baker is funded by a VA Clinical Science Research and Development Career Development Award (IK2 CX000955). Dr. Caplan is supported by the VA Health Service Research and Development Career Development Award (07-221). Dr. Mikuls is funded by a VA Merit Award. J.F. Baker, MD, MSCE, Division of Rheumatology, Philadelphia VA Medical Center, and Division of Rheumatology, and Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania; G.W. Cannon, MD, Salt Lake City VA Medical Center and University of Utah; S. Ibrahim, MD, MPH, Center for Health Equity Research and Promotion, Philadelphia VA Medical Center, and Perelman School of Medicine, University of Pennsylvania; C. Haroldsen, MSPH, Salt Lake City VA Medical Center and University of Utah; L. Caplan, MD, PhD, Department of Medicine, Denver VA Medical Center; T.R. Mikuls, MD, MSPH, Department of Medicine, Nebraska-Western Iowa VA Medical Center.
We thank Kaleb Michaud for helpful comments with regard to the analysis.
References
- 1.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology. 2011;50:101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol. 2003;30:2350–5. [PubMed] [Google Scholar]
- 3.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67:769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 4.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56:3575–82. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 5.Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 6.Munro R, Capell H. Prevalence of low body mass in rheumatoid arthritis: association with the acute phase response. Ann Rheum Dis. 1997;56:326–9. doi: 10.1136/ard.56.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roubenoff R. Rheumatoid cachexia: a complication of rheumatoid arthritis moves into the 21st century. Arthritis Res Ther. 2009;11:108. doi: 10.1186/ar2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J Rheumatol. 1992;19:1505–10. [PubMed] [Google Scholar]
- 9.Summers GD, Deighton CM, Rennie MJ, Booth AH. Rheumatoid cachexia: a clinical perspective. Rheumatology. 2008;47:1124–31. doi: 10.1093/rheumatology/ken146. [DOI] [PubMed] [Google Scholar]
- 10.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66:1316–21. doi: 10.1136/ard.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurgens MS, Jacobs JW, Geenen R, Bossema ER, Bakker MF, Bijlsma JW, et al. Utrecht Arthritis Cohort Study Group. Increase of body mass index in a tight controlled methotrexate-based strategy with prednisone in early rheumatoid arthritis: side effect of the prednisone or better control of disease activity? Arthritis Care Res. 2013;65:88–93. doi: 10.1002/acr.21797. [DOI] [PubMed] [Google Scholar]
- 12.Brown RA, Spina D, Butt S, Summers GD. Long-term effects of anti-tumour necrosis factor therapy on weight in patients with rheumatoid arthritis. Clin Rheumatol. 2012;31:455–61. doi: 10.1007/s10067-011-1863-6. [DOI] [PubMed] [Google Scholar]
- 13.Myrskylä M, Chang VW. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiology. 2009;20:840–8. doi: 10.1097/EDE.0b013e3181b5f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis LA, Cannon GW, Pointer LF, Haverhals LM, Wolff RK, Mikuls TR, et al. Cardiovascular events are not associated with MTHFR polymorphisms, but are associated with methotrexate use and traditional risk factors in US veterans with rheumatoid arthritis. J Rheumatol. 2013;40:809–17. doi: 10.3899/jrheum.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards JS, Cannon GW, Hayden CL, Amdur RL, Lazaro D, Mikuls TR, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res. 2012;64:1864–70. doi: 10.1002/acr.21777. [DOI] [PubMed] [Google Scholar]
- 16.Curtis JR, Baddley JW, Yang S, Patkar N, Chen L, Delzell E, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13:R155. doi: 10.1186/ar3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon GW, Mikuls TR, Hayden CL, Ying J, Curtis JR, Reimold AM, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res. 2011;63:1680–90. doi: 10.1002/acr.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikuls TR, Gould KA, Bynote KK, Yu F, Levan TD, Thiele GM, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12:R213. doi: 10.1186/ar3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis JR, Jain A, Askling J, Bridges SL, Jr, Carmona L, Dixon W, et al. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin Arthritis Rheum. 2010;40:2–14. e1. doi: 10.1016/j.semarthrit.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikuls TR, Padala PR, Sayles HR, Yu F, Michaud K, Caplan L, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res. 2013;65:227–34. doi: 10.1002/acr.21778. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 22.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 23.Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care. 2002;8:37–43. [PubMed] [Google Scholar]
- 24.Michaud K, Wolfe F. The development of a Rheumatic Disease Research Comorbidity Index for use in outpatients patients with RA, OA, SLE, and fibromyalgia (FMS) Arthritis Rheum Suppl. 2007;56:S596. [Google Scholar]
- 25.Wolfe F, Michaud K, Li T, Katz RS. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol. 2010;37:305–15. doi: 10.3899/jrheum.090781. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res. 2012;64:1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 27.Oloritun RO, Ouarda TB, Moturu S, Madan A, Pentland AS, Khayal I. Change in BMI accurately predicted by social exposure to acquaintances. PLoS One. 2013;8:e79238. doi: 10.1371/journal.pone.0079238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20:539–44. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 29.Bodegard J, Sundström J, Svennblad B, Östgren CJ, Nilsson PM, Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabetes Metab. 2013;39:306–13. doi: 10.1016/j.diabet.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Baker JF, Ostergaard M, George M, Shults J, Emery P, Baker DG, et al. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1–2 years. Ann Rheum Dis. 2014;73:1923–8. doi: 10.1136/annrheumdis-2014-205544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–43. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 32.Yuki A, Ando F, Otsuka R, Shimokata H. Low free testosterone is associated with loss of appendicular muscle mass in Japanese community-dwelling women. Geriatr Gerontol Int. 2014 Mar 14; doi: 10.1111/ggi.12278. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Fonseca V, McDuffie R, Calles J, Cohen RM, Feeney P, Feinglos M, et al. ACCORD Study Group. Determinants of weight gain in the action to control cardiovascular risk in diabetes trial. Diabetes Care. 2013;36:2162–8. doi: 10.2337/dc12-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clair C, Rigotti NA, Porneala B, Fox CS, D’Agostino RB, Pencina MJ, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309:1014–21. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–8. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masdottir B, Jónsson T, Manfredsdottir V, Vikingsson A, Brekkan A, Valdimarsson H. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatology. 2000;39:1202–5. doi: 10.1093/rheumatology/39.11.1202. [DOI] [PubMed] [Google Scholar]
- 37.Papadopoulos NG, Alamanos Y, Voulgari PV, Epagelis EK, Tsifetaki N, Drosos AA. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol. 2005;23:861–6. [PubMed] [Google Scholar]
- 38.Mikuls TR, Hughes LB, Westfall AO, Holers VM, Parrish L, van der Heijde D, et al. Cigarette smoking, disease severity and autoantibody expression in African Americans with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2008;67:1529–34. doi: 10.1136/ard.2007.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reife CM. Involuntary weight loss. Med Clin North Am. 1995;79:299–313. doi: 10.1016/s0025-7125(16)30069-4. [DOI] [PubMed] [Google Scholar]
- 40.Gray-Donald K, St-Arnaud-McKenzie D, Gaudreau P, Morais JA, Shatenstein B, Payette H. Protein intake protects against weight loss in healthy community-dwelling older adults. J Nutr. 2014;144:321–6. doi: 10.3945/jn.113.184705. [DOI] [PubMed] [Google Scholar]
- 41.Payette H, Coulombe C, Boutier V, Gray-Donald K. Weight loss and mortality among free-living frail elders: a prospective study. J Gerontol A Biol Sci Med Sci. 1999;54:M440–5. doi: 10.1093/gerona/54.9.m440. [DOI] [PubMed] [Google Scholar]
- 42.Singer LG, Brazelton TR, Doyle RL, Morris RE, Theodore J International Lung Transplant Database Study Group. Weight gain after lung transplantation. J Heart Lung Transplant. 2003;22:894–902. doi: 10.1016/s1053-2498(02)00807-0. [DOI] [PubMed] [Google Scholar]
- 43.Fadl YY, Krumholz HM, Kosiborod M, Masoudi FA, Peterson PN, Reid KJ, et al. Predictors of weight change in overweight patients with myocardial infarction. Am Heart J. 2007;154:711–7. doi: 10.1016/j.ahj.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–6. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]