Abstract
Objective
In contrast to what is observed in the general population, a low body mass index (BMI) has been associated with accelerated mortality in patients with rheumatoid arthritis (RA). The aim of this study was to assess whether weight loss might explain these seemingly paradoxical observations.
Methods
Our study included patients identified from the Veterans Affairs (VA) RA Registry. Dates of death were abstracted from VA electronic medical records. The BMI at each study visit and the change from the previous visit were determined. The maximum BMI of each patient was also obtained from medical records. The annualized rate of BMI loss was determined from the slope of change (per year) in BMI over visits within the preceding 13 months. Cox multivariable proportional hazards models were used to assess associations between BMI measures and mortality.
Results
In a sample of 1,674 patients, 312 deaths occurred over 9,183 person-years. A loss in BMI of ≥1 kg/m2 was associated with a greater risk of death, after adjustment for demographics, comorbidities, BMI, smoking, and RA therapies (hazard ratio [HR] 1.99, 95% confidence interval [95% CI] 1.53–2.59, P < 0.001). This association remained significant in a subsample analysis adjusting for C-reactive protein and physical function (HR 1.81, 95% CI 1.36–2.41, P < 0.001). Weight loss at an annualized rate of ≥3 kg/m2 was associated with the greatest risk of death (HR 2.49, 95% CI 1.73–3.57, P < 0.001). Low BMI (<20 kg/m2) in patients with a history of obesity (>30 kg/m2) was associated with the greatest risk (HR 8.52, 95% CI 4.10–17.71, P < 0.001).
Conclusion
Weight loss is a strong predictor of death in patients with RA. These observations may explain the observed obesity paradox and do not support a biologically protective role of obesity.
Several studies have demonstrated a greater risk of adverse outcomes in rheumatoid arthritis (RA), including a greater risk of death, in patients with low body mass index (BMI) (1,2). These observations might conceivably be explained by greater weight loss over time in patients with more severe disease and worsening health. Weight loss itself has been shown to be associated with a greater risk of death in other populations, including the elderly, and in patients with chronic conditions such as chronic obstructive pulmonary disease or chronic heart failure (3,4). No previous studies, however, have specifically investigated weight loss as a predictor of death in patients with RA.
Greater BMI is associated with the development of comorbid diseases that are associated with greater mortality, such as cardiovascular disease and certain forms of malignancy (5,6). However, several epidemiologic studies in the elderly and in patients with chronic disease states have shown that overweight patients may have lower mortality compared with those with normal body weight (3,7,8). Recent evidence supports the hypothesis that weight loss as the result of the development of chronic illness is a significant confounder explaining these seemingly paradoxical associations between greater body weight and lower mortality (9,10). Illness-induced weight loss also appears to explain findings of an obesity paradox in diabetes (11,12). Therefore, studies that do not consider the weight loss that occurs in chronic illnesses (such as RA) are likely to underestimate causal associations between greater body weight and death. To support this, incorporation of the patient’s BMI earlier in his or her life or the maximum lifetime BMI into analyses has been shown to demonstrate greater estimated risks associated with obesity by accounting for confounding weight loss due to chronic illness (9,13,14).
We sought to examine the association between recent weight loss and risk of death, using a large US cohort of patients with RA. Our aim was to determine whether weight loss was associated with an increased risk of subsequent death after adjusting for potential confounders such as demographics, comorbidities, treatments, and measures of systemic inflammation. We also assessed whether information regarding each patient’s maximum BMI could help to clarify the nature of previously observed associations between low BMI and risk of death in RA (9,10). We hypothesized that weight loss, particularly rapid weight loss, would be associated with greater mortality, and that the greatest risk of death would be observed in patients with a low BMI who had previously been overweight or obese.
PATIENTS AND METHODS
Our study sample consisted of 1,674 patients from the Veterans Affairs RA (VARA) Registry (2,15–21). VARA is a national repository and multicenter RA registry that has been active for more than 10 years (initiated in October, 2002). At the time this study was conducted, there were 12 participating VA sites (Salt Lake City, Washington, DC, Jackson, MS, Philadelphia, Brooklyn, Omaha, Dallas, Iowa City, Denver, Little Rock, Portland, Birmingham). Veterans with RA are identified by the treating physician at individual sites. All veterans who fulfill the American College of Rheumatology 1987 revised criteria for the classification of RA (22) and are older than age 18 years are eligible for VARA enrollment. The physician investigators at each site record clinical data at the time of enrollment and at routine followup visits as part of normal clinical care. Each individual site has institutional review board approval, and all study patients provided informed written consent and Health Insurance Portability and Accountability Act authorization at the time of enrollment.
Ascertainment of BMI and death
Data used in this study included those derived from the VA Corporate Data Warehouse (CDW) and the Decisions Support System accessed and linked with VARA data via the VA Informatics and Computing Infrastructure (23). Weight was extracted from the VA electronic medical record within 14 days of each study visit. Height (taken as the constant modal value) was used to calculate BMI (kg/m2). Modal height was used to avoid changing estimates of BMI due to measurement error in height over time. BMI categories were defined as previously described (underweight, <20 kg/m2; normal weight, 20–25 kg/m2; overweight, >25–30 kg/m2; obese, >30 kg/m2) (2). Changes in BMI between registry visits were determined. Consistent with previously published definitions of weight loss, modest weight loss was defined as an absolute decrease in BMI of >1 kg/m2 since the preceding visit (4,24). For each study observation, the annualized rate of change in the BMI was determined from the slope of the BMI over time over all available visits in the preceding 13 months. The annualized rate of decrease in the BMI was categorized as follows: no weight loss, BMI loss of <2 kg/m2 per year, BMI loss of 2–3 kg/m2 per year, and BMI loss of >3 kg/m2 per year.
The CDW Vital Signs package was also queried for all available body weight measurements for all patients from 1999 to 2013. The maximum BMI recorded during that time was obtained for each patient during this period regardless of when the RA diagnosis was made or when enrollment in the registry occurred. The median age at the time the maximum BMI was recorded was 64 years (interquartile range [IQR] 58–71 years). Patients were assigned to a BMI category based on their maximum BMI, as previously described (9).
Deaths and the dates of death were identified through systematic review of the VA Computerized Patient Record System (CPRS) throughout the period of October 2002 to June 2013 (2). Mortality events were captured by yearly CPRS abstraction, notification of the next of kin, or by periodic regional VA surveillance. All VA beneficiaries are tracked for vital status and death benefit eligibility, using social security numbers, on a quarterly basis.
Other study measures
Demographics and disease-specific characteristics at baseline and during followup were extracted from the VARA Registry database. Results of testing for markers of inflammation (erythrocyte sedimentation rate-, C-reactive protein [CRP]), clinical swollen/tender joint counts, global disease assessments, physician-reported presence of erosions, results from the Multidimensional Health Assessment Questionnaire (MD-HAQ) (25), and current smoking status were extracted from the VARA Registry data. The presence of erosions and current smoking status were considered to be time invariant (presence or absence of exposure at baseline). For analyses incorporating MD-HAQ scores, missing values were imputed using the last observation carried forward method.
The prescription of specific RA therapies within 1 month of the visit date was determined by querying the VA pharmacy records using previously validated algorithms (18). Anti–cyclic citrullinated peptide (anti-CCP) antibodies were measured using a Diastat second-generation anti-CCP2 enzyme-linked immunosorbent assay (Axis-Shield; positive ≥5 units/ml). Baseline comorbid conditions that were considered a priori to be potential confounders were defined based on International Classification of Diseases, Ninth Revision, Clinical Modification codes and Current Procedural Terminology codes identified prior to or within 1 year of enrollment in the registry. Determination of the presence or absence of specific comorbidities at baseline was based on previously validated algorithms (26,27) or algorithms appearing in peer-reviewed publications (15,28), with particular attention to prevalent comorbidities previously identified in the Rheumatic Disease Comorbidity Index (29,30). Comorbidities specifically assessed included cardiovascular disease, diabetes, hypertension, depression, chronic lung disease, any malignancy, and chronic kidney disease. Comorbidities not initially shown to be significant predictors of death were not included in the final models.
Statistical analysis
Patients for whom data for all of the variables of interest were available were included in longitudinal analyses. Skewed variables (the CRP level) were transformed to fit a normal distribution. Observations that were missing necessary values for BMI and change in BMI were not included. Changes in BMI prior to the time of death in patients who died compared with patients who survived to the date of data extraction were illustrated using Lowess curves. Cox proportional hazards models were used to study time-variant and time-invariant predictors of death. BMI category was treated as time-varying, with a unique value for each study visit. Similarly, weight loss and the annualized rate of decrease in the BMI were considered time-varying predictors at each study visit with available data. Potential confounding variables including age, sex, race, smoking, comorbidities, RA treatments, disability (the MD-HAQ score), and systemic inflammation (the CRP level) were included in multivariable models.
In separate models, the maximum recorded BMI category for each patient was included as an independent time-invariant predictor in Cox proportional hazards models. Linear combination was performed to assess the risk of death in patients with all possible weight histories. For example, the hazard ratio (HR) was calculated for a patient who was previously maximally obese and currently had a low BMI compared with a patient with a normal current and normal maximum BMI. For all analyses, the proportional hazards assumptions were evaluated by assessing for the significance of multiplicative interaction terms with time in the model. Data were analyzed with Stata version 12 software.
RESULTS
The baseline characteristics of the study population from VARA are shown in Table 1. BMI data were available for 1,674 of 1,900 patients; 1,639 patients had sequential values that enabled calculation of the change in BMI since the previous assessment. Among the 1,674 patients, 312 deaths occurred during 9,183 person-years of followup. The median followup time was 5.5 years (IQR 3.3–7.9 years), and the median interval between visits was 105 days (IQR 63–175 days).
Table 1.
Characteristics of the 1,674 VARA participants at enrollment*
| Age, mean ± SD years | 63.5 ± 11.1 |
| Male sex | 1,515 (91) |
| Race | |
| White | 1,206 (72) |
| African American | 275 (16) |
| Current smoker | 441 (27) |
| Baseline BMI, mean ± SD kg/m2 | 28.4 ± 5.5 |
| Maximum BMI, mean ± SD kg/m2 | 33.3 ± 12.7 |
| Baseline BMI category | |
| <20 kg/m2 | 50 (3) |
| 20–25 kg/m2 | 415 (25) |
| >25–30 kg/m2 | 635 (38) |
| >30 kg/m2 | 575 (34) |
| Maximum BMI category | |
| <20 kg/m2 | 7 (0.4) |
| 20–25 kg/m2 | 133 (8) |
| >25–30 kg/m2 | 571 (34) |
| >30 kg/m2 | 964 (58) |
| Disease specific | |
| Anti-CCP antibody positive† | 1,191 (79) |
| Disease duration, median (IQR) years | 7.4 (1.6–16.8) |
| DAS28-ESR, mean ± SD | 4.06 ± 1.6 |
| CRP, median (IQR) mg/dl | 0.8 (0.4–1.9) |
| ESR, median (IQR) mm/hour | 22 (10–38) |
| MD-HAQ score, mean ± SD | 0.93 ± 0.60 |
| Current therapy | |
| Prednisone | 646 (39) |
| Methotrexate | 846 (51) |
| TNF inhibitor | 336 (20) |
| Common comorbidities | |
| Diabetes | 437 (26) |
| Chronic kidney disease | 118 (7) |
| Lung disease | 538 (32) |
| Cardiovascular disease | 639 (38) |
| Depression | 523 (31) |
| Any malignancy | 863 (52) |
| Hypertension | 1,167 (70) |
Except where indicated otherwise, values are the number (%). VARA = Veterans Affairs Rheumatoid Arthritis Registry; BMI = body mass index; anti-CCP = anti–cyclic citrullinated peptide; IQR = interquartile range; DAS28-ESR = Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; CRP = C-reactive protein; MD-HAQ = Multidimensional Health Assessment Questionnaire; TNF = tumor necrosis factor.
A total of 1,517 patients were assessed.
At baseline, the mean ± SD age of the patients was 63.5 ± 11.1 years, and 91% of the patients were male, which is consistent with the demographics of the VA population. In the whole cohort, the maximum BMI was significantly higher than the BMI at enrollment (mean ± SD 33.3 ± 12.7 kg/m2 versus 28.5 ± 5.4 kg/m2; P<0.001). Fewer patients were obese, and a greater number of patients had a low or normal BMI based on their BMI at enrollment compared with their maximum BMI.
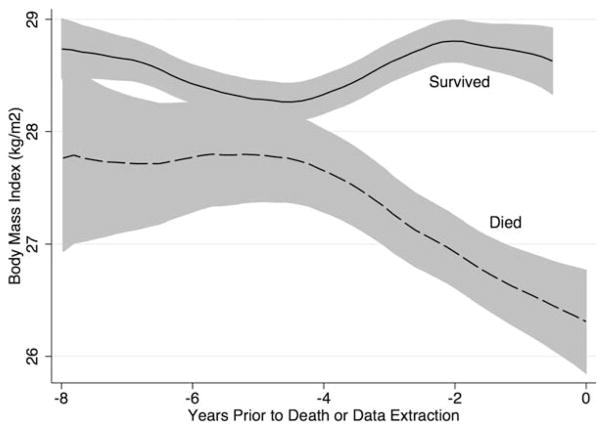
After multivariable adjustment, low BMI (<20 kg/m2) was associated with an increased risk of death over the subsequent followup period (Table 2). A modest weight loss (>1 kg/m2 of BMI) over the preceding observation period was associated with an increased risk of death before and after adjustment for multiple potential confounders (HR 1.99, 95% CI 1.53–2.59, P<0.001) (model 1) (Table 2). Further adjustment for CRP and MD-HAQ (model 2) (Table 2) in a subsample of observation periods with available data (n = 1,453 [13,844 observations]) did not attenuate these associations. Adjustment for length of the preceding interval did not affect these estimates. Figure 1 illustrates the rapid decline in mean BMI over time prior to death in patients who died compared with those who survived until the date of data extraction.
Table 2.
Multivariable-adjusted hazard ratios for death in patients with rheumatoid arthritis*
| Variable | Model 1 (n = 1,634) | Model 2 (n = 1,453) |
|---|---|---|
| Age at baseline | 1.06 (1.04–1.07)† | 1.06 (1.04–1.08)† |
| Female sex | 0.47 (0.23–0.95)† | 0.58 (0.27–1.24) |
| White race versus other | 1.10 (0.82–1.49) | 1.29 (0.88–1.89) |
| BMI category | ||
| <20 kg/m2 | 3.12 (2.12–4.57)† | 2.31 (1.50–3.57)† |
| 20–25 kg/m2 | Reference | Reference |
| >25–30 kg/m2 | 0.91 (0.67–1.23) | 0.92 (0.66–1.29) |
| >30 kg/m2 | 0.87 (0.61–1.24) | 0.74 (0.49–1.11) |
| Weight loss category | ||
| <1 kg/m2 loss | Reference | Reference |
| >1 kg/m2 loss | 1.99 (1.53–2.59)† | 1.81 (1.36–2.41)† |
| Current therapy | ||
| Methotrexate | 0.58 (0.45–0.76)† | 0.62 (0.46–0.82)† |
| Prednisone | 1.37 (1.06–1.77)† | 1.21 (0.91–1.63) |
| TNF inhibitor | 0.76 (0.55–1.05) | 0.85 (0.60–1.21) |
| Baseline comorbidities | ||
| Diabetes | 1.41 (1.09–1.83)† | 1.32 (0.98–1.77) |
| Cardiovascular disease | 1.55 (1.21–1.98)† | 1.57 (1.19–2.09)† |
| Chronic kidney disease | 1.62 (1.12–2.35)† | 1.41 (0.88–2.23) |
| Chronic lung disease | 1.39 (1.09–1.77)† | 1.10 (0.83–1.45) |
| Any malignancy | 1.36 (1.06–1.73)† | 1.59 (1.21–2.10)† |
| Active smoking | 1.50 (1.09–2.05)† | 1.44 (1.03–2.03)† |
| Natural log–transformed CRP | – | 1.29 (1.14–1.46)† |
| MD-HAQ score | – | 1.53 (1.22–1.90)† |
Model 1 includes age, female sex, white race, current body mass index (BMI) category, weight loss over the previous interval (versus no weight loss), use of methotrexate, prednisone, or tumor necrosis factor (TNF) inhibitor, presence of diabetes, cardiovascular disease, chronic kidney disease, or malignancy, and active smoking (17,057 observations, 280 deaths, 8,101 person-years). Model 2 includes the variables in model 1 but with the addition of the natural log–transformed C-reactive protein (CRP) level and the Multidimensional Health Assessment Questionnaire (MD-HAQ) scores (13,844 observations, 222 deaths, 6,417 person-years). Values are the hazard ratio (95% confidence interval).
P <0.05.
Figure 1.
Lowess curve illustrating the mean body mass index and 95% confidence interval prior to the date of death in patients who died compared with those who survived to the date when the database was queried.
There was a dose-dependent increase in the risk of death based on the annualized rate of decrease in BMI (Table 3). A decrease in the BMI of >3 kg/m2 per year was associated with the greatest risk of death over the subsequent observation period, after multivariable adjustment (HR 2.49, 95% CI 1.73–3.57, P < 0.001). There was no difference in the risk of death between patients who lost weight at a rate of <2 kg/m2 per year and those who experienced no weight loss over the preceding interval. The greater risk observed with a BMI decrease of >3 kg/m2 per year was similar after adjusting for CRP levels and MD-HAQ score in the subsample of patients for whom data were available (HR 2.08, 95% CI 1.39–3.10, P < 0.001) (1,424 patients [13,890 observations]) (full model not shown).
Table 3.
Rate of change in BMI over the previous interval and risk of subsequent death*
| Rate of change in BMI (no. of observations) | Risk of death | P |
|---|---|---|
| No weight loss (8,000) | 1 (reference) | |
| 0–<2 kg/m2 loss/year (6,541) | 1.12 (0.85–1.49) | 0.4 |
| 2–3 kg/m2 loss/year (1,067) | 1.65 (1.09–2.50) | 0.02 |
| >3 kg/m2 loss/year (1,421) | 2.49 (1.73–3.57) | <0.001 |
Values are the hazard ratio (95% confidence interval), adjusted for age, sex, white race/ethnicity (versus other), body mass index (BMI) category, diabetes, cardiovascular disease, chronic lung disease, any malignancy, chronic kidney disease, smoking, methotrexate use, prednisone use, and anti–tumor necrosis factor therapy use (1,574 patients, 17,029 observations, 286 deaths, 7,883 person-years).
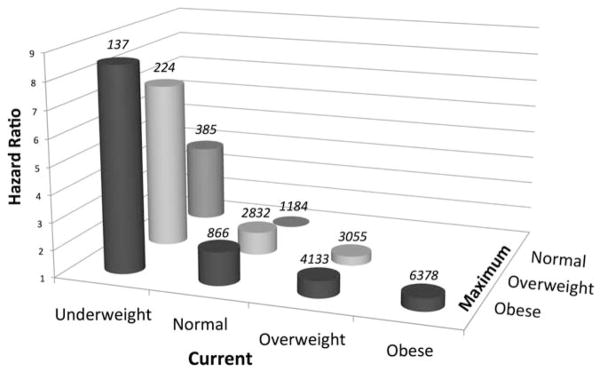
The date of maximum BMI was a median 9.5 years after the diagnosis of RA and a median 1.8 years after enrollment in the registry. In multivariable models, maximum BMI (per 1 kg/m2) was not associated with risk of death (HR 1.00, 95% CI 0.99–1.01, P = 0.80). In models including both current and maximum BMI, a lower current BMI and a higher maximum BMI category were each independently associated with a greater risk of death (Table 4). This model thus demonstrated that in patients with a similar maximum BMI, a low current BMI of <20 kg/m2 was associated with a greater risk of death (HR 3.82, 95% CI 2.51–5.84, P < 0.001). Similarly, among patients with a similar current BMI, those who were previously obese would have an increased risk of death (HR 2.22, 95% CI 1.28–3.58, P = 0.001). This model therefore suggests that a patient with a low BMI at a given visit and a history of obesity would have a dramatically increased risk of death compared with a normal-weight individual who consistently had a normal body weight (HR 8.52, 95% CI 4.10–17.71, P < 0.001) (Figure 2). In addition, among patients who remained in a stable weight category, obese patients had a nonsignificantly higher risk compared with patients in the normal-weight category (HR 1.47, 95% CI 0.89–2.42, P = 0.10). Finally, a decrease in BMI category between the maximum BMI to the current BMI was independently associated with a greater risk of death (HR 1.42, 95% CI 1.03–1.96, P = 0.03) (full model not shown).
Table 4.
Risk of death according to BMI category and maximum available BMI category*
| BMI | Risk of death | P |
|---|---|---|
| Current | ||
| <20 kg/m2 | 3.82 (2.51–5.84) | <0.001 |
| 20–25 kg/m2 | 1 (reference) | – |
| >25–30 kg/m2 | 0.73 (0.54–0.98) | 0.04 |
| >30 kg/m2 | 0.66 (0.45–0.97) | 0.03 |
| Maximum | ||
| <20 kg/m2 | 1.26 (0.40–4.52) | 0.6 |
| 20–25 kg/m2 | 1 (reference) | – |
| >25–30 kg/m2 | 1.84 (1.19–2.86) | 0.007 |
| >30 kg/m2 | 2.22 (1.28–3.58) | 0.001 |
Values are the hazard ratio (95% confidence interval), adjusted for age, sex, white race/ethnicity (versus other), smoking, lung disease, chronic kidney disease, any malignancy, cardiovascular disease, diabetes, and the use of methotrexate, prednisone, and anti–tumor necrosis factor therapies (1,674 patients, 19,348 observations, 312 deaths, 9,183 person-years).
BMI = body mass index.
Figure 2.
Calculated hazard ratios for death in patients with different body weight histories. Values above each column are the number of observations for each weight history. Only 7 patients were maximally underweight, and data for these patients are not shown.
DISCUSSION
This study is the first to comprehensively assess the roles of body weight and weight loss over time in predicting death in RA. We believe these observations are critical to understanding the predictive value of body weight on mortality in RA. This study is also the first to demonstrate a strong and independent association between recent weight loss, as opposed to BMI per se, and the risk of subsequent death in patients with RA. These observations represent an important step in explaining the “obesity paradox” in RA.
A change in weight of 1 kg/m2 over the preceding observation period was associated with an important increase in the risk of death. This study also demonstrated that a greater rate of weight loss was associated with an increased risk of death. Patients who lost weight at a rate of <2 kg/m2 per year did not have an increased risk of death. In contrast, those who lost weight at a rate of >3 kg/m2 per year had the greatest risk. This observation supports the common dogma that more rapid weight loss is a poor prognostic sign, while slower changes should be less alarming.
This study also went further to show that a history of obesity based on a patient’s maximum BMI was associated with an increased risk of death independent of the patient’s current BMI. In other words, for 2 patients who are currently in the same BMI category, the patient whose maximum recorded BMI was consistent with obesity has a higher risk of mortality. We believe that this novel observation, in combination with the previous observations, helps to explain the paradoxical association between obesity and lower mortality observed in RA, namely, that weight loss over time, perhaps related to chronic illness, at least partially explains the inverse association between BMI and mortality in RA. This notion is supported by the observation that in patients whose body weight remained stable over time, obesity was not associated with a decreased risk of death and tended toward an association with an increased risk.
Overall, these observations do not support a biologically protective role of obesity on the mortality risk in RA. Epidemiologic studies to date are likely to have been confounded by disease-related weight loss in patients with the most severe phenotype of the disease and with other comorbid illnesses. However, although the current study suggests that low body weight is not likely to be in the causal pathway to increased mortality in RA, it should be recognized as a profound and important marker of an increased risk of death by identifying those who have experienced significant weight loss.
An association between weight loss and death has been identified in the elderly and in patients with other chronic inflammatory conditions (13,31,32). Weight loss may be associated with the onset and severity of chronic illness and has been cited as an explanation for the “obesity paradox” observed in epidemiologic studies of the risk of mortality in patients in higher body weight categories in the general population (7,11). Weight loss is likely to represent poor functional status, poor nutrition, ongoing inflammation, and/or underlying malignancy, all of which may lead to early death in patients with RA.
Several limitations are worth noting. Although the VA setting was ideal for many aspects of the study, the proportion of men was higher than that in other RA cohorts. Therefore, observations may not be entirely generalizable and should be confirmed in other study populations. We were not able to assess details surrounding the cause of weight loss in individual patients. Therefore, we were not able to distinguish between unintentional and intentional weight loss or to understand how changes in the medical history may have influenced body weight. However, meaningful weight loss in older adults is most commonly associated with illness (33). We also did not assess the cause of death for each individual and therefore whether certain causes of death are more likely to be associated with preceding weight loss. Although the CDW provided a unique opportunity to perform analyses using a maximum BMI, the limited date range for available body weights may have resulted in underestimation of the actual lifetime maximum BMI for individual patients, and the study may therefore have underestimated the observed associations between greater maximum BMI and death. In addition, because only 7 patients had a maximum BMI of <20 kg/m2, this study was limited in the assessment of risk in the group of patients with a low BMI that remained stable. Finally, our study did not have enough power to assess whether there was modification of the effect of weight loss over the range of BMIs. In other words, smaller amounts of weight loss could conceivably be of greater importance in patients with a low BMI.
This study has several critical strengths, including the use of a well-characterized registry sample with highly reliable outcomes for death and survival time. The use of VA electronic medical records in combination with the VARA Registry data allowed for reliable ascertainment of multiple BMI measures over time to support robust and comprehensive analyses in a large cohort of patients with rheumatologist-diagnosed RA with reliable clinical assessments.
In conclusion, in this sample of patients with RA, we observed that weight loss is an important independent predictor of death. A rate of BMI loss of ~3 kg/m2 per year is associated with a significantly increased risk of death. Furthermore, we observed that patients with a low BMI who have a history of being overweight would be expected to have the most dramatic increase in the risk of death. These observations do not support a biologically protective role of obesity in RA and support close monitoring of patients with RA who experience unintentional weight loss.
Acknowledgments
Dr. Baker’s work was supported by a VA Clinical Science Research and Development Career Development Award (IK2 CX000955). Dr. Michaud’s work was supported by a Rheumatology Research Foundation Investigator Award. Dr. Ibrahim’s work was supported by the NIH (grant 1-K24-AR-055259-01 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). Dr. Caplan’s work was supported by a VA Health Service Research and Development Career Development Award (07-221). Dr. Mikuls’ work was supported by a VA Merit Award. The VA Rheumatoid Arthritis Registry is supported by the Nebraska Arthritis Outcomes Research Center at the University of Nebraska Medical Center and by the VA Health Services Research and Development Program of the Veterans Health Administration.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Baker had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Baker, Michaud, Ibrahim.
Acquisition of data. Baker, Caplan, Cannon, Mikuls.
Analysis and interpretation of data. Baker, Billig, Michaud, Ibrahim, Caplan, Stokes, Majithia, Mikuls.
The views expressed herein do not represent those of the Department of Veterans Affairs or the United States Government.
References
- 1.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 2.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford) 2011;50:101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–14. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20:539–44. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson S, Hedblad B, Engstrom G, Nilsson P, Berglund G, Janzon L. Influence of obesity on cardiovascular risk: twenty-three-year follow-up of 22,025 men from an urban Swedish population. Int J Obes Relat Metab Disord. 2002;26:1046–53. doi: 10.1038/sj.ijo.0802060. [DOI] [PubMed] [Google Scholar]
- 6.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624–9. doi: 10.1001/archinte.165.14.1624. [DOI] [PubMed] [Google Scholar]
- 9.Stokes A. Using maximum weight to redefine body mass index categories in studies of the mortality risks of obesity. Popul Health Metr. 2014;12:6. doi: 10.1186/1478-7954-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram DD, Mussolino ME. Weight loss from maximum body weight and mortality: the Third National Health and Nutrition Examination Survey Linked Mortality File. Int J Obes (Lond) 2010;34:1044–50. doi: 10.1038/ijo.2010.41. [DOI] [PubMed] [Google Scholar]
- 11.Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014;25:454–61. doi: 10.1097/EDE.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–44. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somes GW, Kritchevsky SB, Shorr RI, Pahor M, Applegate WB. Body mass index, weight change, and death in older adults: the systolic hypertension in the elderly program. Am J Epidemiol. 2002;156:132–8. doi: 10.1093/aje/kwf019. [DOI] [PubMed] [Google Scholar]
- 14.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–78. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 15.Davis LA, Cannon GW, Pointer LF, Haverhals LM, Wolff RK, Mikuls TR, et al. Cardiovascular events are not associated with MTHFR polymorphisms, but are associated with methotrexate use and traditional risk factors in US veterans with rheumatoid arthritis. J Rheumatol. 2013;40:809–17. doi: 10.3899/jrheum.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards JS, Cannon GW, Hayden CL, Amdur RL, Lazaro D, Mikuls TR, et al. Adherence with bisphosphonate therapy in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1864–70. doi: 10.1002/acr.21777. [DOI] [PubMed] [Google Scholar]
- 17.Curtis JR, Baddley JW, Yang S, Patkar N, Chen L, Delzell E, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther. 2011;13:R155. doi: 10.1186/ar3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon GW, Mikuls TR, Hayden CL, Ying J, Curtis JR, Reimold AM, et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1680–90. doi: 10.1002/acr.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikuls TR, Gould KA, Bynote KK, Yu F, Levan TD, Thiele GM, et al. Anticitrullinated protein antibody (ACPA) in rheumatoid arthritis: influence of an interaction between HLA-DRB1 shared epitope and a deletion polymorphism in glutathione S-transferase in a cross-sectional study. Arthritis Res Ther. 2010;12:R213. doi: 10.1186/ar3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis JR, Jain A, Askling J, Bridges SL, Jr, Carmona L, Dixon W, et al. A comparison of patient characteristics and outcomes in selected European and U.S rheumatoid arthritis registries. Semin Arthritis Rheum. 2010;40:2–14. e1. doi: 10.1016/j.semarthrit.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikuls TR, Padala PR, Sayles HR, Yu F, Michaud K, Caplan L, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:227–34. doi: 10.1002/acr.21778. [DOI] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.United States Department of Veterans Affairs. VA Informatics and Computing Infrastructure. 2014 cited 2014 http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm.
- 24.Myrskyla M, Chang VW. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiology. 2009;20:840–8. doi: 10.1097/EDE.0b013e3181b5f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ) assessment of advanced activities of daily living and psychological status in the patient-friendly Health Assessment Questionnaire format. Arthritis Rheum. 1999;42:2220–30. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 28.Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care. 2002;8:37–43. [PubMed] [Google Scholar]
- 29.Michaud K, Wolfe F. The development of a rheumatic disease research comorbidity index for use in outpatients with RA, OA, SLE, and fibromyalgia (FMS) Arthritis Rheum. 2007;56(Suppl):S597. [Google Scholar]
- 30.Wolfe F, Michaud K, Li T, Katz RS. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol. 2010;37:305–15. doi: 10.3899/jrheum.090781. [DOI] [PubMed] [Google Scholar]
- 31.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PRO-active study population. Int J Cardiol. 2012;162:20–6. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Campbell KL, MacLaughlin HL. Unintentional weight loss is an independent predictor of mortality in a hemodialysis population. J Ren Nutr. 2010;20:414–8. doi: 10.1053/j.jrn.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician. 2014;89:718–22. [PubMed] [Google Scholar]