Abstract
Background
Population-based estimates of cardiac dysfunction and clinical heart failure (HF) remain undefined among Hispanics/Latino adults.
Methods and Results
Participants of Hispanic/Latino origin across the US, aged 45–74 years were enrolled into the Echocardiographic Study of Latinos (ECHO-SOL) and underwent a comprehensive echocardiography exam to define left ventricular systolic dysfunction (LVSD) and left ventricular diastolic dysfunction (LVDD). Clinical HF was defined according to self-report; and those with cardiac dysfunction but without clinical HF were characterized as having subclinical or unrecognized cardiac dysfunction. Of 1,818 ECHO-SOL participants (mean age 56.4 years; 42.6% male) , 49.7% had LVSD and/or LVDD. LVSD prevalence was 3.6%, while LVDD was detected in 50.3%. Participants with LVSD were more likely to be males and current smokers (all p<0.05). Female sex, hypertension, diabetes, higher body-mass index and renal dysfunction were more common among those with LVDD (all p<0.05). In age-sex adjusted models, individuals of Central American and Cuban backgrounds were almost two-fold more likely to have LVDD compared to those of Mexican backgrounds. Prevalence of clinical HF with LVSD (HF with reduced EF) was 7.3%; prevalence of clinical HF with LVDD (HF with preserved EF) was 3.6%. 96.1% of the cardiac dysfunction seen was subclinical or unrecognized. Compared to those with clinical cardiac dysfunction, prevalent coronary heart disease was the only factor independently associated with subclinical or unrecognized cardiac dysfunction (odds ratio: 0.1; 95% confidence interval: 0.1–0.4).
Conclusions
Among Hispanics/Latinos, most cardiac dysfunction is subclinical or unrecognized, with a high prevalence of diastolic dysfunction. This identifies a high-risk population for the development of clinical HF.
Keywords: Hispanics, systolic dysfunction, diastolic dysfunction echocardiography, heart failure, echocardiography
Cardiac dysfunction is an important and independent risk factor for the future development of clinical heart failure (HF).1–4 Early recognition and treatment of American College of Cardiology Foundation (ACCF) / American Heart Association (AHA) stage B HF, defined as cardiac dysfunction without signs or symptoms of HF, is a potentially powerful strategy to prevent progression to ACCF/AHA stage C or D HF, defined as clinical or symptomatic HF.5 Unfortunately, current population-based estimates of cardiac dysfunction are based on studies that did not include Hispanics/Latinos.6, 7
Hispanics/Latinos are particularly vulnerable to cardiac dysfunction for several reasons: 1) Hispanics/Latinos have increased prevalence of HF risk factors (stage A HF) with higher rates of diabetes (both diagnosed and undiagnosed),8, 9 obesity6 and hypertension10; 2) Hispanics/Latinos have an almost two-fold higher prevalence of structural heart disease (stage B HF) with high rates of left ventricular (LV) hypertrophy and abnormal LV geometry.11 A high prevalence of stage A and stage B HF predisposes to higher rates of cardiac dysfunction.12, 13 and 3) Hispanics/Latinos are unfavorably affected by health disparities such as undertreated diabetes14 and hypertension.10 Furthermore, the Hispanic/Latino population >65 years of age is expected to grow 328% between 2000 and 2030.6 As the Hispanic/Latino population ages, it is likely that an epidemic of cardiac dysfunction and clinical HF among Hispanics/Latinos will emerge.15
Due to under-representation in prior community-based HF cohorts, few studies have highlighted the prevalence of cardiac dysfunction among Hispanics/Latinos. Our objective was to establish the prevalence of the two components of cardiac dysfunction –LV systolic dysfunction (LVSD) and LV diastolic dysfunction (LVDD), as well as self-reported clinical HF, in a large representative community-based cohort of US Hispanic/Latino adults.
METHODS
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a prospective, population based study of the prevalence of multiple health conditions and their risk factors among 16,415 diverse Hispanic/Latino individuals ages 18–74 and residing in four U.S. metropolitan areas, the Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA.16, 17 Participants included Hispanics/Latinos who self-identified as Cuban, Central American, Dominican, Mexican, Puerto Rican and South American heritage. Probability sampling was used to ensure a broad representation of the target population and to minimize the various sources of bias that may otherwise enter into the cohort selection and recruitment process. Ineligibility criteria for the HCHS/SOL included being on active military service, not currently living at home, planning to move from the area in the next six months, unable to complete the study in English or Spanish, or unable to attend the clinic examination.
The Echocardiographic Study of Latinos (ECHO-SOL), an ancillary study to the HCHS/SOL, was designed to provide echocardiographic parameters characterizing cardiac structure and function in a representative HCHS/SOL subsample. ECHO-SOL used a stratified random sampling design to assure that ECHO-SOL represents not only the overall HCHS/SOL population, but also the major Hispanic/Latino background group distribution found in HCHS/SOL. A detailed description of the design, rational and methods has been described elsewhere.18 Across all ECHO-SOL sites, enrollment was conducted from October 2011 through June 2014 with participation rates averaging ~80% among eligible participants. The Institutional Review Board at the Wake Forest School of Medicine and at each study site provided approval and oversight of all study materials and activities. All ECHO-SOL participants gave informed consent.
Echocardiographic Measurements
A standardized echocardiography ultrasound examination, including M-mode, two-dimensional (2D), spectral, color flow and tissue Doppler was performed by experienced Registered Diagnostic Cardiac Sonographers at each of the four parent study field sites using Philips IE-33 or Sonos 7500 scanners interfaced with a standard 2.5- to 3.5-MHz phased-array probe, according to American Society of Echocardiography (ASE) recommendations.19, 20 Echocardiograms were analyzed and interpreted centrally at Wake Forest School of Medicine (Winston-Salem, NC). All ECHO-SOL echocardiograms were read by a certified technical reader and over-read by a board-certified cardiologist with expertise in echocardiography (CJR). Over-reads of echocardiograms were performed to confirm the accuracy of key quantitative measurements and to identify clinically important findings.21, 22Inter- and intra-reader reproducibility was assessed and previously reported.18 For inter-reader reproducibility, intra-class correlation (ICC) values ranged from 0.80 to 0.99 with left atrial volume and left ventricular end-diastolic volumes having the highest ICC values (0.97–0.99). ICC values were slightly better from intra-reader assessments for all measures.
LVSD was assessed using LV ejection fraction (LVEF) derived from volumetric assessments and defined as LVEF < 50%. Two-dimensional (2D) imaging of the LV was performed to obtain the best possible images of the LV endocardium without foreshortening of the LV cavity or echo ‘drop out.’ Using the apical 4- and 2-chamber views, LV end-diastolic (EDV) and end-systolic (ESV) volumes were derived using biplane method of discs, as per the ASE recommended methodology.20 The modified Simpson’s rule states that the volume of a three dimensional structure can be determined by dividing the structure into a sequence of 2D slices (or discs) and then summing the product of the cross sectional area and thickness of each disc. EF was calculated from EDV and ESV estimates, using the following formula: LVEF = (EDV - ESV) / EDV. LVEF could not be ascertained in 4.9% of the cohort due to image quality.
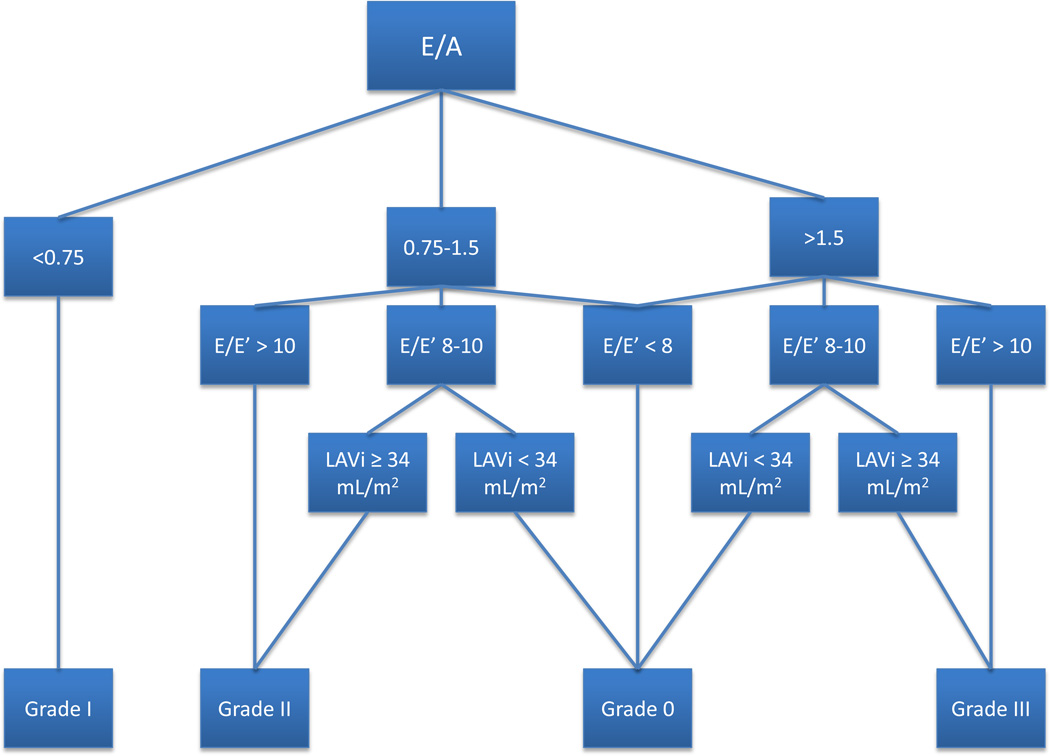
Echocardiographic assessment of LVDD included a) pulse-wave Doppler performed in the apical four chamber view with the sample volume placed in the mitral valve orifice at the level of the leaflet tips to obtain peak early (E) and late (A) diastolic transmitral inflow velocities; b) tissue Doppler imaging to acquire mitral early diastolic (e’) annular velocities from the apical 4-chamber view. We used the average of septal and lateral annular velocities; and c) left atrial volume measured in biplane views indexed (LAVI) to BSA. The grading scheme for LVDD was grade I (mild), grade II (moderate) or grade III (severe) (Figure 1). Our grading algorithm was developed using a combination of published ASE and Redfield definitions19, 23. For the analysis of LVDD we excluded participants with unclassifiable or indeterminate LVDD (n=32; 1.8%), current pregnancy (n=2; 0.1%), EDV indexed to BSA (EDVI) >97mL/m2 (n=1; 0.05%), atrial fibrillation by ECG (n=2; 0.1%), moderate or severe left-sided valvular disease (n=20; 1.1%) or LVEF <50% or missing (n=149; 8.2%), hence participants classified as having LVDD had isolated LVDD and no LVSD. Abnormal isovolumetric relaxation time (IVRT) was defined as IVRT outside of the range of 0.06 –0.1 seconds. Abnormal LV stroke volume (LVSV) was defined as LVSV less than 55 ml and abnormal E/e’ is defined as value greater than 10.
Figure 1.
Algorithm for assessing LVDD via echocardiography based on the Redfield and ASE criteria.
E’=early diastolic mitral annular velocity
For LVDD assessment we excluded participants with atrial fibrillation, more than mild mitral valvular disease, LVEF <50% or LVEDV >97 ml/m2
Clinical covariates
Methods for HCHS/SOL baseline clinical parameters have been previously described.10, 14, 24 Briefly, trained personnel administered a standardized questionnaire assessing participant sociodemographic characteristics, such as age, sex. Socioeconomic status was assessed using information collected on educational attainment and income. Self-report questionnaires were used to assess whether participants have ever smoked and/or were current smokers as well as whether or not they have ever been diagnosed with HF by a health care provider. Coronary heart disease (CHD) was defined as history of angina, myocardial infarction or revascularization as well as ECG evidence of old myocardial infarction. Physical activity levels were assessed using the Global Physical Activity Questionnaire25 to collect information on physical activity participation in three settings (work, travel, leisure). Low, medium and high physical activity categories were defined based on type of physical activity and time spent on each specific activity. Information on both prescription and over-the-counter medications used by participants in the four weeks preceding the examination date was obtained via scanning of medication package bar code symbols, transcription of pill bottle labels and survey interviews.
Trained technicians measured each participant’s height and weight twice and then averaged these two measures to calculate body mass index (BMI=weight (kg) / height (m2)). Participants with BMI of greater than or equal to 30 kg/ m2 are categorized as obese. Medical personnel measured resting systolic and diastolic blood pressures using a standardized protocol. Hypertension was defined as a systolic blood pressure of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or higher, the participant’s self-report of a history of hypertension, or if the patients were on antihypertensive treatment. Participants were classified as having hypercholesterolemia if they were currently using cholesterol-lowering medication, had low density lipoprotein (LDL) levels ≥160 mg/dl and/or total cholesterol (TC) ≥240 mg/dl. Type 2 diabetes was defined based on American Diabetes Association definition26 using one or more of the following criteria: (1) fasting serum glucose ≥126 mg/dl, (2) oral glucose tolerance test ≥200 mg/dl, (3) self-reported diabetes, (4) Hb A1C ≥6.5% or (5) taking anti-diabetic medication or insulin. Renal disease was defined as an eGFR <60 mL/min.
The prevalence of HF was assessed based on self-reported history of physician diagnosed clinical HF. Participants with no HF diagnosis but with either diastolic or systolic cardiac dysfunction at echocardiography were considered to have subclinical or unrecognized diastolic or systolic dysfunction. This designation does not imply that the participant did not have symptoms, only that the participant had not sought evaluation or had not had an evaluation that resulted in a diagnosis of HF. Participants with a clinical HF history and LVEF <50% or >50% were classified as HF with reduced EF (HFrEF) or HF with preserved EF (HFpEF), respectively.
Statistical analysis
Survey methods using sampling weights were used to obtain weighted frequencies of descriptive variables and population estimates of cardiac dysfunction prevalence rates, as well as LVSD and LVDD estimates in the ECHO-SOL target population. All weights were calibrated to the age, gender and Hispanic/Latino background distributions from the 2010 US Census for the four study field centers. The corresponding distribution of all baseline sociodemographic and clinical characteristics was summarized for the overall population using means ± standard errors (SEs) for continuous variables and proportions for categorical variables. The prevalence of LVSD, LVDD and cardiac dysfunction was calculated for the overall cohort as well as across sex, age strata and Hispanic/Latino background group.
The association between the prevalence of LVSD, LVDD and cardiac dysfunction with clinical and sociodemographic variables was investigated using the Rao-Scott chi-square test for univariate associations. Multivariable logistic regression models were constructed for LVSD, LVDD and cardiac dysfunction as outcomes with inclusion of all potential predictor variables to determine independent associations. Covariates of interest included: age, sex, BMI, hypertension, diabetes, hypercholesterolemia, renal disease, prevalent coronary heart disease, physical activity, current tobacco use, current alcohol use, educational level, income level, health insurance status, and study site.
Prevalence estimates of subclinical or unrecognized LVSD, LVDD, and cardiac dysfunction were determined. Logistic regression models assessed the association of different variables with the presence of subclinical or unrecognized LVSD, LVDD and cardiac dysfunction. Sequential logistic regression models (unadjusted; age-sex adjusted; then fully adjusted models) assessed the association of Hispanic/Latino background group as a predictor of LVSD, LVDD and cardiac dysfunction as separate outcomes after adjustment for all covariates of interest listed above. Additional exploratory analysis assessed age-adjusted prevalence estimates of LVSD, LVDD and cardiac dysfunction by center and Hispanic/Latino background groups. Sampling weights and survey statistics were used for all analyses. All analyses were weighted to adjust for sampling probability and non-response. All analyses were conducted using SAS v. 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Seventy-nine percent of the ECHO-SOL study population was under age 65 and predominantly 57.4% were female (Table 1). Almost half were obese. Half of the study participants had hypertension and over two-thirds had either pre-diabetes or diabetes. Renal disease by eGFR was present in 6.4%, while prevalent CHD was reported in 18%. More than two-thirds reported low levels of physical activity. Almost one-fifth of participants were current smokers and over 40% were current drinkers. Over a third reported having less than high school education and over half reported annual incomes below $20,000. (Table 1) Only 6.9% of the participants had abnormal LAVI. With regard to functional indices, e/e’ and LV stroke volume were abnormal in almost half of the cohort, whereas IVRT was abnormal in almost a third of participants.
Table 1.
Baseline Characteristics*
| Age, Mean | 56.36 ± 0.37 |
| Age, Greater than 65 years | 214 (21.0) |
| Male | 631 (42.6) |
| Hypertension | 861 (50.0) |
| Diabetes | 523 (28.4) |
| Hypercholesterolemia | 711 (40.2) |
| Renal disease | 106 (6.40) |
| Heart Disease | 303 (18.00) |
| Heart Failure | 64 (4.30) |
| Low Physical Activity | 1227 (67.30) |
| Body Mass Index, mean | 30.11 ± 0.22 |
| Body Mass Index greater than or equal to 30 kg/m2 | 822 (44.30) |
| Current Alcohol Use | 770 (43.50) |
| Current Tobacco Use | 304 (17.60) |
| Health Insurance | 1042 (60.10) |
| Education, Less than high school | 647 (34.80) |
| Income below 20,000 US Dollars | 900 (54.50) |
| Hispanic Subgroup Distribution | |
| Dominican | 326 (18.20) |
| Central American | 176 (6.40) |
| Cuban American | 356 (31.60) |
| Mexican American | 458 (20.40) |
| Puerto Rican | 348 (17.10) |
| South American | 150 (6.20) |
| Echocardiographic Parameters | |
| Left Atrial Volume Index ≥34 ml/m2 | 122(6.90) |
| Early diastolic mitral annular velocity (E’) < 8 cm/sec | 905 (52.50) |
| E/E’ ratio > 10 | 747(41.0) |
| No LV Diastolic Dysfunction | 812 (49.74) |
| LV Diastolic Dysfunction, Grade 1 | 230 (16.10) |
| LV Diastolic Dysfunction, Grade 2 | 556 (32.69) |
| LV Diastolic Dysfunction, Grade 3 | 31 (1.48) |
| LV Mass (grams) | 153.4 ± 1.51 |
| LV Mass Index (g/m2) | 41.5 ± 0.34 |
| Relative Wall Thickness | 0.40 ± 0.01 |
| Ejection Fraction, % | 59.80 ± 0.20 |
| End Systolic Volume, ml | 33.80 ± 0.37 |
| End Diastolic Volume, ml | 83.50 ± 0.70 |
Data are presented as mean ± SEM or N (%) using weighted row percentages; N’s presented are unweighted counts of total participants in the HCHS-SOL with respective characteristic.
Diabetes - fasting serum glucose >126 mg/dl, oral glucose tolerance test >200 mg/dl, self-reported diabetes, Hemoglobin A1C >6.5% or taking anti-diabetic medication or insulin.
Hypercholesterolemia - Currently using of cholesterol-lowering medication, LDL-C > 160 mg/dl and/or Total Cholesterol > 240 mg/dl.
Heart disease - History of angina, myocardial infarction or revascularization;, abnormal ECG or taking beta blocker or clopidogrel.
Renal disease - eGFR <60 mL/min.
LV –Left Ventricle, LA- Left Atrium, E –Early peak diastolic transmitral inflow velocity.
Systolic Dysfunction
LVEF was obtained in ~95% of participants with a mean LVEF of 59.8% (± 0.2). LVEF was essentially the same among participants with a history of clinical HF vs. those without (59.8% vs. 59.0% respectively). LVSD was prevalent in 60 (3.6%) cohort members. Prevalence of LVSD was significantly higher among men compared to women across all age groups and did not increase significantly with older age. (Table 2) In multivariable models, female sex and current tobacco smoking were the only factors independently associated with LVSD. LVSD did not vary by age, BMI, hypertension, diabetes, high cholesterol, renal disease, prevalent heart disease, physical activity, alcohol use, educational level, income or health insurance status. (Table 3)
Table 2.
Cardiac Dysfunction Prevalence by Age, Sex, and Hispanic Background Group
| Left Ventricular Systolic Dysfunction (LVSD)* |
Left Ventricular Diastolic Dysfunction (LVDD)† | Cardiac Dysfunction (LVSD and/or LVDD)‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 45–54 | 55–64 | 65+ | All | 45–54 | 55–64 | 65+ | All | 45–54 | 55–64 | 65+ | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| All | 60 (3.6) | 34 (3.7) | 21 (3.6) | 5 (3.1) | 817 (50.3) | 310 (33.1) | 358 (57.4) | 149 (80.1) | 877 (49.7) | 344 (34.5) | 379 (55.8) | 154 (75.3) |
| Male | 37 (5.6) | 19 (5.2) | 14 (7.0) | 4 (4.7) | 246 (45.5) | 77 (25.8) | 103 (50.1) | 66 (76.9) | 283 (46.8) | 96 (28.5) | 117 (52.5) | 70 (75.8) |
| Female | 23 (2.0) | 15 (2.7) | 7 (1.1) | 1 (1.6) | 571 (53.7) | 233 (38.2) | 255 (61.7) | 83 (83.3) | 594 (51.8) | 248 (38.8) | 262 (58.1) | 84 (74.8) |
| Mexican | 8 (2.0) | 6 (2.4) | 2 (2.4 | 0 (0) | 184 (42.3) | 74 (30.5) | 76 (44.4) | 34 (74.4) | 192 (41.2) | 80 (30.0) | 78 (43.7) | 34 (73.1) |
| Dominican | 14 (4.4) | 7 (3.8) | 5 (4.8 | 2 (6.0) | 141 (48.4) | 54 (31.5) | 58 (57.6) | 29 (93.5) | 155 (48.5) | 61 (33.3) | 63 (55.3) | 31 (87.0) |
| Cuban | 12 (3.7) | 6 (4.0) | 4 (3.4 | 2 (3.8) | 162 (53.8) | 57 (33.7) | 71 (61.0) | 34 (74.8) | 174 (53.5) | 63 (35.6) | 75 (60.0) | 36 (71.4) |
| Puerto Rican | 15 (3.6) | 7 (3.7) | 8 (6.3 | 0 (0) | 178 (54.4) | 58 (31.6) | 83 (67.4) | 37 (90.1) | 193 (54.2) | 65 (33.4) | 91 (64.4) | 37 (89.1) |
| South American | 4 (2.9) | 3 (4.2) | 1 (1.6 | 0 (0) | 61 (44.3) | 23(33.3) | 33 (54.3) | 5 (74.3) | 65 (43.1) | 26 (35.0) | 34 (50.5) | 5 (63.1) |
| Central American | 6 (5.1) | 4 (6.3) | 1 (1 | 1 (7.5) | 90 (58.1) | 44 (50.4) | 36 (64.6) | 10 (69.0) | 96 (54.2) | 48 (51.3) | 37 (63.8) | 11 (48.2) |
LVSD - Left ventricular systolic dysfunction,
LVSD could not be determined in n=89 (4.9%). Total participants = 1729
LVDD - Left ventricular diastolic dysfunction
Excluded LVDD unclassifiable (n=32; 1.8%), current pregnancy (n=2; 0.1%), End Diastolic Volume Index >97mL/m2 (n=1; 0.05%), Atrial Fibrillation (n=2; 0.1%), ≥moderate left-sided valvular disease (n=20; 1.1%) or Left Ventricular Ejection Fraction <50% or missing (n=149; 8.2%). Total participants = 1629.
based only on individuals were LVSD or LVDD could be determined (n=59 were excluded). Total participants = 1762
N’s presented are unweighted counts of total participants in the HCHS-SOL with respective characteristic. Weighted row percentages.
Table 3.
Factors Associated with Cardiac Dysfunction
| Left Ventricular Systolic Dysfunction (LVSD)* |
Left Ventricular Diastolic Dysfunction (LVDD)† |
Cardiac Dysfunction (LVSD and/or LVDD) |
||||
|---|---|---|---|---|---|---|
| OR (95% CIs)* | OR (95% CIs)*** |
OR (95% CIs)* | OR (95% CIs)*** |
OR (95% CIs)* | OR (95% CIs)*** | |
| Age (years) | 1.00 (0.96–1.04) | 1.11 (1.09–1.13) | 1.10 (1.07–1.13) | 1.09 (1.07–1.12) | 1.08 (1.06–1.11) | |
| Gender (Female vs. Male) | 0.34 (0.18–0.65) | 0.34 (0.14–0.79) | 1.39 (1.03–1.87) | 1.57 (1.15–2.13) | 1.22 (0.96–1.60) | |
| BMI (kg/m2) | 0.97 (0.92–1.03) | 1.08 (1.05–1.11) | 1.08 (1.05–1.11) | 1.07 (1.04–1.10) | 1.07 (1.05–1.10) | |
| Hypertension | 1.06 (0.60–1.88) | 3.45 (2.61–4.55) | 2.02 (1.48–2.77) | 3.12 (2.39–4.08) | 2.02 (1.47–2.78) | |
| Diabetes | 1.60 (0.74–3.46) | 4.85 (3.39–6.95) | 2.17 (1.38–3.39) | 4.10 (2.91–5.77) | 1.97 (1.32–2.96) | |
| High Cholesterol | 0.94 (0.54–1.66) | 1.34 (0.98–1.84) | 1.26 (0.93–1.69) | |||
| Renal Disease | 1.33 (0.45–3.95) | 3.12 (1.75–5.58) | 1.91 (1.05–3.47) | 2.54 (1.51–4.28) | ||
| Heart Disease*** | 2.06 (0.82–5.20) | 1.94 (1.14–3.31) | 1.57 (0.96–2.55) | |||
| Physical Activity (Low vs. mod/high) | 0.84 (0.47–1.53) | 1.42 (0.99–2.04) | 1.37 (0.98–1.93) | |||
| Current Tobacco | 2.32 (1.27–4.23) | 1.97 (0.99–3.95) | 0.86 (0.61–1.22) | 0.93 (0.67–1.28) | ||
| Current Alcohol | 1.47 (0.67–3.23) | 0.63 (0.41–0.96) | 0.67 (0.45–0.99) | |||
| Education*** | 1.20 (0.60–2.35) | 1.43 (1.00–2.03) | 1.39 (1.01–1.94) | |||
| Income*** | 2.17 (0.76–6.18) | 1.21 (0.77–1.90) | 1.22 (0.80–1.86) | |||
| Health Insurance | 0.98 (0.56–1.71) | 0.64 (0.49–0.84) | 0.68 (0.52–0.88) | |||
LVSD - Left ventricular systolic dysfunction, LVDD - Left ventricular diastolic dysfunction
Unadjusted (crude) models
Adjusted models (include age, sex, BMI, hypertension, diabetes, hypercholesterolemia, renal disease, prevalent coronary heart disease, physical activity, current tobacco use, current alcohol use, educational level, income level, and health insurance status);
Heart disease - History of angina, myocardial infarction or revascularization; or abnormal ECG with prior MI
Income - ≤ 40,000 versus greater than 40,000
Education <HS versus >HS
CIs denote confidence intervals
Diastolic Dysfunction
Diastolic function was classified as abnormal in 817 (50.3%) participants; 16.1% had mild (grade I), 32.7% had moderate (grade II), and 1.5% had severe (grade III) LVDD (Table 1). The prevalence of LVDD increased significantly with age and was significantly higher in women vs. men. Sex differences persisted across age groups, except in those 65+ years of age LVDD prevalence became similar among men and women (Table 2). In fully adjusted models, age, male gender, BMI, hypertension, diabetes and renal disease were independently associated with the presence of LVDD. (Table 3)
Cardiac Dysfunction (LVSD and/or LVDD)
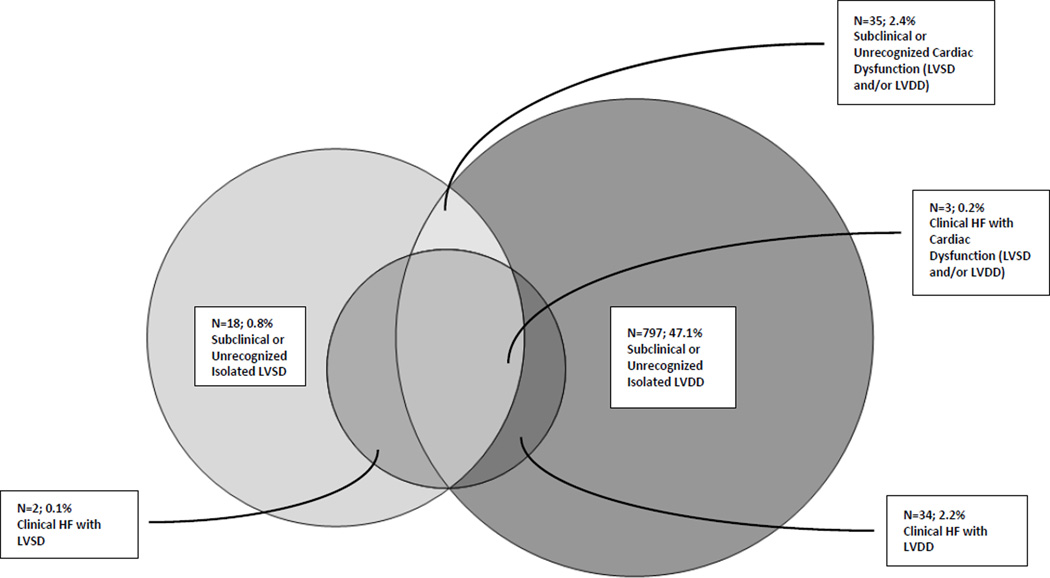
Since some participants with LVSD also had LVDD, there is overlap seen within participants classified as having LVSD, LVDD and cardiac dysfunction. (Figure 2a) Among our community cohort of Hispanics/Latinos, the prevalence of cardiac dysfunction was high, with 49.7% of participants having either LVSD and/or LVDD. The prevalence of cardiac dysfunction was somewhat higher in women and increased with older age, particularly among men compared to women. (Table 2) Having health insurance was associated with less prevalent cardiac dysfunction in unadjusted models. Only age, hypertension, BMI and diabetes were independently associated with the presence of cardiac dysfunction (all p<0.05). (Table 3)
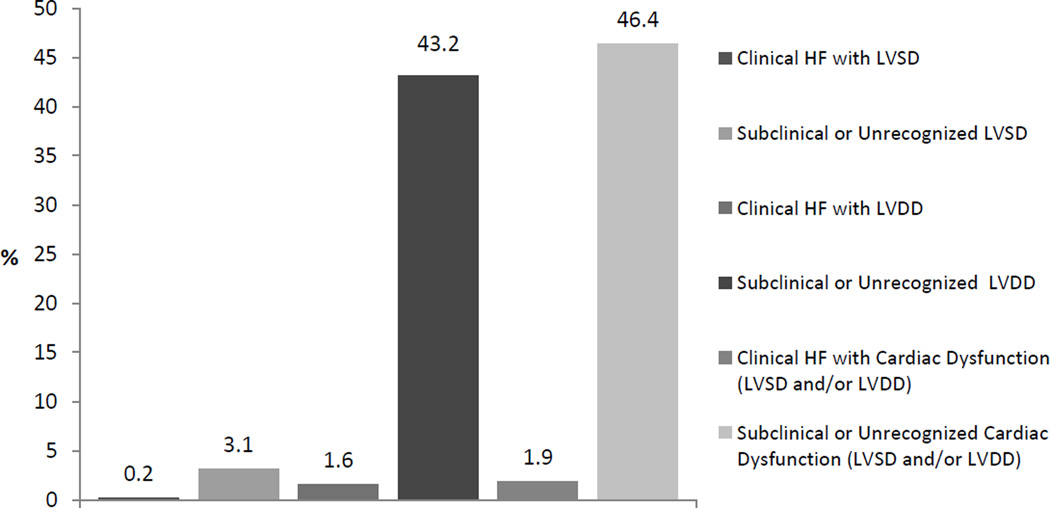
Figure 2.
a. Overlap of Subclinical or Unrecognized versus Clinical Cardiac Dysfunction
b. Prevalence of Subclinical or Unrecognized versus Clinical Cardiac Dysfunction
Subclinical or Unrecognized Cardiac Dysfunction
Self-reported clinical HF prevalence was 4.4% (n=64). Among participants with cardiac dysfunction, Figure 2b shows the prevalence of clinical and subclinical cardiac dysfunction. Even though self-reported clinical HF was more common among those with an abnormal LVEF (HFrEF) than those with a LVEF >50% (HFpEF) (7.3% vs. 3.6% respectively), the more prevalent clinical HF was HFpEF. Among those with self-reported HF, 53 (93.6%) participants had an EF >50%. The prevalence of self-reported HF did not significantly vary with age, sex or Hispanic/Latino background group (all p >0.19), but did vary by insurance status (p<0.01). Of all participants with cardiac dysfunction, 94.7% had subclinical or unrecognized cardiac dysfunction. The proportion of subclinical or unrecognized cardiac dysfunction did not differ when looking at LVSD or LVDD separately. (Table 4) Of all participants with subclinical or unrecognized cardiac dysfunction, 19.5% were taking angiotensin converting enzyme inhibitor (ACE I), 14.2% were on a beta blocker, and 15.6% on diuretics; as opposed to 33.5%, 36.9% and 30.0% respectively among those with clinical cardiac dysfunction (all p<0.01). In multivariable models, only having prevalent CHD was independently inversely associated (OR: 0.1; CIs 0.1–0.3) with having subclinical or unrecognized cardiac dysfunction.
Table 4.
Proportion of Subclinical or Unrecognized versus Clinical Cardiac Dysfunction Among all Participants
| Subclinical | Clinical | |||
|---|---|---|---|---|
| Sample size (N)* | % (95% CIs)** | Sample size (N)* | % (95% CIs)** | |
| Cardiac Dysfunction (LVSD and/or LVDD) | 838 | 96.1 (94.6–97.6) | 38 | 3.8 (2.4–5.4) |
| LVSD | ||||
| EF <50 | 55 | 92.7 (85.8–99.6) | 5 | 7.3 (0.4–14.2) |
| LVDD | ||||
| Grade 1 | 219 | 94.6 (90.5–98.7) | 11 | 5.4 (1.3–9.5) |
| Grade 2 | 534 | 97.2 (95.7–98.7) | 21 | 2.8 (1.3–4.3) |
| Grade 3 | 30 | 97.6 (92.8–100) | 1 | 2.4 (0–7.2) |
LVSD - Left ventricular systolic dysfunction, LVDD - Left ventricular diastolic dysfunction, EF –Ejection Fraction
N’s presented are unweighted counts of total participants in the HCHS-SOL with respective characteristic.
Weighted row percentages.
CIs denote confidence intervals
Hispanic/Latino subpopulation and Cardiac Dysfunction
Individuals of Mexican background consistently had the lowest unadjusted prevalence of cardiac dysfunction, whereas the prevalence was higher among those of Central American backgrounds. (Tables 2) Among individuals of Central American backgrounds, more prevalent cardiac dysfunction was seen among younger age groups. (Tables 2) These differences were mostly driven by LVDD. In age-adjusted analysis, only participants of Central American background (OR: 2.0; CIs 1.2–3.2) had an increased odds of having LVDD compared to those of Mexican heritage. Differences among Central Americans persisted on multivariable models for LVDD (OR: 1.7; CIs 1.0- 2.8). In additional models adjusted by study site, these differences were no longer significant. However, age-adjusted models stratified by site and Hispanic/Latino background revealed that LVDD prevalence among participants of Central American heritage did not significantly vary by site (p=0.2).
DISCUSSION
To our knowledge, ECHO-SOL is the largest community-based cohort to date representing diverse Hispanic/Latino groups that has been systematically examined with standardized echocardiography. No prior study has evaluated the prevalence of cardiac dysfunction, both systolic and diastolic, as well as the presence of subclinical or unrecognized cardiac dysfunction, in a community-based cohort of Hispanic/Latino adults representative of the six major Hispanic/Latino background groups. Previous population-based studies of US Hispanics/Latinos were smaller, single-site, and/or provided only limited information on LV systolic and diastolic dysfunction and clinical HF. In ECHO-SOL, the prevalence of HF risk factors was high. Half of the participants were obese or hypertensive, and two-thirds being diabetic or pre-diabetic. Cardiac dysfunction was present in almost half of the cohort and due predominantly to diastolic dysfunction. This is important given the epidemic of HFpEF which is projected to increase in the US.27, 28 Moreover, of all cardiac dysfunction, upwards of 95% was unrecognized or subclinical. Lastly, there was a suggestion of differentially higher LVDD prevalence among certain Hispanic/Latino groups, in particular Central Americans, which may be more at risk.
Systolic Dysfunction and Diastolic Dysfunction
Prevalence of LVDD in the ECHO-SOL cohort was higher compared to previously published data for European cohorts: 11%29; 37%.30 In a community-based sample of 2,042 non-Hispanic white residents of Olmsted County, Minnesota, Redfield et al.,31 reported an LVDD prevalence of 28%, clinical HF prevalence of 2.2%, and LVSD prevalence of 6% (EF 50% or less). In comparison, although the Olmstead cohort had study design similar to ECHO-SOL and also included participants aged 45 years or older, ECHO-SOL comprises exclusively of Hispanics/Latinos with greater proportion of women, higher mean BMI (28.4 vs 30.1), diabetes prevalence (4.5% vs 28.4%) and current tobacco use (17.6% vs 8.9%). Importantly, longitudinal follow-up of the Olmstead cohort showed LVDD prevalence and severity worsened over four years, predicting the occurrence of incident clinical HF.4 This finding has notable implications given the higher prevalence of LVDD in ECHO-SOL compared to the Olmstead County. Interestingly; the prevalence of LVSD was higher in the Olmstead cohort, perhaps reflecting the fact that LVDD and possibly HFpEF are more significant problems in Hispanics/Latinos compared to whites. This would be consistent with the findings by Russo et al32 who reported significantly worse echocardiographic diastolic dysfunction characteristics among Hispanics/Latinos compared to non-Hispanic whites mostly attributable to a worse cardiovascular risk factor profile.
A study by Halley et al33 using retrospective analysis of a hospital database of more than 36,000 residents from Cleveland, Ohio reported prevalence of LVDD was 65.2% in patients referred for an outpatient cardiac echocardiogram. The high prevalence was expected given the higher probability for cardiac abnormalities in a population referred for testing by a physician. Thus the high prevalence of LVDD in the ECHO-SOL community based study population is surprising. Castro et al34 reported on LVDD prevalence among Hispanics/Latinos in southwest Texas by doing a retrospective analysis of 166 consecutive echocardiogram from a hospital database. 129 of 166 (77.8%) were Hispanics/Latinos; 87 of 129 (67.4%) has some degree of LVDD. Although the LVDD prevalence in ECHO-SOL cohort was similar to the data published by Castro et al., the southwest Texas cohort was older, with a higher prevalence of diabetes and hypertension as would be expected from hospitalized patients.
Clinical heart failure among Hispanic/Latino adults
Our analysis suggests that the prevalence of self-reported clinical HF in the Hispanic/Latino community is relatively low, despite the high prevalence of stage A and stage B HF. The majority of HF in our Hispanic/Latino study population appears to be stage B HF which includes asymptomatic LVSD or asymptomatic LVDD. In predominantly Caucasian cohorts, the prevalence of asymptomatic LVSD and LVDD is estimated at 3–6%35 and 26%36, respectively. Prior estimates of projected clinical HF prevalence in US Hispanics/Latinos have been low.37 However, these estimates are only based on Hispanics of one particular background group. When one examines a representative sample of all Hispanics/Latinos, such as in our study, the projected HF prevalence, particularly for future HFpEF, appears high. Furthermore, only ~one-third of Hispanics/Latinos with clinical cardiac dysfunction were on an ACEI, beta blocker or diuretic. This prevalence of cardiac medication use among those with clinical cardiac dysfunction is lower compared to previous publications38 and is even lower among ECHO-SOL participants with stage A and stage B HF. Since antecedent cardiac dysfunction (systolic or diastolic) is associated with increased incidence of clinical HF,4, 27, 39 the increased prevalence of subclinical or unrecognized functional abnormalities (stage B HF) coupled with a high burden of HF risk factors (stage A HF) makes the ECHO-SOL study population highly vulnerable to progression to clinically overt HF (stage C or stage D HF). Income, educational level or insurance status were not significantly associated with subclinical or unrecognized cardiac dysfunction suggesting that the reasons for the high presence of stage B HF among Hispanics/Latinos goes beyond these variables and deserves further attention.
The available information regarding HF in Hispanics/Latinos from community-based cohorts is insufficient and conflicting. For example, national statistics from National Health and Nutrition Examination Survey (NHANES) show HF prevalence as being the lowest in Hispanics/Latinos, followed by non-Hispanic Whites.40 However, others reported that HF incidence was greater in Hispanics/Latinos than in non-Hispanic Whites.41 Vivo et al42 reported on the prevalence of HFrEF (54%) and HFpEF (46%) in a registry of 6,117 Hispanic/Latino patients hospitalized with HF. Hospitalized Hispanics/Latinos were younger, more obese and more likely to have diabetes, hypertension compared to non-Hispanics/Latinos in the registry. Despite these differences, compared to non-Hispanic Whites, the prevalence of HFpEF (46% vs. 55%) was lower and the prevalence of HFrEF was higher (54% vs. 45%) in these Hispanic/Latino patients.42 Our study found the prevalence of subclinical or unrecognized cardiac dysfunction was lower among those with clinical CHD. Thus, having the comorbid condition of clinical CHD increases the likelihood of having received attention of the health care system so that their cardiac dysfunction is less likely to be subclinical or unrecognized.
Hispanic/Latino subpopulation differences
We observed a differential prevalence of LVDD by Hispanic/Latino subpopulation where participants of Central American background had an almost two-fold chance having LVDD compared to those of Mexican background. In additional models adjusted by site, these differences were no longer significant. However, age-adjusted analysis stratified by site and Hispanic/Latino background group revealed that LVDD prevalence among Central Americans did not vary by site. As the correlation between study site and Hispanic/Latino background group in ECHO-SOL is high, it becomes impossible to distinguish their effects separately. While it is notable in our study that individuals of Central American background in Chicago and the Bronx were similar with respect to LVDD prevalence, it doesn’t help clearly distinguish whether the differentially higher LVDD prevalence in Central American background is due to site factors (such as environmental differences) or to intrinsic Hispanic/Latino background group differences (such as genetic ancestry).
Comparisons with other populations in the context of the Hispanic Paradox
Hispanics in ECHO SOL have higher prevalence of HF risk factors (diabetes, hypertension, and obesity) compared to non-Hispanic whites in MESA, despite being younger.43 Non-Hispanic blacks in the MESA and ARIC cohorts had similar prevalence of these risk factors when compared to ECHO-SOL Hispanics.43, 44 Prevalence of asymptomatic LVSD in MESA was 1.7% among Whites, 2.7% among Non-Hispanic blacks and 1.6% among Hispanics 45; however, MESA excluded prevalent CHD and clinical HF which were included in ECHO-SOL. When compared to non-Hispanic white cohorts of similar age, prevalence of LVSD in ECHO SOL (3.6%) is lower than that of Framingham (5%),46 Heart of England Screening study (5.3%)47 and Mayo cohorts (6%)31 despite a higher level of HF risk factors among ECHO-SOL participants.
Using only transmitral Doppler in defining LVDD, the prevalence of asymptomatic LVDD was 25% amongst Non-Hispanic blacks in ARIC.48 ECHO-SOL Hispanics, have a similarly high prevalence of HF risk factors as non-Hispanic blacks in ARIC, but a much higher prevalence of asymptomatic LVDD (47.1%). Prevalence of LVDD in population-based, non-Hispanic white cohorts, mean age was 53–65 years of age, has varied from 11% to 35%.4, 29, 30, 36 In comparison, ECHO-SOL LVDD prevalence is higher, with a higher prevalence of HF risk factors compared to non-Hispanic whites, but ECHO-SOL participants are younger or of similar age as these cohorts implying a worse HF risk factor profile and LVDD prevalence among ECHO-SOL Hispanics despite a younger or similar age.
The Hispanic Paradox states that despite a high prevalence of risk factors, Hispanics seem to have a more favorable cardiovascular morbidity and mortality experience than non-Hispanic whites.49 Despite the increased prevalence of HF risk factors, ECHO-SOL Hispanics have a similar or lower prevalence of LVSD as non-Hispanic whites. These findings provide support for the Hispanic Paradox as it applies to the LVSD. However, LVDD prevalence is higher in ECHO-SOL with a higher prevalence of HF risk factors, despite a younger or similar age which does not support the Hispanic paradox. The higher LVDD prevalence can be partly attributed to our comprehensive criteria for defining LVDD, which may be more sensitive than prior studies. Predominantly, this also speaks to the complexity of the Hispanic Paradox and how it has been understudied in the context of cardiac dysfunction and HF.
Strengths and Limitations
Our study has several strengths. Our measures of LVDD are comprehensive, do not rely on a single component and are inclusive of tissue Doppler assessment and LAVI measurements as per the ASE and Redfield criteria.19, 31 Previous LVDD criteria based solely on diastolic relaxation velocity has its own limitations.50 ASE has recommended that the best assessment of LVDD is when a combination of several diastology components are used.19 Our study tries to move closer to an ideal assessment by incorporating various components into our LVDD algorithm. (Figure 1) A related strength is that LVDD could not be classified in less than 2% of our sample. This is a testament to our LVDD algorithm and the quality of our measures. We used modified Simpson’s bi-plane method, the ASE recommended method for calculating LVEF,20 in all ECHO-SOL participants whereas many prior studies calculated LVEF using the Teichholz method, an older M-mode method based on fractional shortening which is no longer recommend.
Some study limitations do exist. ECHO-SOL, by design, recruited specific Hispanic/Latino background groups by site since US Hispanic/Latino background groups have differential geographic immigration patterns. Second, echocardiographic studies were not performed simultaneously with other HCHS-SOL data. To alleviate this, all efforts were made to keep the time interval between baseline HCHS-SOL measurements and ECHO-SOL assessments as negligible as possible; additionally, key covariates (hypertension, diabetes, self-reported HF) were updated using the most recent HCHS-SOL annual follow-up assessment. Some participants could have had a reduced LVEF when they first self-reported HF, and the LVEF may have recovered by the time the echocardiography exam was performed, possibly limiting our HFpEF and HFrEF estimates. We obtained only the list of medications but not the actual indication for those medications. Various schemes have been used to define LVDD in prior studies, which make pure comparisons difficult. Lastly, the diagnosis of clinical HF may be inaccurate since it is based on self-report and has not been validated by review of medical records.
Conclusions
The prevalence of cardiac dysfunction, especially LVDD, is high among the Hispanics/Latinos population. Even though the prevalence of HFpEF is higher than HFrEF among Hispanics/Latinos, overall cardiac dysfunction (LVDD or LVSD) is primarily subclinical or unrecognized. Our study substantiates the need for aggressive risk factor modification in this at risk population as recommended in the guidelines to prevent further cardiac remodeling and progression to clinical HF.51
Clinical Perspective.
Echocardiographic Study of Latinos (ECHO-SOL) is the largest study to date of US Hispanic/Latino adults. This manuscript presents the left ventricular dysfunction and heart failure data from this cohort. Our key findings indicate that the prevalence of heart failure (HF) risk factors (stage A HF) and LV diastolic dysfunction (LVDD) is high among Hispanics/Latinos. Prevalence of clinical heart failure is low and most of the cardiac dysfunction is subclinical or unrecognized (stage B HF). Despite the increased prevalence of HF risk factors, ECHO-SOL Hispanics have a similar or lower prevalence of LVSD as non-Hispanic whites. These findings provide support for the Hispanic Paradox as it applies to the LVSD. However, LVDD prevalence is higher in ECHO-SOL with a higher prevalence of HF risk factors, despite a younger or similar age which does not support the Hispanic Paradox. The findings from our study raise awareness of the increased prevalence of LVDD and subclinical HF among Hispanics/Latinos. Our study substantiates the need for aggressive risk factor modification in this at risk population.
Acknowledgments
The authors acknowledge the investigators, the staff, and the participants of HCHS-SOL and ECHO-SOL for their dedication and commitment to the success of this study. Investigators website - http://www.cscc.unc.edu/hchs/
Sources of Funding: The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01- HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
ECHO-SOL was supported by a grant from the National Heart, Lung, and Blood Institute (R01 HL104199, Epidemiologic Determinants of Cardiac Structure and Function among Hispanics: Carlos J. Rodriguez, MD, MPH Principal Investigator).
Footnotes
Disclosures: None
References
- 1.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 3.Stevens SM, Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imaging. 2009;2:11–20. doi: 10.1016/j.jcmg.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Pina IL, Ramirez SM, Rodriguez B, Sims M. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Census Bureau. 65+ in the United States:2005. Current Population Reports. 2005 Dec [Google Scholar]
- 8.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. Jama. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 9.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 10.Sorlie PD, Allison MA, Aviles-Santa ML, Cai J, Daviglus ML, Howard AG, Kaplan R, Lavange LM, Raij L, Schneiderman N, Wassertheil-Smoller S, Talavera GA. Prevalence of hypertension, awareness, treatment, and control in the Hispanic community health study/study of Latinos. Am J Hypertens. 2014;27:793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez CJ, Diez-Roux AV, Moran A, Jin Z, Kronmal RA, Lima J, Homma S, Bluemke DA, Barr RG. Left ventricular mass and ventricular remodeling among Hispanic subgroups compared with non-Hispanic blacks and whites: MESA (Multi-ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;55:234–242. doi: 10.1016/j.jacc.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 13.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–747. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 14.Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Giachello AL, Heiss G, Kaplan RC, LaVange LM, Teng Y, Villa-Caballero L, Aviles-Santa ML. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2014;37:2233–2239. doi: 10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivo RP, Krim SR, Cevik C, Witteles RM. Heart Failure in Hispanics. Journal of the American College of Cardiology. 2009;53:1167–1175. doi: 10.1016/j.jacc.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, Lavange L, Chambless LE, Heiss G. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez CJ, Dharod A, Allison MA, Shah SJ, Hurwitz B, Bangdiwala S, Gonzalez F, Kitzman D, Gillam L, Spevack D, Dadhania R, Langdon S, Kaplan R. Rationale and Design of the Echocardiographic Study of Hispanics / Latinos (ECHO-SOL) Ethnicity and Disease. 2015;25:180–186. [PMC free article] [PubMed] [Google Scholar]
- 19.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 21.Byrd BF, Finkbeiner W, Bouchard A, Silverman NH, Schiller NB. Accuracy and reproducibility of clinically acquired two-dimensional echocardiographic mass measurements. American Heart Journal. 1989;118:133–137. doi: 10.1016/0002-8703(89)90083-5. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left-ventricular hypertrophy - comparison to necropsy findings. American Journal of Cardiology. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 23.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures - A comparative simultaneous Doppler-Catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 24.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health. 2014;14:1255. doi: 10.1186/1471-2458-14-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. [Google Scholar]
- 29.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Doring A, Broeckel U, Riegger G, Schunkert H. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–328. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 30.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 32.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Race/ethnic disparities in left ventricular diastolic function in a triethnic community cohort. Am Heart J. 2010;160:152–158. doi: 10.1016/j.ahj.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 34.Castro CE, Lyapin A, Pattathan M, Negrin J, Mukherjee D. Prevalence and predictors of left ventricular diastolic dysfunction in a Hispanic patient population. Int J Angiol. 2013;22:229–234. doi: 10.1055/s-0033-1353240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 36.Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, Gonzalez A, Herregods MC, Fagard RH, Diez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 37.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 39.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 41.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vivo RP, Krim SR, Krim NR, Zhao X, Hernandez AF, Peterson ED, Pina IL, Bhatt DL, Schwamm LH, Fonarow GC. Care and outcomes of Hispanic patients admitted with heart failure with preserved or reduced ejection fraction: findings from get with the guidelines-heart failure. Circ Heart Fail. 2012;5:167–175. doi: 10.1161/CIRCHEARTFAILURE.111.963546. [DOI] [PubMed] [Google Scholar]
- 43.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Blecker S, Matsushita K, Fox E, Russell SD, Miller ER, 3rd, Taylor H, Brancati F, Coresh J. Left ventricular dysfunction as a risk factor for cardiovascular and noncardiovascular hospitalizations in African Americans. Am Heart J. 2010;160:488–495. doi: 10.1016/j.ahj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeboah J, Rodriguez CJ, Stacey B, Lima JA, Liu S, Carr JJ, Hundley WG, Herrington DM. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA) Circulation. 2012;126:2713–2719. doi: 10.1161/CIRCULATIONAHA.112.112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 48.Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW, Solomon SD. Heart failure with preserved ejection fraction in African Americans: The ARIC (Atherosclerosis Risk In Communities) study. JACC Heart Fail. 2013;1:156–163. doi: 10.1016/j.jchf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortes-Bergoderi M, Goel K, Murad MH, Allison T, Somers VK, Erwin PJ, Sochor O, Lopez-Jimenez F. Cardiovascular mortality in Hispanics compared to non-Hispanic whites: a systematic review and meta-analysis of the Hispanic paradox. Eur J Intern Med. 2013;24:791–799. doi: 10.1016/j.ejim.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 50.LJ Rasmussen-Torvik LC, Lima J, Jacobs D, Rodriguez CJ, Lloyd-Jones D, Shah S. Widely Varying Prevalence of Diastolic Dysfunction by Different Classification Criteria: The CARDIA study. Circulation. 2014;130:A15955. [Google Scholar]
- 51.Frigerio M, Oliva F, Turazza FM, Bonow RO. Prevention and management of chronic heart failure in management of asymptomatic patients. Am J Cardiol. 2003;91:4F–9F. doi: 10.1016/s0002-9149(02)03335-0. [DOI] [PubMed] [Google Scholar]