Abstract
Background
Age is the foremost risk factor for atrial fibrillation (AF) and AF has a rising prevalence in older adults. How AF may contribute to decline in physical performance in older adults has had limited investigation. We examined the associations of incident AF and 4-year interval declines in physical performance at ages 70, 74, 78, and 82 years in the Health, Aging, and Body Composition (Health ABC) Study.
Methods and Results
Health ABC is a prospective cohort of community-dwelling older adults (n=3075). The study conducted serial assessments of physical performance with the Health ABC physical performance battery (PPB, scored 0-4), grip strength, 2-minute walk distance, and 400-meter walking time. Incident AF was identified from the Center for Medicare and Medicaid Services, and related to 4-year interval decline in physical performance. Following exclusions, the analysis included 2753 Health ABC participants (52% women, 41% black race). Participants with AF had a significantly greater 4-year PPB decline than those without AF at age 70, 74, 78, and 82, with mean estimated decline ranging from −.08 to −0.10 units (95% confidence interval, −0.18 to −0.01; P<0.05 for all estimates) following multivariable adjustment. Grip strength, walk distance, and walk time similarly showed significantly greater declines at each 4-year age interval in participants with AF.
Conclusions
In community-based cohort older adults, incident AF was associated with increased risk of decline in physical performance. Further research is essential to identify mechanisms and preventive strategies for how AF may contribute towards declining physical performance in older adults.
Keywords: atrial fibrillation, aging, epidemiology, physical exercise
Atrial fibrillation (AF) has a significant public health burden and conveys profound morbidity and mortality. AF is largely a disease of advancing age, as risk doubles with each progressive decade of gaining and exceeds 20% by age 80 years.1,2 The aging of the population and accompanying rise in prevalence have magnified its morbidity and health care costs.
Older adults are at increased risk for both AF and decline in physical performance. AF has been related to decline in physical function, yet most studies have consisted primarily in individuals of younger age (i.e. <70 years) and limited quantification of physical performance to exercise testing.3-5 Further assessment of the relation between AF and physical function is needed to identify the relations between AF and age-related mobility. Understanding how AF is interrelated with non-cardiac pathophysiology in aging may guide AF management and likewise prompt interventions to prevent physical performance decline. In addition, the contributions of AF towards the frailty syndrome6 – a complex and dynamic clinical syndrome marking progressive functional decline7,8 – have not been well established.
We sought to identify the associations of AF and prospective decline in physical performance in the Health, Aging, and Body Composition Study (Health ABC). The associations of AF and insults in aging – silent cerebral infarcts, diastolic and systolic heart failure, decreased exertional stamina, and others – may contribute to decline in physical performance. We hypothesized that AF would exacerbate progressive decline in physical performance measured in this community-based cohort of older adults.
Methods
Study cohort
Health ABC is a longitudinal study of community-dwelling, older adults designed to examine aging-related health outcomes.9,10 Cohort participants were recruited by randomly sampling white and all black Medicare beneficiaries around Memphis, Tennessee, and Pittsburgh, Pennsylvania. Eligibility criteria included age 70-79 years, white or black race, and well-functioning, defined as not having difficulty walking a quarter mile, climbing a flight of stairs, and performing activities of daily living without assistance. Study participants underwent the initial, baseline examination in 1997-98 (n=3,075; 52% women, 42% black race), followed by 6-month interim telephone contacts with annual examinations through year 6 and then at years 8 and 10. Examinations included standardized assessments (interview, interim history and medications, physical examination and anthropometry, motor and cognitive testing, and blood tests). The present study used data from the baseline exam and years 4, 6, 8, and 10.
The present analysis excluded participants lacking Center for Medicare and Medicaid Services (CMS) data (n=36) pertinent to AF status, those with prevalent AF at the Health ABC baseline visit identified by CMS coding (n=128), missing follow-up assessments of physical function (n=3), or lacking covariates (n=155). Health ABC study protocols were approved by Institutional Review Boards at the University of Tennessee and the University of Pittsburgh, and participants provided informed consent at all study visits.
Determination of incident atrial fibrillation in Health ABC
Incident AF was obtained by linkage of unique participant identifiers with the CMS database.11 CMS data were obtained from the baseline exam through 10 years. Individuals with International Classification of Diseases, Ninth Revision, codes 427.31 or 427.32 from either a single inpatient or 2 outpatient claims within 365 days were identified as having AF. The use of 2 outpatient claims has been reported as improving diagnostic specificity.11,12 The earliest date of ICD-9 coding assignment was deemed the date of incident AF. Participant electrocardiograms or hospital records pertinent to identifying AF were not available for review; AF is not an adjudicated study end-point in Health ABC. Ascertainment of AF using ICD-9 coding has been reported to have 84% sensitivity and 98% specificity for AF identification.13 Duration of AF was determined from the date of incident AF to the date of subsequent exams.
Physical performance assessment in Health ABC
A primary objective of Health ABC is to examine changes in physical performance in older adults concomitant with aging. The Health ABC Physical Performance Battery (PPB) combines measures modified from the lower extremity performance tests employed in the Established Populations for the Epidemiologic Studies of the Elderly battery14 and is well-validated for the repeated measure of physical performance in older adults.15,16 The Health ABC PPB is determined from: (1) standing balance, assessed by 30-seconds in the semi-tandem, tandem, and one-leg stands; (2) chair stands, measured as the time to rise 5 times unassisted from a seated position; (3) gait speed, time to walk 6 meters at usual pace; and (4) a narrow walk balance test using a 6-meter long, 20- centimeter wide path for balance assessment. The methods for conducting the PPB assessments and their baseline distributions in Health ABC have been previously detailed.9,15 Scoring of the Health ABC PPB ranges continuously from 0 to 4, where a higher score indicates superior performance.
Measures in addition to the PPB comprised hand grip strength, 2-minute walking distance, and time to complete a 400-meter walk. Grip strength was performed using a Jamar Hydraulic Hand Dynamometer (Sammons-Preston, Jackson, MI) in two trials of both hands. Participants were excluded for wrist or hand pain an upper-extremity procedure in the 3 months prior to testing. Walking assessments were conducted by completion of the 2-minute walk, 30-second pause, and then the timed 400-meter walk. Participants were instructed to walk as far as possible during the 2-minute walk and to complete the 400-meter walk as quickly as possible at a pace they could maintain. Participants were excluded from the walking test for having systolic blood pressure >199 mm Hg, diastolic blood pressure >109 mm Hg, resting heart rate <40 or >110 beats/minute, or specified electrocardiographic abnormalities (pre-excitation, idioventricular rhythm, ventricular tachycardia, third degree or complete heart block, or evidence of acute injury, ischemia, or marked T-wave abnormality). The Health ABC PPB was performed at baseline and exam years 4, 6, and 10. The remaining physical performance assessments were performed at these years and exam year 8.
Baseline study measurements and interim events
Race (black or white) and smoking status (current/former or never) were determined by self-report. Moderate-to-heavy alcohol consumption was defined as men consuming ≥14 drinks weekly and women ≥7 drinks weekly. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Systolic and diastolic blood pressures were determined from the average of two measurements obtained in a seated position. Hypertension was determined by self-report and initiation of new antihypertensive medication. Diabetes was ascertained from self-reported history, fasting glucose ≥126 mg/dL, or use of oral hypoglycemic or insulin medications. Blood samples were assayed for serum creatinine and after an 8-hour fast for total and high-density lipoprotein cholesterol measures (Ortho-Clinical Diagnostics, Rochester, NY). Abnormal cholesterol was defined as total cholesterol≥200 or high-density lipoprotein <40 mg/dL. Medications were classified using the Iowa Drug Information System. Prevalent cardiovascular disease was defined in Health ABC as including coronary artery disease (determined by angioplasty, bypass graft, myocardial infarction, or electrocardiogram showing a major Q-wave abnormality) and stroke by self-report and review of medications. Prevalent heart failure was established by self-report or medications.
Interim covariates were incident hypertension, diabetes, cardiovascular disease, and cancer. Incident cardiovascular disease was defined as hospitalization for myocardial infarction or angina; stroke; or congestive heart failure with overnight hospitalization, as determined by report or documentation and adjudicated as previously detailed.17-19 Incident cancer was determined by malignancy history (excluding melanoma) and following adjudication of a cancer diagnosis.20 Interim incident covariates were ascertained at annual examinations or 6-month telephone contacts with Health ABC participants or their proxies. Deaths were identified using obituaries, the Social Security Death Index, or telephone contact with participant representative; death certificates, interviews with next of kin, and hospital records were reviewed by the study's Diagnosis and Disease Ascertainment Committee to adjudicate cause of death. Follow-up duration was determined from the baseline visit through 10 years with censoring at the date of death or last known study contact.
Statistical analyses
We examined categorical variables for their distributions and continuous variables for their mean and standard deviations. We examined Health ABC PPB and additional measures of physical performance (grip strength, walking distance, and time to complete a 400-meter walk) and their 4-year decline in HABC participants with and without incident AF. We employed a mixed-effects model using a random intercept and slope of age. Age was considered a time-varying variable. We developed models to estimate the 4-year decline at ages 70, 74, 78, and 82 years for each of the physical performance assessments. We determined interactions by examining the statistical significance of interaction terms. Specifically, we examined the fixed effect of age and then examined statistical interaction terms to determine effect modification by sex, race, study site, body mass index, systolic and diastolic blood pressure, smoking, alcohol use, cholesterol, and creatinine. We used fixed effects on age, the product of age*AF duration, age*incident AF, and age2 (centered at 74 years). The initial model (Model 1) adjusted for baseline age, sex, race, study site, and the interaction of age*sex. Model 2 included all Model 1 covariates and additionally adjusted for body mass index, systolic and diastolic blood pressure, smoking, moderate/heavy alcohol use, cholesterol, creatinine, and interactions of age*body mass index and age*creatinine, and the following covariates as time-varying exposures: hypertension, diabetes, cardiovascular disease, and cancer. Models examining grip strength, walking distance, and 400-meter walk time were constructed using the same random effects as the 4-year decline in Health ABC PPB. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). A 2-sided P<0.01 was deemed statistically significant.
Results
Following exclusions, the analysis consisted in 2753 Health ABC participants (52% women, 41% black race; Table 1). Many study participants had risk factors for AF (mean body mass index in the overweight range; prevalence of current/former smoking, 56%; hypertension, 39%; and diabetes, 39%). Over the 10-year follow-up, 262 participants both developed AF and had subsequent physical performance assessments. Table 2 summarizes participation, AF status, and the number of participants completing physical performance assessments across exam years. Supplemental Table 1 presents the distributions of assessments across Health ABC participants at ages 70, 74, 78, and 82 years.
Table 1.
Baseline characteristics for the Health, Aging, and Body Composition Study cohort from the initial, 1997-98 exam (n=2753).
| Clinical characteristics | |
| Age, years | 73.6 ± 2.9 |
| Women | 1427 (52) |
| Black race | 1140 (41) |
| Memphis, TN site | 1390 (51) |
| Smoking, current/former | 1540 (56) |
| Moderate or heavy drinking | 451 (16) |
| Body mass index, kg/m2 | 27.4 ± 4.8 |
| Systolic blood pressure, mm Hg | 136 ± 21 |
| Diastolic blood pressure, mm Hg | 71 ± 12 |
| Creatinine, mg/dL | 1.10 ± 0.40 |
| Abnormal cholesterol | 183 (7) |
| Hypertension | 1067 (39) |
| Diabetes | 1067 (39) |
| Prevalent cardiovascular disease | 702 (26) |
| Prevalent cancer | 473 (17) |
| Measures of physical performance | |
| Health ABC PPB, scored 0-4 | 2.20 ± 0.54 |
| Grip strength, kg | 32.7 ± 10.9 |
| Mean walking distance, meters | 153.4 ± 27.2 |
| 400-meter walk time, seconds | 331 ± 61 |
Continuous variables expressed as mean ± sd, categorical variables as n (%). PPB indicates Physical Performance Battery.
Table 2.
Health ABC exam participation in across exam years, AF status, and physical function assessment in the present analysis.
| Health ABC Exam | Calendar Year | Total Attendees | Age | No. with AF at exam | Health ABC PPB | Grip strength | Two-minute walk distance | 400-meter walk time |
|---|---|---|---|---|---|---|---|---|
| Year 1 (baseline) | 1997-98 | 2753 | 73.6±2.9 | 0 | 2649 | 2711 | 2375 | 2100 |
| Year 4 | 2000-01 | 2504 | 76.6±2.9 | 109 | 2131 | 2167 | 1845 | 1522 |
| Year 6 | 2002-03 | 2381 | 78.5±2.9 | 152 | 1910 | 1926 | 1630 | 1316 |
| Year 8 | 2004-05 | 2098 | 80.5 ±2.8 | 164 | N/A | 1629 | 1271 | 1003 |
| Year 10 | 2006-07 | 1870 | 82.3±2.9 | 184 | 1318 | 1462 | 1001 | 772 |
Continuous values expressed as mean±standard deviation. AF indicates atrial fibrillation; PPB, physical performance battery.
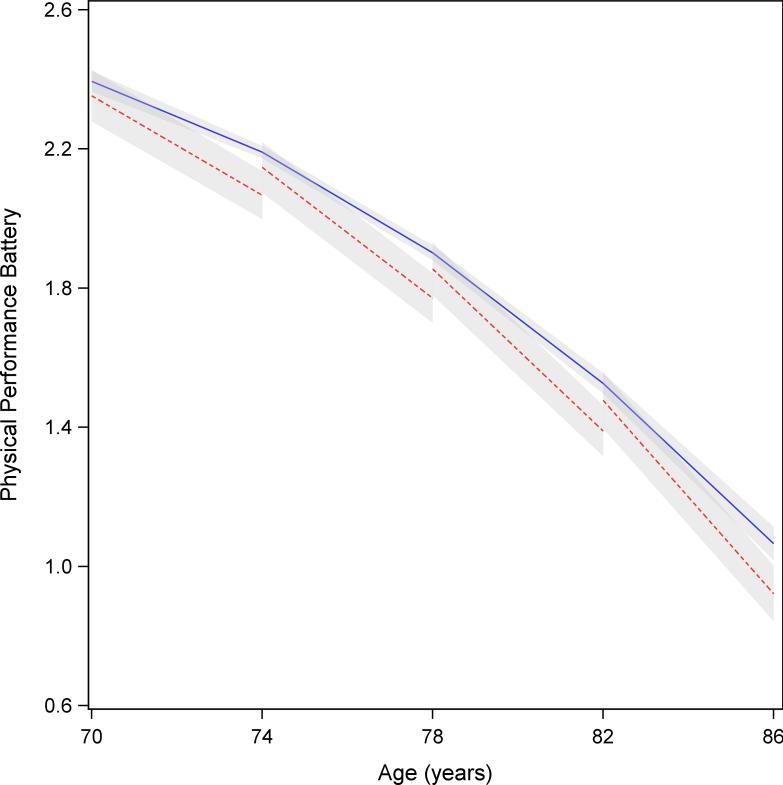
The estimates of 4-year declines in Health ABC PPB scores at ages 70, 74, 78, and 82 years, are shown in Table 3. The table presents the mean estimated 4-year decline in Health ABC PPB scores in participants without AF, those with AF, and the difference in PPB decline between those with and without AF at the specified age. Participants with AF at each of the 4-year age categories had significantly greater decline in Health ABC PPB compared to those without AF. For instance, at age 70 in the multivariable and interim-adjusted model 3, those without AF had a PPB decline of 0.20, whereas those with AF had a decline of 0.28, for an absolute difference in decline of 0.08. The decline in PPB in participants with AF was accelerated by approximately 4 years compared to those without AF. This significantly accelerated decline in PPB in participants with AF compared to those without remained present following adjustment for baseline and time-varying covariates. Figure 1 presents the differences in the estimates of PPB decline across progressive 4-year age categories graphically. The figure demonstrates distinct trajectories of Health ABC PPB decline between participants with and without AF from age 70 years onwards.
Table 3.
Estimates of 4-year decline in Health ABC physical performance battery* by AF status at ages 70, 74, 78, and 82.
| AF status | Age 70 Est (95% CI) | P | Age 74 Est (95% CI) | P | Age 78 Est (95% CI) | P | Age 82 Est (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | No prior AF | −0.21 (−0.23, −0.18) | −0.29 (−0.30, −0.28) | −0.38 (−0.39, −0.36) | −0.46 (−0.49, −0.43) | ||||
| Incident AF | −0.29 (−0.37, −0.21) | −0.38 (−0.46, −0.30) | −0.47 (−0.55, −0.39) | −0.555 (−0.64, −0.47) | |||||
| Difference† | −0.08 (−0.16, −0.01) | 0.026 | −0.09 (−0.164, −0.010) | 0.027 | −0.09 (−0.17, −0.01) | 0.027 | −0.10 (−0.18, −0.01) | 0.027 | |
| Model 2 | No prior AF | −0.20 (−0.22, −0.17) | −0.28 (−0.30, −0.27) | −0.37 (−0.39, −0.35) | −0.46 (−0.48, −0.43) | ||||
| Incident AF | −0.28 (−0.36, −0.21) | −0.37 (−0.45, −0.30) | −0.46 (−0.54, −0.38) | −0.55 (−0.64, −0.47) | |||||
| Difference | −0.08 (−0.16, −0.01) | 0.023 | −0.09 (−0.16, −0.01) | 0.023 | −0.09 (−0.17, −0.01) | 0.023 | −0.10 (−0.18, −0.01) | 0.024 | |
Model 1, adjusted baseline age, sex, race, site, and interaction of age-by-female. Model 2, additionally adjusted on baseline BMI, SBP, DBP, smoking, moderate/heavy drinking, abnormal cholesterol, creatinine, and interactions of age with BMI and creatinine, and for hypertension, diabetes, cardiovascular disease, and cancer as time-varying covariates. Est indicates estimate.
Health ABC physical performance battery, scored 0-4, employed as a continuous measure.
Difference is the difference in 4-year physical function decline between Health ABC participants with and without incident AF at the specified age.
Figure 1.
Model predicted trajectory of mean Health ABC physical performance battery scores. The 4-year decline in Health ABC participants’ physical performance battery at ages 70, 74, 78, and 82 years, comparing participants with incident atrial fibrillation (red-dashed line) and those without (blue solid line; shaded, 95% confidence intervals).
Table 4 describes the estimates for 4-year decline in grip strength with and without incident AF at ages 70, 74, 78, and 82 years. Similar to the PPB estimates, participants with AF had a significantly greater grip strength decline relative to those without. The decline remained significant across age group and progressive multivariable adjustment. Likewise, Table 5 presents the differences in 2-minute walking distance and 400-meter walking time. Like the other measures, there are progressive declines in 2-minute walking distance and 400-meter walk time with aging, but those with AF have a significantly greater 4-year decrease compared to those without.
Table 4.
Estimates of 4-year decline in grip strength* in Health ABC participants by AF status at ages 70, 74, 78, and 82.
| AF status | Age 70 Est (95% CI) | Age 74 Est (95% CI) | Age 78 Est (95% CI) | Age 82 Est (95% CI) | |
|---|---|---|---|---|---|
| Model 1 | No prior AF | −1.67 (−1.89, −1.44) | −1.97 (−2.09, −1.84) | −2.27 (−2.42, −2.12) | −2.57 (−2.83, −2.31) |
| With AF | −1.73 (−1.96, −1.50) | −2.03 (−2.16, −1.91) | −2.33 (−2.48, −2.19) | −2.63 (−2.89, −2.38) | |
| Difference† | −0.06 (−0.09, −0.03)‡ | −0.06 (−0.09, −0.03)‡ | −0.06 (−0.09, −0.03)‡ | −0.06 (−0.09, −0.03)‡ | |
| Model 2 | No prior AF | −1.59 (−1.84, −1.35) | −1.91 (−2.05, −1.76) | −2.22 (−2.38, −2.06) | −2.53 (−2.80, −2.27) |
| With AF | −1.65 (−1.90, −1.41) | −1.97 (−2.11, −1.82) | −2.28 (−2.44, −2.12) | −2.59 (−2.85, −2.33) | |
| Difference | −0.06 (−0.09, −0.03)‡ | −0.06 (−0.09, −0.03)‡ | −0.06 (−0.09, −0.03)‡ | −0.06 (−0.09, −0.03)‡ | |
Est indicates estimate. Model 1 adjusted for baseline age, sex, race, site, and interaction of age*sex. Model 2, additionally adjusted for smoking, moderate/heavy drinking, baseline BMI, SBP, DBP, abnormal cholesterol, creatinine, and interactions of age with BMI and creatinine, and for hypertension, diabetes, cardiovascular disease, and cancer as time-varying covariates.
Grip strength, scored 0-4, employed as a continuous measure.
Difference is the difference in 4-year grip strength decline between Health ABC participants with and without AF by the specified age.
P<.001.
Table 5.
Estimates of 4-year decline in mean, 2-minute walking distance (meters) and 400-meter walk time (seconds)* in Health ABC participants by AF status at ages 70, 74, 78, and 82 years.
| Age 70 Est (95% CI) | Age 74 Est (95% CI) | Age 78 Est (95% CI) | Age 82 Est (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | AF status | Walking distance (meters) | 400-meter walk time (seconds) | Walking distance (meters) | 400-meter walk time (seconds) | Walking distance (meters) | 400-meter walk time (seconds) | Walking distance (meters) | 400-meter walk time (seconds) |
| Model 1 | No prior AF | −1.20 (−2.32, −0.13) |
9.9 (7.24, 12.53) |
−6.20 (−6.81, −5.58) |
22.1 (20.5, 23.7) |
−11.16 (−11.9, −10.4) |
34.4 (32.3, 36.4) |
−16.13 (−17.5, −14.8) |
46.6 (43.2, 50.0) |
| With AF | −1.46 (−2.55, −0.36) |
10.5 (7.87, 13.19) |
−6.42 (−7.04, −5.81) |
22.8 (21.2, 24.3) |
−11.39 (−12.2, −10.6) |
35.0 (33.0, 37.0) |
−16.36 (−17.7, −15.0) |
47.2 (43.8, 50.6) |
|
| Difference† |
−0.23 (−0.39, −0.08)‡ |
0.7 (0.3, 1.0)§ |
−0.23 (−0.39, −0.08)‡ |
0.7 (0.3, 1.0)§ |
−0.23 (−0.39, −0.08)‡ |
0.7 (0.3, 1.0)§ |
−0.23 (−0.39, −0.08)‡ |
0.7 (0.3, 1.0)§ |
|
| Model 2 | No prior AF | −1.15 (−2.31, 0.02) |
9.7 (6.8, 12.5) |
−6.18 (−6.88, −5.47) |
22.1 (20.3, 23.9) |
−11.23 (−12.0, −10.4) |
34.5 (32.4, 36.6) |
−16.25 (−17.6, −14.9) |
46.9 (43.5, 50.4) |
| With AF | −1.36 (−2.53, −0.18) |
10.3 (7.4, 13.1) |
−6.39 (−7.09, −5.69) |
22.7 (20.9, 24.5) |
−11.44 (−12.2, −10.6) |
35.2 (33.0, 37.3) |
−16.46 (−17.8, −15.1) |
47.6 (44.1, 51.0) |
|
| Difference |
−0.21 (−0.37, −0.06)‡ |
0.6 (0.3, 1.0)§ |
−0.21 (−0.37, −0.06)‡ |
0.6 (0.3, 1.0)§ |
−0.21 (−0.37, −0.06)‡ |
0.6 (0.3, 1.0)§ |
−0.21 (−0.37, −0.06)‡ |
0.6 (0.3, 1.0)§ |
|
Est indicates estimate. Model 1 adjusted for baseline age, sex, race, site, and interaction of age*sex. Model 2, additionally adjusted for smoking, moderate/heavy drinking, baseline BMI, SBP, DBP, abnormal cholesterol, creatinine, and interactions of age with BMI and creatinine, and for hypertension, diabetes, cardiovascular disease, and cancer as time-varying covariates.
Walking distance in meters employed as a continuous measure.
Difference is the difference in 4-year walking distance decline between Health ABC participants with and without incident AF at the specified age.
P<0.01.
P<0.001.
Discussion
In a community-based, biracial cohort of older adults, we observed that participants with AF had a significantly higher 4-year decline in physical performance compared to those without AF The results reported here were consistent for each measure employed in Health ABC to evaluate physical performance (including PPB, grip strength, 2-minute walking distance, and 400-meter walk time). The accelerated decline in physical performance with AF was not substantively attenuated by comorbidities such as stroke, congestive heart failure, cancer and non-cardiac diseases associated with morbidity in older adults. Figure 1 summarizes the accelerated, progressive decline of physical performance in cohort participants with AF.
Health ABC enrolled community-dwelling older adults with a primary objective of studying determinants of the onset of mobility impairment, and hence is an important opportunity to examine the relations of AF with physical performance in healthy older adults.
Small changes in physical function in older adults have been shown to be clinically meaningful,21 particularly because of the relation of decline in physical function to clinical events. Our work describes the modest but important associations between AF and progressive decline in physical performance in Health ABC participants. For example, PPB is measured continuously from 0 to 4, and a 4-year decline of 0.12 has been determined as a meaningful change in physical function.22 We identified that cohort participants with AF experience an additional decline of 0.10 (95% CI 0.18 to 0.01) at age 82 after accounting for relevant covariates.
In the context of the literature
The literature has documented the relations of physical performance measures and adverse outcomes in older adults. Subtle changes may contribute towards adverse outcomes in older adults. Gait speed – a highly reproducible measure, central to the Health ABC PPB, and integral to 2-minute distance and 400-meter walk time – has been associated with prospective disability, loss of capacity for activities of daily living, cognitive decline, dementia, institutionalization, and mortality.23-26 Chair stands, also part of the Health ABC PPB, have similarly been associated with disability and mortality in Health ABC participants.27 Hand grip strength has been associated with prospective disability28 and functional decline,29 and is a core measure for assessing frailty, in turn related to cardiovascular outcomes.30-32 In our analysis AF was related to two of the proposed criteria for frailty33 – reduced grip strength and slower walking speed. A robust literature has confirmed the clinical importance of the assessments of physical performance performed here. Our findings support the hypothesis that AF contributes towards degenerative processes of physical performance in aging.
The relation of AF and physical function has had repeated assessments in community-based studies and clinical trials.34 In general the literature has been challenged by limited inclusion of adults beyond the eighth decade. Prior investigations examining AF and frailty in older adults remain limited. A cross-sectional study of community-dwelling older adults, younger than Health ABC, identified an association between AF and reduced gait speed,35 but did not assess the prospective relation of AF to gait speed decline. Studies of hospitalized patients have reported increased disability and frailty in older adults with AF, but relied on self-reported disability and did not use a validated definition of frailty.36,37 Perception of frailty has been reported as a fall risk in anticoagulation for stroke prevention in AF,38 but again not using validated criteria for frailty. Our analyses extend these prior findings that AF is associated with a decline in physical performance in aging, and underscore the importance of more formal evaluations of the contributions of AF towards frailty and longitudinal adversity in aging.
Interrelated mechanisms of AF and declining physical performance
We posit that the associations between AF and decline in physical performance are interrelated. AF is a clinical syndrome with both cardiovascular and non-cardiovascular manifestations. Our analysis adjusted for standard AF risk factors that may relate to diminished physical performance. It is further possible that incident AF is an intermediate outcome resulting from exposures related to physiologic decline in aging. Cardiovascular disease, a chief risk factor for AF, is associated with the development of frailty.39 Obesity and diabetes merit particular attention for their separate associations with AF and the development of sarcopenia and progression to frailty.40,41 Novel risk factors for AF in older adults (e.g., hypogonadism42) have also been related to sarcopenia.43 Subclinical cardiovascular disease and inflammation may also be associated with AF and declining physical performance.44-47 Silent cerebral infarcts secondary to AF48 were not accounted for in our analysis and may also affect physical performance in older adults. Essential questions entail investigating the reciprocal associations of sarcopenia and AF; the relation of inflammation to AF in aging; the burden of silent cerebral infarcts; and the association of AF patterns (i.e. paroxysmal, persistent, chronic) with physical performance.
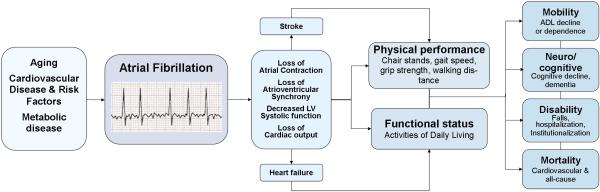
AF may also have a more direct contribution towards the decline in physical performance. AF alters atrial structure and performance, modifies atrial refractoriness, reduces atrial transport (systole), and promotes atrial fibrosis.49 Irregular heart rates diminish left ventricular filling and cardiac output.50 Structural and electrical cardiac remodeling may impair peripheral perfusion and ultimately diminished capacity for activities of daily living.51,52 We present a summary of selected clinical pathways for the association between AF and physical function in Figure 2.
Figure 2.
Proposed relation between atrial fibrillation, loss of physical performance capacity and functional status, and morbidity in older adults. The figure shows proposed pathways relating atrial fibrillation – with augmented risk in older adults with cardiovascular disease and its risk factors – to loss of atrial mechanics and decreased ventricular function. Heart failure, stroke, and decreased atrial function may contribute towards the decline in physical performance as observed in our analysis. Poor physical performance and functional status in older adults have been related to declining mobility, loss of independence, cognitive decline, disability, institutionalization, and increased mortality.
Our analysis has multiple strengths. First, the measures of physical performance were administered in a standardized format. Second, we conducted our study in a cohort of older adults with preserved functional capacity at baseline, enhancing the relevance of our findings for community-dwelling older adults. Third, our baseline age range was 70-79 years. As age is the foremost risk factor for both AF and for decline in physical performance, conducting our study in this demographic enhanced the generalizability and relevance of our findings for older adults.
Our study has some limitations. Health ABC had selected geographic enrollment, and generalizability to other races, ethnicities, or geographic regions may be limited. Second, our analysis may have limited generalizability to the growing number of older adults with marginally limited physical performance who reside in transitional settings with supportive care. Such individuals may have unrecognized AF and likely experience more accelerated decline in physical performance than the cohort examined here. We expect that our findings likely underestimate the relation of AF to functional decline by selecting a healthier cohort of older adults. Third, comorbidity is highly common in late adulthood and our analyses accounted only for major comorbid conditions and risk factors. AF and physical performance, both complex phenotypes, have multiple potential physiologic pathways. We cannot exclude residual confounding, and recognize that unaccounted for comorbidities may affect both risk of AF and decline in physical performance. Silent cerebral infarcts in particular may have an unrecognized role in mediating the relation of AF and decline in physical function. Fourth, AF status was obtained solely from CMS coding with potential for misclassification of AF status. Even more importantly, Health ABC participants may have had incident AF, developed frailty or other comorbidities, and either not attended subsequent exams or been unable to participate in physical performance assessments. Hence, our results are biased by including only those with AF who were able to perform the physical performance assessments. Fifth, our study is observational; no inference can be made about whether AF causes decline in physical performance. Further, our work cannot establish how treatment of AF may limit age-related changes in physical performance or functional decline. Sixth, there may be non-random missing data. Health ABC participants who were ill or who died would not have attended examinations, yet may have had an interim change in physical performance that we did not measure or identify.
Conclusions
We demonstrated that incident AF in independent older adults was associated with significantly greater 4-year decline in physical performance, as determined by validated, clinically relevant measures. We hypothesize that AF is a marker of the frailty syndrome and exacerbates the multisystem decline in physical performance in aging-related pathophysiology. It is possible that AF and physical decline share underlying neuroendocrine or neuromuscular causes. Further research is essential to delineate specific mechanisms relating the onset of AF to decline in physical performance. As well, investigating how training may impact declining physical performance in individuals with AF merits investigation.
Supplementary Material
WHAT IS KNOWN
Studies have related atrial fibrillation (AF) and reduced physical function, but most investigations have not included adults beyond the eighth decade, the age group at highest risk for both AF and decline in physical performance.
Decline in physical performance in older adults is an important marker of increased risk of cardiovascular and non-cardiovascular morbidity and mortality.
WHAT THE STUDY ADDS
In this study of community-dwelling older adults, participants with AF experienced a greater 4-year decline in physical performance across multiple measures than those without AF.
The study demonstrates that AF contributes towards the multisystem decline in physical performance as part of aging-related pathophysiology.
The study expands our understanding of the insults associated with AF, and raises the hypothesis that interventions may attenuate the contribution of AF towards the decline in physical strength and performance in vulnerable older adults.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Funding Sources: This research was funded by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grants R01AG028050 and R03AG045075, NINR grant R01-NR012459, 1RC1HL101056; 1R01HL102214; 1R01AG028321; NIH grants 1RO1HL092577(EJB and PTE) and 1K24HL105780 (PTE); and awards from the American Heart Association (13EIA14220013) and the Fondation Leducq 14CVD01(PTE). This work was supported Grant 2015084 from the Doris Duke Charitable Foundation.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam Study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 3.Atwood JE, Myers JN, Tang XC, Reda DJ, Singh SN, Singh BN. Exercise capacity in atrial fibrillation: A substudy of the Sotalol-Amiodarone atrial Fibrillation Efficacy Trial (SAFE-T). Am Heart J. 2007;153:566–572. doi: 10.1016/j.ahj.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Ueshima K, Myers J, Ribisl PM, Morris CK, Kawaguchi T, Liu J, Froelicher VF. Exercise capacity and prognosis in patients with chronic atrial fibrillation. Cardiology. 1995;86:108–113. doi: 10.1159/000176850. [DOI] [PubMed] [Google Scholar]
- 5.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD, Jr., Lopez B, Raisch DW, Ezekowitz MD. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: A Veterans Affairs Cooperative Studies Program substudy. J Am Coll Cardiol. 2006;48:721–730. doi: 10.1016/j.jacc.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 8.Lang PO, Michel JP, Zekry D. Frailty syndrome: A transitional state in a dynamic process. Gerontology. 2009;55:539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- 9.Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Kritchevsky SB, Tylavsky FA, Harris T, Everhart J, Simonsick EM, Rubin SM, Newman AB. Weight-loss intention in the well-functioning, community-dwelling elderly: Associations with diet quality, physical activity, and weight change. Am J Clin Nutr. 2004;80:466–474. doi: 10.1093/ajcn/80.2.466. [DOI] [PubMed] [Google Scholar]
- 11.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic variation in diagnosis frequency and risk of death among medicare beneficiaries. JAMA. 2011;305:1113–1118. doi: 10.1001/jama.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. NEJM. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Kritchevsky SB, Tylavsky F, Harris T, Simonsick EM, Rubin SM, Newman AB. Weight change, weight change intention, and the incidence of mobility limitation in well-functioning community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2005;60:1007–1012. doi: 10.1093/gerona/60.8.1007. [DOI] [PubMed] [Google Scholar]
- 17.Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: The Health, Aging and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175:410–419. doi: 10.1001/jamainternmed.2014.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan H, Kunutsor S, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Bibbins-Domingo K, Kauhanen J, Gheorghiade M, Fonarow GC, Kritchevsky SB, Laukkanen JA, Butler J. Resting heart rate and risk of incident heart failure: Three prospective cohort studies and a systematic meta-analysis. J Am Heart Assoc. 2015;4:e001364. doi: 10.1161/JAHA.114.001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC Study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 20.Thorpe RJ, Jr., Koster A, Bosma H, Harris TB, Simonsick EM, van Eijk JT, Kempen GI, Newman AB, Satterfield S, Rubin SM, Kritchevsky SB. Racial differences in mortality in older adults: Factors beyond socioeconomic status. Ann Behav Med. 2012;43:29–38. doi: 10.1007/s12160-011-9335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieves JW, Li T, Zion M, Gussekloo J, Pahor M, Bernabei R, Simon H, Williams GR, Lapuerta P. The clinically meaningful change in physical performance scores in an elderly cohort. Aging Clin Exp Res. 2007;19:484–491. doi: 10.1007/BF03324735. [DOI] [PubMed] [Google Scholar]
- 22.Perera S, Studenski S, Newman A, Simonsick E, Harris T, Schwartz A, Visser M. Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (health abc study). J Gerontol A Biol Sci Med Sci. 2014;69:1260–1268. doi: 10.1093/gerona/glu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 24.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait speed predicts decline in attention and psychomotor speed in older adults: The health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, Brach JS, Tylavsky FA, Satterfield S, Bauer DC, Rubin SM, Visser M, Pahor M. Added value of physical performance measures in predicting adverse health-related events: Results from the health, aging and body composition study. J. Am. Geriatr. Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giampaoli S, Ferrucci L, Cecchi F, Lo Noce C, Poce A, Dima F, Santaquilani A, Vescio MF, Menotti A. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 29.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: Results from the women's health and aging study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 30.Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E. Pre-frailty and risk of cardiovascular disease in elderly men and women: The pro VA Study. J Am Coll Cardiol. 2015;65:976–983. doi: 10.1016/j.jacc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 31.Boeckxstaens P, Vaes B, Legrand D, Dalleur O, De Sutter A, Degryse JM. The relationship of multimorbidity with disability and frailty in the oldest patients: A cross-sectional analysis of three measures of multimorbidity in the belfrail cohort. Eur J Gen Pract. 2015;21:39–44. doi: 10.3109/13814788.2014.914167. [DOI] [PubMed] [Google Scholar]
- 32.Sanchis J, Bonanad C, Ruiz V, Fernandez J, Garcia-Blas S, Mainar L, Ventura S, Rodriguez- Borja E, Chorro FJ, Hermenegildo C, Bertomeu-Gonzalez V, Nunez E, Nunez J. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–791. doi: 10.1016/j.ahj.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, Van Gelder IC, Ellinor PT, Benjamin EJ. Symptoms and functional status of patients with atrial fibrillation: State of the art and future research opportunities. Circulation. 2012;125:2933–2943. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donoghue OA, Jansen S, Dooley C, De Rooij S, Van Der Velde N, Kenny RA. Atrial fibrillation is associated with impaired mobility in community-dwelling older adults. J Am Med Dir. 2014;15:929–933. doi: 10.1016/j.jamda.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Gerontol Geriatr. 2013;57:325–327. doi: 10.1016/j.archger.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Fumagalli S, Tarantini F, Guarducci L, Pozzi C, Pepe G, Boncinelli L, Valoti P, Baldasseroni S, Masotti G, Marchionni N. Atrial fibrillation is a possible marker of frailty in hospitalized patients: Results of the GIFA Study. Aging Clin Exp Res. 2010;22:129–133. doi: 10.1007/BF03324785. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, Thomas LE, Ezekowitz MD, Mahaffey KW, Chang P, Piccini JP, Peterson ED. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2014;168:487–494. doi: 10.1016/j.ahj.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Chaves PH, Kuller LH, O'Leary DH, Manolio TA, Newman AB. Subclinical cardiovascular disease in older adults: Insights from the cardiovascular health study. Am J Geriatr Cardiol. 2004;13:137–151. doi: 10.1111/j.1076-7460.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 40.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 41.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469, vi. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD. Association of sex hormones, aging, and atrial fibrillation in men: The Framingham Heart Study. Circ Arrhythm Electrophysiol. 2014;7:307–312. doi: 10.1161/CIRCEP.113.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, Stefanick ML, Cummings SR, Orwoll ES. Sex hormones and frailty in older men: The Osteoporotic Fractures in Men (MROS) Study. J Clin Endocrinol Metab. 2009;94:3806–3815. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: The Inchianti Study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 45.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 46.Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, Keaney JF, Jr., Wang TJ, Vasan RS, Benjamin EJ. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104:92–96. doi: 10.1016/j.amjcard.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman AB, Arnold AM, Naydeck BL, Fried LP, Burke GL, Enright P, Gottdiener J, Hirsch C, O'Leary D, Tracy R. “Successful aging”: Effect of subclinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 48.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O'Donnell CJ, Yoshita M, D'Agostino RB, Sr., DeCarli C, Wolf PA. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 50.Lau CP, Leung WH, Wong CK, Cheng CH. Haemodynamics of induced atrial fibrillation: A comparative assessment with sinus rhythm, atrial and ventricular pacing. Eur Heart J. 1990;11:219–224. doi: 10.1093/oxfordjournals.eurheartj.a059687. [DOI] [PubMed] [Google Scholar]
- 51.Groenveld HF, Crijns HJ, Van den Berg MP, Van Sonderen E, Alings AM, Tijssen JG, Hillege HL, Tuininga YS, Van Veldhuisen DJ, Ranchor AV, Van Gelder IC. The effect of rate control on quality of life in patients with permanent atrial fibrillation: Data from the RACE II (RAte Control Efficacy in permanent atrial fibrillation II) Study. J Am Coll Cardiol. 2011;58:1795–1803. doi: 10.1016/j.jacc.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 52.van den Berg MP, Hassink RJ, Tuinenburg AE, Lefrandt JD, de Kam PJ, Crijns HJ. Impaired autonomic function predicts dizziness at onset of paroxysmal atrial fibrillation. Int J Cardiol. 2001;81:175–180. doi: 10.1016/s0167-5273(01)00564-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.