Abstract
Background
Tumor characteristics affect surgical complexity and outcomes of partial nephrectomy (PN).
Objective
To develop an Arterial Based Complexity (ABC) scoring system to predict morbidity of PN.
Design, Setting, and Participants
Four readers independently scored contrast-enhanced computed tomography images of 179 patients who underwent PN.
Intervention
Renal cortical masses were categorized by the order of vessels needed to be transected/dissected during PN. Scores of 1, 2, 3S, or 3H were assigned to tumors requiring transection of interlobular and arcuate arteries, interlobar arteries, segmental arteries, or in close proximity of the renal hilum, respectively during PN.
Outcome Measurements and Statistical Analysis
Interobserver variability was assessed with kappa values and percentage of exact matches between each pairwise combination of readers. Linear regression was used to evaluate the association between reference scores and ischemia time, estimated blood loss, and estimated glomerular filtration rates (eGFR) at 6 wk and 6 mo after surgery adjusted for baseline eGFR. Fisher’s exact test was used to test for differences in risk of urinary fistula formation by reference category assignment.
Results and Limitations
Pairwise comparisons of readers’ score assignments were significantly correlated (all p <0.0001); average kappa = 0.545 across all reader pairs. The average proportion of exact matches was 69%. Linear regression between the complexity score system and surgical outcomes showed significant associations between reference category assignments and ischemia time (p <0.0001) and estimated blood loss (p = 0.049). Fisher’s exact test showed a significant difference in risk of urinary fistula formation with higher reference category assignments (p = 0.028). Limitations include use of a single institutional cohort to evaluate our system.
Conclusions
The ABC scoring system for PN is intuitive, easy to use, and demonstrated good correlation with perioperative morbidity.
Patient Summary
The ABC scoring system is novel anatomy-reproducible tool developed to help patients and doctors understand the complexity of renal masses and predict the outcomes of kidney surgery.
Keywords: kidney neoplasms, nephrometry, observer variation, outcome assessment, partial nephrectomy
INTRODUCTION
In recent years, several nephrometry scoring systems have been developed to provide clinicians with standardized, reproducible, and possibly quantitative tools to describe relevant anatomical aspects of renal tumors and allow comparisons of published studies [1–5]. Increasing incorporation of such scores into clinical practice has improved both communication between treating physicians and physician counseling of patients in regards to risk quantification. Nephrometry systems have also been used to predict surgical complexity, risk of perioperative complications, and, in some instances, oncologic outcomes of partial nephrectomy (PN) [6–9].
Despite reported high levels of agreement among readers with similar levels of clinical expertise, the available nephrometry scoring systems have limitations [10–12]. In a recent study, we found that increasing radiological and clinical experience reduced the interobserver variability of these systems and that only subscales measuring tumor size and distance to intrarenal structures had clinical significance [13]. Other contemporary studies confirmed that reproducibility of nephrometry scores is affected by the level of the reader’s training and experience [14,15]. Furthermore, each system’s ability to predict clinical outcomes of PN has been inconsistent between studies [4,12,16–20]. Finally, morphologic descriptors such as the exophytic rate and anterior/posterior descriptor in the RENAL nephrometry scoring system and the anterior or posterior face, longitudinal, and rim tumor location, and exophytic rate in the preoperative aspects and dimensions used for an anatomical (PADUA) scoring system, appear clinically irrelevant for predicting clinical outcomes of PN [5,13]
We hypothesize that surgical complexity and outcomes of PN are mainly driven by the size of the renal arterial branches needing to be dissected/transected to achieve complete excision of the renal tumor with negative surgical margins. To test our hypothesis, we developed an Arterial Based Complexity (ABC) scoring system and determined its correlation with postoperative outcomes of PN.
MATERIALS AND METHODS
After obtaining institutional review board approval, we retrospectively reviewed the records of 1016 patients with a single, sporadic renal cortical neoplasm and a normal contralateral kidney who underwent open or laparoscopic (with or without robot-assistance) PN at Memorial Sloan Kettering Cancer Center (MSKCC) between January, 2002 and August, 2009. Standardization of all computed tomography (CT) imaging evaluated in this study was necessary to determine all the measurements and features needed for our surgical complexity system. Therefore, of the 1016 patients, we included 179 patients who underwent preoperative CT using a dedicated renal mass imaging protocol at our institution and excluded patients who underwent preoperative CT at an outside institution. Our imaging protocol included triple-phase images through the kidneys in the transverse plane and coronal reconstructions. To rule out potential selection bias that could affect the results of the study, we investigated the differences between patients who had preoperative CT at MSKCC and those who had preoperative CT elsewhere.
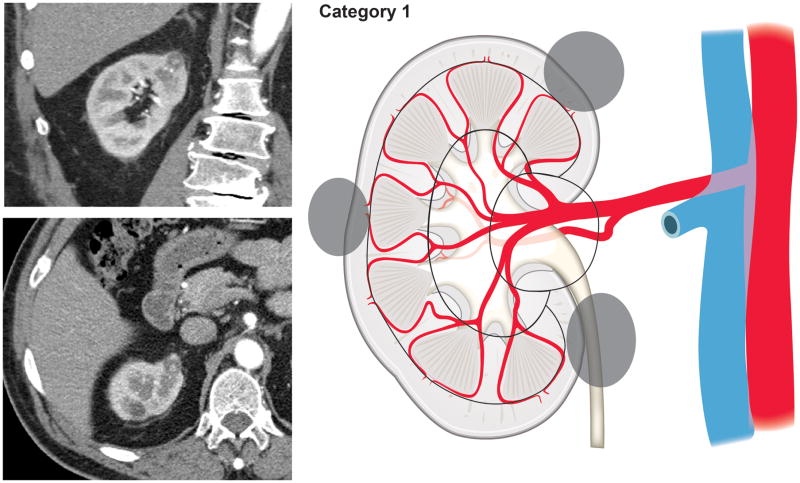
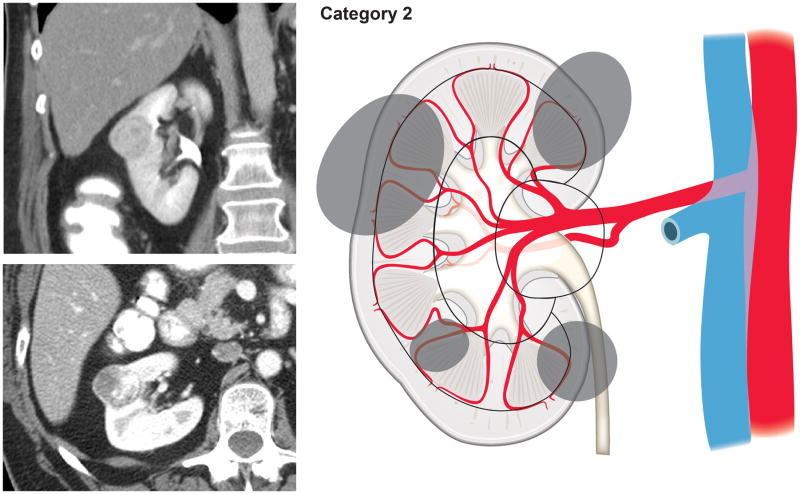
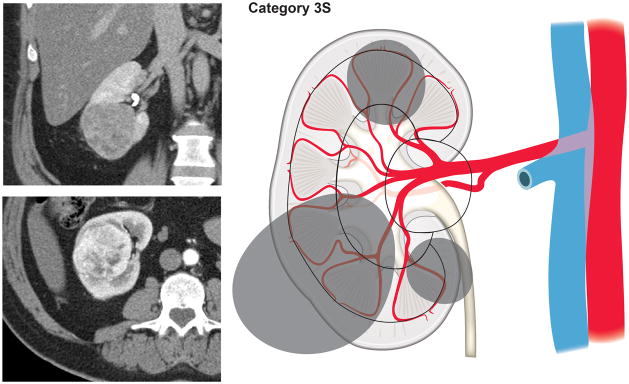
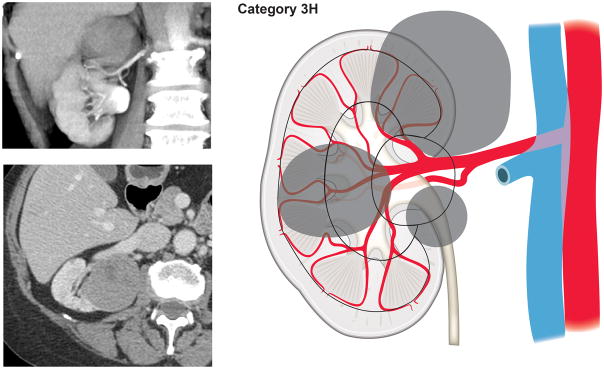
Using our newly developed surgical complexity assessment system based on the arterial vascular anatomy of the kidney, CT images of the renal lesions were reviewed and each lesion was assigned to a category of either 1, 2, 3S (sinus), or 3H (hilum). Category 1 included tumors involving only the renal cortex, thus encompassing interlobular and arcuate arteries (Fig. 1). Category 2 included tumors originating from or extending to the renal medulla and reaching the virtual line connecting the tip of the renal papillae, therefore requiring transection of the interlobar arteries (Fig. 2). Renal masses extending into the renal sinus towards the central collecting system and involving the segmental arteries and their branches were categorized as 3S (Fig. 3). Tumors in proximity of or involving the renal hilar vessels were categorized as 3H (Fig. 4).
Figure 1.
Tumors involving only the renal cortex, thus encompassing interlobular and arcuate arteries (Category 1).
Figure 2.
Tumors originating from or extending to the renal medulla and reaching the line connecting the tip of the renal papillae, therefore requiring transection of the interlobar arteries (Category 2).
Figure 3.
Renal masses extending into the renal sinus towards the central collecting system and involving the segmental arteries and their branches (Category 3S).
Figure 4.
Tumors in proximity of or involving the renal hilum (Category 3H).
CT images of the 179 renal masses were independently reviewed by four readers, including two urologists, one radiologist, and one radiologist in training. Readers were blinded to patient characteristics, surgical approach, clinical outcomes, and other readers’ score assignments. The category assignments of the reader with the most radiologic experience (radiologist) were used as the reference standard.
The kappa value between each pairwise combination of the four readers was calculated to assess the level of agreement amongst readers when using the ABC scoring system. Kappa values ranging from −1 to 1 were used to measure the degree of agreement. A kappa of 1 represented perfect agreement between readers; a kappa of zero represented as much agreement as expected by chance; and a kappa less than zero represented less agreement than expected by chance. The level of agreement between the readers was also determined by calculating the average percentage of times the readers matched each other exactly in pairwise combinations when scoring the same patient, as well as when readers differed by no more than 1 point.
We assessed whether tumors confined to the kidney cortex or medulla were associated with less surgical complexity than tumors extending to the renal sinus and involving either the collecting systems or the renal hilum. This was performed using linear regression to determine the association between the reference category assignments and estimated blood loss, ischemia time, and estimated glomerular filtration rate (eGFR) at 6 wk (+− 4 weeks) and 6 mo (+− 1 mo). These analyses were adjusted for open or minimally invasive approach except for estimated blood loss. The amount of blood loss for minimally invasive surgery is far less compared to open surgeries, and, therefore, the estimated blood loss analyses were conducted separately for each type of surgery. Analyses of eGFR were additionally adjusted for baseline eGFR. Furthermore, the ABC scoring system was evaluated for its ability to predict urinary fistula formation from reference category assignments using Fisher’s exact test. Surgical approach was not adjusted for in the analysis of fistula formation due to the low events rate.
All analyses were conducted using Stata 12 (Stata Corp., College Station, TX).
RESULTS
Patient characteristics are presented in Table 1. In our cohort, the median tumor size on CT was 3 cm and the median preoperative baseline eGFR was 66 mL/min/1.73m2. When compared with patients excluded from the study, patients included in the study did not have any significant differences other than a slightly larger median tumor size (2.8 cm vs. 3.1 cm, respectively; p = 0.009). Of the 179 patients included in the study, histopathology revealed a renal cortical malignancy in 92% and a benign renal lesion in 8% of patients. Urologic complications within 30 d of PN included grade 1–2 events in 13 (7%) patients (six urinary fistulas, four peri-incisional cellulitis, one perirenal hematoma, one renal infarction, and one urinary tract infection) and grade 3 events in 11 (6%) patients (six urinary fistulas, three perirenal hematomas, and two arteriovenous fistulas).
Table 1.
Patient characteristics
| Characteristics | |
|---|---|
| No. of patients | 179 |
| Age at surgery, yr, median (IQR) | 60 (53, 67) |
| Gender, male, no. (%) | 116 (65%) |
| Body mass index, kg/m2, median (IQR) | 29 (25, 32) |
| Preoperative eGFR, mL/min/1.73m2, median (IQR) | 66 (59, 74) |
| ASA score, no. (%) | |
| Class I, II | 106 (59%) |
| Class III | 73 (41%) |
| Maximum tumor size, cm, median (IQR) | 3 (2, 4) |
| R.E.N.A.L. score, median (IQR) | 8 (6, 9) |
| P.A.D.U.A. score, median (IQR) | 9 (7, 10) |
| Partial nephrectomy – approach, no. (%) | |
| Open | 102 (57%) |
| Laparoscopic | 69 (39%) |
| Robot-assisted laparoscopic | 8 (4%) |
| Pathologic stage, no. (%) | |
| Benign | 15 (8%) |
| pT1 | 139 (78%) |
| pT2 | 4 (2%) |
| pT3 | 21 (12%) |
| Ischemia time, min, median (IQR) | 35 (25, 45) |
| Estimated blood loss, mL, median (IQR) | 200 (100, 350) |
| Urinary fistula formation, no. (%) | 14 (8%) |
| eGFR at 6 ± 4 wk, n = 100, mL/min/1.73m2, median (IQR) | 60 (54, 69) |
| eGFR at 6 ± 1 mo, n = 49, mL/min/1.73m2, median (IQR) | 62 (54, 72) |
ASA = American Society of Anesthesiologists; eGFR = estimated glomerular filtration rate; IQR = interquartile range.
Kappa values are shown in Table 2. The average kappa value for all readers was 0.545 and all p values for each of the pairwise comparisons were significant. These findings were confirmed by the calculation of the average percentage of cases in which the readers’ category assignments matched each other exactly in pairwise combinations. The average percentage of near matches (1 point difference or less) amongst readers was 98% and the average percentage of exact matches was 69% for the four categories of the ABC scoring system (Table 3).
Table 2.
Kappa values for each pairwise combination using the Arterial Based Complexity (ABC) scoring system. All values are significantly different from 0 at p <0.0001.
| Radiologist (Reference Standard) | Urologist 1 | Urologist 2 | Radiologist In-Training | |
|---|---|---|---|---|
| Radiologist | - | |||
| Urologist 1 | 0.531 | - | ||
| Urologist 2 | 0.489 | 0.554 | - | |
| Radiologist In-Training | 0.569 | 0.595 | 0.530 | - |
Table 3.
Proportion of agreement and disagreement in complexity scoring for each pair of readers. For example, urologist 1 and the radiologist agreed on the complexity score for 68% of patients, 28% of patients were scored as 3S by both readers, and 3% were scored as 3S by the radiologist and 3H by the urologist
| Radiologist | Urologist 1 | Urologist 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3S | 3H | 1 | 2 | 3S | 3H | 1 | 2 | 3S | 3H | ||
| Urologist 1 | 1 | 4% | 3% | 0% | 1% | ||||||||
| 2 | 3% | 27% | 7% | 1% | |||||||||
| 3S | 0% | 4% | 28% | 9% | |||||||||
| 3H | 0% | 1% | 3% | 8% | |||||||||
| Total Agreement | 68% | ||||||||||||
| Urologist 2 | 1 | 6% | 4% | 0% | 0% | 4% | 6% | 0% | 0% | ||||
| 2 | 2% | 21% | 8% | 0% | 3% | 22% | 6% | 0% | |||||
| 3S | 0% | 7% | 27% | 9% | 1% | 7% | 33% | 2% | |||||
| 3H | 0% | 2% | 3% | 11% | 1% | 2% | 3% | 10% | |||||
| Total Agreement | 65% | Total Agreement | 70% | ||||||||||
| Radiologist In-Training | 1 | 3% | 3% | 0% | 0% | 4% | 3% | 0% | 0% | 6% | 1% | 0% | 0% |
| 2 | 4% | 26% | 7% | 1% | 4% | 28% | 5% | 0% | 4% | 23% | 8% | 3% | |
| 3S | 0% | 4% | 27% | 5% | 0% | 6% | 29% | 1% | 0% | 6% | 28% | 2% | |
| 3H | 0% | 1% | 4% | 13% | 0% | 0% | 8% | 11% | 0% | 1% | 7% | 11% | |
| Total Agreement | 70% | Total Agreement | 73% | Total Agreement | 68% | ||||||||
The ABC scoring system showed a significant ability to predict surgical complexity from reference category assignments. A higher category assignment was associated with higher estimated blood loss, longer cold ischemia time, longer warm ischemia time, and longer overall ischemia time (all p <0.05) as shown in Table 4. Furthermore, patients with a reference category of 3S and 3H were more likely to develop a urinary fistula compared to patients with a reference category of 1 or 2 (p = 0.028). Of note, no urinary fistula formation occurred in patients with reference category 1, thus patients in categories 1 and 2 were combined as the reference group in this analysis. However, no evidence of correlation between the reference category assignments and eGFR at 6 wk or 6 mo after surgery was found.
Table 4.
Mean estimates of surgical outcomes by reference category assignment
| Estimated Blood Loss (mL) | ||||
|---|---|---|---|---|
| N=179 | Coef. | 95% C.I. | p-value | |
| 1 | - | Ref. | 0.049 | |
| 2 | 86.25 | −68.18, 240.68 | ||
| 3S | 115.41 | −37.66, 268.47 | ||
| 3H | 208.05 | 43.62, 372.49 | ||
| N=179 | Ischemia Time (min) | |||
| 1 | - | Ref. | <0.0001 | |
| 2 | 13.49 | 3.84, 23.15 | ||
| 3S | 22.89 | 13.34, 32.45 | ||
| 3H | 27.24 | 16.96, 37.51 | ||
| N=67 | Warm Ischemia Time (min) | |||
| 1 | - | Ref. | 0.014 | |
| 2 | 14.73 | 1.92, 27.54 | ||
| 3S | 21.03 | 7.98, 34.09 | ||
| 3H | 21.84 | 5.34, 38.34 | ||
| N=112 | Cold Ischemia Time (min) | |||
| 1 | - | Ref. | 0.001 | |
| 2 | 1.43 | −11.54, 14.40 | ||
| 3S | 11.29 | −1.46, 24.04 | ||
| 3H | 14.79 | 1.74, 27.85 | ||
| N=100 | 6 wk eGFR (mL/min/1.73 m2) | |||
| 1 | - | Ref. | 0.8 | |
| 2 | −1.64 | −12.74, 9.46 | ||
| 3S | −2.63 | −13.53, 8.26 | ||
| 3H | 0.53 | −11.47, 12.52 | ||
| N=49 | 6 mo eGFR (mL/min/1.73 m2) | |||
| 1 | - | Ref. | 0.8 | |
| 2 | 6.83 | −13.18, 26.84 | ||
| 3S | 7.49 | −12.73, 27.71 | ||
| 3H | 3.95 | −17.16, 25.06 | ||
| Urinary Fistula | ||||
| N=179 | Risk | Risk Difference | 95% C.I. | p-value |
| 1 or 2 | 2 (2.7%) | Ref. | Ref. | 0.028 |
| 3S | 6 (8.7%) | 6% | −1.6%, 14% | |
| 3H | 6 (17%) | 14% | 1.5%, 27% | |
Coef. = coefficient; CI = confidence interval; eGFR = estimated glomerular filtration rate; Ref: reference.
DISCUSSION
We developed a simple ABC scoring system to assess the surgical complexity of renal cortical masses, taking into consideration the relationship between renal tumor depth and the renal arterial vascular anatomy, and in particular the order of arterial branches that needed to be dissected/transected during PN. In our analysis, the ABC scoring system correlated well with the risk of increased estimated blood loss and ischemia time, as well as urinary fistula formation. The ABC scoring system is intuitive and simple to use, as categories are assigned by interpreting cross sectional CT images. From the surgical complexity assessment perspective, it depends on the size and importance of the arteries to be controlled in order to achieve complete surgical excision with negative surgical margins and maximum parenchymal preservation using the shortest ischemia time. Although used as predictors of surgical complexity, tumor morphology or size are indirect indicators of the relationship between the tumor depth and size/importance of the renal arterial branches to be addressed during PN.
Existing nephrometry score systems were initially designed to assess complexity and compare renal masses treated at various institutions and have been individually validated and incorporated into clinical practice. However, reports have shown each of those systems to be inconsistent in predicting PN outcomes, rendering their universal applicability and reproducibility difficult [4,12,16–18]. The inherent characteristics of existing nephrometry systems may partially be responsible for their limitations. The RENAL and PADUA scoring systems were built on the morphologic attributes of renal tumors primarily to facilitate communication of tumor anatomy [1,2]. However, cutoff values for some of these systems’ components, for example, maximal tumor size or nearness to the renal sinus structures were chosen based on simplicity rather than proven clinical relevance in predicting surgical outcomes [1,13]. In a recent critical evaluation of the RENAL and PADUA scoring systems and centrality index, only four predictors of complexity (radius, nearness to the collecting system, location relative to the lateral rim, and centrality) were found to be clinically significant variables on multivariable analysis [21]. A RENAL-based, simplified numeric scoring system was recently created to overcome some of the limitations of the other nephrometry systems; however, no superiority to RENAL was demonstrated [5]. A different approach was taken with the centrality index, a mathematical method that assesses the technical complexity of PN by measuring the proximity of the renal lesion center to the kidney center; however, it does not provide information on tumor characteristics [3]. The Diameter-Axial-Polar nephrometry system is centrality index-refined and integrates the relevant and optimized components of the RENAL scoring system, showing improved performance and interpretability [4]. However, the variables of both these methods may be difficult to accurately measure in a time-efficient manner, particularly in the outpatient clinic setting, and possibly difficult to explain to patients.
The ABC scoring system assigns higher grades of surgical complexity to renal tumors in close proximity to higher-order branches of the renal artery. Morphologic descriptors and associated numeric values or points included in existing nephrometry scoring systems were deliberately omitted during development, as they were unnecessary to determine the surgical complexity of renal tumors. For example, a category 1 renal mass limited to the renal cortex was regarded as one of low complexity irrespective of size, percent of exophytic component, or polar lines. Such a lesion would only require transection of the interlobular and arcuate arteries, which may be controlled by minimal suture repair, cauterization, or a combination thereof during PN. In the case of a category 3S renal mass involving the segmental arteries or a 3H renal mass involving the hilum, vascular system involvement/proximity was regarded as the key determinant of surgical complexity, although entry of the collecting system during PN increases the risk of postoperative urinary fistula. The ABC scoring system does not account for tumor proximity of the collecting system because the collecting system anatomically follows the arterial branching within the parenchyma and is therefore indirectly incorporated. The increase in ischemia time, blood loss, and risk of urinary fistula formation associated with renal masses of greater complexity in the current study seem to confirm such assumption. Furthermore, the ABC scoring system did not require difficult measurements of variables or definition of observer-dependent lines; it was easy to learn and straightforward to use in all clinical scenarios encountered in the studied cohort. However, the ABC system requires good quality CT or MRI scans with triple-phase images to allow for a delineation of the renal arterial branches. It also requires reading both transverse and multi-planar image reconstructions for adequate category assignment. Multi-planar image reconstruction capability is widely available in currently used clinical scanners.
We acknowledge that our study was only tested on a single institution dataset and will require external validation. Another important limitation that the ABC scoring system shares with all other nephrometry scoring systems is the inability to predict long-term renal functional outcomes, which are important clinical aspects for patients undergoing PN and renal surgery in general. However, the kidney’s ability to tolerate the loss of nephron units and to partially or completely recover its baseline function after a surgery appears to be multifactorial and highly dependent on baseline renal function and medical comorbidities. Hence, it is unlikely that such prediction could be precisely made by a scoring system unless it is part of a predictive model combining surgical and functional aspects of renal tumors.
CONCLUSIONS
Similar to other nephrometry scoring systems, our anatomic system is associated with the surgical complexity and perioperative morbidity of PN. However, its intuitiveness and ease of learning is an advantage when compared to existing nephrometry systems. While these initial results are promising, further evaluation of our new scoring system and direct comparisons to existing nephrometry systems are required. Efforts to incorporate this surgical complexity system into a predictive model for PN are currently underway at our institution.
Acknowledgments
To Terry Helms for the preparation of the figures; to Melanie L. Bernstein for data management; and to Joyce Tsoi, Michael Newman, and Michael J. McGregor for their assistance in the preparation of this manuscript.
The study was supported in part by NIH/NCI Cancer Center Support Grant to MSKCC (award number P30 CA008748) and the Sidney Kimmel Center for Prostate and Urologic Cancers.
References
- 1.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–53. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–93. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C Index method. J Urol. 2010;183:1708–13. doi: 10.1016/j.juro.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Simmons MN, Hillyer SP, Lee BH, et al. Diameter-Axial-Polar nephrometry: integration and optimization of R.E.N.A.L. and centrality index scoring systems. J Urol. 2012;188:384–90. doi: 10.1016/j.juro.2012.03.123. [DOI] [PubMed] [Google Scholar]
- 5.Hakky TS, Baumgarten AS, Allen B, et al. Zonal nephro scoring system: a superior renal tumor complexity classification model. Clin Genitourin Cancer. 2014;12:e13–8. doi: 10.1016/j.clgc.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Simhan J, Smaldone MC, Tsai KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011;60:724–30. doi: 10.1016/j.eururo.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canter D, Kutikov A, Manley B, et al. Utility of the R.E.N.A.L. nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology. 2011;78:1089–94. doi: 10.1016/j.urology.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumors categorized by renal nephrometry score. BJU Int. 2013;114:708–18. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]
- 9.Tobert CM, Kahnoski RJ, Thompson DE, et al. Renal nephrometry score predicts surgery type independent of individual surgeon’s use of nephron-sparing surgery. Urology. 2012;80:157–61. doi: 10.1016/j.urology.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Kolla SB, Spiess PE, Sexton WJ. Interobserver reliability of the renal nephrometry scoring system. Urology. 2011;78:592–94. doi: 10.1016/j.urology.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Montag S, Waingankar N, Sadek MA, et al. Reproducibility and fidelity of the R.E.N.A.L. nephrometry score. J Endourol. 2011;25:1925–28. doi: 10.1089/end.2011.0217. [DOI] [PubMed] [Google Scholar]
- 12.Okhunov Z, Rais-Bahrami S, George AK, et al. The comparison of three renal tumor scoring systems: C-index, P.A.D.U.A., and R.E.N.A.L. nephrometry scores. J Endourol. 2011;25:1921–24. doi: 10.1089/end.2011.0301. [DOI] [PubMed] [Google Scholar]
- 13.Spaliviero M, Poon B, Aras O, et al. Interobserver variability of R.E.N.A.L., PADUA, and centrality index nephrometry score systems. World J Urol. 2014 Aug 24; doi: 10.1007/s00345-014-1376-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benadiba S, Verin AL, Pignot G, et al. Are urologists and radiologists equally effective in determining the renal nephrometry score? Ann Surg Oncol. 2015;22:1618–24. doi: 10.1245/s10434-014-4152-1. [DOI] [PubMed] [Google Scholar]
- 15.Monn MF, Gellhaus PT, Masterson TA, et al. R.E.N.A.L. nephrometry scoring: how well correlated are urologist, radiologist, and collaborator scores? J Endourol. 2014;28:1006–10. doi: 10.1089/end.2014.0166. [DOI] [PubMed] [Google Scholar]
- 16.Hew MN, Baseskioglu B, Barwari K, et al. Critical appraisal of the PADUA classification and assessment of the R.E.N.A.L. nephrometry score in patients undergoing partial nephrectomy. J Urol. 2011;186:42–6. doi: 10.1016/j.juro.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Lavallee LT, Desantis D, Kamal F, et al. The association between renal tumour scoring systems and ischemia time during open partial nephrectomy. Can Urol Assoc J. 2012;15:1–8. doi: 10.5489/cuaj.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehrazin R, Palazzi KL, Kopp RP, et al. Impact of tumour morphology on renal function decline after partial nephrectomy. BJU Int. 2013;111:E374–82. doi: 10.1111/bju.12149. [DOI] [PubMed] [Google Scholar]
- 19.Reddy UD, Pillai R, Parker RA, et al. Prediction of complications after partial nephrectomy by renal nephrometry score. Ann R Coll Surg Engl. 2014;96:475–9. doi: 10.1308/003588414X13946184903522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake H, Furukawa J, Hinata N, et al. Significant impact of R.E.N.A.L. nephrometry score on changes in postoperative renal function early after robot-assisted partial nephrectomy. Int J Clin Onc. 2014 Sep 17; doi: 10.1007/s10147-014-0751-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Tobert CM, Shoemaker A, Kahnoski RJ, et al. Critical appraisal of first-generation renal tumor complexity scoring systems: Creation of a second-generation model of tumor complexity. Urol Oncol. 2015;33:167, e1–6. doi: 10.1016/j.urolonc.2014.12.016. [DOI] [PubMed] [Google Scholar]