Abstract
The mesolimbic dopaminergic reward system is responsible for the negative affective symptomatology of schizophrenia, which may be related to a low dopamine tonus within the ventral striatum. The monetary incentive delay (MID) task can be used to study the response of the ventral striatum to incentive stimuli. We show that activation of the ventral striatum is low in patients with schizophrenia, and that this low activation is related to primary and secondary negative symptoms induced by neuroleptics, also known as antipsychotics. Switching from first-(typical) to second-generation (atypical) antipsychotics increased activation of the ventral striatum due to less blocking of dopamine D2 receptors. This and similar studies show that functional magnetic resonance imaging (fMRI) tasks are suitable to investigate important aspects of antipsychotic mechanisms.
Keywords: D2 receptor, dopamine, fMRI, MID task, first- and second-generation antipsychotic, reward system
Abstract
El sistema de recompensa dopaminérgico mesolímbico es responsable de la sintomatología afectiva negativa de la esquizofrenia, la cual puede estar relacionada con un tono dopaminérgico bajo dentro del estriado ventral. La prueba del retardo del incentivo monetario (RIM) se puede emplear para estudiar la respuesta del estriado ventral al estímulo incentivador. Se muestra que la activación del estriado ventral es baja en pacientes con esquizofrenia y esta reducción de la activación está relacionada con síntomas negativos primarios y secundarios inducidos por los neurolépticos, también conocidos como antipsicóticos. El cambio de los antipsicóticos típicos o de primera generación a los atípicos o de segunda generación aumentó la activación del estriado ventral debido a un menor bloqueo de los receptores dopaminérgicos D2. Este y otros estudios similares muestran que las pruebas con imágenes de resonancia magnética funcional son adecuadas para investigar importantes aspectos de los mecanismos de los antipsicóticos.
Abstract
Le système de récompense dopaminergique mésolimbique est responsable de la symptomatologie affective négative de la schizophrénie, qui peut être liée à un faible tonus dopaminergique dans le striatum ventral. Le test du retard à l'incitation financière peut être utilisé pour étudier la réponse du striatum ventral aux stimuli incitatifs. Nous montrons que l'activation du striatum ventral est faible chez les schizophrènes et que cette faible activation est liée aux symptômes négatifs primaires et secondaires induits par les neuroleptiques, connus aussi comme antipsychotiques. Le passage des antipsychotiques de première génération (typiques) aux antipsychotiques de seconde génération (atypiques) augmente l'activation du striatum ventral due au moindre blocage des récepteurs de la dopamine D2. Cette étude et d'autres études similaires montrent que l'lRM fonctionnelle permet d'examiner les aspects importants des mécanismes antipsychotiques.
The mesolimbic dopaminergic reward system in schizophrenia
A dysfunction of the dopaminergic reward, or motivation, system has been postulated in patients with schizophrenia.1 Evidence of a dopamine hypothesis in schizophrenia has been primarily provided by studies of the effects of antipsychotics. Furthermore, positron emission tomography (PET) and single-photon emission computed tomography (SPECT) studies have revealed dysfunction of the striatal dopaminergic system, noting an increase in the presynaptic synthesis of dopamine in patients with schizophrenia.2 A hyper dopaminergic state within the ventral striatum in patients with schizophrenia may be associated with enhanced ideas and thoughts in the context of delusions. A hypodopaminergic state may be related to an amotivational syndrome characterized by apathy, anhedonia, and other negative symptoms. In the hypodopaminergic state, few or no external stimuli are motivating and important for the organism. However, the administration of antipsychotics has to be taken into account, because antipsychotics may affect motivational salience. The main problem in this regard is the blockade of dopamine D2 receptors, especially by typical neuroleptics, also known as first-generation antipsychotics (FGAs). This results in clinical depression in 20% to 40% of patients taking FGAs, which block D2 receptors. In terms of secondary negative symptomatology, such blockade results in loss of drive, energy, and motivation; apathy, anhedonia, and patient noncompliance. The advantage of the new atypical neuroleptics, also called second-generation antipsychotics (SGAs), is that they only partially block the D2 receptors in the ventral striatum (especially clozapine.3). Particularly with regard to the heteroreceptor mechanism involving antagonism of the presynaptic serotonin 5-hydroxytryptamine (5-HT2) receptor, the administration of SGAs results in a sufficient and permanent flow of dopamine within the ventral striatum, thus maintaining affectivity and drive.
The “monetary incentive delay” task in functional magnetic resonance imaging
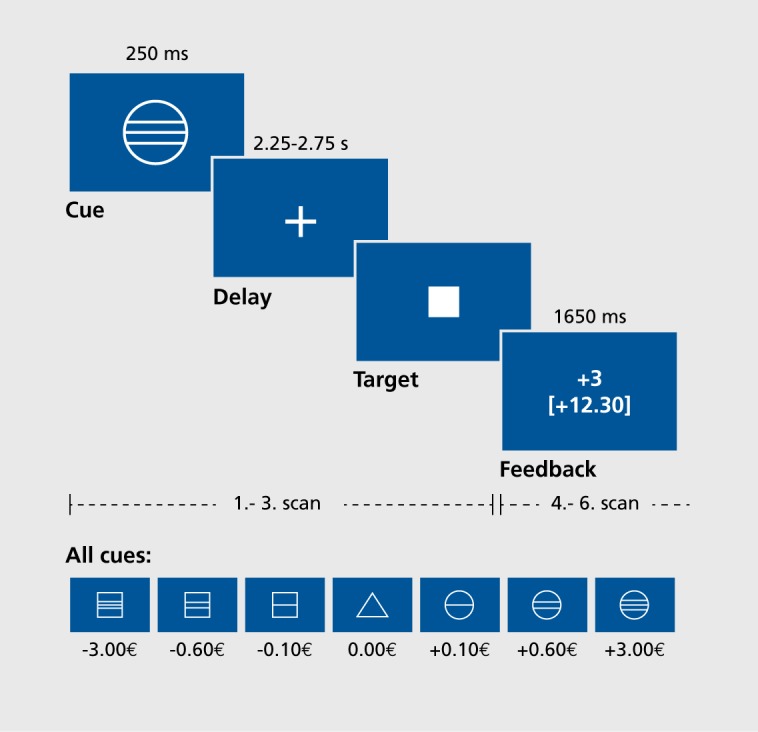
The function of the reward system can be visualized with functional magnetic resonance imaging (fMRI); for this purpose, Knutson et al4 developed the monetary incentive delay (MID) task. This is a simple monetary-gain game that requires subjects to respond to a sequence of geometric shapes that are projected onto the screen of the MRI scanner during fMRI measurement. This game (Figure 1) consists of two runs of 72 trials each, at the beginning of which a geometric shape informs the volunteer about the following: that money can be gained (indicated by a circle), that loss of money can be avoided (square), or that it is a neutral trial (triangle) with no monetary consequence. After these cues have been given, a delay phase follows during which the volunteer waits for the appearance of a target. The task is to press a button with the thumb when the briefly presented target is visible. Immediately after this, success or failure is indicated, and the cumulative total of money won is shown. The chance of winning is set to 66% by means of an adaption mechanism, so that the volunteer anticipates the receipt of monetary gain on appearance of a reward-indicating cue. After the game, the money is paid out immediately.
Figure 1. The monetary incentive delay task, developed by Brian Knutson. 4In this monetary-gain game, the subject presses a button when the target stimulus is presented in order to gain or avoid losing money, the amount indicated by the cue stimulus. During delay and feedback phases, magnetic resonance imaging scans are recorded in order to visualize brain activity, ms, milliseconds; s, seconds. Reproduced from reference 6: Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl). 2006:187(21:222-228. Copyright @ Springer-Verlag, 2006.
It is well-known that the occurrence of the dopaminergic signal is to be expected during the anticipation phase. The game was administered while subjects underwent scanning with a 1.5-tesla scanner (MAGNETOM Vision, Siemens) with an echo-planar imaging (EPI)-sequence (repetition time [TR]=1.9 s, echo time [TE]=40 ms, flip angle=90°, voxel size=4x4x3.3 mm). Data were analyzed with SPM2 (Statistical Parameter Mapping software version 2; http://www.fil.ion.ucl.ac.uk/spm). Using the normalized preprocessed data (slice timing, motion correction, spatial normalization, and spatial smoothing with 8-mm full width at half maximum [FWHM]), we developed single models — according to the general linear model — for each volunteer, by defining the different gain, loss, and neutral cues as single conditions. Using random effects analysis, we examined the contrast between “anticipation of gain” and “neutral consequence” (for detailed descriptions, see refs 5 and 6).
First-versus second-generation antipsychotics: a cross-sectional study
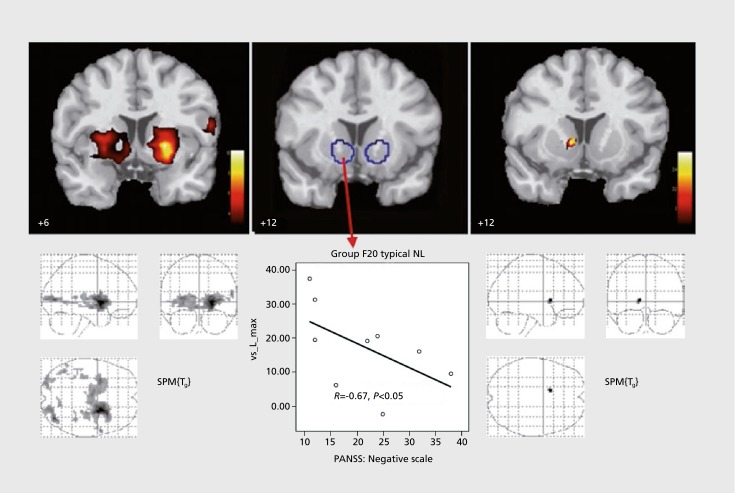
A few years ago, we examined the influence of FGAs and SGAs on the reward system in patients with schizophrenia.6 Three groups were tested: a group of 10 schizophrenic patients who received an FGA (4 received flupenthixol 12±4 mg; 4, haloperidol 10±5 mg; and 2, fluphenazine 12+4 mg), a group of 10 schizophrenic patients who were treated with an SGA (4 received risperidone 5±1 mg; 4, olanzapine 19±6 mg; 1, aripiprazole 30 mg; and 1, amisulpride 300 mg) and a group of 10 healthy control subjects. There were no significant differences between the three groups for age, gender, and total weight gain. There were no significant differences in psychopathology in both patient groups (total Positive and Negative Syndrome Scale [PANSS] score in patients taking an FGA, 70.11±20.37; those taking an SGA, 64.44+22.59). In the healthy control subjects, we observed activation for the contrast between gain anticipation versus neutral consequence at both sides of the ventral striatum (left: [x y z] = [-21 5 -3], t=9.53; right: [x y z] = [12 2 -10], t=4.25). Whereas no activation in response to reward-indicating stimuli was observed in the schizophrenic patients taking an FGA, activation was seen in the patients taking an SGA, in the right ventral striatum ([x y z] = [12 12 -1], t=3.58) (Figure 2). In group comparisons, there was a significant difference between the healthy subjects and the schizophrenic patients taking an FGA ([x y z] = [-21 9 -3], t=3.39), but there was no difference between healthy subjects and patients taking an SGA. The reduction in activation in response to reward- indicating stimuli was inversely correlated with the severity of negative symptomatology (Spearman's R = -0.67, P<0.05) in the group of patients being treated with an FGA, as well as in the group of nonmedicated schizophrenic patients.
Figure 2. Effects of first-generation (middle) vs second-generation antipsychotics (right) on ventral striatum in patients with schizophrenia (cross-sectional study), as compared with healthy controls (left), group F20 typical NL, patients with schizophrenia treated with a typical neuroleptic; PANSS, Positive and Negative Syndrome Scale; SPM, Statistical Parametric Mapping. Reproduced from reference 6: Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl). 2006:187(2):222-228. Copyright@Springer-Verlag, 2006.
Switch from first- to second-generation antipsychotics: reactivation of the ventral striatum in patients with schizophrenia in longitudinal studies
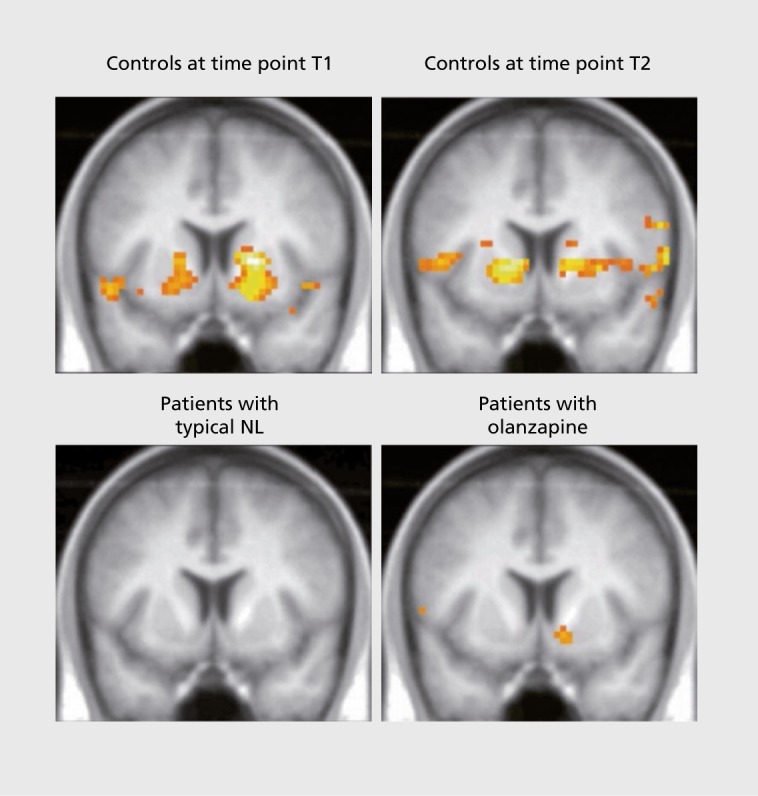
As cross-sectional studies are not sufficient to evaluate the effects of antipsychotic medication on ventral striatum activation during the MID task, further studies using a longitudinal design have been carried out in which schizophrenic patients were treated with an FGA (such as haloperidol, flupenthixol, or fluphenazine) for at least 2 weeks, and then with an SGA (such as risperidone, olanzapine, or aripiprazole) for another 2 weeks. In such studies, after 2 weeks of the respective treatments, the patients were examined by fMRI while carrying out the MID task. They were then compared with healthy subjects, who were examined twice by fMRI at similar time intervals. In a study of changes associated with a switch to olanzapine,7 10 schizophrenic patients treated with an FGA (4 received haloperidol: 10.8+4.3 mg/d; 5, flupenthixol 7.0+5.1 mg/d; 1, fluphenazine 15 mg/d) were first examined by fMRI while performing the MID task and were afterwards switched to olanzapine (18.5±7.5 mg/d). The mean treatment period was 17.8 days (±15 .0) for FGAs and 20.2 days (±6.7) for olanzapine. The repeat examination by fMRI after olanzapine treatment was carried out 31.7±17.3 days after the first fMRI examination (which followed FGA treatment). Ten healthy subjects were also examined twice, the repeat examination performed 32.7±15.5 days after the first fMRI, in order to control for a potential time effect. In anticipation of monetary gain, healthy subjects showed a significant activation of the ventral striatum (left: [x y z] = [-18 6 -9], t=3.87; right: [x y z] = [18 6 -3], t=4.70), whereas schizophrenic patients treated with an FGA did not show any activity (Figure 3). However, after switching to olanzapine treatment, activation of the ventral striatum was observed on the right side ([x y z] = [15 6 -12], t4.36). In the healthy control subjects, activation of the ventral striatum, due to the anticipation of a reward, remained quite stable over time. Presumably due to secondary negative symptomatology in the schizophrenic patients, there was a significant correlation between the activation of the ventral striatum and the PANSS negative score (R=-0.721, P=0.019), though only after treatment with an FGA.
Figure 3. Functional magnetic resonance imaging showing activation of ventral striatum in patients with schizophrenia after several weeks of treatment with a second-generation antipsychotic (SGA; olanzapine) after switching from a first-generation antipsychotic (FGA; also known as typical neuroleptic; most often, haloperidol or flupenthixol), compared with activation in healthy subjects with two recordings at a similar time interval, typical NL, typical neuroleptic; T1, after treatment with an FGA; T2, after switching to the SGA olanzapine. Reproduced from reference 7: Schlagenhauf F, Juckel G, Koslowski M, et al. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology. 2008:196(4):673-684. Copyright © Springer-Verlag, 2006.
In a pilot study (GJ et al, unpublished data, 2015) investigating the change in activation associated with a switch from treatment with an FGA to an SGA, 8 male schizophrenic patients under treatment with either haloperidol or flupentixol (FGAs) at baseline, were switched to treatment with risperidone (an SGA). The average age of these patients was 35±13 years; the healthy controls were aged 33.6±12.5 years. At baseline, fMRI scans carried out during performance of the MID task in FGA-treated schizophrenic patients showed a significantly lower activation of the ventral striatum than healthy controls (T-values 2.5 to 3.0, t=0.03 to 0.04, corrected for ventral striatum region of interest false discovery rate [ROI FDR]). After changing the medication to the SGA risperidone, fMRI scans — carried out nearly 2 months after the baseline scans in order to minimize any residual FGA effects, known to be long-acting — showed a visible increase in activity over that seen under FGA treatment on both sides of the ventral striatum for gain anticipation (left: t=3.6, P=0.04 corrected for ROI; right: t=3.6, P=0.02 corrected for ROI). This implies a possible regeneration of the dopaminergic reward system in the ventral striatum after the change to an SGA. In another study, switching from an FGA to the SGA aripiprazole,8 also resulted in increased activation of the ventral striatum (t=2.17, P<0.05).
Conclusions
Dysfunction within the dopaminergic reward system can be explored in schizophrenic patients, with or without treatment by FGAs and SGAs, by fMRI during performance of the MID task. Schizophrenic patients have been shown to have less activation of the ventral striatum, a core region of the reward system, than healthy controls; the severity of the (primary) negative symptomatology is associated with reduced activation of the ventral striatum.6 The findings about the effects of FGAs in the cross-sectional and switch-longitudinal studies can be interpreted as expressions of the secondary FGA-induced negative symptoms caused by a flattening of ventral striatal dopaminergic tone, symptoms that were not present, or that disappeared, after switching to an SGA.
The findings of the two study types about the influence of FGAs and SGAs on the reward system may be summarized as follows: (i) patients with schizophrenia treated with FGAs, but not with SGAs, had less activity in the ventral striatum than healthy controls and (ii) the severity of the negative symptomatology correlated with the reduction in activity in the ventral striatum in patients treated with FGAs The better effectiveness of SGAs on negative symptomatology, shown in clinical studies,9 potentially correlates with lower impairment of the reward system. SGAs, for example, risperidone or olanzapine, cause relatively low blockade of the striatal dopamine D2 receptors and are not bound so tightly to these receptors. Moreover, they interact with other transmitter systems, such as the serotonergic system (through effects on the 5-HT2A receptor).
The following limitations for the interpretation of the results need to be noted. Firstly, only a relatively small number of cases were examined. Secondly, the exact mechanism of the dopaminergic reward dysfunction cannot be clarified by fMRI. The application of multimodal methods with a combination of dopamine-PET and fMRI could possibly explain which mechanisms cause the dysfunction of the reward system in patients with schizophrenia. In order to compare FGAs and SGAs, a study design with an intrasubject comparison seems more adequate. Therefore, the results discussed here require replication in a better and longer longitudinal study in patients in which treatment with FGAs is then switched to treatment with SGAs. Alternatively, a controlled before-and-after study of patients receiving no treatment and then receiving a specific antipsychotic monotherapy would also help to elucidate the relationship between different antipsychotic treatments and the mesolimbic dopaminergic reward system in schizophrenia. To this end, one study has compared 23 antipsychotic-naive patients before and after a 6-week treatment with amisulpride.10 Similarly to our findings, patients without any medication showed clear attenuation of brain fMRI activation during reward anticipation in the ventral striatum during the MID task, with activation normalized after a 6-week treatment with an SGA.
REFERENCES
- 1.Juckel G., Sass L., Heinz A. Anhedonia, self-experience in schizophrenia, and implications for treatment. Pharmacopsychiatry. 2003;36(suppl 3):176–180. doi: 10.1055/s-2003-45127. [DOI] [PubMed] [Google Scholar]
- 2.Grunder G., Muller MJ., Andreas J., et al Occupancy of striatal D(2)-like dopamine receptors after treatment with the sigma ligand EMD 57445, a putative atypical antipsychotic. Psychopharmacology (Berl). 1999;146(1):81–86. doi: 10.1007/s002130051091. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 4.Knutson B., Adams CM., Fong GW., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juckel G., Schlagenhauf F., Koslowski M., et al Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Juckel G., Schlagenhauf F., Koslowski M., et al Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl). 2006;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 7.Schlagenhauf F., Juckel G., Koslowski M., et al Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology. 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- 8.Schlagenhauf F., Dinges M., Beck A., et al Switching schizophrenia patients from typical neuroleptics to aripiprazole: effects on working memory dependent functional activation. Schizophr Res. 2010;118(1-3):189–200. doi: 10.1016/j.schres.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Leucht S., Pitschel-Walz G., Abraham D., Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35(1):51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen MO., Rostrup E., Wulff S., et al Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69(12):1195–1204. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]