Abstract
Corticostriatal connections play a central role in developing appropriate goal-directed behaviors, including the motivation and cognition to develop appropriate actions to obtain a specific outcome. The cortex projects to the striatum topographically. Thus, different regions of the striatum have been associated with these different functions: the ventral striatum with reward; the caudate nucleus with cognition; and the putamen with motor control. However, corticostriatal connections are more complex, and interactions between functional territories are extensive. These interactions occur in specific regions in which convergence of terminal fields from different functional cortical regions are found. This article provides an overview of the connections of the cortex to the striatum and their role in integrating information across reward, cognitive, and motor functions. Emphasis is placed on the interface between functional domains within the striatum.
Keywords: basal ganglia, cognition, dopamine, dorsal striatum, functional integration, motor control, prefrontal cortex, reward, ventral striatum
Abstract
Las conexiones cortico-estriatales juegan un papel central en el desarrollo de conductas adecuadas dirigidas a un objetivo, incluyendo la motivación y los aspectos cognitivos para desarrollar acciones apropiadas para obtener un resultado específico. La corteza se proyecta hacia el cuerpo estriado topográficamente. En consecuencia, se han asociado diferentes regiones del cuerpo estriado con estas diversas funciones: el estriado ventral con la recompensa, el núcleo caudado con la cognición y el putamen con el control motor. Sin embargo, las conexiones cortico-estritales son más complejas y las interacciones entre los territorios funcionales son amplias. Estas interacciones se producen en regiones específicas en las cuales se encuentra la convergencia de los campos terminales de diferentes regiones corticales funcionales. Este artículo proporciona una panorámica acerca de las conexiones de la corteza con el cuerpo estriado y su papel en la integración de la información en las funciones de recompensa, cognitiva y motora. Se hace hincapie en la interfaz entre los dominios funcionales dentro del cuerpo estriado.
Abstract
Les connexions cortico-striatales jouent un rôle central dans l'élaboration de comportements pertinents axés sur des objectifs, dont la motivation et la cognition, pour mettre en oeuvre des actions adaptées en vue d'un résultat spécifique. Le cortex se projette sur le striatum de façon topographique. Différentes régions du striatum ont donc été associées à ces différentes fonctions: le striatum ventral pour la récompense ; le noyau caudé pour la cognition ; et le putamen pour le contrôle moteur. Les connexions cortico-striatales sont néanmoins plus complexes et les interactions entre les territoires fonctionnels sont larges. Ces interactions surviennent dans des régions spécifiques où il y a convergence de territoires terminaux issus de régions corticales fonctionnelles différentes. Cet article offre une mise au point sur les connexions du cortex vers le striatum et sur leur rôle d'intégration de L'information par le biais des fonctions motrices, cognitives et de récompense. L'accent est mis sur L'interface entre les domaines fonctionnels au sein du striatum.
Introduction
The basal ganglia work in concert with the cortex, thalamus, and brain stem centers to orchestrate and execute planned, motivated behaviors requiring motor, cognitive, and limbic circuits. Historically, the basal ganglia are best known for their motor functions and their association with the neuropathology in the neurodegenerative disorders affecting the control of movement, such as Parkinson disease and Huntington disease. While a role for the basal ganglia in the control of movement is clear, our concept of basal ganglia function has dramatically changed in the last 40 years, from a purely motor or sensory-motor one to one of a more complex and complicated set of functions that mediate the full range of goal-directed behaviors. Thus, in addition to their involvement in the expression of goal-directed behaviors through movement, the basal ganglia are also involved in the processes that lead to movement—that is, the elements that drive actions, including emotions, motivation, and cognition. Indeed, regions within each of the basal ganglia nuclei have been identified as serving not only a sensory-motor function, but also limbic and cognitive ones as well. Overall, ventral regions of the basal ganglia play a key role in reward and reinforcement1-3 and are important in the development of addictive behaviors and habit formation.4-6 More central basal ganglia areas are involved in cognitive functions, such as procedural learning and working memory tasks.7 Importantly, diseases of the basal ganglia are not only linked to neurological disorders, but also psychiatric illnesses, including schizophrenia, drug addiction, depression, and obsessive-compulsive disorder. Thus, the role of the basal ganglia in cognitive and emotional behaviors is now as well accepted as its role in motor control.
Although several new theories of general function have emerged from the enormous progress in understanding the anatomy, physiology, and behaviors associated with the basal ganglia,7-10 the actual role of the basal ganglia in executing goal-directed behaviors remains elusive. What is clear from the recent progress is that this set of subcortical nuclei work in tandem with the cortex (particularly the frontal cortex) via a complex cortico-basal ganglia network to develop and carry out complex behaviors. The concept of parallel cortico-subcortical channels through the basal ganglia has been prominent in how the field has viewed the organizational understanding of basal ganglia circuits.11 However, we now know that cortico-basal ganglia connections are more complicated and that interactions between functional territories are extensive.12-15 These two organizational schemes (separate functional channels and integration across function) probably work together, allowing the coordinated behaviors to be maintained and focused, but also to be modified according to appropriate external and internal stimuli. Indeed, both the ability to maintain and to focus on the execution of specific behaviors, as well as the ability to adapt appropriately to external and internal cues, are key deficits in basal ganglia diseases that affect these aspects of motor control, cognition, and motivation. The present review focuses on the primate cortico-striatal circuits from the perspective of cortical function. The focus is on the striatum as it is the main input structure of the basal ganglia. Particular emphasis is on the integrative nature of this system in carrying out goal-directed actions.
Figure 1 provides an overview of the cortico-basal ganglia circuit. The striatum is comprised of the dorsal striatum (DS) and ventral striatum (VS). The VS is a ventral extension of the DS and, based on its connections, cell morphology, and histochemistry, it includes the nucleus accumbens, the medial and ventral portions of the caudate and putamen, the striatal cells of the olfactory tubercle, and the anterior perforated substance.16,17 Afferent projections to the striatum are derived from three major sources: (i) a massive and topographic input from the cerebral cortex; (ii) input from the thalamus; and (iii) and input from the brain stem, primarily from the brain stem dopaminergic cells. Inputs from the cortex and thalamus terminate in a general topographic manner, such that the dorsolateral striatum receives cortical input from sensory-motor areas, central striatum receives input from associative cortical areas, and the ventromedial striatum receives input from limbic areas.14,18-20 The striatum, in turn, projects topographically to the pallidal complex and the substantia nigra (SN), both pars reticulata (SNr), and pars compacta(SNc).21-24
Figure 1. Schematic illustrating key structures and pathways of the basal ganglia. Dark blue arrows represent the direct pathway; light blue arrows represent the indirect pathway. Amy, amygdala; DS, dorsal striatum; GPi, globus pallidus, internal segment; GPe, globus pallidus, external segment; Hipp, hippocampus; SN, substantia nigra STN, subthalamic nucleus; Thal, thalamus; VP, ventral pallidum; VS, ventral striatum; VTA, ventral tegmental area.
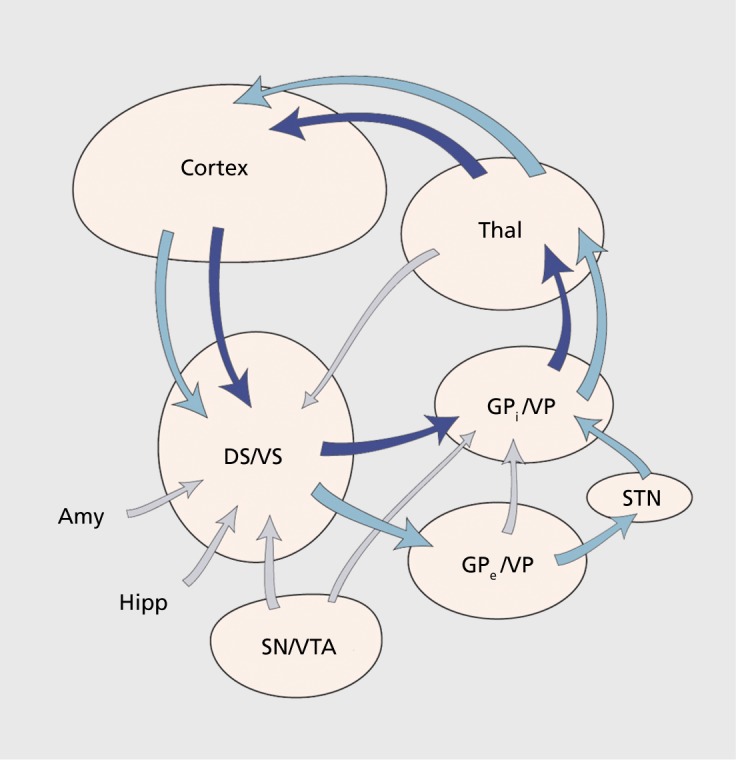
The pallidal complex includes the external (GPe) and internal segments (GPi) of the globus pallidus and the ventral pallidum (VP). The GPi and GPe receive inputs from the DS, both the caudate nucleus and putamen. The VP, generally located ventral to the anterior commissure, receives its input from the VS.18,25,26 The outputs from the GPi and SNr are to the thalamus, which then projects back to the cortex, completing the “direct” cortico-basal ganglia circuit. The GPe and parts of the VP project to the subthalamic nucleus (STN). The STN, in turn, provides inputs to the GPi. This pathway is considered the indirect pathway. Importantly, the STN also projects back to the GPe. In addition to connections with the basal ganglia, the STN also receives a direct projection from all parts of the frontal cortex.27 This is referred to as the hyperdirect pathway. The dopaminergic system includes the SNc, the ventral tegmental area (VTA), and the retrorubral group. Dopamine (DA) neurons from the SNc and the VTA project to the DS and VS, respectively.
Organizational features of the striatum
The DS is comprised of the caudate nucleus and putamen, which are separated in primates by the internal capsule. The rostroventral border of the caudate nucleus and putamen merge with the dorsal nucleus accumbens, making the region ventral to the internal capsule continuous. Caudally the putamen merges to varying degrees with the tail of the caudate. The nucleus accumbens and the rostroventral-most aspects of the caudate and putamen form the VS. The VS includes the ventral extension of the putamen, which reaches the surface of the brain in the region of the anterior perforated space. This ventral region, which had been included in the substantia innominata, is now considered part of the VS on the basis of its histochemistry and connections. In addition, the olfactory tubercle and the rostrolateral portion of the anterior perforated space adjacent to the lateral olfactory tract in primates are also included in the VS.
The striatum (both dorsal and ventral) contains several cell types that are generally divided into two general groups: projection neurons and interneurons. Projection neurons are the most common cell type and are the medium spiny neurons (MSN) or the principal neurons: in nonprimate species they account for over 90% of the cells, and probably far less in the primates.28,29 These cells project to both segments of the globus pallidus, the VP, and the SN21,22,30-32 Their axon collaterals also terminate within the striatum, onto other MSNs and interneurons forming symmetrical GABAergic synaptic contacts.33-35 On the basis of this data and electrophysiological data, the projection cells of the striatum are considered inhibitory.36 There are two general types of MSNs: one that co-contains substance P and GABA; and one that co-contains enkephalin and GABA. Substance P-containing MSNs project primarily to the GPi and SN, while the enkephalin-containing cells project primarily to the GPe. 37-38
The main inputs to the MSNs are derived from the cortex, thalamus, and brain stem. Cortical fibers primarily project to the dendritic spines, form asymmetric terminals, and are glutamatergic.39 Thalamic projections terminate primarily onto dendrites, and are excitatory and also glutamatergic.40,41 Cortical and thalamic inputs are distinguished by their transporter molecules and by their regulation of striatal circuitry.42-44 Extracellular physiological recordings in awake, behaving monkeys show that the MSNs are phasically active neurons (PANs). These cells have a very low spontaneous discharge rate (0.5-1 spike/s), but a relatively high firing rate (10-40 spikes/s) associated with behavioral tasks, including movement, preparation for movement, and the performance of learned tasks.45-47 Brain stem projections from the dopaminergic cells of the SNc and the VTA terminate onto the spines as well as the dendritic shafts of the MSNs.48-50 However, recent studies of the relationship between dopaminergic terminals and cortical and thalamic inputs show that all cellular structures in the striatum are within approximately 1 um of a tyrosine-hydroxlyase-positive synapse, suggesting a nonselective dopaminergic axon lattice in the striatum.44 In addition to projections from the cortex, thalamus, and brain stem, MSNs also receive input from interneurons and from local collaterals of other MSNs.35,51,52
While overall the striatum is a relatively homogenous structure, striatal neurons do form clusters, referred to as cell islands.53 Moreover, histochemical and tracing studies show a patchy organization of transmitter-related molecules and afferent terminals.54,55 For example, acetylcholinesterase (AChE)-poor striatal regions (termed striosomes or patches) are surrounded by a densely stained “matrix.” The striosomes, embedded in the AChE-rich matrix, correspond to opiate-receptor patches as well as to several other transmitter-related compounds.55 While several hypotheses have been put forth concerning the significance of the compartmental organization in the striatum, it continues to present a challenge in understanding the functional significance of this complex arrangement.
Unique features of the ventral striatum
Several features of the VS stand out as particularly unique. Firstly, embedded within parts of the VS are the islands of Calleja or cell islands, identifiable in all mammalian species by their dense core of granule cells,56 the largest of which (Calleja magna) forms the medial border of the nucleus accumbens. Additional, albeit smaller, islands (referred to as interface islands, 57) are located in the basal forebrain in primates, including humans. The neurochemical features of these islands suggest that they may function as a type of “endocrine striatopallidal system”. 58 Of particular interest is the fact that the cells in the islands are thought to contain quiescent, immature cells that remain in the adult brain.59 In support of this idea, studies show that bcl2 protein—which, though abundant in fetal neurons, persists in limited regions of the adult primate brain—marks these islands.60,61 Secondly, a subterritory of the VS, the shell region, divides the VS into two parts, a medial/ventral shell region and a central core region.62 Experiments aimed at delineating the functional significance of these two regions have been instrumental in understanding the circuitry underlying goal-directed behaviors, behavioral sensitization, and changes in affective states. Studies in rodents have been particularly important in demonstrating the organization of the shell and core and their relationship to distinct ventral striatal afferent and efferent projections.63,64 Whereas several transmitter and receptor distribution patterns distinguish the shell/core subterritories, lack of calbindin-positive staining is the most consistent marker for the shell across species.65 Thirdly, while the basic cortical basal ganglia loop is similar in all basal ganglia circuits, the VS alone receives an additional subcortical input from the amygdala and from the hippocampus, for which there is no comparable input to the other basal ganglia territories.61,66 Finally, the DA transporter is relatively low throughout the VS, including the core. This pattern is consistent with the fact that the dorsal-tier DA neurons that project to the VS express relatively low levels of messenger RNA for the DA transporter, compared with the ventral tier.67
Corticostriatal connections
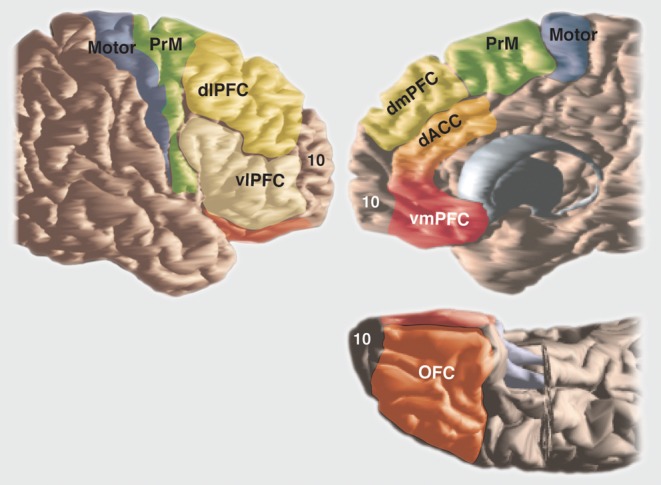
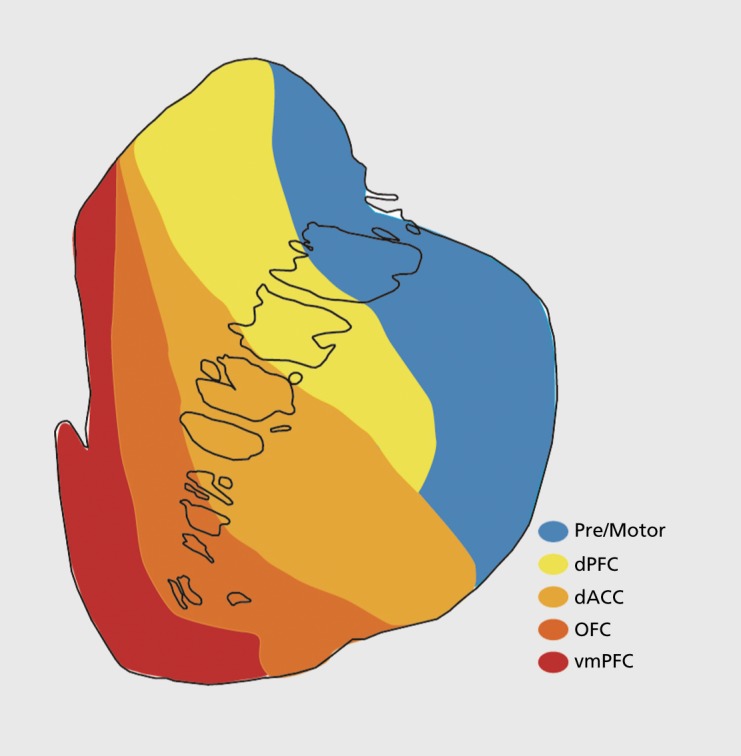
The organization of basal ganglia circuitry is best appreciated within the context of frontal cortical organization. The basal ganglia and frontal cortex operate together to learn optimal behavior policy and to execute goal-directed behaviors. This requires not only the execution of motor plans, but also the behaviors that lead to this execution, including emotions and motivation that drive behaviors, cognition that organizes and plans the general strategy, motor planning, and finally, the execution of that plan. The components of the frontal cortex that mediate behaviors, including the motivation and emotional drive, coupled with the planning and cognitive components to plan the action, and finally, the movement itself, are reflected in the organization, physiology, and connections between areas of frontal cortex and in their projections to the striatum. The frontal cortex can be divided into several general functional regions (Figure 2): the orbital and medial prefrontal cortex (PFC), involved in emotions and motivation; the dorsolateral PFC, involved in higher cognitive processes or “executive functions”; the premotor areas, involved in different aspects of motor planning; and motor cortex, involved in the execution of those plans. Although the striatum also receives input from other cortical areas, we focus on the frontal cortex because it receives the main basal ganglia output via the thalamus. Inputs from other cortical areas are also functionally organized, such that they overlap with frontal projections in the striatum, which are both functionally related and closely connected with specific frontal regions.19,68,69
Figure 2. Schematic illustrating key areas of frontal cortex. dACC, dorsal anterior cingulate cortex; dIPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; OFC, orbitofrontal cortex; PrM, premotor cortex; vIPFC, ventrolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
Corticostriatal terminals, which are primarily from the layer-five and deep-layer-three pyramidal neurons are distributed in a patchy manner.70,71 This patchy pattern is consistent with patterns found with other afferent projections as well as with transmitter-related compounds. Whereas the VS is associated with emotion, the caudate nucleus with cognition, and the putamen with sensorimotor function, it is important to remember that there is no clear boundary between the VS and DS, and the separation between the caudate nucleus and the putamen is merely a structural one, based solely on the IC separation, not a functional one. The distribution of terminals from the cortex is generally organized topographically (Figure 3). Thus, prefrontal areas project primarily to the VS and rostral caudate nucleus and putamen. Sensorimotor areas project more caudally, primarily to the putamen. These distributions form the basis for the idea of parallel and segregated cortico-basal ganglia circuits. However, when terminals from various cortical areas are examined closely, it clearly demonstrates extensive convergence of inputs from different functional regions. This actually creates a complex interface between inputs from functionally distinct regions. Indeed, each cortical region, while projecting topographically to the striatum, does not maintain the narrow funneling that would be required to “fit” a large cortex into a far smaller striatal volume. For example, the volume occupied by the collective dense terminal fields from the ventromedial PFC (vmPFC), dorsal anterior cingulate cortex (dACC), and orbitofrontal cortex (OFC) is approximately 22% of the striatum, a larger cortical input than would be predicted by the relative cortical volume of these areas.14
Figure 3. Schematic illustrating the general topography of frontal inputs to the rostral striatum. dACC, dorsal anterior cingulate cortex; dPFC, dorsal prefrontal cortex; OFC, orbitofrontal ccrtex; vmPFC, ventromedial prefrontal cortex.
Motor and premotor corticostriatal projections
The projections from motor and premotor cortices were some of the first to be identified.72 Projections from M1 terminate almost entirely in the putamen, in the dorsolateral and central region. There are some terminals, albeit few, rostral to the anterior commissure; however, the main projection lies caudal to the anterior commissure. The caudal premotor area projects to a striatal region that is just adjacent to M1 projections. These terminals extend only slightly into the caudate nucleus. The rostral premotor areas terminate in both the caudate and putamen, bridging the two with a continuous projection. These terminals extend more rostrally than those from the motor cortex, although they do not extend into the rostral pole of the striatum. Both motor and premotor areas extend through much of the putamen caudally. This dorsolateral sector of the striatum also receives overlapping projections from parietal areas associated with somatosensory function. Furthermore, these parietal projections follow the same somatotopic organization.73 Thus, the dorsolateral striatum is linked to motor function.71 Physiological studies support this idea, demonstrating somatotopic maps and neuronal responses to specific movement.74,75 Moreover, imaging studies in humans show that activity in the lateral putamen is associated with repetitive and well-learned movements that require little cognitive effort. In contrast, activity recorded during the learning of sequential movements in both monkey physiological experiments, as well as in human imaging studies, demonstrate activity in the rostral motor regions (pre-supplementary motor area [SMA]) and more anterior striatal areas including the caudate nucleus.76,77 The frontal eye fields send projections to the striatum that terminate in the central lateral part of the head and body of the caudate nucleus. This area of the cortex also contains a rostral region, referred to as the supplementary eye fields, which has a relationship to the frontal eye fields, with respect to learned acquisition of oculomotor behaviors, similar to that which the rostral and caudal motor regions have to learned behavioral sequences of hand movements. 78 Finally, the motor cingulate cortex also sends an overlapping projection to the sensorimotor region of the striatum.71,79 Taken together, the motor and premotor areas (and the frontal eye fields) mediate different aspects of motor behavior, including planning, learning, and execution, which are in turn reflected both anatomically and physiologically in the central and lateral caudate nucleus and in the central, dorsal, and lateral putamen, respectively. The pattern of neuronal discharge in these striatal regions that accompanies these behaviors has been proposed to underlie procedural learning.7,80
Prefrontal and anterior cingulate-striatal projections
Together, projections from PFC and ACC cortical areas terminate primarily in the rostral striatum, including the VS, caudate, and putamen. Whereas terminals are mostly located rostral to the anterior commissure, a small portion continues caudally for some distance. 14,19 The PFC and ACC are comprised of several regions that are associated with reward, motivation, and cognition. According to specific roles for mediating different aspects of these behaviors, these regions can be functionally grouped into: (i) the OFC; (ii) the dACC; (iii) the vmPFC, which includes medial OFC, the subgenual ACC, and area 10; and (iv) the dorsal PFC (dPFC).
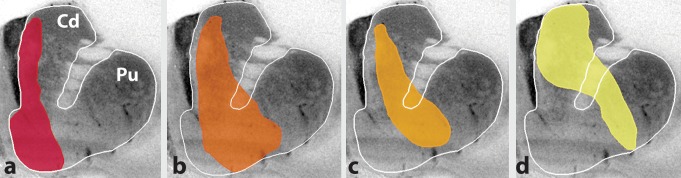
Overall, the ACC and OFC project primarily to the rostral striatum and include terminals in the medial caudate nucleus, the medial and ventral rostral putamen, and the nucleus accumbens (both the shell and the core). This projection extends caudally and occupies the ventromedial portion of the caudate nucleus and the most ventral and medial part of the putamen.79,81-83 Indeed, the caudal, ventral putamen has been suggested to be the caudal extension of the VS.84 The vmPFC is closely associated with visceral and emotional functions and has strong connections to the hypothalamus, amygdala, and hippocampus. These cortical cells track values in the context of internally generated states, such as satiety85 Moreover, this area plays a role, not only in valuation of stimuli, but also in selecting between these values. However, perhaps the most remarkable feature of the vmPFC valuation signal is its flexibility. While several other brain regions rely on experience to estimate values, vmPFC can encode values that must be computed quickly, often just prior to or during an action.86 The shell receives the densest innervation from the vmPFC and from the agranular insular cortex, areas involved in monitoring the internal milieu. vmPFC projections also extend dorsally into the medial wall of the caudate nucleus.81 Nonetheless, the vmPFC projection field to the striatum is the most limited of the frontal cortex and is concentrated within the shell and a narrow column along the medial border of the caudate nucleus adjacent to the ventricle (Figure 4a).
Figure 4. Combined chartings of labeled fibers following injections into different prefrontal regions, (a) vmPFC, ventromedial prefrontal cortex; orbitofrontal cortex; (c) dACC, dorsal anterior cingulate cortex; (d) dIPFC, dorsolateral prefrontal cortex. Cd, caudate; Pu, putamen.
A key function of the OFC is to link sensory representations of stimuli to outcomes, which is consistent with its connections to both sensory and reward-related regions.87,88 The OFC's unique access to both primary and highly processed sensory information, coupled with connections to the amygdala and ACC, explain many of the functional properties of the region. The two cardinal tests of OFC function are reward devaluation paradigms and stimulus-outcome reversal learning.89,90
In contrast to projections from the vmPFC to the striatum, the OFC projects to the central and lateral parts of the VS.81 Here, these projections extend more centrally and overlap with inputs from the lateral aspects of the dPFC. OFC projections also continue dorsally along the medial regions of the caudate nucleus and ventromedial part of the putamen (Figure 4b). However, they are located lateral to those from the vmPFC Within the OFC projections, there also is some topography such that medial OFC regions terminate medial to those from more lateral OFC areas. Nonetheless, the overlap between the OFC terminals is large.
The dACC is tightly linked with many PFC areas, including most areas of the dPFC, regions associated with cognitive control, and more caudally, with motor control areas Thus, the dACC sits at the connectional intersection of the brain's reward and action networks. Unlike OFC, dACC appears not to be involved in learning reward reversals based on sensory cues. However, it is associated with reward that is tied to two different actions (such as “turn” or “push”).91,92 Imaging studies show ACC activation in a wide variety of tasks, many of which can be explained in the context of selecting between different actions.93 Projections from the dACC to the striatum are extensive and stretch from its rostral pole to the anterior commissure. Terminals are located in both the central caudate nucleus and putamen. Moreover, there are some patches of dACC terminals also found in the VS. However, they primarily avoid the shell region. Overall, these fibers terminate somewhat lateral to those from the OFC. Thus, the OFC terminal fields are positioned between the vmPFC and dACC (Figure 4c). However, projections from the dACC extend dorsally and somewhat more centrally into the caudate nucleus and overlap with inputs from the medial parts of the dPFC (particularly rostral area 9). Importantly, that part of the cingulate cortex that is closely connected to motor areas projects to the dorsolateral striatum. These terminals do not extend into the VS.94
The dPFC is engaged when working memory is required for monitoring incentive-based behavioral responses. It also encodes reward amount and becomes active when anticipated rewards signal future outcomes.88,95,96 The dPFC projects primarily to the rostral central region of the caudate nucleus.14 However, the rostrocaudal extent of this projection is quite large and is one of the largest to the striatum and, thus, interfaces with several other cortical areas.19,97 This innervation extends from the rostral pole, through the rostral, central putamen, and much of the length of the caudate nucleus.14,19 These projections are organized somewhat topographically such that, for example, input from the rostral pole of the frontal cortex terminates most densely in the rostral part of the striatum, including both the caudate and putamen. There are few terminals in the central and caudal putamen. In contrast to the vmPFC, OFC, and dACC, terminals from the dPFC are located primarily in the head of the caudate and the medial, ventral, and central parts of the rostral putamen (figure 4d). The dorsolateral portion of the putamen contains fewer terminating axons. Consistent with input from this cortical area, cells in the head of the caudate nucleus demonstrate spike-firing activity during the delayed portion of the task. This activity resembles that observed in the dPFC.98 Furthermore, imaging studies support the idea that the head of the caudate is instrumental in delayed tasks, particularly in specific working memory tasks.99 Finally, lesions of the caudate nucleus in both human and nonhuman primates produce deficits in working memory, as measured by delayed response tasks.100 Taken together, the caudate nucleus—in particular, the head of the caudate—is involved in working memory and strategic planning processes, working together with the dPFC in mediating this function.
Fewer studies have focused on projections from other cortical areas to the striatum. As with the frontal lobe, temporal lobe projections terminate throughout wide areas of the striatum.19,68,101,102 The superior temporal gyrus terminates primarily in the central half of the caudate nucleus and overlaps and is interdigitated with inputs from dPFC. Inferior temporal areas terminate more ventrally including the caudal and ventral putamen. These inputs overlap to some extent with those from the medial and orbital prefrontal areas. Visual areas in the temporal lobe terminate primarily in the caudal part of the striatum. Projections from the parietal lobe terminate in striatal areas that complement those that are interconnected with frontal lobe projections.19
The amygdala and hippocampal projections to the ventral striatum
The amygdala is a prominent limbic structure that plays a key role in emotional coding of environmental stimuli and provides contextual information used for adjusting motivational level that is based on immediate goals. The basolateral nuclear group processes higher-order sensory inputs in all modalities except olfactory, and responds to highly palatable foods, and “multimodal” cues. This is the primary source of amygdaloid input to the VS outside the shell.61,66 The lateral nucleus has a relatively minor input to the VS, while the basal and accessory basal nuclei prominently innervate both the shell and VS striatum outside the shell. The shell is set apart from the rest of the VS by a specific set of connections from the central nucleus, periamygdaloid cortex, and the medial nucleus of the amygdala. The amygdala has few inputs to the DS in primates. In contrast to the amygdala, the hippocampal formation (specifically the subiculum) projects to a more limited region of the VS and is primarily confined to the shell region. Some afferent fibers are also derived from the parasubiculum and part of CA1.103
Integration between prefrontal corticostriatal projections
While the topographic organization of corticostriatal projections is well documented, there is increasing evidence for regions of interface between terminals from different cortical areas, suggesting functional integration. For example, early studies showed that corticostriatal terminals from sensory and motor cortex converge within the striatum.69 Here, axons from each area synapse onto single fast-spiking GABAergic interneurons. Interestingly, these interneurons are more responsive to cortical input than the MSNs.104-106 This suggests a potentially critical role for interneurons in integrating information from different cortical areas before passing that information on to the medium spiny projection cells.
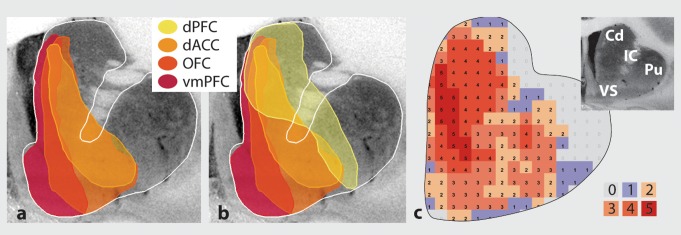
Despite the general PFC topographic input to the striatum described above, dense terminal fields from the vmPFC, OFC, and dACC show a complex interweaving and convergence, providing an anatomical substrate for modulation between circuits14 (Figure 5). For example, dense projections from the dACC and OFC regions converge extensively at rostral levels. By contrast, there is greater separation of projections between terminals from the vmPFC and the dACC/OFC, particularly at caudal levels. These regions of convergence between the dense terminal fields of the vmPFC, OFC, and dACC provide an anatomical substrate for integration between different reward processing circuits within specific striatal areas and may represent critical “hubs” for integrating reward value, predictability, and salience (Figure 5a).
Figure 5. Schematics demonstrating convergence of cortical projections from different reward-related regions and dorsal prefrontal areas, (a) Convergence between dense projections from vmPFC, OFC, and dACC. (b) Convergence between dense projections from vmPFC, OFC, dACC, and dPFC. (c) Probabilistic map of areas of convergence in the striatum. Color in each section indicates voxels that receive projections from 0 to 5 prefrontal cortical regions (ie, vmPFC, OFC, dACC, dPFC, vIPFC). Cd, caudate; dACC, dorsal anterior cingulate cortex; dPFC, dorsal prefrontal cortex; IC, internal capsule; OFC, orbitofrontal cortex; Pu, putamen; vmPFC, ventromedial prefrontal cortex; VS, ventral striatum. Modified from ref 15: Averbeck BB, Lehman J, Jacobson M, Haber SN. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J. Neurosci. 2014:34(29):3497-9505. Copyright © Society for Neuroscience 2014.
In addition to convergence between vmPFC, dACC, and OFC dense terminals, projections from dACC and OFC also converge with inputs from the dPFC, demonstrating that functionally diverse PFC projections also converge in the striatum (Figure 5b). At rostral levels, dPFC terminals converge with those from both the dACC and OFC, although each cortical projection also occupies its own territory. Here, projections from all PFC areas occupy a central region, the different cortical projections extending into nonoverlapping zones. Convergence is less prominent caudally, with almost complete separation of the dense terminals from the dPFC and dACC/OFC just rostral to the anterior commissure. Importantly, convergence does not take place only at the boundaries or edges of different functional domains. Rather, there are dense clusters of invading terminals from the dPFC embedded deep within the dense projection field of the OFC14,94 Since MSNs have a dendritic field spanning 0.5 mm,107 the area of convergence is likely to be larger than that estimated solely on the basis of the relationship between the afferent projections.
Using a computational model to characterize the consistency of cortical projection zones into the striatum, investigators can predict striatal locations that receive specific cortical inputs. These data demonstrate that there is an exponential decay in overlap as a function of distance. 15 Thus, convergence of corticostriatal terminals from cortical areas that are separated by 5 mm is 50% in nonhuman primates. The overlap decreased to below 20% from regions separated by 30 mm. However, this distance between cortical regions can span several functional domains. For example, there is a region within the rostral, anterior striatum that receives projections from across the prefrontal cortical regions, including the vmPFC, OFC, dACC, and dorsal and lateral PFC (Figure 5c).
A similar pattern of both topographic and integrative connectivity of corticostriatal projections has been demonstrated in the human brain by diffusion magnetic resonance imaging (MRI). These data show a similar overall organization of the different cortical regions and the striatum, providing a strong correlation between monkey anatomical tracing studies and human diffusion MRI studies. 108 Taken together, a coordinated activation of dPFC, dACC, and/or OFC terminals in these subregions could produce a unique combinatorial activation at the specific sites for channeling reward-based incentive drive in selecting between different valued options.
There has been growing interest in the idea of broad associative cortical networks, which feature nodes (or connection hubs) that integrate and distribute information across multiple systems.109,110 These studies indicate that there are specific regions that receive inputs from multiple cortical areas that crosslink between functional systems. The above data suggest that these hubs exist in the striatum as well as the cortex. In particular, this rostral striatal region may serve as a hub for vmPFC, OFC, and dACC to connect with dorsal and lateral PFC regions that integrate motivational, reward, and cognitive control information. It is possible that this convergence of cortical areas on specific locations in the striatum may thus facilitate value computations across diverse domains into a common currency. Thus, a coordinated activation of dPFC, dACC, and/or OFC terminals in specific striatal regions would produce a unique combinatorial activation at the specific sites, suggesting specific subregions for reward-based incentive drive to impact on long-term strategic planning and habit formation. In contrast to this, some striatal regions, particularly posterior and lateral portions, receive inputs from only a few prefrontal regions, and therefore they may serve more specialized computational roles.
Relationship of the substantia nigra/ventral tegmental area connections to the corticostriatal organization
The midbrain DA neurons play a unique role in basal ganglia and cortical circuits modulating a broad range of behaviors from learning and working memory to motor control. These neurons play a key role in the acquisition of newly acquired behaviors. While the role of DA in reward has been well established,111,112 its primary function is to direct attention to important stimuli likely to bring about a desired outcome.113 Behavioral and pharmacological studies of DA pathways have lead to the association of the mesolimbic pathway and nigrostriatal pathway with reward and motor activity, respectively; more recently, both of these cell groups have been associated with encoding of reward or saliency prediction errors. In this respect, the DA system has been an intense focus of productive research in the development of reward-based learning, drug addiction, and plasticity.114,115
A dorsal group of cells (retrorubral) are loosely arranged and extend dorsolaterally, circumventing the superior cerebellar peduncle and the red nucleus. These cells merge with the immediately adjacent VTA to form a continuous mediodorsal band of cells. The dendrites of this dorsal group stretch in a mediolateral direction, and do not extend into the ventral parts of the pars compacta or into the pars reticulata. Calbindin (CaBP), a calcium binding protein, is an important phenotypic marker for both the VTA and the dorsal SNc. This group is referred to as the dorsal tier. In contrast, a ventral cell group (the ventral tier) is calbindin-negative, with dendrites oriented ventrally and extending deep into the SNr.67,116,117 The main connections of the SNc are to the striatum and cortex. In addition, there are connections with the pallidum STN, thalamus, hippocampus, amygdala, and brain stem structures. The DA synapses are in a position on the dendrites of the MSNs to modulate incoming cortical information or exert a generalized volume effect in the striatum. Therefore, with relatively few synapses, the impact of the dopaminergic innervation on corticostriatal modulation is large.
While projections from the different DA cell groups are not as topographically organized as inputs from the cortex, there is an important organization to this input that underlies a mechanism for modulating the different corticostriatal regions, especially across functional domains. This complex system includes both the DA-striatal pathway as well as the striatal input to the DA neurons. There is a general inverse dorsal-ventral topographic organization to the midbrain projection to the striatum. The dorsal DA cells project to the ventral parts of the striatum, while the ventrally placed cells project to the dorsal parts of the striatum.13,118 Thus, the dorsal tier projects to the VS, and the ventral tier projects centrally and throughout different regions of the striatum, with the most ventral part of this cell group projecting primarily to the dorsolateral (sensorimotor) striatum. The shell region of the VS receives the most limited input, primarily derived from the VTA. The rest of the VS receives input from the entire dorsal tier and from the most medial and dorsal part of the ventral tier. In contrast to the VS, the central striatal area (the region innervated by the dPFC) receives input from a wide region of the ventral tier. In addition to an inverse topography, there is also a differential ratio of DA projections to the different striatal areas. The input to the VS is derived from the most limited midbrain region, whereas the input to the dorsolateral striatum is derived from the largest midbrain area.13
Projections from the striatum to the midbrain are also arranged in an inverse dorsal-ventral topography. Thus, the dorsal aspects of the striatum terminate in the ventral regions of the midbrain, whereas the ventral areas terminate dorsally.22,119 Moreover, inputs from the ventromedial striatum, including the shell, terminate throughout an extensive dorsal region, including the VTA and the medial SNc, along with the medial SNr. Thus, the VS projects widely throughout the rostrocaudal extent of the SN, including a large mediolateral area of dopaminergic cells. Taken together, the VS innervates a large area of the midbrain, but receives a relatively limited afferent midbrain input. At central and caudal levels, this projection field extends laterally and includes much of the ventral tier cells. The central striatum projection terminates more ventrally, primarily in the ventral tier (and associated SNr). The dorsolateral striatum projections to the midbrain terminate in the ventrolateral midbrain in the pars reticulata, which includes some of the dopaminergic cell columns that extend into this region. Thus, in contrast to the VS, the dorsolateral striatum projects to a limited midbrain region, but receives a relatively extensive midbrain input.13
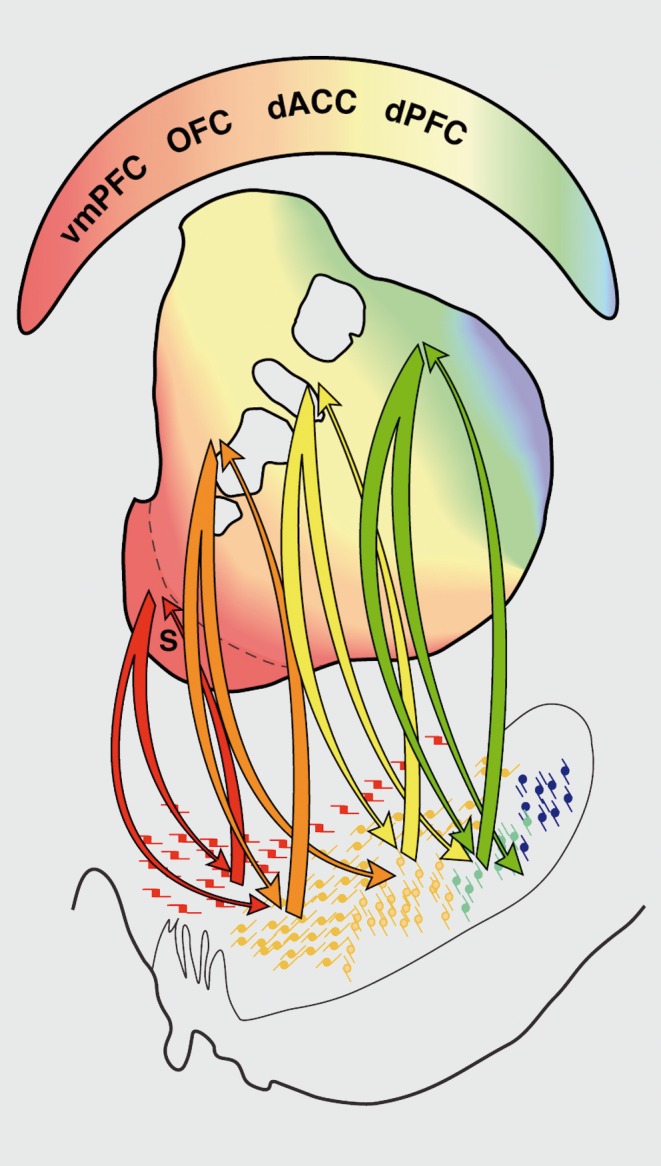
Thus, although the striato-nigro-striatal projection system demonstrates a loose topographic and functional organization—the VTA and medial SN are associated with the limbic system, and the lateral and ventral midbrain are related to the associative and motor striatal regions, respectively—there is a complexity of the inputs and outputs that influences those functions. Specifically, the data show that the VS influences a wide range of DA neurons, but is itself influenced by a relatively limited group of DA cells. On the other hand, the dorsolateral striatum influences a limited midbrain region, but is affected by a relatively large midbrain region. Thus, the size and position of the afferent and efferent connections for each system allow information from the limbic system to reach the motor system through a series of connections.13 The dorsal tier projects to the VS. However, the VS efferent projection to the midbrain extends beyond the tight ventral striatal/dorsal tier/ventral striatal circuit, terminating lateral and ventral to the dorsal tier. This area of terminal projection does not project back to the VS. Rather, cells in this region project more dorsally, into the striatal area that receives input from the dPFC. Through this connection, the same cortical information that influences the dorsal tier through the VS also modulates parts of the ventral tier region that projects to the central striatum. This central striatal region is reciprocally connected to the central part of the ventral tier. But it also projects to its more ventral parts. Thus, projections from the dPFC, via the striatum, are in a position to influence cells that project to motor control areas of the striatum. The dorsolateral striatum is reciprocally connected to the ventral tier. The confined distribution of efferent dorsolateral striatal fibers limits the influence of the motor striatum to a relatively small region involving the primarily dopaminergic cells embedded within the SNr. Taken together, the interface between different striatal regions via the midbrain DA cells is organized in an ascending spiral interconnecting different functional regions of the striatum (Figure 6). Through this spiral of inputs and outputs between the striatum and midbrain DA neurons, information can flow from limbic to cognitive to motor circuits, providing a mechanism by which motivation and cognition can influence motor decision-making processes and appropriate responses to environmental cues.
Figure 6. Striato-nigro-striataI connections. Through the organization of striato-nigro-striatal projections, the ventral striatum can influence the dorsal striatum. Midbrain projections from the shell target both the ventral tegmental area (VTA) and ventromedial substantia nigra, pars compacta (SNc). Projections from the VTA to the shell form a “closed” reciprocal loop, but also project more laterally to impact en dopamine cells projecting to the rest of the ventral striatum, forming the first part of a feedforward loop (or spiral). The spiral continues through the striato-nigro-striatal projections, through which the ventral striatum impacts on cognitive and motor striatal areas via the midbrain dopamine cells. dACC, dorsal anterior cingulate cortex; dPFC, dorsal prefrontal cortex; OFC, orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex. Red, vmPFC pathways; dark orange, OFC pathways; light orange, dACC pathways; yellow, dPFC pathways; green, output to motor control areas. Modified from ref 13: Haber SN, Fudge JL, McFarland NR. Striato-nigro-striatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369-2382. Copyright © Society for Neuroscience 2000.
Functional considerations
Most models of basal ganglia function rely on the concept of parallel processing. This is based on the idea that each cortical area mediates a parallel and segregated circuit through each of the basal ganglia structures. Therefore, these models do not address how information flows between circuits, thereby developing new learned behaviors (or actions) from a combination of inputs from emotional, cognitive, and motor cortical areas. Although the frontal cortex is indeed divided according to specific functions, expressed behaviors are the result of a combination of complex information processing that involves all the frontal cortex. The development and execution of appropriate responses to environmental stimuli require continual updating, and learning and adjustments of the response. Thus, response learning does not represent a separate motor, cognitive, and motivational tract; rather, it is the combination of these areas working together that forms a smoothly executed, goal-directed behavior. Although the anatomical pathways appear to be generally topographic from cortex through basal ganglia circuits and there are some physiological correlates to the functional domains of the striatum, there are major points that argue against a strict parallel processing system. A number of studies show that these pathways are not as segregated as once thought.13,120-123 The dendrites and axons of the striatal interneurons often cross functional boundaries and have been suggested to be one mechanism that integrates information across regions. The fact that adjacent areas overlap in function is not surprising. Many cortical areas are tightly linked to the immediately adjacent cortex. Nonetheless, the interface between functional circuits increases with the complexity of interconnections within the intrinsic basal ganglia circuitry and with the compression of pathways to successively smaller structures.14,124
However, interaction at the interface of functions may not be sufficient to link across circuits to accommodate adaptive responses during the learning process or in response to new environmental cues. As such, mechanisms by which information “flows” through functional circuits are less well understood, but are fundamental for understanding how the execution of goal-directed actions evolve from reward and cognitive inputs. The striato-nigro-striatal network is an example of one such network. Within each of these sets of connected structures, there are both reciprocal connections linking up regions associated with similar functions However, in addition, there are nonreciprocal connections linking up regions that are associated with different cortical basal ganglia circuits. Each component of information (from limbic to motor outcome) sends both feedback and feedforward signals, allowing the transfer of information.
Conclusion
Goal-directed behaviors require processing a complex chain of events, beginning with motivation and proceeding through cognitive processing that shapes final motor outcomes. This sequence is reflected in the feedforward organization of both the striate-nigral connections and the thalamo-cortical connections. Information is channeled from limbic, to cognitive, to motor circuits. Action decision-making processes are thus influenced by motivation and cognitive inputs, allowing the individual to respond appropriately to environmental cues.
Acknowledgments
This work was supported by NIH grant MH045573.
Selected abbreviations and acronyms
- ACC
anterior cingulate cortex
- DA
dopamine
- dACC
dorsal anterior cingulate cortex
- dPFC
dorsal prefrontal cortex
- DS
dorsal striatum
- GPe
external segment of the globus pallidus
- GPi
internal segment of the globus pallidus
- MSN
medium spiny neurons
- OFC
orbitofrontal cortex
- PFC
prefrontal cortex
- SN
substantia nigra
- SNc
substantia nigra, pars compacta
- SNr
substantia nigra, pars reticulate
- STN
subthalamic nucleus
- vmPFC
ventromedial prefrontal corte
- VP
ventral pallidum
- VS
ventral striatum
- VTA
ventral tegmental area
REFERENCES
- 1.Wise RA. Addictive drugs and brain stimulation reward. Ann Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 2.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 3.Robbins TW., Everitt BJ. Motivation and reward. In: Squire LR, Roberts JL, Spitzer NC, Zigmond MJ, McConnell SK, Bloom FE, eds. Fundamental Neuroscience. 2nd ed. San Diego, CA: Academic Press; 1999:1245–1259. [Google Scholar]
- 4.Koob GF. Drug reward and addiction. In: Fundamental Neuroscience. New York, NY: Academic Press; 1999:1261–1279. [Google Scholar]
- 5.Kalivas PW., Churchill L., Klitenick MA. The circuitry mediating the translation of motivational stimuli into adaptive motor responses. In: Kalivas PW, Barnes CD, eds. Limbic Motor Circuits and Neuropsychiatry. Boca Raton, FL: CRC Press, Inc; 1993:237–275. [Google Scholar]
- 6.Nestler EJ., Hope BT., Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11(6):995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 7.Jog MS., Kubota Y., Connolly CI., Hillegaart V., Graybiel AM. Building neural representations of habits. Science. 1999;286(26):1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 8.Bergman H., Feingold A., Nini A., et al Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21(1):32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MX., Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199(1):141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander GE., Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 12.Percheron G., Filion M. Parallel processing in the basal ganglia: up to a point. Trends Neurosci. 1991;14(2):55–59. doi: 10.1016/0166-2236(91)90020-u. [DOI] [PubMed] [Google Scholar]
- 13.Haber SN., Fudge JL., McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorso lateral striatum. J Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber SN., Kim KS., Mailly P., Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Averbeck BB., Lehman J., Jacobson M., Haber SN. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci. 2014;34(29):9497–9505. doi: 10.1523/JNEUROSCI.5806-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haber SN., McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann NY Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 17.Heimer L., Alheid GF., Zahm DS. Basal forebrain organization: an anatomical framework for motor aspects of drive and motivation. In: Kalivas PW, Barnes CD, eds. Limbic Motor Circuits and Neuropsychiatry. Boca Raton, FL: CRC Press, Inc; 1993:1–43. [Google Scholar]
- 18.Parent A. Comparative Neurobiology of the Basal Ganglia. New York, NY: John Wiley and Sons; 1986 [Google Scholar]
- 19.Selemon LD., Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland NR., Haber SN. The thalamostriataI projection and its relation to corticostriatal and cortiocthalamic inputs. Soc Neurosci Abst. 1996;22:412. [Google Scholar]
- 21.Haber SN., Lynd E., Klein C., Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293(2):282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- 22.Lynd-Balta E., Haber SN. Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol. 1994;345(4):562–578. doi: 10.1002/cne.903450407. [DOI] [PubMed] [Google Scholar]
- 23.Smith Y., Shink E., Sidibe M. Neuronal circuitry and synaptic connectivity of the basal ganglia. Neurosurg Clin N Am. 1998;9(2):203–222. [PubMed] [Google Scholar]
- 24.Parent A., Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20(1):91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 25.Haber SN., Lynd-Balta E., Mitchell SJ. The organization of the descending ventral pallidal projections in the monkey. J Comp Neurol. 1993;329(1):111–128. doi: 10.1002/cne.903290108. [DOI] [PubMed] [Google Scholar]
- 26.Lyons D., Friedman DP., Nader MA., Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16(3):1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes Wl., Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neurosci. 2013;33(11):4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiFiglia M., Carey J. Large neurons in the primate neostriatum examined with the combined Golgi-electron microscopic method. J Comp Neurol. 1986;244(1):36–52. doi: 10.1002/cne.902440104. [DOI] [PubMed] [Google Scholar]
- 29.Graveland GA., DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327(1-2):307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- 30.Preston RJ., Bishop GA., Kitai ST. Medium spiny neuron projection from the rat striatum: an intracellular horseradish peroxidase study. Brain Res. 1980;183(2):253–263. doi: 10.1016/0006-8993(80)90462-x. [DOI] [PubMed] [Google Scholar]
- 31.Szabo J. The course and distribution of efferents from the tail of the caudate nucleus in the monkey. Exp Neurol. 1972;37(3):562–572. doi: 10.1016/0014-4886(72)90099-4. [DOI] [PubMed] [Google Scholar]
- 32.Chang HT. Single neostriatal efferent axons in the globus pallidus: a light and electron microscopic study. Science. 1981;213(4510):915–918. doi: 10.1126/science.7256286. [DOI] [PubMed] [Google Scholar]
- 33.Smith Y., Bevan MD., Shink E., Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 34.Bolam JP., Ingham CA., Izzo PN., et al Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res. 1986;397(2):279–289. doi: 10.1016/0006-8993(86)90629-3. [DOI] [PubMed] [Google Scholar]
- 35.Pickel VM., Chan J. Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. J Neurosci Res. 1990;25(3):263–280. doi: 10.1002/jnr.490250302. [DOI] [PubMed] [Google Scholar]
- 36.Rav-Acha M., Sagiv N., Segev I., Bergman H., Yarom Y. Dynamic and spatial features of the inhibitory pallidal GABAergic synapses. Neuroscience. 2005;135(3):791–802. doi: 10.1016/j.neuroscience.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 37.Haber S., Elde R. Correlation between Met-enkephalin and substance P immunoreactivity in the primate globus pallidus. Neuroscience. 1981;6(7):1291–1297. doi: 10.1016/0306-4522(81)90188-3. [DOI] [PubMed] [Google Scholar]
- 38.Reiner A., Medina L., Haber SN. The distribution of dynorphinergic terminals in striatal target regions in comparison to the distribution of substance P-containing and enkephalinergic terminals in monkeys and humans. Neuroscience. 1999;88(3):775–793. doi: 10.1016/s0306-4522(98)00254-1. [DOI] [PubMed] [Google Scholar]
- 39.Somogyi P., Bolam JP., Smith AD. Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscopic study using the Golgi-peroxidase transport-degeneration procedure. J Comp Neurol. 1981;195(4):567–584. doi: 10.1002/cne.901950403. [DOI] [PubMed] [Google Scholar]
- 40.Sadikot AF., Parent A., Smith Y., Bolam JP. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriataI projection in relation to striatal heterogeneity. J Comp Neurol. 1992;320(2):228–242. doi: 10.1002/cne.903200207. [DOI] [PubMed] [Google Scholar]
- 41.Sidibé M., Smith Y. Differential synaptic innervation of striatofugal neurones projecting to the internal or external segments of the globus pallidus by thalamic afferents in the squirrel monkey. J Comp Neurol. 1996;365(3):445–465. doi: 10.1002/(SICI)1096-9861(19960212)365:3<445::AID-CNE8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Smith Y., Raju DV., Pare JF., Sidibé M. The thalamostriataI system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27(9):520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Ding J., Peterson JD., Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28(25):6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss J., Bolam JP. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci. 2008;28(44):11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura M., Kato M., Shimazaki H., Watanabe K., Matsumoto N. Neural information transferred from the putamen to the globus pallidus during learned movement in the monkey. J Neurophysiol. 1996;76(6):3771–3786. doi: 10.1152/jn.1996.76.6.3771. [DOI] [PubMed] [Google Scholar]
- 46.Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63(6):1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- 47.Crutcher MD., DeLong MR. Single cell studies of the primate putamen II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res. 1984;53(2):244–258. doi: 10.1007/BF00238154. [DOI] [PubMed] [Google Scholar]
- 48.Freund TF., Powell JF., Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13(4):1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 49.Kung L., Force M., Chute DJ., Roberts RC. Immunocytochemical localization of tyrosine hydroxylase in the human striatum: a postmortem ultrastructural study. J Comp Neurol. 1998;390(1):52–62. doi: 10.1002/(sici)1096-9861(19980105)390:1<52::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 50.Smith Y., Bennett BD., Bolam JP., Parent A., Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344(1):1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- 51.DiFiglia M. Synaptic organization of cholinergic neurons in the monkey neostriatum. J Comp Neurol. 1987;255(2):245–258. doi: 10.1002/cne.902550208. [DOI] [PubMed] [Google Scholar]
- 52.Yung KK., Smith AD., Levey AI., Bolam JP. Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evident from dopamine receptro and neuropeptide immostaining. Eur Journal Neurosci. 1996;8(5):861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 53.Goldman-Rakic PS. Cytoarchitectonic heterogeneity of the primate neostriatum: subdivision into island and matrix cellular compartments. J Comp Neurol. 1982;205(4):398–413. doi: 10.1002/cne.902050408. [DOI] [PubMed] [Google Scholar]
- 54.Goldman PS., Nauta Wl. An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol. 1977;72(3):369–386. doi: 10.1002/cne.901710305. [DOI] [PubMed] [Google Scholar]
- 55.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 56.Meyer G., Gonzalez-Hernandez T., Carrillo-Padilla F., Ferres-Torres R. Aggregations of granule cells in the basal forebrain (islands of Calleja): Golgi and cytoarchitectonic study in different mammals, including man. J Comp Neurol. 1989;284(3):405–428. doi: 10.1002/cne.902840308. [DOI] [PubMed] [Google Scholar]
- 57.Heimer L., De Olmos JS., Alheid GF., et al The human basal forebrain. Part II. In: Bloom FE, Bjorkland A, Hokfelt T, eds. Handbook of Chemical Neuroanatomy. Amsterdam, The Netherlands: Elsevier; 1999:57–226. [Google Scholar]
- 58.Mai JK., Stephens PH., Hopf A., Cuello AC. Substance P in the human brain. Neuroscience. 1986;17(3):709–739. doi: 10.1016/0306-4522(86)90041-2. [DOI] [PubMed] [Google Scholar]
- 59.Heimer L. Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev. 2000;31(2-3):205–235. doi: 10.1016/s0165-0173(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 60.Bernier PJ., Parent A. Bcl-2 protein as a marker of neuronal immaturity in postnatal primate brain. J Neurosci. 1998;18(7):2486–2497. doi: 10.1523/JNEUROSCI.18-07-02486.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fudge JL., Kunishio K., Walsh P., Richard C., Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110(2):257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- 62.Zaborszky L., Alheid GF., Beinfeld MC., Eiden LE., Heimer L., Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14(2):427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- 63.Brog JS., Salyapongse A., Deutch AY., Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 64.Heimer L., Zahm DS., Churchill L., Kalivas PW., Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 65.Meredith GE., Pattiselanno A., Groenewegen HJ., Haber SN. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin-D28k. J Comp Neurol. 1996;365(4):628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Russchen FT., Bakst I., Amaral DG., Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329(1-2):241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- 67.Haber SN., Ryoo H., Cox C., Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol. 1995;362(3):400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- 68.Yeterian EH., Van Hoesen GW. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res. 1978;139(1):43–63. doi: 10.1016/0006-8993(78)90059-8. [DOI] [PubMed] [Google Scholar]
- 69.Flaherty AW., Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13(3):1120–1137. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldman-Rakic PS., Selemon LD. Topography of corticostriatal projections in nonhuman primates and implications for functional panellation of the neostriatum. In: Jones EG, Peters A, eds. Cerebral Cortex. Vol 5. New York, NY: Plenum Publishing Corporation; 1986:447–466. [Google Scholar]
- 71.McFarland NR., Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20(10):3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- 73.Flaherty AW., Graybiel AM. Input-output organization of the sensorimotor striatum in the squirrel monkey. J Neurosci. 1994;14(2):599–610. doi: 10.1523/JNEUROSCI.14-02-00599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kimura M. The role of primate putamen neurons in the association of sensory stimulus with movement. Neurosci Res. 1986;3(5):436–443. doi: 10.1016/0168-0102(86)90035-0. [DOI] [PubMed] [Google Scholar]
- 75.Yin HH., Mulcare SP., Hilario MR., et al Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12(3):333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boecker H., Dagher A., Ceballos-Baumann AO., et al Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J Neurophysiol. 1998;79(2):1070–1080. doi: 10.1152/jn.1998.79.2.1070. [DOI] [PubMed] [Google Scholar]
- 77.Kermadi I., Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. J Neurophysiol. 1995;74(3):911–933. doi: 10.1152/jn.1995.74.3.911. [DOI] [PubMed] [Google Scholar]
- 78.Chen LL., Wise SP. Supplementary eye field contrasted with the frontal eye field during acquisition of conditional oculomotor associations. J Neurophysiol. 1995;73(3):1122–1134. doi: 10.1152/jn.1995.73.3.1122. [DOI] [PubMed] [Google Scholar]
- 79.Kunishio K., Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350(3):337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- 80.Hikosaka O., Rand MK., Miyachi S., Miyashita K. Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol. 1995;74(4):1652–1661. doi: 10.1152/jn.1995.74.4.1652. [DOI] [PubMed] [Google Scholar]
- 81.Haber SN., Kunishio K., Mizobuchi M., Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15(7 pt 1):4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chikama M., McFarland NR., Amaral DG., Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17(24):9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandya DN., Van Hoesen GW., Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42(3-4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 84.Fudge JL., Haber SN. Defining the caudal ventral striatum in primates: cellular and histochemicaI features. J Neurosci. 2002;22(23):10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bouret S., Richmond BJ. Ventromedial and orbital prefrontal neurons differentially encode internally and externally driven motivational values in monkeys. J Neurosci. 2010;30(25):8591–8601. doi: 10.1523/JNEUROSCI.0049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Behrens TE., Hunt LT., Woolrich MW., Rushworth MF. Associative learning of social value. Nature. 2008;456(7219):245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Padoa-Schioppa C., Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallis JD., Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18(7):2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 89.Fellows LK., Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 90.Izquierdo A., Suda RK., Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24(34):7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudebeck PH., Behrens TE., Kennerley SW., et al Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28(51):13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camille N., Tsuchida A., Fellows LK. Double dissociation of stimulusvalue and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. J Neurosci. 2011;31(42):15048–15052. doi: 10.1523/JNEUROSCI.3164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexander WH., Brown JW. Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci. 2011;14(10):1338–1344. doi: 10.1038/nn.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calzavara R., Mailly P., Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 2007;26(7):2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldman-Rakic PS., Funahashi S., Bruce CJ. Neocortical memory circuits. Cold Spring Harb Symp Quant Biol. 1990;55:1025–1038. doi: 10.1101/sqb.1990.055.01.097. [DOI] [PubMed] [Google Scholar]
- 96.Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriatr. 1987;6(3):299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- 97.Arikuni T., Kubota K. The organization of prefrontocaudate projections and their laminar origin in the macaque monkey - a retrograde study using HRP-gel. J Comp Neurol. 1986;244(4):492–510. doi: 10.1002/cne.902440407. [DOI] [PubMed] [Google Scholar]
- 98.Hikosaka O., Sakamoto M., Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol. 1989;61(4):814–832. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- 99.Elliott R., DoIan RJ. Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. J Neurosci. 1999;19(12):5066–5073. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Partiot A., Verin M., Pillon B., Teixeira-Ferreira C., Agid Y., Dubois B. Delayed response tasks in basal ganglia lesions in man: further evidence for a striato-frontal cooperation in behavioural adaptation. Neuropsychologia. 1996;34(7):709–721. doi: 10.1016/0028-3932(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 101.Van Hoesen GW., Yeterian EH., Lavizzo-Mourey R. Widespread corticostriate projections from temporal cortex of the rhesus monkey. J Comp Neurol. 1981;199(2):205–219. doi: 10.1002/cne.901990205. [DOI] [PubMed] [Google Scholar]
- 102.Yeterian EH., Pandya DN. Corticostriatal connections of the superior temporal region in rhesus monkeys. J Comp Neurol. 1998;399(3):384–402. [PubMed] [Google Scholar]
- 103.Friedman DP., Aggleton JP., Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the macaque brain. J Comp Neurol. 2002;450(4):345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- 104.Ramanathan S., Hanley JJ., Deniau JM., Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22:8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mallet N., Le Moine C., Charpier S., Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25(15):3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takada M., Tokuno H., Nambu A., Inase M. Corticostriatal input zones from the supplementary motor area overlap those from the contra-rather than ipsilateral primary motor cortex. Brain Res. 1998;791(12):335–340. doi: 10.1016/s0006-8993(98)00198-x. [DOI] [PubMed] [Google Scholar]
- 107.Wilson CJ. The basal ganglia. In: Shepherd GM, ed. Synaptic Organization of the Brain. 5th ed. New York, NY: Oxford University Press; 2004:361–413. [Google Scholar]
- 108.Draganski B., Kherif F., Kloppel S., et al Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. 2008;28(28):7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buckner RL., Andrews-Hanna JR., Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 110.Power JD., Schlaggar BL., Lessov-Schlaggar CN., Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wise RA., Rompre PP. Brain dopamine and reward. Ann Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 112.Fibiger HC., Phillips AG. Reward, motivation, cognition: psychobiology of mesotelencephalic dopamine systems. In: Mountcastle VB, Bloom F, Geiger SR, eds. Handbook of Physiology. Section I: the Nervous System. Vol 4. Bethesda,MD: American Physiological Society; 1986:647–674. [Google Scholar]
- 113.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 114.Koob GF., Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nestler EJ., Malenka RC. The addicted brain. Sci Am. 2004;290(3):78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- 116.Lavoie B., Parent A. Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport. 1991;2(10):601–604. doi: 10.1097/00001756-199110000-00012. [DOI] [PubMed] [Google Scholar]
- 117.McRitchie DA., Halliday GM. Calbindin D28k-containing neurons are restricted to the medial substantia nigra in humans. Neuroscience. 1995;65(1):87–91. doi: 10.1016/0306-4522(94)00483-l. [DOI] [PubMed] [Google Scholar]
- 118.Lynd-Balta E., Haber SN. The organization of midbrain projections to the striatum in the primate: sensor imotor-re late d striatum versus ventral striatum. Neuroscience. 1994;59(3):625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 119.Parent A., Hazrati LN. Multiple striatal representation in primate substantia nigra. J Comp Neurol. 1994;344(2):305–320. doi: 10.1002/cne.903440211. [DOI] [PubMed] [Google Scholar]
- 120.Bevan MD., Clarke NP., Bolam JP. Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. J Neurosci. 1997;17(1):308–324. doi: 10.1523/JNEUROSCI.17-01-00308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Somogyi P., Bolam JP., Totterdell S., Smith AD. Monosynaptic input from the nucleus accumbens-ventral striatum region to retrograde ly labelled nigrostriatal neurones. Brain Res. 1981;217(2):245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- 122.Nauta WJ., Smith GP., Faull RLM., Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3(4-5):385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 123.McFarland NR., Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22(18):8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bar-Gad I., Havazelet-Heimer G., Goldberg JA., Ruppin E., Bergman H. Reinforcement-driven dimensionality reduction—a model for information processing in the basal ganglia. J Basic Clin Physiol Pharmacol. 2000;11(4):305–320. doi: 10.1515/jbcpp.2000.11.4.305. [DOI] [PubMed] [Google Scholar]