Abstract
We used eye-tracking to investigate the roles of enhanced discrimination and peripheral selection in superior visual search in autism spectrum disorder (ASD). Children with ASD were faster at visual search than their typically developing peers. However, group differences in performance and eye-movements did not vary with the level of difficulty of discrimination or selection. Rather, consistent with prior ASD research, group differences were mainly the effect of faster performance on target-absent trials. Eye-tracking revealed a lack of left-visual-field search asymmetry in ASD, which may confer an additional advantage when the target is absent. Lastly, ASD symptomatology was positively associated with search superiority, the mechanisms of which may shed light on the atypical brain organization that underlies social-communicative impairment in ASD.
Keywords: autism, attention, visual search, eye-tracking
We used eye-tracking to investigate the roles of enhanced discrimination and peripheral selection in superior visual search in autism spectrum disorder (ASD). Children with ASD were faster at visual search than their typically developing peers. However, group differences in performance and eye-movements did not vary with the level of difficulty of discrimination or selection. Rather, consistent with prior ASD research, group differences were mainly the effect of faster performance on target-absent trials. Eye-tracking revealed a lack of left-visual-field search asymmetry in ASD, which may confer an additional advantage when the target is absent. Lastly, ASD symptomatology was positively associated with search superiority, the mechanisms of which may shed light on the atypical brain organization that underlies social-communicative impairment in ASD.
Introduction
Visual search requires an observer to locate a target presented within an array of distractors. Determining the presence or absence of the target, at least in more difficult searches, requires the observer to make a series of eye-movements, and knowledge about these eye movements is important to understanding the attentional and perceptual processes involved in search. At each fixation, a viewer must 1) discriminate foveal information (i.e., determine whether foveated object(s) match the target item), and 2) extract peripheral information necessary to plan subsequent saccadic eye-movements (i.e., select the next item(s) for foveal analysis). Prior research has demonstrated that the duration of each fixation is dependent on the difficulty of foveal discrimination and not peripheral selection (Hooge & Erkelens, 1999; Shen, Reingold, Pomplun, & Williams, 2003). However, during a fixation, analysis of peripheral information permits subsequent saccades to be directed towards the target or target-like distractors that share features with the target (i.e., saccadic selectivity) (Findlay, 1997; Findlay, Brown, & Gilchrist, 2001; Hooge & Erkelens, 1999; Shen et al., 2003).
Individuals with autism spectrum disorder (ASD) excel at visual search (see Kaldy, Giserman, Carter, & Blaser, 2013, for review), performing better than their typically developing (TD) peers across the lifespan (Kaldy, Kraper, Carter, & Blaser, 2011; O’Riordan, 2004). This disparity in performance generally becomes larger as target-distractor similarity increases (O’Riordan, 2004; O’Riordan & Plaisted, 2001; O’Riordan, Plaisted, Driver, & Baron-Cohen, 2001; Plaisted, O’Riordan, & Baron-Cohen, 1998; see Hessels, Hooge, Snijders, & Kemner, 2014, for conflicting findings), as set size increases, and when the target is absent (Kaldy et al., 2013). Evidence of augmented ASD search superiority with increasing target-distractor similarity has been used to support the hypothesis that enhanced discrimination contributes to faster search (O’Riordan & Plaisted, 2001), which is consistent with the more general concept of enhanced perceptual functioning (Mottron, Dawson, Soulieres, Hubert, & Burack, 2006). Others have suggested that faster search may be due in part to more focused attention rather than enhanced lower-level perceptual processing (Blaser, Eglington, Carter, & Kaldy, 2014; Kaldy et al., 2013). Additionally, evidence of increased perceptual capacity in ASD (Remington, Swettenham, Campbell, & Coleman, 2009; Remington, Swettenham, & Lavie, 2012) has been invoked as a possible mechanism supporting superior search (Milne, Dunn, Freeth, & Rosas-Martinez, 2013), and may contribute to enhanced peripheral selection in ASD.
Kemner and colleagues (2008a) were the first to use eye-tracking to examine visual search performance in adults with ASD. They reported decreased fixation frequency, and concluded that fewer fixations (of similar duration) were indicative of enhanced discrimination in ASD. A subsequent study of children and adolescents with ASD by Joseph and colleagues (2009) showed that superior visual search abilities in ASD were associated with shorter rather than fewer fixations, and concluded that enhanced performance was due to non-search factors such as enhanced discrimination or speeded post-selection decision-making. Toddlers with ASD have also been shown to be more successful at finding the target in a search task (Kaldy et al., 2011). Eye-tracking results from this study showed no difference in fixation frequency or duration; rather, the authors suggested that better performance was due to a larger number of items examined at each fixation in ASD as compared to TD toddlers (Kaldy et al., 2011). Thus, while individuals with ASD excel at visual search from toddlerhood to adulthood, evidence from eye-tracking studies has been limited and has failed to provide a more coherent picture of how individuals with ASD achieve faster search.
We used eye tracking to further investigate how advantages in stimulus discrimination and peripheral selection might contribute to superior search performance in ASD using a paradigm developed by Hooge and Erkelens (1999) in which distractors were varied on two dimensions, discriminability and ease of selection. However, given the well-documented search advantage on target absent trials among individuals with ASD, we added a target absent condition to investigate the processes involved in the pronounced ASD absent advantage. We reasoned that if enhanced discrimination underlies visual search, then the ASD reaction time advantage would become greater as discrimination difficulty increases (similar to O’Riordan & Plaisted, 2001), and that individuals with ASD would also evidence shorter fixation durations, which would be less affected by increasing discrimination difficulty (Joseph, Keehn, Connolly, Wolfe, & Horowitz, 2009). Alternatively, if faster search arises from increased perceptual capacity (Remington et al., 2009; Remington et al., 2012), which arguably affords individuals with ASD access to an increased number of distractors, the ASD search advantage would be expected to increase with greater selection difficulty, resulting from greater saccadic selectivity and a reduced number of fixations during search. Further, we hypothesized that enhanced discrimination (i.e., shorter fixation durations) or peripheral selection (i.e., fewer fixations) would have a disproportionally greater effect for target absent compared to target present trials, as participants are required to complete a more extensive search of the array to confirm that the target is absent. Lastly, given that prior studies have demonstrated that search-related indices are associated with increased ASD symptom severity (Gliga, Bedford, Charman, Johnson, & The BASIS Team, 2015; Joseph et al., 2009; Keehn & Joseph, 2008; Keehn, Shih, Brenner, Townsend, & Müller, 2013), we examined the relationship between response time and eye-tracking measures and ASD symptomatology.
Methods
Participants
Participants were 22 school-age children and adolescents with ASD (16 males) and an age- and non-verbal IQ-matched comparison group of 30 typically developing children (26 males). All ASD participants were confirmed to meet DSM-5 (APA, 2013) criteria for ASD using both the Autism Diagnostic Interview – Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003), a comprehensive parent interview focusing on the age of 4–5 years when ASD symptoms are typically most severe, and the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999), a semi-structured, interactive observation protocol that assesses current ASD symptoms. All TD participants had no reported history of ASD and were confirmed to be free of ASD-related symptoms and any other neurological or psychiatric conditions via parent report and expert clinical observation. As shown in Table 1, groups were matched on age and non-verbal IQ, but not on verbal IQ, as measured with the Kaufman Brief Intelligence Test – II (Kaufman & Kaufman, 2004). Informed consent was obtained from all research participants in accordance with the IRB.
Table 1.
Participant Characteristics
| ASD (n = 22) | TD (n = 30) | t-value | p** | |
|---|---|---|---|---|
| Age (years) | 13.7 (3); 9–20* | 13.5 (2); 9–18 | 0.21 | 0.829 |
| Verbal IQ | 104 (20); 63–135 | 115 (16); 81–152 | −2.1 | 0.035 |
| Nonverbal IQ | 114 (12); 85–131 | 113 (10); 95–135 | 0.3 | 0.765 |
| ADI-R | ||||
| Communication | 17 (4); 7–22 | - | - | - |
| Social Interaction | 21 (5); 11–30 | - | - | - |
| Repetitive Behavior | 6 (2); 2–10 | - | - | - |
| ADOS | ||||
| Communication | 4 (2); 1–6 | - | - | - |
| Social Interaction | 10 (2); 5–13 | - | - | - |
| Repetitive Behavior | 3 (1); 0–6 | - | - | - |
Mean (SD); Range
Independent-samples t-test
Apparatus
The experiment was presented using E-Prime 1.1 on a 19-inch LCD monitor. Participants were seated approximately 57cm from the monitor. Test responses were registered using a PST serial response box. Participants’ point of visual regard was tracked throughout the experiment using an ISCAN Model ETL-500 head-mounted eye monitoring system. Point of regard was calibrated for each participant prior to the start of the experiment and between experimental blocks as necessary by registering the distance between the pupil and corneal reflection while gaze was directed at five fixation points, in succession, at the center of the computer screen and at light emitting diodes placed at each of the four corners of the screen. In order to count as a fixation, POR had to be maintained for at least 5 continuous data samples (approximately 80 – 85 ms at a sample rate of 60Hz) within an area of 1° of visual angle.
Stimuli
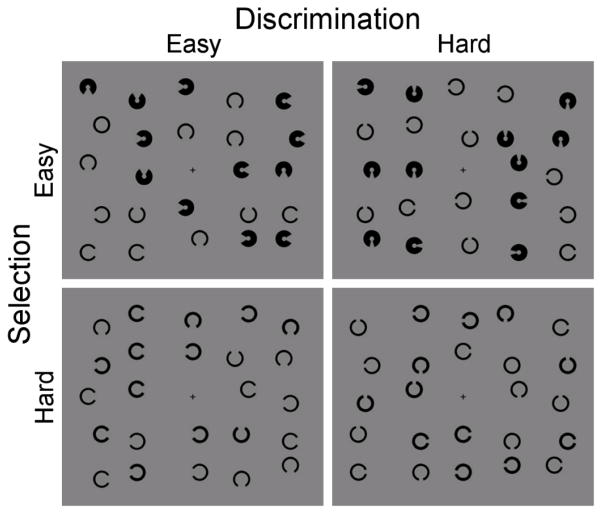
Stimuli were modeled after Hooge and Erkelens (1999). The stimulus array had a fixed set size of 24 items. The target was a circle and the distractors were “C” shaped figures. At a viewing distance of 57cm, shapes had a diameter of 1.18° visual angle. The target (circle) and thin distractors had line widths of .30°. Peripheral selection was varied by increasing the line-width of thick distractors (0.45 or 0.75°), and discrimination between the target and distractors was varied by increasing the size of the gap in the distractor Cs (0.30° or 0.60°). Manipulation of thick distractor width (selection) and gap size (discrimination) were used to create four conditions: discrimination easy, selection easy (DESE), discrimination easy, selection hard (DESH), discrimination hard, selection easy (DHSE), and discrimination hard, selection hard (DHSH). On a given trial, thin and thick distractors had the same gap size, and thick distractors all shared the same line width (see Figure 1). Target absent arrays contain 12 thin and 12 thick distractors and target present arrays contained 11 thin and 12 thick distractors plus a single target (circle). Target and distractors were drawn in black on a gray background. Distractor Cs were randomly displayed in any one of four cardinal orientations. Stimuli were randomly positioned on a 5 × 5 array of 2.2º × 2.2º squares. Elements were randomly positioned within each square to produce layout irregularity.
Figure 1.
Illustration of search array for each trial type. The level of discrimination between target and distractors was varied by increasing the size of the gap in the distractor Cs, whereas peripheral selection was varied by increasing the line-width of thick distractors. Manipulation of gap size (discrimination) and thick distractor width (selection) were used to create four conditions: discrimination easy, selection easy (DESE; upper left), discrimination easy, selection hard (DESH; lower left), discrimination hard, selection easy (DHSE); upper right, and discrimination hard, selection hard (DHSH; lower right). The target (circle) is present for DESE and DHSH conditions, target absent for DHSE and DESH conditions.
Design
The experiment was divided into four blocks, each consisting of 40 trials. Within each block, the target was present on 50% of trials. In addition to target absence or presence, each trial was defined by the size of the gap in the distractor circles (0.30 or 0.60°) and by the line-width of the thick distractor Cs (0.45 or 0.75°). Thus trials were defined according to three parameters (target presence: present, absent; discrimination: easy [0.60], hard [0.30]; selection: easy [0.75], hard [0.45]), yielding a total of eight (2 × 2 × 2) possible trial conditions. All trial conditions were presented an equal number of times within each block and across the experiment.
A trial began with a fixation cross (“+”) presented alone for 1000ms. Next, with the fixation cross remaining on the screen, the stimulus array was presented until a response was made or until 7500ms had elapsed. Children responded with their dominant hand via a two-choice button box on which one button represented target present and the other target absent. The examiner first modeled the task and then participants completed a block of 16 practice trials. Feedback was provided for practice, but not for test trials. Participants were instructed to respond as quickly as possible without making errors.
Results
Medians were used in all RT analyses to reduce the influence of outliers. Accuracy and RT data for correct responses were analyzed using mixed-model repeated-measures ANOVA with between-subject factor group (ASD, TD) and within-subjects factors target presence (absent, present), discrimination (easy, hard), and selection (easy, hard).
Search Performance
Accuracy
As illustrated in Figure 2, accuracy was greater for target present than absent trials, F(1, 50) = 48.5, p < .001, ηp2 = 0.49, easy compared to hard discrimination, F(1, 50) = 23.1, p < .001, ηp2 = 0.32, and easy compared to hard selection, F(1, 50) = 15.7, p < .001, ηp2 = 0.24. There was no significant main effect of group, F(1, 50) = 0.8, p = .388, ηp2 = 0.02, nor were there significant interactions between group and target presence, discrimination, selection, or a three-way interaction between group, discrimination, and selection (all p > .05). Correlations between condition-specific accuracy and RT measures for both groups revealed no evidence of a speed-accuracy trade offs. To ensure that group differences in RT did not reflect a response bias, c-criterion and likelihood ratio beta were calculated (Stanislaw & Todorov, 1999); positive values for c-criterion and values greater than one for beta ratio reflect a bias towards a “no” response, whereas negative c-criterion and beta values less than 1 reflect a “yes” response bias. Both groups showed a “no” response bias (i.e., “no target present” reflecting greater likelihood to miss the target when present, rather than inaccurately report the presence of a target that does not exist). There was no difference between groups for c-criterion (ASD = 0.32; TD = 0.29), t(50) = 0.42, p = .678, d = 0.12, or beta vales (ASD = 4.95; TD = 3.95), t(50) = 0.90, p = .727, d = 0.25.
Figure 2.
Mean response time (right y-axis) for ASD (gray) and TD (white) for target present (left graph) and target absent (right graph) trials. Line graphs reflect error rate (right y-axis) for ASD (circles) and TD (squares). DESE = discrimination easy, selection easy; DHSE = discrimination hard, selection easy; DESH = discrimination easy, selection hard; DHSH = discrimination hard, selection hard. Error bars represent ± 1 SEM.
Reaction time
As expected based on previous visual search findings, within-subjects main effects of target presence, discrimination, and selection were significant. Response time were faster to target present than target absent trials, F(1, 50) = 328.5, p < .001, ηp2 = 0.87, easy compared to hard discrimination, F(1, 50) = 402.2, p < .001, ηp2 = 0.89, and easy compared to hard selection, F(1, 50) = 125.4, p < .001, ηp2 = 0.72. There was also a significant interaction between discrimination and selection, F(1, 50) = 44.7, p < .001, ηp2 = 0.47, such that differences in RT for easy compared to hard selection were greater on hard, t(51) = −12.6, p < .001, d = 1.83, versus easy, t(51) = −8.3, p < .001, d = 1.21, discrimination. Response time differences between groups approached statistical significance, F(1, 50) = 3.5, p = .068, ηp2 = 0.07, reflecting faster RT in the ASD (M = 2224ms) compared to the TD (M = 2516ms) group. However, there were no significant interactions between group and discrimination, F(1, 50) = 0.14, p = .707, ηp2 = 0.00, selection, F(1, 50) = 0.26, p = .614, ηp2 = 0.01, or a three-way interaction between group, discrimination, and selection, F(1, 50) = 0.47, p = .498, ηp2 = 0.01. There was a significant group by target presence interaction, F(1, 50) = 6.7, p = .013, ηp2 = 0.12. Follow-up independent samples t-tests revealed that individuals with ASD were significantly faster in target absent (all p < .05), but not target present trials (all p > .4) for all search conditions (see Figure 2).
Eye-Movement
Eye-movement data were successfully collected for 18 of the 22 ASD participants and 27 of 30 TD participants; missing data for ASD (n = 4) and TD (n = 3) participants resulted from unsuccessful calibration. The subsample of participants with eye-movement data remained matched on age and non-verbal IQ (all p > .05). Eye-tracking data were recorded from the onset of the search array until participant response and were analyzed for correct trials only. The ASD (M = 89%) and TD (M = 91%) groups did not differ in their percentage of looking time for each trial, t(43) = 1.1, p = .273, d = .33. For each fixation, we determined whether the closest element (within 2.5°) was a target or a thin or thick distractor (note: thin distractors were always the same width [i.e., the width of the target], and thick distractors varied as a function of selection). Fixation frequency and duration data were analyzed using mixed-model repeated-measures ANOVA with between-subject factor group (ASD, TD) and within-subjects factors target presence (absent, present), discrimination (easy, hard), selection (easy, hard), and element fixated (thin, thick).
Fixation frequency
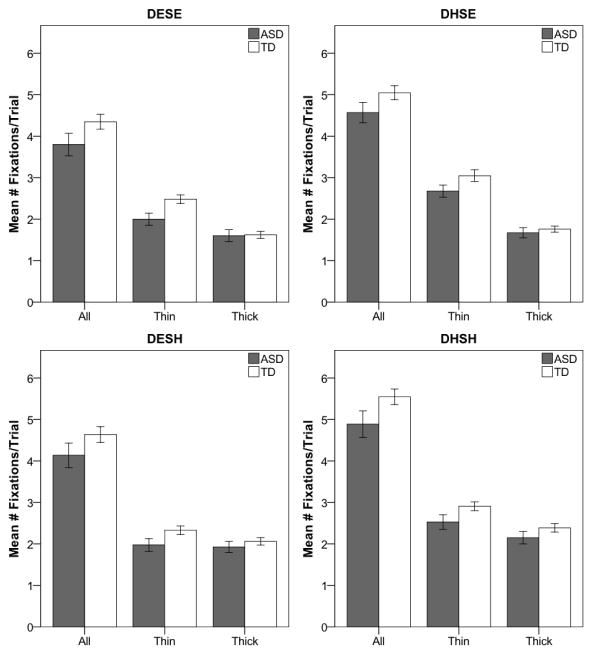
Mean number of fixations per trial was significantly greater for target absent compared to target present, F(1, 43) = 377.8, p < .001, ηp2 = 0.90, hard compared to easy discrimination, F(1, 43) = 171.5, p < .001, ηp2 = 0.80, hard compared to easy selection, F(1, 43) = 34.1, p < .001, ηp2 = 0.44, and thin compared to thick distractors, F(1, 43) = 102.7, p < .001, ηp2 = 0.71. Fixations on thin and thick distractors varied as a function of both selection, F(1, 43) = 70.4, p < .001, ηp2 = 0.62, and discrimination, F(1, 43) = 53.7, p < .001, ηp2 = 0.56. For selection, easy selection resulted in greater saccadic selectivity (i.e., more fixations to thin than thick distractors), while hard selection resulted in an even distribution of fixations to thin and thick. For discrimination, hard discrimination resulted in more fixations to thin versus thick distractors, whereas easy discrimination resulted in similar numbers of fixations to thin and thick distractors. There was no difference between the groups in the number of fixations per trial, F(1, 43) = 2.9, p = .096, ηp2 = 0.06; however, there was a significant interaction between group and fixated element, F(1, 43) = 5.7, p = .022, ηp2 = 0.12. As can be seen in Figure 3, group differences in fixation frequency resulted from fewer fixations to thin elements by the ASD than the TD group.
Figure 3.
(a) Fixation frequency to all distractors, thin distractors, and thick distractors for each condition (DESE, DHSE, DESH, and DHSH). Fraction of saccades to thin distractors for easy versus hard discrimination (b) and easy versus hard selection (c). Dashed line reflects 50% of saccades to thin distractors. Error bars represent ± 1 SEM.
Fixation duration
The average fixation duration was longer for target present (511 ms) compared to target absent (434 ms) trials, F(1, 43) = 129.2, p < .001, ηp2 = 0.75, hard (488 ms) compared to easy (457 ms) discrimination, F(1, 43) = 30.2, p < .001, ηp2 = 0.41, and hard (497 ms) compared to easy (448 ms) selection, F(1, 43) = 47.0, p < .001, ηp2 = 0.52; however, there was no significant main effect of element fixated (thin = 475ms; thick = 470ms), F(1, 43) = 0.8, p = .375, ηp2 = 0.02, or significant interactions between element fixated and discrimination or selection (all p > .05). There was no main effect of group (ASD = 464 ms; TD = 481 ms), F(1, 43) = 0.5, p = .490, ηp2 = 0.01, nor were there any significant interactions between group and any other factor (all p > .05).
Latency and error of initial saccade
In addition to frequency and duration of fixations, we also examined latency of initial saccade (i.e., time from onset of search array until first eye-movement) and the distance between the endpoint of that eye-movement and the location of the target (i.e., saccadic error) for target present trials. Latencies were longer for hard compared to easy discrimination, F(1, 43) = 17.6, p < .001, ηp2 = 0.29, and hard compared to easy selection, F(1, 43) = 5.4, p = .025, ηp2 = 0.11. However, there was no main effect of group or interaction between group and any other variable (all p > .05). Saccadic error was greater in hard versus easy discrimination, F(1, 43) = 4.8, p = .035, ηp2 = 0.10, and hard versus easy selection, F(1, 43) = 5.7, p = .021, ηp2 = 0.12. There was also a main effect of group, F(1, 43) = 4.6, p = .037, ηp2 = 0.10, as individuals with ASD (M = 6.7°) had smaller saccadic errors than their TD peers (M = 7.4°). However, there were no interactions between group and discrimination or selection (all p > .05).
Understanding the ASD Absent Advantage
We conducted a series of analyses similar to Chun and Wolfe (1996) in an attempt to elucidate the search characteristics that confer a unique advantage in target absent trials to individuals with ASD. It should be noted, however, that because these analyses focus on specific trial types (e.g., misses or targets appearing in unique locations) not all participants are included in each analysis (e.g., when they did not make any errors); nevertheless, all analyses include a majority of participants and do not exclude a disproportionate number of participants from either group.
Frequency of and reaction time for miss errors
We first examined RT to miss errors (i.e., a “no target present” response on a target present trial). Miss errors may reflect a “quitting threshold,” in which the decision that a target is absent is made before the target is found. Frequency of miss errors was analyzed using a 2 (group: ASD, TD) × 2 (discrimination: easy, hard) × 2 (selection: easy, hard) mixed-model repeated-measures ANOVA. Miss errors were more frequent for hard compared to easy discrimination, F(1, 50) = 5.5, p = .024, ηp2 = 0.10, and selection, F(1, 50) = 11.6, p = .001, ηp2 = 0.19. However, ASD (M = 7.1) and TD (M = 6.0) groups did not differ on the frequency of miss errors, F(1, 50) = 0.6, p = .440, ηp2 = 0.01. Additionally, χ2 tests showed that a similar proportion of ASD and TD participants made miss errors (all p > .05). As shown in Figure 4a, miss error RT for the ASD group was significantly faster for the DESE condition, t(31) = 2.3, p = .029, d = 0.83, but not for DESH, DHSE, or DHSH conditions (all p > .05).
Figure 4.
Average RT for miss trials for ASD and TD participant (a). The deviation of RT associated with post-miss slowing on target absent trials (b). Error bars represent ± 1 SEM.
The effect of errors on target absent RT
After a miss, RT to target absent trials is slowed relative to pre-miss absent trials (although this is not the case for target present trials; Chun & Wolfe, 1996). Post-miss slowing likely reflects the use of a more conservative search strategy, involving a more comprehensive examination of items in the search array. Reduced or absent post-miss slowing in ASD, potentially related to a lack of error awareness; (Bogte, Flamma, van der Meere, & van Engeland, 2007; Russell & Jarrold, 1998; Sokhadze et al., 2010), could also contribute to accelerated absent RT. Thus, we examined target-absent trial RT as a function of serial position relative to a miss trial. The average of target absent RT was computed for three correct target absent trials preceding and following a miss. For each participant, RT for each correct target-absent trial was computed as the difference between the trial RT and the average RT for the target absent condition, and then the mean of the three pre- and post-miss trials was calculated. RT difference scores were analyzed using a 2 (group: ASD, TD) × 3 (trial position: pre-miss, miss, post-miss) mixed-model repeated-measures ANOVA. There was a significant main effect of trial position, F(2, 92) = 13.0, p < .001, ηp2 = 0.22, but there was no difference between groups, F(1, 46) = 0.3, p = .574, ηp2 = 0.01, nor was there a significant interaction between group and position, F(2, 92) = 0.1, p = .877, ηp2 = 0.00, suggesting that post-miss slowing was not different between groups and did not contribute to faster target absent RT in the ASD group (see Figure 4b).
Target location and RT
Rates of visual acuity decline with retinal eccentricity, thus increased misses and slower RT for correct responses should be present for trials where the target appears farther from the fixation cross (i.e., the location where search should begin). We examined target present RT for correct trials as a function of target location. Misses were infrequent and thus could not be analyzed in this manner. Mean RT was calculated for each of 24 potential target locations for each condition and then averaged within participant.
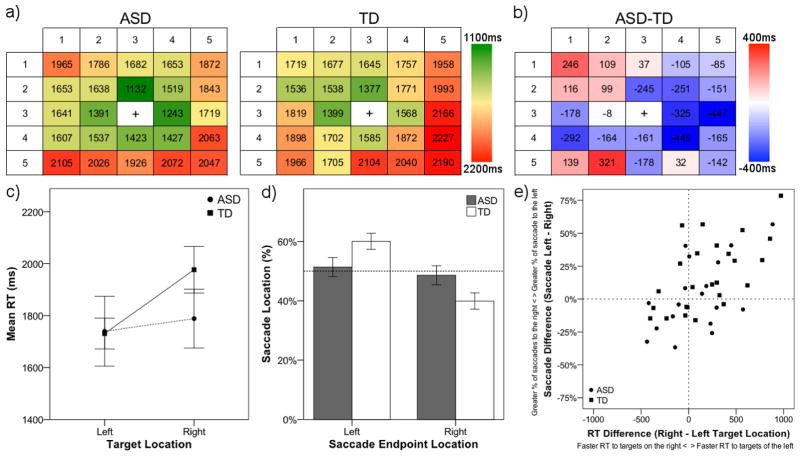
Prior analysis of target present RT (collapsed across target location) revealed similar search speed for ASD and TD groups (see Figure 2); however, as illustrated in Figure 5a, RT varied depending on the position of the target. The search array was divided into left (columns 1 and 2 in Figure 5a) and right (columns 4 and 5) locations, and RT was averaged across locations. RT was analyzed using a 2 (group: ASD, TD) × 2 (target location: left, right) mixed-model repeated-measures ANOVA. There was a main effect of location, F(1, 50) = 5.2, p = .027, ηp2 = 0.09, but no main effect of group, F(1, 50) = 0.53, p = .469, ηp2 = 0.01, or group by location interaction, F(1, 50) = 2.3, p = .133, ηp2 = 0.05. As illustrated in Figure 5c, while the ASD group was slower at finding targets appearing on the left compared to the TD group, they were faster at right-sided targets. Exploratory post-hoc paired-samples t-tests showed that TD children were significantly faster to targets appearing on the left compared to the right side of the array, t(29) = 2.7, p = .011, d = 0.51, but that there was no difference between RT for left and right targets in children with ASD, t(21) = 0.6, p = .581, d = 0.12.
Figure 5.
Heat maps representing RT for ASD and TD group (a). The difference between ASD and TD (ASD-TD) for RT at each possible target location (b). Cool colors (blues) represent target locations with faster RT for the ASD group; Warm colors (reds) represent target locations with faster RT for the TD group. Average RT for ASD and TD groups for targets appearing in left and right locations (c). Endpoint of initial saccades for ASD and TD groups on the left or right side of the search array (d). Scatter plot of RT and saccadic left versus right difference scores for ASD and TD individuals (e). Error bars represent ± 1 SEM.
Endpoint of initial saccade
Lastly, given RT results showing faster responses to left than right targets in the TD but not the ASD group, we examined the endpoint of initial saccades. Based on our response time findings and previous eye-tracking visual search studies (e.g., Zelinsky, 1996), we expected that TD individuals would be more likely to shift attention to the left side of the array. We divided the search array into left and right regions of interest by splitting the array down the center and calculated the percentage of saccades directed to either the left or the right side of the array. Saccadic endpoint was analyzed using a 2 (group: ASD, TD) × 2 (location: left, right) mixed-model repeated measures ANOVA. There was a significant main effect of location, F(1, 42) = 7.3, p = .010, ηp2 = 0.15, and a significant group by location interaction, F(1, 42) = 4.22, p = .046, ηp2 = 0.09, but no significant main effect of group, F(1, 42) = 0.0. As can be seen in Figure 5d, TD individuals were more likely to direct their initial saccade to the left side of the array, t(25) = 3.7, p = .001, d = 0.72, however, individuals with ASD did not show a bias to saccade to either the left or the right, t(17) = 0.4, p = .674, d = 0.10. Furthermore, one-sample t-tests showed that the percentage of leftward saccades was significantly greater than 50% for the TD, t(25) = 3.7, p = .001, d = 0.72, but not ASD group, t(17) = 0.4, p = .674, d = 0.10. Leftward bias in TD individuals was accompanied by significantly more saccades directed to the left in the TD compared to the ASD group, t(42) = 2.1, p = .046, d = 0.63. Leftward bias of initial saccades was associated with shorter RT to targets on the left versus the right side of the array, r(44) = .627, p < .001, (see Figure 5e) and decreased saccadic error, r(44) = .462, p = .002, among both TD and ASD participants.
Correlations with Symptom Severity
We examined the relationship between response time and eye-tracking measures and ADI-R and ADOS scores. Correlational analyses focused on target absent search, as this condition was uniquely sensitive to ASD search superiority, as well as measures of reduced search bias that may underlie accelerated search in ASD. We were interested in whether our measures of target absent search were related to social and communicative symptoms in children with ASD. Faster target absent RT (collapsed across discrimination/selection conditions) was inversely related to scores in the ADI-R social, r(22) = −.49, p = .021, and communication domains, r(22) = −.55, p = .008, indicating that greater social and communication impairment was associated with faster target absent search. Eye-tracking but not response time measures of left versus right search asymmetry were also correlated with social and communication deficits (see Table 2); greater percentages of initial leftward saccades (i.e., the pattern present in TD individuals) were associated with decreased ASD symptomatology (especially for target absent trials), whereas a bilateral pattern or rightward bias was associated with greater ASD symptomatology. Additionally, decreased saccadic error was also associated communication, r(18) = −.475, p = .046, and to a lesser extent, social, r(18) = −.424, p = .080, domains on the ADI-R. Partial correlations controlling for verbal IQ showed similar or more robust findings. There were no significant correlations for the ADOS.
Table 2.
ADI-R Correlations with Visual Search Measures
| Absent RT | RT Left vs. Right difference score | Saccade left vs. right difference score | Absent saccade left vs. right difference score | Saccadic error | |
|---|---|---|---|---|---|
| Social | −.49* (−.31) | −.30 (−.32) | −.36 (−.76*) | −.40 (−.81*) | −.42 (−.43) |
| Communication | −.55* (−.48*) | −.15 (−.27) | −.47 (−.65*) | −.53* (−.73*) | −.48* (−.46) |
| Repetitive Behaviors | −.28 (−.18) | −.29 (−.32) | −.06 (−.16) | .01 (−.09) | −.30 (−.28) |
Correlation coefficient (partial correlation controlling for VIQ)
p < .05
Discussion
Contrary to our hypotheses, differences in performance between ASD and TD individuals did not vary with the difficulty of discrimination or selection of search items. Rather, consistent with the bulk of the ASD visual search research, children and adolescents with ASD were faster than their TD peers on target absent trials. Analyses probing the mechanisms underlying accelerated absent search showed that faster responses were not due to speed-accuracy trade-off, response bias, lower quitting thresholds, or lack of post-error slowing in ASD. Instead, our findings indicate that absent advantage is related to a lack of search asymmetry, which may confer a specific advantage when the target is not present. Lastly, faster target absent search and measures of search asymmetry were related to ASD symptom severity.
Effects of Discrimination and Selection
In the current study, we varied discriminability (gaps of distractors Cs) and selectivity (width of distractor Cs) to determine whether enhanced lower-level discrimination or peripheral selection (potentially due to increased perceptual load) were related to superior visual search in ASD. Responses were slower for hard compared to easy discrimination and selection, indicating that our task manipulations affected performance as intended. However, there were no interactions between these factors and group, such that varying levels of discrimination and selection did not result in relatively faster ASD performance in any condition. These results contradict previous studies, which have shown that the ASD visual search advantage increases with increasing target-distractor similarity (O’Riordan & Plaisted, 2001), but do confirm previous findings of superior target absent search (discussed in greater detail below).
Effects of discrimination and selection on patterns of eye-movements also did not vary across TD and ASD groups. However, consistent with the findings of Kemner and colleagues (2008a), we did find that individuals with ASD make fewer fixations, and in particular fewer fixations to thin, more target-like distractors. In contrast to the hypothesis outlined by Kemner and colleagues (2008b), fewer fixations (of similar duration) could be indicative of enhanced selection processes. More recently, Remington and colleagues (2009; 2012) have demonstrated that individuals with ASD may have increased perceptual capacity. If perceptual capacity is increased in individuals with ASD, they may be able to process more task (ir)relevant information in the search array, explaining their superior performance at larger set sizes and in target absent conditions (Milne et al., 2013).
Reduced saccadic error in the ASD group found in the current study may also support the increased perceptual capacity hypothesis. That is, if immediately after the onset of the search array more items are processed, then the target is more likely to be encoded and the initial saccade directed towards that location. Moreover, the proximity of the target to foveal vision is an important factor in determining whether subsequent saccades are directed towards the target (Findlay et al., 2001), and could also explain why we and others have found reduced number of fixations. Both decreased fixation frequency and saccadic error lend support to the hypothesis that enhanced perceptual capacity may contribute to accelerated search in ASD. Greater perceptual capacity may allow individuals with ASD to process more distractors, resulting in greater probability of locating the target and directing attention towards the location, decreasing saccadic error and the number of fixations necessary to locate target, and thus reducing search times.
Why are Individuals with ASD Consistently Faster on Absent Trials?
That accelerated search is more pronounced in absent trials has been noted in the earliest research examining visual search in ASD (O’Riordan et al., 2001), and while multiple studies have since reported a similar finding (Hessels et al., 2014; Joseph et al., 2009; O’Riordan, 2000; O’Riordan & Plaisted, 2001), it remains unknown as to why this is the case. What is it about autistic attentional or perceptual processes that convey this advantage for target absent trials? O’Riordan and colleauges (2001) proposed that the target-absent advantage may be due to removal of ceiling effects; the ASD visual search advantage is commonly augmented in more difficult search conditions, including those with larger set sizes, greater target-distractor similarity, and when the target is absent. Alternatively, these authors hypothesized that if the presence of the target is more salient, then the absence of the target may also be clearer to individuals with ASD, speeding target-absent decisions. A third proposal, made by Milne and colleagues (2013), contends that attention to irrelevant information (i.e., distractors) may be due to increased perceptual capacity, and could potentially result in accelerated absent search (and faster RT at larger set sizes) as each item in the array will be processed faster.
Understanding superior performance in target absent search is not trivial as absent trials are particularly hard to interpret. Responses in target present trials reflect the fact the participants have located the target; however, how and when do individuals decide to terminate search? Chun and Wolfe (1996) have proposed an activation threshold model of target absent search. Briefly, their model posits that an activation threshold is used as a cutoff criterion for ending search. During target present search, observers examine distractors that exceed average target activation until the target is located; for target absent search, individuals visit all distractors above some activation threshold and then terminate search. Using this model, we examined factors that might explain why visual search performance in ASD is particularly enhanced for target absent trials.
Similar to previous ASD visual search studies, we did not find differences in error rates or the presence of correlations between error rate and RT, thus ruling out speed-accuracy trade-offs. We found no difference in measures that reflect response biases, suggesting that differences in target absent RT are not due to a greater predisposition to respond “no” (i.e., that the target is absent) in ASD. We further examined error trials focusing on response time associated with miss errors (i.e., responding that the target is absent, when a target is present). Miss errors occur when the observer quits too soon, i.e., when a quitting threshold is reached prior to target identification. Frequency of miss errors was not consistently higher, and while overall miss RT was faster in individuals with ASD compared to TD individuals, this difference was not robust and was only significant for one condition (i.e., DESE). This suggests that faster target absent RT is not due to a lower quitting threshold in ASD (which would also predict that individuals with ASD would make more miss errors, which was not the case).
Chun and Wolfe (1996) demonstrated that visual search RTs are not universally slower following an error, but that RT after a miss trial RT is slowed for target absent but not present trials. Prior studies have shown impaired error monitoring and the absence of post-error slowing of RT (Bogte et al., 2007; Russell & Jarrold, 1998; Sokhadze et al., 2010). However, contrary to these findings, we did not find evidence of reduced post-miss slowing in ASD.
An interesting and unexpected pattern emerged when we examined target present RT by target location and direction of initial saccade. Similar to previous studies, we found better performance to targets appearing on the left side of the array for our TD group (Fecteau, Enns, & Kingstone, 2000; Zelinsky, 1996). However, this left-side bias was not present in individuals with ASD. Furthermore, prior visual search eye-tracking studies have also shown that a disproportionate number of initial saccades are directed towards the (upper) left quadrant (Zelinsky, 1996), and that initial saccades to the left are more common on difficult, inefficient searches compared to easy, efficient searches (Williams & Reingold, 2001). Consistent with our response time results, TD individuals, but not individuals with ASD, showed a leftward bias in saccadic endpoint. The absence or reduction of this attentional bias may allow individuals with ASD to conduct a faster search for both target absent trials and during searches in which target activation is limited (e.g., increasing target-distractor similarity). As discussed above, because target proximity is an important factor for determining whether the target is fixated on the subsequent eye-movement (Findlay et al., 2001), if non-biased saccades bring the target within greater proximity, it is more likely to be found, facilitating search in ASD. For target absent trials, unbiased search may result in a quicker decision to reject the presence of a distractor as areas above activation threshold may be visited faster in ASD.
One potential mechanism for reduced asymmetry is increased perceptual capacity. In TD individuals, the frequency of saccades directed to the left-side of the array increased in harder search trials (Williams & Reingold, 2001), which may indicate that in the absence of sufficient target activation TD individuals default to shift left. Greater perceptual capacity in ASD may result in increased processing of (ir)relevant information within the array and potential greater target activation despite harder search conditions, thus resulting in less spatial bias of saccades and equivalent RT for targets appearing on the left or the right side of the array. With respect to target absent trials because more items within the array can be processed, individuals with ASD may be more likely to direct their attention to regions that are above the activation threshold. Because individuals with ASD are more likely to visit these areas upon initial inspection, they may terminate search faster.
Associations with ASD Symptomatology
In agreement with previous studies that have shown an association between ASD search superiority and ASD symptom severity (Gliga et al., 2015; Joseph et al., 2009; Keehn, Shih, et al., 2013), our correlational analyses demonstrated that faster absent search, reduced leftward bias, and decreased saccade error were associated with increased social and communicative deficits in ASD. This result adds to the small but growing body of evidence that suggests that non-social visual spatial processing strengths are related to sociocommunicative impairments in ASD (see also, Joseph, Tager-Flusberg, & Lord, 2002). However, we cannot determine if the attentional and perceptual mechanisms involved in superior search contribute directly to the development of the core symptoms in ASD or if they reflect relatively independent manifestations of the atypical brain architecture that underlies ASD.
Recently, Gliga and colleagues (2015) demonstrated that visual search abilities at 9-months were predictive of ASD symptoms at 15- and 24-months for both low- and high-risk infants, and concluded that differences in domain-general processes of attention and perception may contribute to the emergence of ASD. Although certain developmental pathways have been proposed to result in enhanced visual search abilities in ASD (Keehn, Müller, & Townsend, 2013), our results do not directly test these hypotheses.
An alternative argument is that enhanced visual search and social and communicative deficits associated with ASD may reflect a more general disruption of brain development, which affects multiple domains (Tager-Flusberg & Joseph, 2003). Atypical early brain growth trajectories may interfere with the specialization of lateralized networks responsible for social and communicative function in ASD (e.g., Eyler, Pierce, & Courchesne, 2012; Keehn, Vogel-Farley, Tager-Flusberg, & Nelson, 2015). In addition, prior research has demonstrated that the distribution of attention is not equal to left and right sides of space (Foulsham, Gray, Nasiopoulos, & Kingstone, 2013), reflecting a right-lateralized attention network. Typically developing individuals in the present study demonstrated this bias, more consistently directing their attention to the left side of the array. However, this attentional bias was not present in individuals with ASD, potentially reflecting reduced hemispheric specialization of attention in ASD. Importantly, correlations between the endpoint of initial saccades and ASD symptom severity showed that a lack of leftward visual-attentional asymmetry is related to greater sociocommunicative deficits. This result is consistent with the finding that individuals with higher autistic traits show reduced lateralization on a greyscales task (English, Maybery, & Visser, 2015), and may suggest that the association between visual-spatial processing strengths and sociocommunicative weaknesses result from generalized reduction in the hemispheric specialization of neurocognitive networks in ASD.
Limitations
Although there were no difference in the percentage of looking time to search arrays between ASD and TD groups, our data collection methods precluded examination of group differences in calibration precision and accuracy. Given that group differences in calibration and data quality may impact eye-movement measures (Nystrom, Andersson, Holmqvist, & van de Weijer, 2013; Wass, Forssman, & Leppanen, 2014), future eye-tracking studies should include metrics of calibration accuracy and data quality.
Conclusion
Consistent with previous reports, we found accelerated target-absent search in ASD. Analyses investigating ASD absent advantage demonstrated that faster RT to target absent trials was not due to speed-accuracy trade-off, response bias, quitting threshold, or post-error slowing. Rather, absent advantage may be due to reduced leftward search bias in ASD. These findings together with fewer fixations and reduced saccadic error in target present trials lends support to the hypothesis that enhanced perceptual load may contribute to superior search in ASD. Additionally, indices of target absent search and reduced search bias were related ASD symptom severity, indicating that mechanisms underlying absent search advantage may have explanatory significance with regard to the development of ASD social and communicative impairments.
Acknowledgments
This research was funded by NIDCD grant U19 DC 03610 and by NIMH grant K01 MH 073944. The authors are especially grateful to the children and families who participated in this study.
References
- APA. Diagnostic and statistical manual of mental disorders. 5. Washington D.C: American Psychological Association; 2013. [Google Scholar]
- Blaser E, Eglington L, Carter AS, Kaldy Z. Pupillometry reveals a mechanism for the Autism Spectrum Disorder (ASD) advantage in visual tasks. Sci Rep. 2014;4:4301. doi: 10.1038/srep04301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogte H, Flamma B, van der Meere J, van Engeland H. Post-error adaptation in adults with high functioning autism. Neuropsychologia. 2007;45(8):1707–1714. doi: 10.1016/j.neuropsychologia.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Chun MM, Wolfe JM. Just say no: How are visual searches terminated when there is no target present? Cognitive Psychology. 1996;30(1):39–78. doi: 10.1006/cogp.1996.0002. [DOI] [PubMed] [Google Scholar]
- English MC, Maybery MT, Visser TA. Individuals with Autistic-Like Traits Show Reduced Lateralization on a Greyscales Task. J Autism Dev Disord. 2015 doi: 10.1007/s10803-015-2493-7. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(Pt 3):949–960. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Enns JT, Kingstone A. Competition-induced visual field differences in search. Psychol Sci. 2000;11(5):386–393. doi: 10.1111/1467-9280.00275. [DOI] [PubMed] [Google Scholar]
- Findlay JM. Saccade target selection during visual search. Vision Res. 1997;37(5):617–631. doi: 10.1016/s0042-6989(96)00218-0. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Brown V, Gilchrist ID. Saccade target selection in visual search: the effect of information from the previous fixation. Vision Res. 2001;41(1):87–95. doi: 10.1016/s0042-6989(00)00236-4. [DOI] [PubMed] [Google Scholar]
- Foulsham T, Gray A, Nasiopoulos E, Kingstone A. Leftward biases in picture scanning and line bisection: a gaze-contingent window study. Vision Res. 2013;78:14–25. doi: 10.1016/j.visres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Gliga T, Bedford R, Charman T, Johnson MH The BASIS Team. Enhanced Visual Search in Infancy Predicts Emerging Autism Symptoms. Curr Biol. 2015;25(13):1727–1730. doi: 10.1016/j.cub.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessels RS, Hooge IT, Snijders TM, Kemner C. Is there a limit to the superiority of individuals with ASD in visual search? J Autism Dev Disord. 2014;44(2):443–451. doi: 10.1007/s10803-013-1886-8. [DOI] [PubMed] [Google Scholar]
- Hooge IT, Erkelens CJ. Peripheral vision and oculomotor control during visual search. Vision Res. 1999;39(8):1567–1575. doi: 10.1016/s0042-6989(98)00213-2. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Dev Sci. 2009;12(6):1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002;43(6):807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldy Z, Giserman I, Carter AS, Blaser E. The Mechanisms Underlying the ASD Advantage in Visual Search. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Dev Sci. 2011;14(5):980–988. doi: 10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test (2nd edition) Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Keehn B, Joseph RM. Impaired prioritization of novel onset stimuli in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2008;49(12):1296–1303. doi: 10.1111/j.1469-7610.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- Keehn B, Müller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Shih P, Brenner LA, Townsend J, Müller RA. Functional connectivity for an “island of sparing” in autism spectrum disorder: an fMRI study of visual search. Hum Brain Mapp. 2013;34(10):2524–2537. doi: 10.1002/hbm.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder. Autism Res. 2015;8(2):187–198. doi: 10.1002/aur.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, van Ewijk L, van Engeland H, Hooge I. Brief report: eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. Journal of Autism and Developmental Disorders. 2008a;38(3):553–557. doi: 10.1007/s10803-007-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, van Ewijk L, van Engeland H, Hooge I. Brief report: eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. J Autism Dev Disord. 2008b;38(3):553–557. doi: 10.1007/s10803-007-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Obervation Schedule - WPS (ADOS-WPS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Milne E, Dunn SA, Freeth M, Rosas-Martinez L. Visual search performance is predicted by the degree to which selective attention to features modulates the ERP between 350 and 600ms. Neuropsychologia. 2013;51(6):1109–1118. doi: 10.1016/j.neuropsychologia.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Nystrom M, Andersson R, Holmqvist K, van de Weijer J. The influence of calibration method and eye physiology on eyetracking data quality. Behav Res Methods. 2013;45(1):272–288. doi: 10.3758/s13428-012-0247-4. [DOI] [PubMed] [Google Scholar]
- O’Riordan M. Superior modulation of activation levels of stimulus representations does not underlie superior discrimination in autism. Cognition. 2000;77(2):81–96. doi: 10.1016/s0010-0277(00)00089-5. [DOI] [PubMed] [Google Scholar]
- O’Riordan M. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, Plaisted K. Enhanced discrimination in autism. Q J Exp Psychol A. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- O’Riordan M, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. J Exp Psychol Hum Percept Perform. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: a research note. Journal of Child Psychology and Psychiatry. 1998;39(5):777–783. [PubMed] [Google Scholar]
- Remington A, Swettenham J, Campbell R, Coleman M. Selective attention and perceptual load in autism spectrum disorder. Psychol Sci. 2009;20(11):1388–1393. doi: 10.1111/j.1467-9280.2009.02454.x. [DOI] [PubMed] [Google Scholar]
- Remington A, Swettenham JG, Lavie N. Lightening the load: perceptual load impairs visual detection in typical adults but not in autism. J Abnorm Psychol. 2012;121(2):544–551. doi: 10.1037/a0027670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Jarrold C. Error-correction problems in autism: evidence for a monitoring impairment? J Autism Dev Disord. 1998;28(3):177–188. doi: 10.1023/a:1026009203333. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview - Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Shen J, Reingold EM, Pomplun M, Williams DL. Saccadic selectivity during visual search: The influence of central processing difficulty. In: Hyona J, Radach R, Deubel H, editors. The Mind’s Eye: Cognitive and Applied Aspects of Eye Movement Research. Amsterdam, Netherlands: Elsevier; 2003. [Google Scholar]
- Sokhadze E, Baruth J, El-Baz A, Horrell T, Sokhadze G, Carroll T, …Casanova MF. Impaired Error Monitoring and Correction Function in Autism. J Neurother. 2010;14(2):79–95. doi: 10.1080/10874201003771561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods Instruments & Computers. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass SV, Forssman L, Leppanen J. Robustness and Precision: How Data Quality May Influence Key Dependent Variables in Infant Eye-Tracker Analyses. Infancy. 2014;19(5):427–460. [Google Scholar]
- Williams DE, Reingold EM. Preattentive guidance of eye movements during triple conjunction search tasks: the effects of feature discriminability and saccadic amplitude. Psychon Bull Rev. 2001;8(3):476–488. doi: 10.3758/bf03196182. [DOI] [PubMed] [Google Scholar]
- Zelinsky GJ. Using eye saccades to assess the selectivity of search movements. Vision Res. 1996;36(14):2177–2187. doi: 10.1016/0042-6989(95)00300-2. [DOI] [PubMed] [Google Scholar]