Abstract
Inosine is an endogenous purine nucleoside that is produced by catabolism of adenosine. Adenosine has a short half-life (approximately 10 s) and is rapidly deaminated to inosine, a stable metabolite with a half-life of approximately 15 h. Resembling adenosine, inosine acting through adenosine receptors (ARs) exerts a wide range of anti-inflammatory and immunomodulatory effects in vivo. The immunomodulatory effects of inosine in vivo, at least in part, are mediated via the adenosine A2A receptor (A2AR), an observation that cannot be explained fully by in vitro pharmacological characterization of inosine at the A2AR. It is unclear whether the in vivo effects of inosine are due to inosine or a metabolite of inosine engaging the A2AR. Here, utilizing a combination of label-free, cell-based, and membrane-based functional assays in conjunction with an equilibrium agonist-binding assay we provide evidence for inosine engagement at the A2AR and subsequent activation of downstream signaling events. Inosine-mediated A2AR activation leads to cAMP production with an EC50 of 300.7 μM and to extracellular signal-regulated kinase-1 and -2 (ERK1/2) phosphorylation with an EC50 of 89.38 μM. Our data demonstrate that inosine produces ERKl/2-biased signaling whereas adenosine produces cAMP-biased signaling at the A2AR, highlighting pharmacological differences between these two agonists. Given the in vivo stability of inosine, our data suggest an additional, previously unrecognized, mechanism that utilizes inosine to functionally amplify and prolong A2AR activation in vivo.
Keywords: Inosine, A2AR, adenosine, signaling bias, cAMP, ERK1/2
1. Introduction
Inosine is an endogenous purine nucleoside formed by deamination of adenosine. As with adenosine, it is produced and released into the extracellular space during normal cell metabolism. Adenosine has a short half-life of less than 10 s in vivo [1] and is rapidly deaminated to inosine by adenosine deaminase. Inosine has a much longer in vivo half-life than adenosine of approximately 15 h [2]. In interstitial fluids, the basal level of inosine is in the micromolar range and can be two to seven times that of adenosine [3-6], During ischemia and sepsis interstitial inosine levels rise dramatically and in certain disease states can be greater than 1 mM [7] and remain elevated longer than those of adenosine [8], Consequently, inosine levels become markedly greater than those of adenosine under disease conditions (>10-fold; [3-6]). The biological significance of the high levels of inosine in vivo is poorly understood.
As has been observed for adenosine, inosine exerts a wide variety of anti-inflammatory and immunomodulatory effects in vivo and in vitro. These include inhibition of proinflammatory cytokine and chemokine production [9-11], stimulation of anti-inflammatory cytokine production [9], protection from TNBS-induced colitis [12], LPS-induced endotoxemia [13,14], LPS-induced acute lung injury [10], glycodeoxycholic acid-induced acute pancreatitis [15], streptozotocin-induced and non-obese type 1 diabetes [16] as well as improvement of islet transplant survival [17]. However, the molecular mechanisms underlying the anti-inflammatory and immunomodulatory effects of inosine are incompletely understood. They do not require cellular uptake of inosine, suggesting involvement of cell surface receptors in this process. However, the presence of cell-surface receptors specific for inosine has not been documented. It is widely stated that the biological effects of inosine are mediated through ligation of specific membrane-bound G protein-coupled receptors (GPCRs) termed P1-purinoceptors, also known as adenosine receptors (AR; [18]).
There are four pharmacologically distinct adenosine receptor subtypes, termed A1R, A2AR, A2BR and A3R [19], A2AR and A2BR are coupled to the stimulatory G protein Gαs while A1R and A3R are coupled to inhibitory G protein Gi [19]. Thus, adenosine occupation at the A2AR and A2BR leads to an increase in intracellular cAMP levels, whereas adenosine ligation at the A1R and A3R results in a reduction in intracellular cAMP levels. Among the four AR subtypes, A2AR is the most effective in downregulating inflammation through modulation of intracellular cAMP levels. Although inosine is a functional agonist for A1R and A3R, with EC50 values of 290 μM and 0.25 μM, respectively, inosine-mediated anti-inflammatory effects resemble those of the activated A2AR. In vivo studies utilizing receptor knockout mice demonstrate that the anti-inflammatory properties of inosine are mediated in part through the A2AR [20,21], Additionally, inosine-mediated inhibition of the production of the pro-inflammatory cytokine TNF-α following LPS-stimulation of peritoneal macrophages is partially reversed by the A2AR-selective antagonist DMPX in vitro [9]. Nevertheless, efforts directed toward a comprehensive demonstration of inosine as a functional agonist at the A2AR at the molecular and cellular level or inosine’s ability to bind directly to the A2AR have been unsuccessful. Hence, inosine is largely considered to be inactive at the A2AR [22] in spite of a large body of in vivo data suggesting otherwise.
To understand the molecular mechanisms underlying the inosine-mediated anti-inflammatory and immunomodulatory effects, we examined inosine agonism at both the human and mouse A2ARs utilizing a variety of cell-based and membrane-based assays. Here we demonstrate that inosine not only binds to the A2AR but also activates A2AR -mediated cAMP production and ERK1/2 phosphorylation. To the best of our knowledge, this is the first comprehensive demonstration of inosine functional agonism at the A2AR. Our data suggest the existence of a sustained A2AR receptor signaling mechanism that is driven by inosine long after its more potent predecessor, adenosine, has been degraded, adding a new dimension to the anti-inflammatory and immunomodulatory role of the A2AR.
2. Materials and Methods
2.1. Materials
NECA, CGS 21680, and ZM241385 were purchased from Tocris Biosciences. [3H]CGS 21680 was purchased from PerkinElmer Corporation. Rolipram, adenosine, inosine, inosine monophosphate, xanthine, hypoxanthine, adenosine 5′ [α,β-methylene] diphosphate and dipyridamole were purchased from Sigma-Aldrich.
2.2. Cell culture
CHO-K1 cells stably expressing human A2AR (CHO-hA2AR; [23]) and human A2BR (CHO-hA2BR; [23]) were grown in DMEM/F-12 (1:1) supplemented with 10 % FBS, 2 mM glutamine and G418 (0.2 mg/ml). CHO-K1 cells transiently expressing mouse A2AR (mA2AR) were grown in the same medium without G418. All cells were maintained at 37 °C in a 5 % CO2 incubator.
2.3. CHO-K1 transfection
CHO-K1 cells were transiently transfected with either a pCMV6 vector bearing the mA2AR gene or the pCMV6 vector alone using Lipofectamine 3000 (Life Technologies) according to the manufacturer’s suggested protocol. Transfected cells were cryopreserved in DMEM/F-12 (1:1) containing 5 % DMSO at −195 °C (liquid nitrogen).
2.4. Dynamic mass redistribution (DMR) assay
DMR responses were assessed using an EnSpire multimode plate reader (PerkinElmer) according to a manufacturer-suggested protocol. Briefly, CHO-K1 and CHO-hA2AR cells were seeded onto EnSpire-LFC, 96-well microplates (PerkinElmer) and cultured for 18 h at 37 °C in a 5% CO2 incubator. Before the assay, cells were washed with assay buffer (HBSS with 20 mM HEPES containing either 1 % DMSO alone or 100 nM ZM 241385 and 1 % DMSO) and allowed to equilibrate for 2 h at room temperature near the EnSpire multimode plate reader. Initial baseline DMR measurements were collected for 5 min, agonists and inverse agonist were added and inducible DMR measurements were collected for an additional 15 min.
2.5. Cell-based cAMP assay
CHO-hA2AR [23], CHO-hA2BR [23] and CHO-K1 cells transiently expressing mA2AR were seeded in 96-well half-area white plates (Greiner bio-one; 5 × 103 cells/well for hA2AR and hA2BR; 1.5 × 104cells/well for mA2AR) in the absence of G418 20 h prior to assay. CHO-hA2AR cells and CHO-K1 cells transiently expressing mA2AR were washed twice with Hanks’ balanced salt solution (HBSS) and incubated in HBSS containing adenosine deaminase (ADA; 3 U/ml) and adenosine 5′ [α,β-methylene] diphosphate (50 μM) for 15 min at 37 °C. Cells were washed twice with HBSS to remove ADA and incubated with rolipram (50 μM), adenosine 5′ [α,β-methylene] diphosphate (50 μM), adenosine, and inosine in the presence or in the absence of dipyridamole at indicated concentration(s) for 10 min at 37 °C. Assay conditions for CHO-hA2BR cells were essentially the same as above with the exception of exclusion of ADA and adenosine 5′ [α,β-methylene] diphosphate pretreatment. Pretreatment was not required for cells expressing the A2BR since no baseline level cAMP signal was detected in the absence of exogenously added adenosine, likely due to the low affinity of the A2BR towards adenosine and the small amount of adenosine produced by the cells during the assay which was not sufficient to activate the A2BR. Intracellular cAMP levels were quantified using an HTRF assay kit (Cisbio).
2.6. Cell-free membrane-based cAMP assay
HEK293-hA2AR cell membranes (PerkinElmer) were incubated in HBSS, containing adenosine 5′ [α,β-methylene] diphosphate (50 μM) and ADA (3 U/ml) at 37 °C for 20 min. Membranes were washed twice with 33mM HEPES containing 0.1 % Tween 20 and stimulated with the same buffer containing 100 μM ATP, 2 μM GTP, 10 μM GDP, 2 μM MgCl2, 150 mM NaCl, 50 μM adenosine 5′ [α,β-methylene] diphosphate, 50 μM rolipram, and inosine (0–300 μM) or CGS 21680 (100 nM) in half-area white plates (Greiner bio-one; 4.5 μg protein/well) for 30 min at 37 °C. cAMP levels were quantified using an HTRF assay kit (Cisbio).
2.7. ERK1/2 phosphorylation assay
CHO-hA2AR cells [23] were seeded in 96-well plates (Greiner bio-one; 2.5 × 104 cells/well) in the absence of G418 20 h prior to assay. Medium was replaced with medium without serum and incubated for an additional 3 h. CHO-hA2AR cells were washed twice with HBSS and incubated in HBSS containing ADA (3 U/ml) and adenosine 5′ [α,β-methylene] diphosphate (50 μM) for 15 min at 37 °C. Cells were washed twice with HBSS to remove ADA and incubated with rolipram (50 μM), adenosine 5′ [α,β-methylene] diphosphate (50 μM), adenosine or inosine at indicated concentration(s) for 20 min at 37 °C. Assay was terminated by aspirating the assay buffer and incubating cells with lysis buffer (50 (μl/well) at room temperature with shaking for 10 min. Phospho ERK1/2 levels were detected using an Alphascreen Surefire kit (PerkinElmer) according the manufacturer suggested protocol. Briefly, 4 μl of the lysate was transferred to a ProxiPlate-384 (PerkinElmer) and incubated with 7 μl of assay detection mixture at room temperature in the dark for 2 h. Fluorescent emissions were quantified using an EnSpire multimode plate reader (PerkinElmer).
2.8. Radioligand displacement assay
The binding of inosine to hA2AR at functional concentrations (cell- and membrane-based cAMP and ERK1/2 phosphorylation) was evaluated using the [3H]-labeled A2AR-selective agonist CGS 21680. HEK293-hA2AR cell membranes (PerkinElmer; 10 μg/well) were pre-incubated in binding buffer (50 mM Tris, 10 mM MgCl2, 1 mM EDTA, 4 U/ml ADA, pH 7.4) in a 96-well MultiScreen plate (Millipore) at 27 °C for 15 min. After addition of varying concentrations of inosine and [3H]CGS 21680 (100 nM), plates were incubated for an additional 75 min followed by washing with cold PBS. Binding was quantified using a MicroBeta TriLux microplate scintillation counter (PerkinElmer). Specific binding was calculated by subtracting counts obtained in the presence of NECA (50 μM) from total counts.
2.9. Data analysis
Data were analyzed using GraphPad Prism Software. Dose response curves were generated by non-linear regression with a variable slope. Equilibrium binding of [3H]CGS 21680 was analyzed using one-way ANOVA with alpha equal to 0.05. Pooled EC50 values for cAMP production and ERK1/2 activation were compared using a paired t test.
3. Results
3.1. Inosine generates an A2AR-selective, inverse agonist-sensitive dynamic mass redistribution (DMR) signal in CHO-hA2AR cells
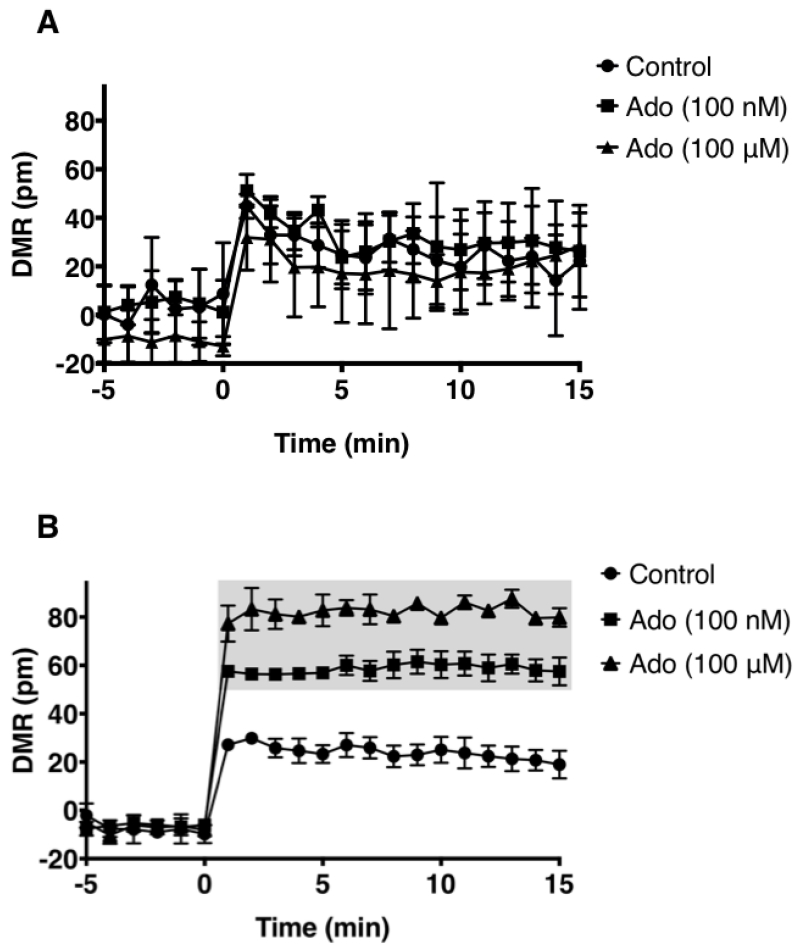
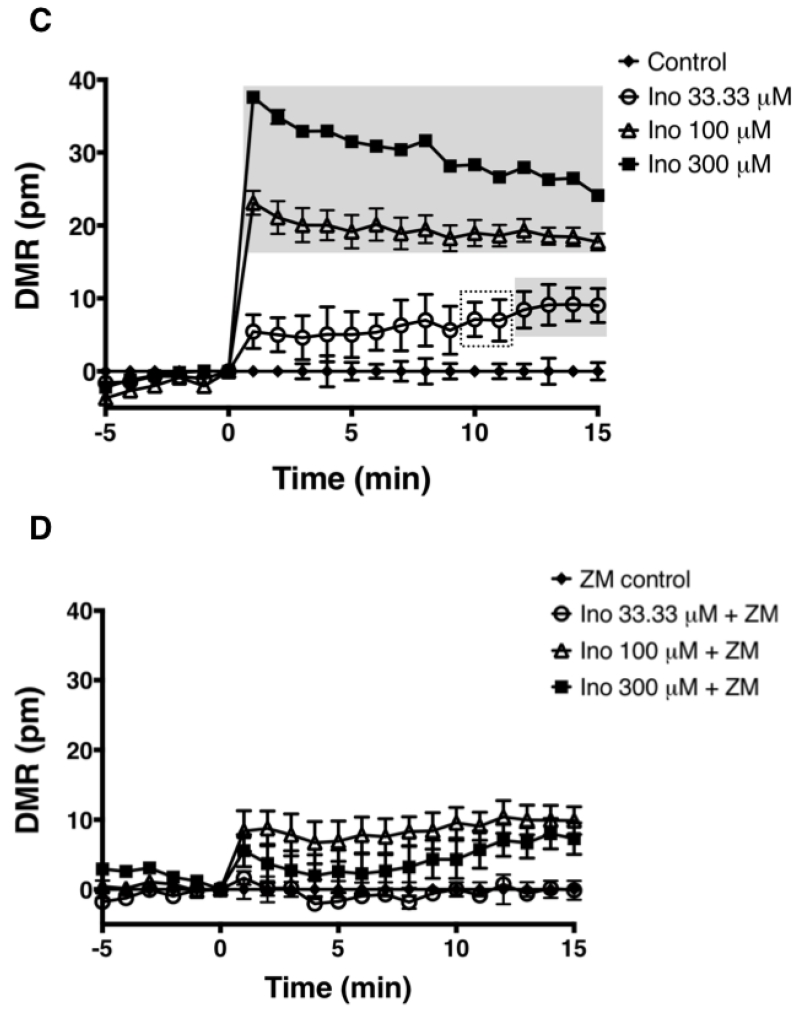
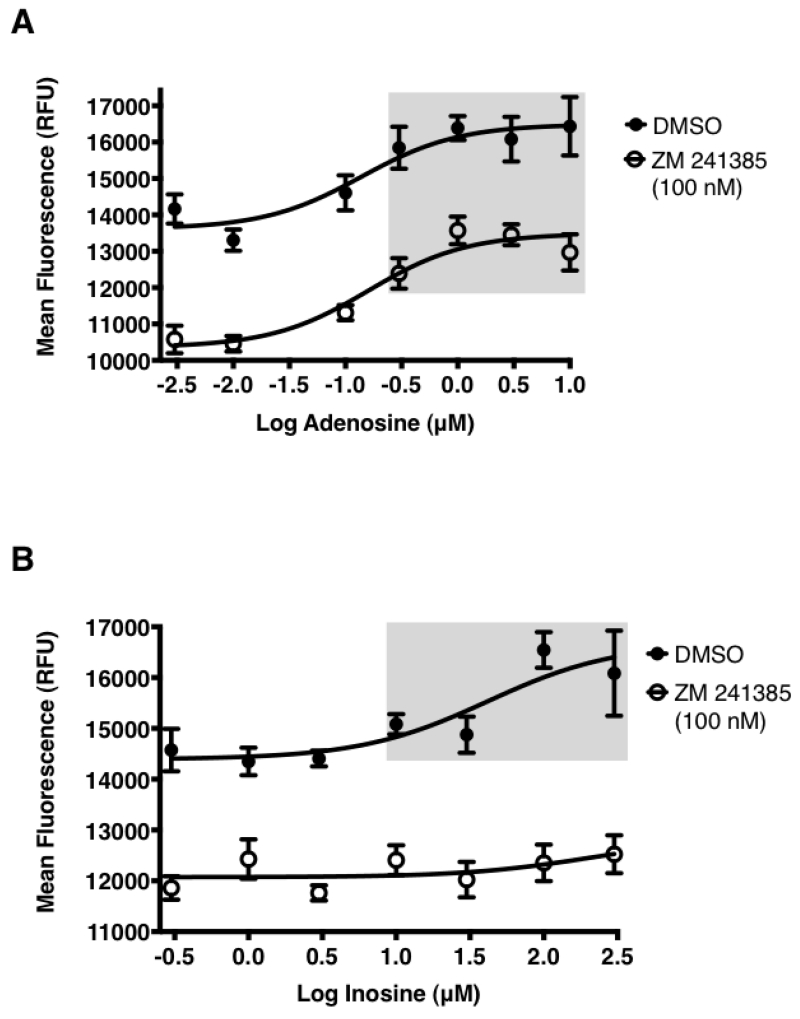
Signaling through the GPCR family of receptors is complex. Receptor coupling to more than one G protein subunit leading to different signaling outcomes is common. The A2AR couples to two different classes of Gα subunits. A2AR coupling to the Gαs and Gαolf subunits that belong to the Gs class stimulates adenylate cyclase to increase intracellular cAMP upon agonist engagement at the receptor [23], Agonist engagement at the A2AR also leads to ERK1/2 phosphorylation via either cAMP dependent or independent mechanisms [24], However, there are no reports demonstrating that inosine stimulates the A2AR to produce cAMP or ERK1/2 phosphorylation. This may be suggestive of weak stimulation by inosine, hence small differences in cAMP production and ERK1/2 phosphorylation are not readily detectable by the analyte-specific assay technologies utilized. We reasoned that an inosine-mediated, pathway-independent, global and integrated signal would be more detectable than individual, isolated, pathway-specific signals such as cAMP or phosphorylated ERK1/2. Thus we utilized a label-free, biosensor-enabled DMR assay capable of capturing and translating agonist-stimulated signaling events into real-time, quantitative, whole cell phenotypic responses. Applying DMR technology to characterize GPCR signaling in living cells has been well established [25]. Since the human A2AR (hA2AR) is the most pharmacologically characterized A2AR [26] relative to other species, we utilized the hA2AR for interrogating whole cell phenotypic responses emanating from inosine agonism at the A2AR. New knowledge gained here on the hA2AR will serve as a guide to the A2ARs of other species as this receptor is evolutionarily conserved in terms of sequence, structure and function. In CHO-K1 cells stably transfected with hA2AR (CHO-hA2AR), both adenosine (Fig. 1B) and inosine (Fig. 1C) dose-dependently triggered a whole cell DMR signal that was qualitatively different from that produced by the CHO-K1 cells (Fig. 1A). The A2AR-selective inverse agonist, ZM 241385, inhibited the inosine-induced DMR signal (Fig. 1D) indicating that inosine is capable of generating whole cell phenotypic responses through the A2AR.
Fig. 1.
Dynamic mass redistribution analysis of ARs on CHO-K1 and CHO-hA2AR cells.
DMR response traces of CHO-K1 (A) and CHO-hA2AR (B, C & D) stimulated with indicated concentrations of adenosine (Ado; A & B), inosine (Ino; C & D) and 100 nM ZM 241385 (ZM; D). All data are mean values ± SEM of a representative independent experiment (n=3). Data with p < 0.001 and p < 0.05 relative to control are indicated in shaded and dotted boxes, respectively.
3.2. Inosine dose-dependently enhances hA2AR- but not hA2BR-mediated cAMP production
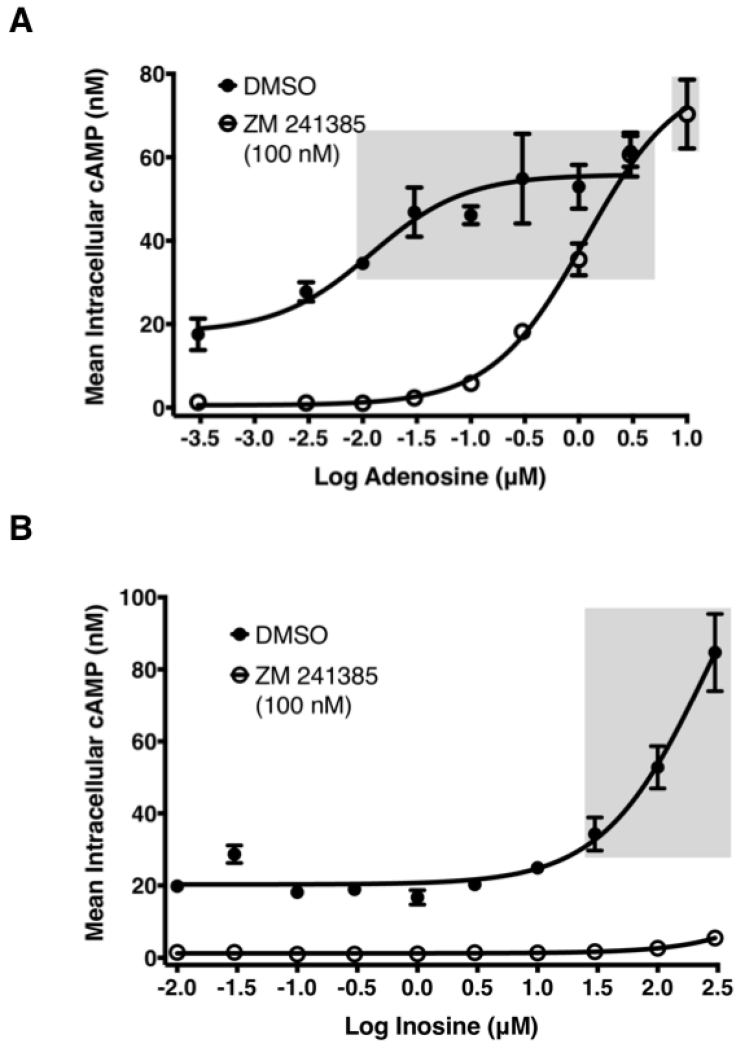
To examine whether inosine-mediated, A2AR-dependent whole cell phenotypic responses are manifestations of increased cAMP production, we evaluated the effects of inosine on cAMP production by CHO-hA2AR. We developed a highly sensitive cell-based cAMP assay by utilizing adenosine deaminase (ADA) to rid the cells of endogenously produced adenosine and adenosine 5′ [α,β-methylene] diphosphate, an inhibitor of ecto-5′-nucleotidase, to halt endogenous/exogenous production of adenosine and inosine. Using this approach we isolate and decrease background signaling and thereby enhance the signal generated by the addition of inosine. In this assay, adenosine increased cAMP production with an EC50 (half maximal effective concentration) of 6 ± 1 nM and an Emax (maximal stimulation) of 48 nM (Fig. 2A; Table 1). Similarly, inosine dose-dependently increased cAMP production with an EC50 of 300.7 ± 48.9 μM (Table 1) and Emax of 80 nM (Fig. 2B; Table 1). However, EC50 values for adenosine and inosine were significantly different (p < 0.0001). To rule out the unlikely possibility that hA2AR stimulation was due to the presence of adenosine as an impurity, we analyzed an 800 μM inosine stock solution by HPLC and found no trace of adenosine (≤ 0.025%). The inosine-induced increase in cAMP production was completely inhibitable by the A2AR inverse agonist ZM 241385, demonstrating the specificity of the inosine-hA2AR interaction (Fig. 2B; Table 1). As expected, ZM 241385 also inhibited adenosine-induced cAMP production however, the inhibition was partial (Fig 2A; Table 1; EC50=2.45 ± 1.29 μM). These results suggest a potency and/or a pharmacological difference between inosine and adenosine at the A2AR, and rule out the remote possibility of hA2AR stimulation by undetectable amounts of adenosine that may be present in inosine.
Fig. 2.
Effect of adenosine and inosine on hA2AR- and hA2BR-mediated intracellular cAMP production.
Adenosine stimulated hA2AR-mediated (A) and hA2BR-mediated (C) cAMP production, while inosine stimulated hA2AR-mediated (B) but not hA2BR-mediated (C) cAMP production. CHO-hA2AR (A and B) and CHO-hA2BR (C) cells were incubated with adenosine (A and C) and inosine (B and C) in the presence or in the absence of the hA2AR-selective inverse agonist ZM 241385 (A and B) for 10 min. Mean intracellular cAMP levels ± SEM of a representative experiment are shown (n=3). Shaded boxes indicate data with p < 0.001 relative to control. EC50 values with 95% confidence intervals (in parentheses) were 12(4–33) nM and 259.9(104–651) μM for adenosine and inosine respectively.
Table 1.
Summary of EC50 ± SEM for adenosine- and inosine-stimulated cAMP production and ERK1/2 phosphorylation at the hA2AR and mA2AR.
| Agonist/ Inverse Agonist |
EC50 (μM) | ||
|---|---|---|---|
| hA2AR | mA2AR | ||
| cAMP Assay |
ERK1/2 Phosphorylation Assay |
cAMP Assay |
|
| Adenosine | 0.006 ± 0.001 | 0.211 ± 0.036 | 0.059 ± 0.02 |
| Inosine | 300.7 ± 48.9 | 89.38 ± 15.12 | 709 ± 135.2 |
| Adenosine + ZM 241385 (100 nM) |
2.45 ± 1.29 | 1.17 ± 0.10 | 7.34 ± 4.56 |
| Inosine + ZM 241385 (100 nM) |
> 300 | > 300 | >1000 |
The A2BR also couples to Gαs, hence it stimulates cAMP production upon activation. Therefore, we addressed whether inosine increased cAMP production by CHO-K1 cells stably expressing the human A2BR (CHO-hA2BR). Although adenosine increased cAMP production by CHO-hA2BR cells, inosine did not, even at concentrations equal to and above those that stimulated cAMP production by CHO-hA2AR bearing cells (Fig. 2C). These results demonstrate that inosine engagement at the A2AR, but not at the A2BR, leads to stimulation of adenylate cyclase and a rise in intracellular cAMP levels.
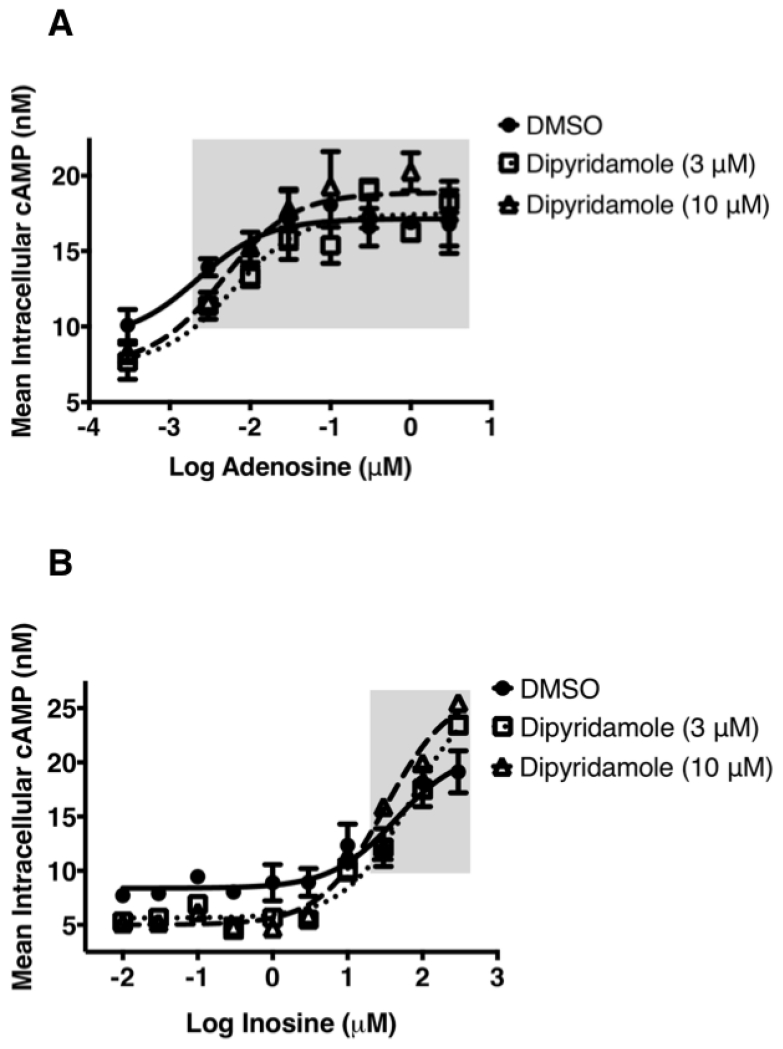
To examine the notion that inosine actions at the A2AR are indirect and that inosine influences the extracellular concentration of adenosine through equilibrative nucleoside transporter 1 (ENT1) and ENT2 to activate the A2AR, we utilized saturating concentrations of an ENT1 and ENT2 inhibitor, dipyridamole. Inhibition of ENT1 and ENT2 with dipyridamole slightly reduced the baseline while slightly increasing the maximal cAMP production in response to adenosine (Fig. 3A) indicating that this cell-based assay a) is minimally influenced by endogenously produced adenosine and b) faithfully reports A2AR activation by the exogenous agonist. Similarly, dipyridamole marginally reduced baseline cAMP levels while enhancing maximum inosine-mediated cAMP production (Fig. 3B) demonstrating that inosine directly and dose-dependently activates the A2AR.
Fig. 3.
Effect of ENT1/ENT2 inhibition on hA2AR-mediated intracellular cAMP production.
The ENT1 and ENT2 inhibitor dipyridamole marginally reduces baseline cAMP production and marginally enhances adenosine- and inosine-mediated cAMP production. CHO-hA2AR cells were incubated with adenosine (A) and inosine (B) in the presence or in the absence of the indicated concentrations of dipyridamole for 10 min. Mean intracellular cAMP levels ± SEM of a representative experiment are shown (n=4). Shaded boxes indicate data with p < 0.001 relative to control.
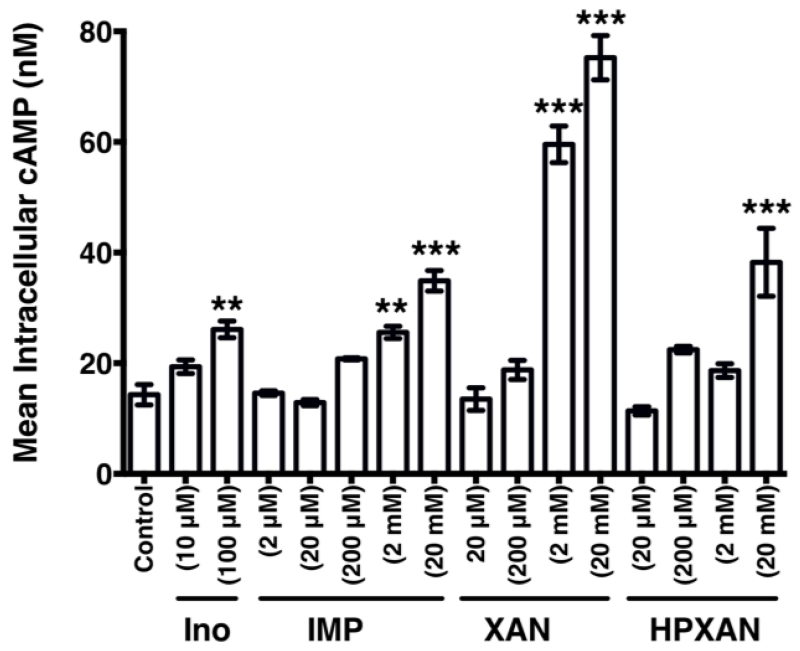
We also evaluated the effects of metabolites of inosine on A2AR function. As shown in Fig. 4, inosine monophosphate is approximately 20-fold less potent than inosine in activating the A2AR. We also observed that xanthine is more potent than inosine monophosphate whereas hypoxanthine is equipotent to inosine monophosphate at the A2AR (Fig. 4) indicating that inosine metabolites are poor agonists relative to adenosine (EC50=0.006 ± 0.001 μM) and inosine (EC50= 300.7 ± 48.9 μM) at the receptor.
Fig. 4.
Metabolites of inosine are less potent in inducing hA2AR-mediated intracellular cAMP production than inosine.
CHO-hA2AR cells were incubated with the indicated concentrations of inosine (Ino), inosine monophosphate (IMP), xanthine (XAN) and hypoxanthine (HPXAN). Mean intracellular cAMP levels ± SEM of a representative experiment are shown (n=3; **, P < 0.01; *** p < 0.001 vs control).
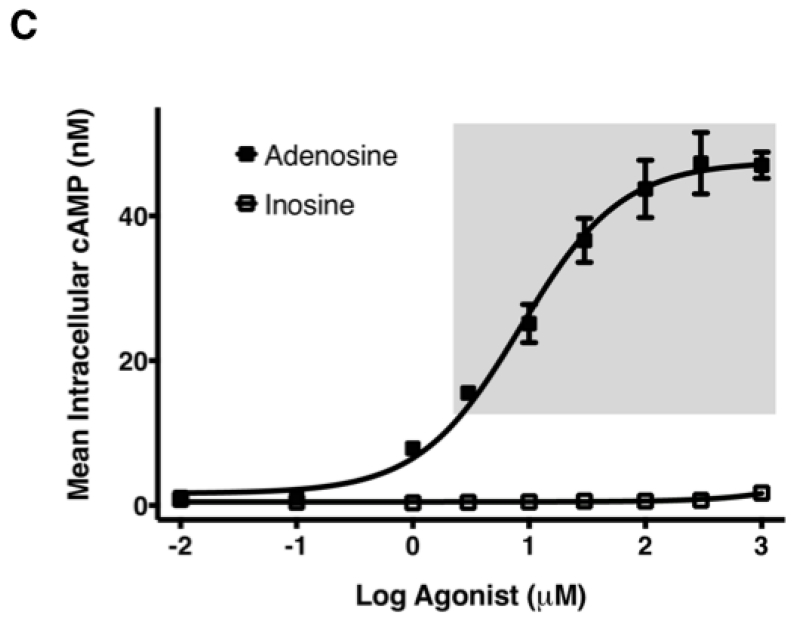
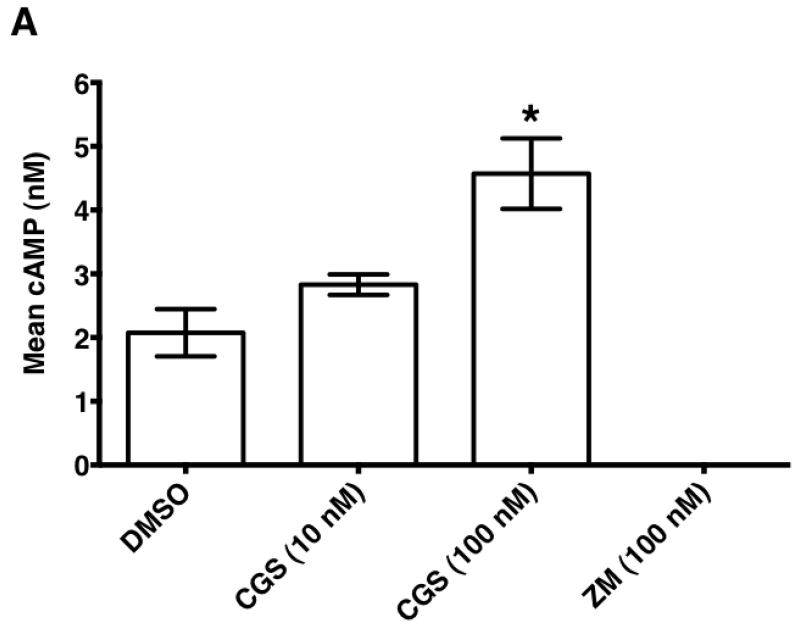
3.3. Inosine dose-dependently increases mouse A2AR (mA2AR)-mediated intracellular cAMP production
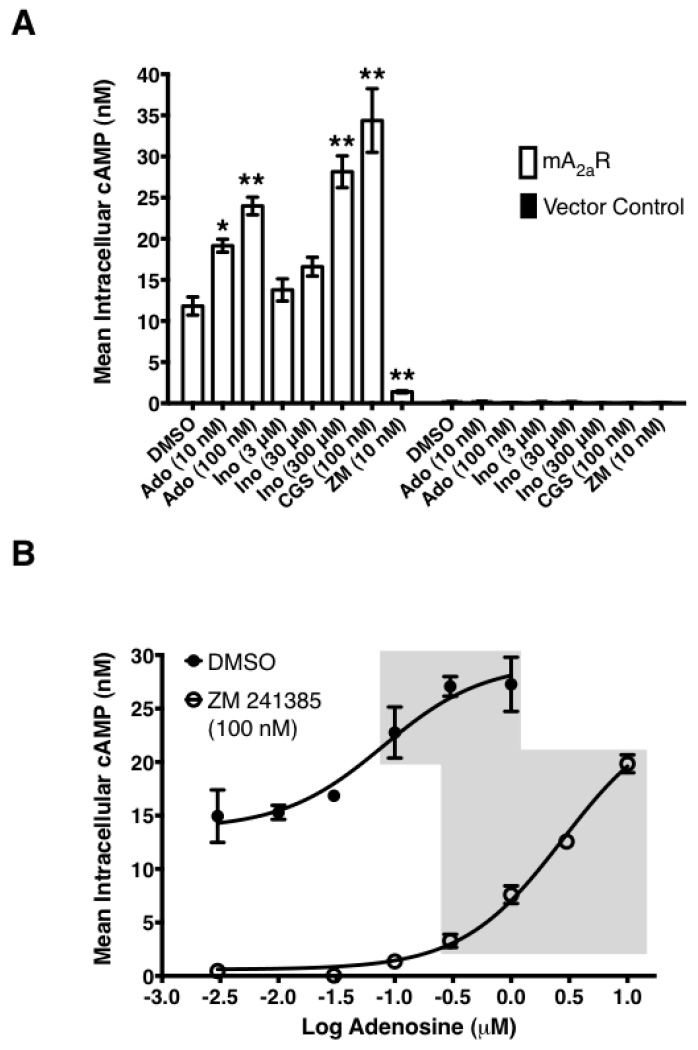
In mouse models of human disease, the role of the A2AR in inosine-mediated inhibition of inflammation [12-15] and pain [27] has been convincingly demonstrated utilizing gene ablation and pharmacological tools. As inosine can be readily converted to adenosine in vivo, it is not clear whether the in vivo therapeutic effects of inosine mediated via the A2AR are direct or indirect in nature. We therefore investigated the ability of inosine to activate the mA2AR in CHO-K1 cells transiently expressing the receptor to produce cAMP. CHO-K1 cells were chosen as the host cells as they do not have endogenous expression of any adenosine receptor subtype [23]. Both adenosine and the A2AR -selective agonist CGS 21680 increased the intracellular production of cAMP by CHO-K1 cells transiently expressing mA2AR, while the A2AR-selective inverse agonist ZM 241385 inhibited it (Fig. 5A). In contrast, vector-transfected CHO-K1 cells did not yield measurable cAMP levels under any condition. As seen with adenosine, inosine dose-dependently increased cAMP production (Fig. 5A). Moreover, dose response analyses indicated that the EC50 values of adenosine and inosine were 0.059 ± 0.02 μM and 709 ± 135.2 μM, respectively (Fig. 5B and 5C; Table 1). Relative to adenosine, inosine-induced cAMP production appeared to be more sensitive to the A2AR inverse agonist ZM 241385. Taken together, these results suggest that inosine, although not as potent as adenosine, is a functional agonist at the mA2AR.
Fig. 5.
Inosine induces mA2AR-mediated intracellular cAMP production.
(A) CHO-K1 cells transiently transfected with mA2AR but not with vector alone exhibit agonist-inducible cAMP production. Transiently transfected CHO-K1 cells were incubated with various agonists and an inverse agonist for 10 min. Mean intracellular cAMP levels ± SEM of representative experiments are shown (n=3; *, p < 0.05; **, p < 0.01 vs DMSO). Adenosine (Ado), inosine (Ino), A2AR-selective agonist CGS 21680 (CGS) and A2AR-selective inverse agonist ZM 241385 (ZM). Dose response of adenosine (B) and inosine (C) to mA2AR. CHO-K1 cells transiently transfected with mA2AR were incubated with various concentrations of adenosine and inosine. Mean cAMP levels ± SEM of a representative experiment in triplicates are shown. Shaded and dotted boxes indicate data with p < 0.001 and p < 0.05 relative to control, respectively. EC50 values with 95% confidence intervals (in parentheses) were 79(21.5–293) nM and 773(249.5–2396) μM for adenosine and inosine respectively.
3.4. Inosine dose-dependently induces hA2AR-mediated ERK1/2 activation
Agonist engagement at the A2AR leads to ERK1/2 phosphorylation and subsequent activation [28,29]. Depending on the cell type, the signaling pathways leading to A2AR-mediated ERK1/2 activation can vary. Gαs-dependent ERK1/2 activation is reported in CHO-K1 cells, whereas Gs-independent ERK1/2 activation is reported in HEK293 cells [28]. Analysis of receptor truncations indicates that Gαs engagement/cAMP production and ERK1/2 phosphorylation/activation are functionally separable [30]. To investigate whether inosine agonism at the A2AR results in ERK1/2 activation, we evaluated the effects of inosine on ERK1/2 phosphorylation in CHO-hA2AR cells. As shown in Fig. 6A and Table 1, adenosine stimulated ERK1/2 phosphorylation with an EC50 of 0.211 ± 0.036 μM. Similarly, inosine dose-dependently enhanced ERK1/2 phosphorylation with an EC50 of 89.38 ± 15.12 μM (Fig. 6B; Table 1). Both adenosine- and inosine-induced ERK1/2 phosphorylation were inhibited by the A2AR inverse agonist ZM 241385 (Fig. 6A & Fig. 6B; Table 1). Comparison of EC50 values of adenosine indicated a 35-fold preference for cAMP production than for ERK1/2 phosphorylation (p < 0.001). In contrast, inosine exhibited a 3.3-fold preference for ERK1/2 phosphorylation over cAMP production (p < 0.005). These results further demonstrate that inosine is an agonist at the A2AR and that the inosine-engaged receptor has a different pharmacological profile relative to the adenosine-engaged receptor.
Fig. 6.
Both adenosine and inosine dose-dependently induce hA2AR-mediated ERK1/2 phosphorylation.
Adenosine- (A) and inosine- (B) stimulated ERK1/2 phosphorylation by CHO-hA2AR cells. CHO-hA2AR cells were incubated with various concentrations of adenosine or inosine for 20 min. Phospho ERK1/2 levels were quantified using a FRET-based detection kit. Mean FRET ratios ± SEM of representative experiments are shown (n=3). Shaded boxes indicate data with p < 0.001 relative to control. EC50 values with 95% confidence intervals (in parentheses) were 139 (28–680) nM and 41.9 (5.8–303) μM for adenosine and inosine respectively. The A2AR-selective inverse agonist ZM 241385 (ZM) blocked adenosine- and inosine-stimulated ERK1/2 phosphorylation.
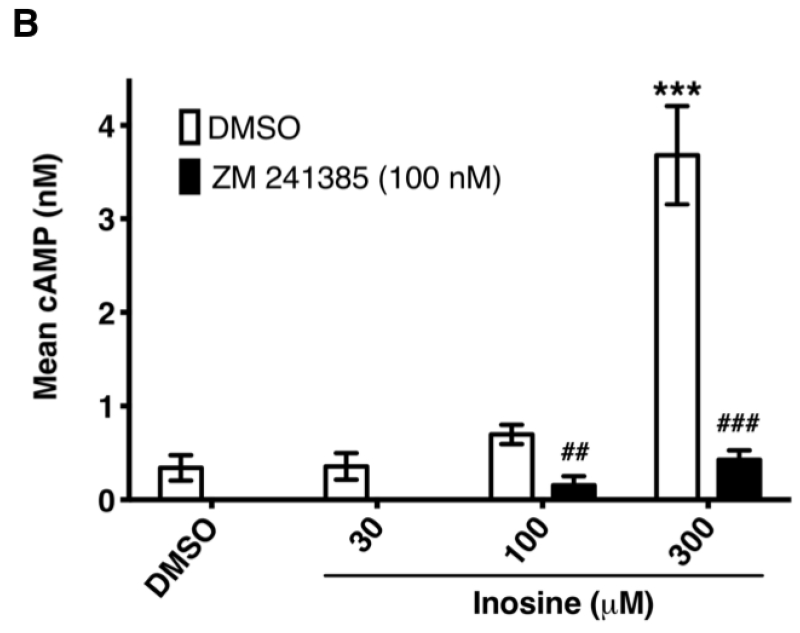
3.5. Inosine dose-dependently stimulates hA2AR-mediated cAMP production in a cell-free, membrane-based system
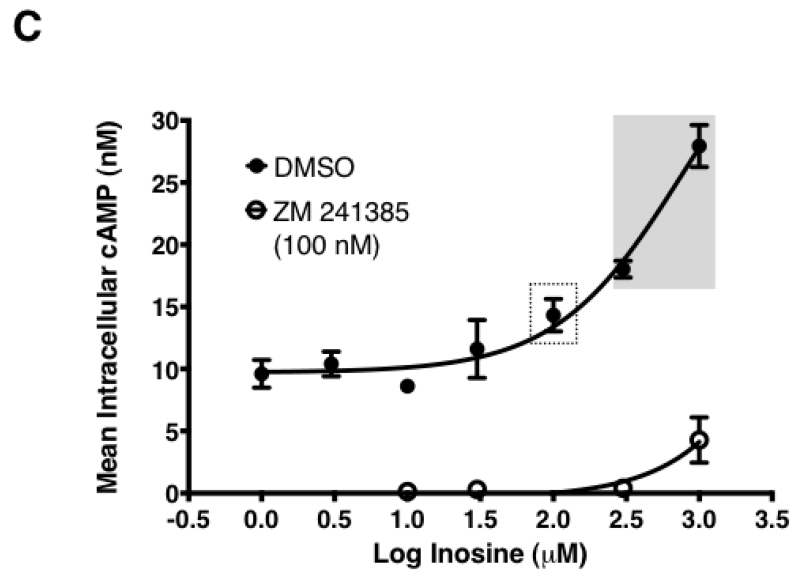
In the cell-based A2AR functional assay, one can conceptualize a scenario where the exogenously added inosine is taken up, converted to adenosine intracellularly and exported back out to engage and activate the receptor. Such a scenario is highly unlikely given the time scale (10 min) of the cell-based assay and maintenance of a cellular response in the presence of the ENT1 and ENT2 inhibitor dipyridamole in intact cell assays. However, to further rule out the possibility, we evaluated the ability of inosine to activate the A2AR in HEK293-hA2AR cytosol-free membrane preparations. To this end, we established a sensitive, cell-free, membrane-based assay for the hA2AR. The A2AR-selective agonist CGS 21680 increased cAMP production, while the A2AR-selective inverse agonist ZM 241385 reduced cAMP production to levels that were not detectable by the assay, demonstrating that this assay reports bona fide A2AR receptor pharmacology (Fig. 7A). Inosine dose-dependently stimulated cAMP production while ZM 241385 inhibited inosine-induced cAMP production in this cell-free, membrane-based assay (Fig. 7B), indicating that the inosine effects at the A2AR are direct, and thus providing further evidence for inosine agonism at this receptor.
Fig. 7.
Inosine dose-dependently induces hA2AR-mediated cAMP production in cell-free membrane preparations.
CHO-hA2AR cell membranes were incubated with indicated concentrations of CGS 21680 (CGS) and ZM 241385 (ZM) (A) and with inosine (B) in the presence and in the absence of the A2AR-selective inverse agonist ZM 241385 for 30 min. Mean cAMP production ± SEM of representative experiments are shown (n=6; * and ***, p < 0.05 and p < 0.001 vs DMSO respectively; ## and ###, p < 0.001 and p < 0.001 vs inosine respectively).
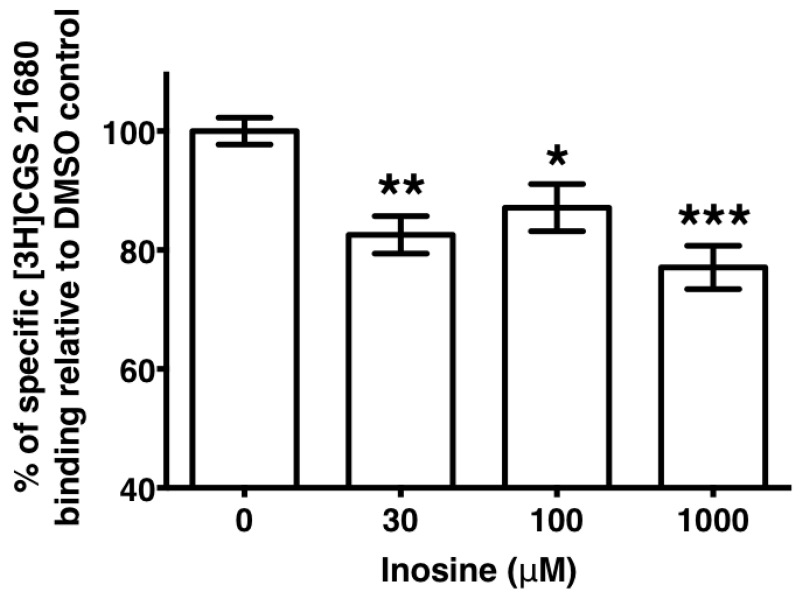
3.6. Inosine displaces the hA2AR-selective agonist, CGS 21680, binding to the receptor
In general, agonist-mediated GPCR stimulation stems from agonist binding at the receptor that is detectable by saturation binding experiments. On the basis of our observations of inosine-mediated hA2AR activation and subsequent generation of cAMP and ERK1/2 responses in the cell-based assays, we hypothesized that inosine may bind to the hA2AR with high enough affinity to be detectable in an equilibrium binding assay. As there are no commercially available radiolabeled inosine analogs to evaluate direct binding, we determined whether inosine could displace binding of the high affinity hA2AR agonist, CGS 21680. In equilibrium binding assays, the specific binding of [3H]CGS 21680 to the A2AR was reduced by approximately 20% regardless of the inosine concentration (F [4, 73] = 7.287; P<0.0001) in the presence of inosine (Fig. 8) supporting the notion that inosine competes with the high affinity adenosine analog CGS 21680 for the same binding site at the A2AR. The low-magnitude displacement of the [3H]CGS 21680 is consistent with inosine being a low affinity agonist.
Fig. 8.
Displacement of hA2AR-bound [3H]CGS 21680 by inosine.
Equilibrium binding of [3H]CGS 21680 to hA2AR in the presence of indicated concentrations of inosine and vehicle control. CHO-hA2AR membranes were incubated with ADA and [3H]CGS 21680 in the presence and absence of inosine. Values are mean ± SEM of results of 3 independent experiments performed in triplicate; overall p < 0.0001; *, p < 0.05; ** < 0.01; *** < 0.001.
4. Discussion
Inosine is a ubiquitous purine nucleoside that exerts anti-inflammatory and immunomodulatory effects. Inosine and metabolically stable analogs of inosine [31,32] utilize adenosine receptors to modulate anti-inflammatory effects. There are four adenosine receptor (AR) subtypes termed A1R, A2AR, A2BR and A3R. Among them, A2AR is the most effective receptor in downregulating inflammation [33,34]. The role of the A2AR, however, is less clear in the context of inosine-mediated anti-inflammation and immunomodulation. There is a discrepancy between postulating inosine-mediated in vivo anti-inflammatory effects requiring the A2AR [20,21] when in vitro studies report no functional activation of the A2AR [22] or displacement of a high-affinity agonist bound to the A2AR in the presence of inosine [7], Here we examined the possibility of inosine-mediated A2AR activation in vitro using a variety of highly sensitive and selective experimental approaches not applied previously to the evaluation of inosine receptor activation.
Our investigations produced several lines of experimental evidence supporting inosine as a specific A2AR agonist. First, in CHO-K1 cells that do not express any endogenous adenosine receptor subtypes [23], inosine dose-dependently increased cAMP production upon exogenous expression of both mouse (mA2AR) and human A2ARs (hA2AR). This inosine-induced cAMP production was inhibited by the A2AR-selective inverse agonist ZM 241385. Second, inosine-induced cAMP production by CHO-hA2AR cells was not altered in the presence of the ENT1 and 2 inhibitor dipyridamole. Third, in CHO-hA2AR cells, inosine exhibited a dose-dependent enhancement of ERK1/2 phosphorylation that was sensitive to ZM 241385. Fourth, consistent with A2AR activation in the cell-based assays, inosine exhibited a ZM 241385-sensitive induction of cAMP production in cell-free membrane preparations containing the hA2AR. This further supports the notion that it is inosine, and not a cellular metabolic byproduct of inosine, that is responsible for the stimulation of the A2AR. Finally, in equilibrium binding assays, inosine inhibited the binding of the A2AR-selective agonist CGS 21680 to CHO-hA2AR membranes.
Our data indicate that inosine is a less potent agonist than adenosine at the A2AR. In cell-based cAMP assays, inosine concentrations that activate the mA2AR and the hA2AR are in the high micromolar range, hence generation of accurate EC50 values are not possible. The approximate EC50 values of inosine for cAMP production for the hA2AR and mA2AR s are 300.7 ± 48.9 μM and 709 ± 135.2 μM, respectively, indicating that, relative to adenosine, inosine is approximately four orders of magnitude less potent at the A2AR. In ERK1/2 phosphorylation assays, EC50 values for adenosine and inosine are 0.211 ± 0.036 μM and 89.38 ± 15.12 μM, respectively, a potency difference of 423-fold. Although the EC50 values of inosine appear relatively high, they are well within the physiological levels achieved in disease settings [7,8].
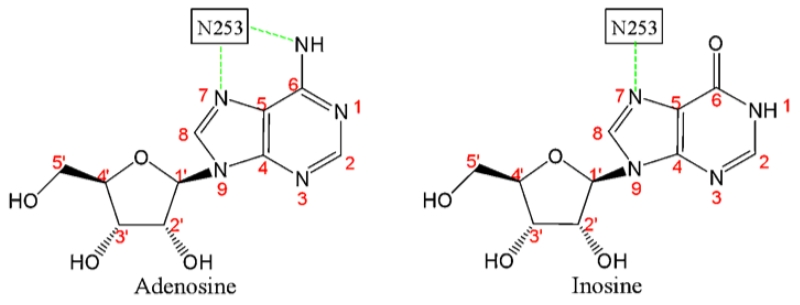
The low potency of inosine relative to adenosine at the A2aR may be explained by the nature of its interactions with the critical residues that constitute the agonist-binding pocket of the receptor. Crystal structure predictions in combination with site-directed mutagenesis indicate that Asn253 in the sixth transmembrane domain of the hA2AR is the critical residue that defines the hydrophobic pocket in which the adenine ring of the agonist rests [35]. In the case of adenosine, two hydrogen bond interactions of Asn253 with N6 and N7 of adenine (Fig. 9; [36]) stabilize adenosine binding at the A2AR. Inosine, a metabolite of adenosine, differs from adenosine by having only N7 but not N6 in the adenine ring, and thus is capable of having only one hydrogen bond interaction with Asn253 in the agonist-binding pocket. This may explain at least in part the observed low affinity interaction between inosine and the receptor relative to that of adenosine as evident from the agonist displacement and functional assays.
Fig. 9.
Schematic representation of hydrogen bonding interaction between adenosine/inosine and Asn253 in the sixth transmembrane domain of the A2AR agonist-binding pocket.
We have found that adenosine and inosine possess uniquely different pharmacological profiles. The EC50 values for cAMP production and ERK1/2 activation reveal that both adenosine and inosine exhibit an agonist-specific signaling bias. Adenosine is 35-fold more potent in stimulating hA2AR-mediated cAMP production relative to hA2AR-mediated ERK1/2 phosphorylation, thus adenosine is a cAMP-biased agonist. In contrast, inosine is 3.3-fold more potent in stimulating hA2AR-mediated ERK1/2 phosphorylation than hA2AR-mediated cAMP production, hence it exhibits an ERK1/2 signaling bias. Additionally, inosine-stimulated ERK1/2 phosphorylation is sensitive to the A2AR reverse agonist ZM 241385 to a greater extent than adenosine-stimulated ERK1/2 phosphorylation. These findings suggest that adenosine and its primary metabolite, inosine, are uniquely different agonists with signaling biases capable of producing overlapping as well as distinct pharmacological outcomes.
Using an equilibrium competitive binding assay, we demonstrate that inosine displaces the binding of the A2AR-selective agonist CGS 21689 to the hA2AR. This observation differs, however, from a previous report [7] wherein inosine failed to displace binding of the A1R/A3R agonist N6-(3-iodo-4-aminobenzyl)adenosine [37,38] to the rat A2AR. Differences in species and in affinity of the agonist utilized in these studies may explain in part the differing outcomes.
Adenosine activates both the A2AR and A2BR. The A2AR is the high affinity receptor that is coupled to Gs and Golf and plays a functionally non-redundant role in down modulating inflammation. The A2BR is the low affinity receptor that is coupled to Gs and Gq, shows Gs-dependent ERK1/2 activation [24] and produces both pro- and anti-inflammatory effects [39], The anti-inflammatory and immunomodulatory roles of both A2AR and A2BRs have been largely attributed to Gs-dependent production of cAMP [33,34], Although the significance of A2AR-mediated ERK1/2 activation in the context of inflammation and immunomodulation is largely unknown, A2AR-mediated ERK1/2 activation promotes cell death [40], cell proliferation [40] and inhibits osteoclast formation [41]. Our finding that inosine, the primary metabolite of adenosine, is a signaling-biased agonist at the A2AR but not at the A2BR suggests a wide variety of functional outcomes that are dependent upon adenosine and inosine levels and relative receptor abundance in vivo.
The major outcome of our investigation is the identification of inosine, the first metabolite of the breakdown of adenosine, as a functional agonist of the A2AR. The demonstration of inosine as a functional agonist at the A2AR increases our understanding of A2AR signaling with respect to the regulation of inflammation and immunomodulation. The current paradigm of A2AR is based on the notion that the endogenous immunomodulator, adenosine, is the sole natural agonist of the A2AR. Adenosine, having a half-life <10 s in vivo [1], is rapidly deaminated to inosine, rendering it inactive at the A2AR [22]. Hence, adenosine-mediated A2AR signaling may be short-lived. Identifying inosine as an agonist at the A2AR expands upon this concept and supports the notion that deamination does not render adenosine completely inactive at the A2AR, but rather produces an agonist, inosine, with a relatively longer half-life (15 h; [2]) and elevated interstitial levels that may prolong A2AR signaling to sustain anti-inflammatory and immunomodulatory responses. We envision that these two endogenous modulators acting together produce prolonged and accentuated activation of the A2AR, an effect that neither one can achieve individually. Adenosine engages the A2AR to generate initial rapid and robust cAMP-biased responses that are subsequently shifted towards ERK1/2 biased responses by inosine. Such dual agonist-mediated signaling may be crucial for mounting a more robust and effective immunomodulatory response in order to limit the extent of tissue damage in inflammatory diseases. Adenosine-driven immunomodulation may be more focused at the disease site where adenosine is produced, released and degraded. In contrast, inosine, being relatively more stable, accumulates, functions locally and due to its stability may diffuse more broadly. Understanding the pharmacological intricacies of interplay between these two natural anti-inflammatory and immunomodulatory agonists and the A2AR may provide new insights into potential therapeutic interventions of inflammation and immune-mediated diseases. Additionally, quantification of the relative potency and efficacy of inosine and adenosine at the A2AR will aid our understanding of agonist-receptor interactions at the binding pocket that in turn may lead to the design of agonists with beneficial pharmacological profiles.
Highlights.
Inosine directly and physically engages the A2AR.
Inosine dose-dependently stimulates cAMP production mediated through the A2AR.
Inosine dose-dependently induces A2AR-mediated ERK1/2 phosphorylation.
Adenosine and inosine differ in their signaling biases at the A2AR.
Acknowledgements
We gratefully acknowledge Dr. Karl-Norbert Klotz for providing CHO-hA2AR and CHO-hA2BR cells and Dr. Jan Rydzewski for HPLC analysis. This work was supported in part by NIH grant 5R21AI105518 from the National Institute of Allergy and Infectious Diseases.
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI105518. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ADA
adenosine deaminase
- Ado
adenosine
- AR
adenosine receptor
- A1R
adenosine A1 receptor
- A2AR
adenosine A2A receptor
- A2BR
adenosine A2A receptor
- A3R
adenosine A3 receptor
- cAMP
cyclic adenosine monophosphate
- CGS 21680
4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl] benzenepropanoic acid hydrochloride
- DMPX
3,7-Dimethyl-l-propargylxanthine
- DMSO
dimethyl sulfoxide
- DMR
dynamic mass redistribution
- ENT1
equilibrative nucleoside transporter 1
- ENT2
equilibrative nucleoside transporter 2
- ERK1/2
extracellular signal-regulated kinase-1 and -2
- GPCR
G protein-coupled receptor
- HBSS
Hanks’ balanced salt solution
- HEPES
4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid
- HTRF
homogeneous time resolved fluorescence
- Ino
inosine
- LPS
lipopolysaccharide
- NECA
5′-N-Ethylcarboxamidoadenosine
- PBS
phosphate buffered saline
- SD
standard deviation
- SEM
standard error of the mean
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- ZM 241385
4-(2-[7-Amino-2-(2-furyl)[l,2,4]triazolo[2,3-a][l,3,5]triazin-5-ylamino]ethyl)phenol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- [1].Möser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256:C799–806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- [2].Viegas TX, Omura GA, Stoltz RR, Kisicki J. Pharmacokinetics and pharmacodynamics of peldesine (BCX-34), a purine nucleoside phosphorylase inhibitor, following single and multiple oral doses in healthy volunteers. J Clin Pharmacol. 2000;40:410–20. doi: 10.1177/00912700022008991. [DOI] [PubMed] [Google Scholar]
- [3].Phillis JW, Walter GA, O’Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab. 1987;7:679–86. doi: 10.1038/jcbfm.1987.122. [DOI] [PubMed] [Google Scholar]
- [4].Phillis JW, O’Regan MH, Walter GA. Effects of nifedipine and felodipine on adenosine and inosine release from the hypoxemic rat cerebral cortex. J Cereb Blood Flow Metab. 1988;8:179–85. doi: 10.1038/jcbfm.1988.47. [DOI] [PubMed] [Google Scholar]
- [5].Phillis JW, O’Regan MH, Walter GA. Effects of deoxycoformycin on adenosine, inosine, hypoxanthine, xanthine, and uric acid release from the hypoxemic rat cerebral cortex. J Cereb Blood Flow Metab. 1988;8:733–41. doi: 10.1038/jcbfm.1988.121. [DOI] [PubMed] [Google Scholar]
- [6].Kékesi V, Zima E, Barát E, Huszár E, Nagy A, Losonczi L, Merkely B, Horkay F, Juhász-Nagy A. Pericardial concentrations of adenosine, inosine and hypoxanthine in an experimental canine model of spastic ischaemia. Clin Sci (Lond) 2002;103(Suppl 48):198S–201S. doi: 10.1042/CS103S198S. [DOI] [PubMed] [Google Scholar]
- [7].Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–57. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, Clark RS, Dixon CE, Marion DW, Jackson E. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma. 1998;15:163–70. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- [9].Haskó G, Kuhel DG, Németh ZH, Mabley JG, Stachlewitz RF, Virág L, Lohinai Z, Southan GJ, Salzman AL, Szabó C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–9. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- [10].Liaudet L, Mabley JG, Pacher P, Virág L, Soriano FG, Marton A, Haskó G, Deitch EA, Szabó C. Inosine exerts a broad range of antiinflammatory effects in a murine model of acute lung injury. Ann Surg. 2002;235:568–78. doi: 10.1097/00000658-200204000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mabley JG, Pacher P, Liaudet L, Soriano FG, Haskó G, Marton A, Szabo C, Salzman AL. Inosine reduces inflammation and improves survival in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G138–44. doi: 10.1152/ajpgi.00060.2002. [DOI] [PubMed] [Google Scholar]
- [12].Rahimian R, Fakhfouri G, Daneshmand A, Mohammadi H, Bahremand A, Rasouli MR, Mousavizadeh K, Dehpour AR. Adenosine A(2A) receptors and uric acid mediate protective effects of inosine against TNBS-induced colitis in rats. Eur J Pharmacol. 2010;649:376–81. doi: 10.1016/j.ejphar.2010.09.044. [DOI] [PubMed] [Google Scholar]
- [13].Liaudet L, Mabley JG, Soriano FG, Pacher P, Marton A, Haskó G, Szabó C. Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture. Am J Respir Crit Care Med. 2001;164:1213–20. doi: 10.1164/ajrccm.164.7.2101013. [DOI] [PubMed] [Google Scholar]
- [14].Darlington DN, Gann DS. Inosine Infusion Prevents Mortality in Endotoxic Shock. The Journal of Trauma: Injury, Infection, and Critical Care. 2005;59:1432–1435. doi: 10.1097/01.ta.0000196007.34175.46. [DOI] [PubMed] [Google Scholar]
- [15].Schneider L, Pietschmann M, Hartwig W, Marcos SS, Hackert T, Gebhard MM, Uhl W, Büchler MW, Werner J. Inosine reduces microcirculatory disturbance and inflammatory organ damage in experimental acute pancreatitis in rats. Am J Surg. 2006;191:510–4. doi: 10.1016/j.amjsurg.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [16].Mabley JG, Rabinovitch A, Suarez-Pinzon W, Haskó G, Pacher P, Power R, Southan G, Salzman A, Szabó C. Inosine protects against the development of diabetes in multiple-low-dose streptozotocin and nonobese diabetic mouse models of type 1 diabetes. Mol Med. 2003;9:96–104. doi: 10.2119/2003-00016.mabley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schneider S, Klein HH. Inosine improves islet xenograft survival in immunocompetent diabetic mice. Eur J Med Res. 2005;10:283–6. [PubMed] [Google Scholar]
- [18].Haskó G, Sitkovsky MV, Szabó C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–7. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [19].Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–92. [PubMed] [Google Scholar]
- [20].Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–8. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- [21].da Rocha Lapa F, de Oliveira AP, Accetturi BG, de Oliveira Martins I, Domingos HV, de Almeida Cabrini D, de Lima WT, Santos AR. Anti-inflammatory effects of inosine in allergic lung inflammation in mice: evidence for the participation of adenosine A2A and A3 receptors. Purinergic Signal. 2013;9:325–36. doi: 10.1007/s11302-013-9351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–8. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- [23].Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1998;357:1–9. doi: 10.1007/pl00005131. [DOI] [PubMed] [Google Scholar]
- [24].Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–27. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- [25].Schröder R, Schmidt J, Blattermann S, Peters L, Janssen N, Grundmann M, Seemann W, Kaufel D, Merten N, Drewke C, Gomeza J, Milligan G, Mohr K, Kostenis E. Applying label-free dynamic mass redistribution technology to frame signaling of G protein–coupled receptors noninvasively in living cells. Nat Protoc. 2011;6:1748–1760. doi: 10.1038/nprot.2011.386. [DOI] [PubMed] [Google Scholar]
- [26].Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptorsan update. Pharmacological Reviews. 2011;63:1. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ, Lima DA, Almeida RC, Ostroski RM, Rodrigues AL, Santos AR. Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther. 2010;334:590–8. doi: 10.1124/jpet.110.166058. [DOI] [PubMed] [Google Scholar]
- [28].Seidel MG. Activation of Mitogen-activated Protein Kinase by the A2A-adenosine Receptor via a rap 1-dependent and via a p2 lras-dependent Pathway. Journal of Biological Chemistry. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- [29].Germack R, Dickenson JM. Characterization of ERK1/2 signalling pathways induced by adenosine receptor subtypes in newborn rat cardiomyocytes. British Journal of Pharmacology. 2004;141:329–339. doi: 10.1038/sj.bjp.0705614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klinger M, Kuhn M, Just H, Stefan E, Palmer T, Freissmuth M, Nanoff C. Removal of the carboxy terminus of the A 2A -adenosine receptor blunts constitutive activity: differential effect on cAMP accumulation and MAP kinase stimulation. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;366:287–298. doi: 10.1007/s00210-002-0617-z. [DOI] [PubMed] [Google Scholar]
- [31].Mabley JG, Pacher P, Murthy KG, Williams W, Southan GJ, Salzman AL, Szabo C. The novel inosine analogue, INO-2002, protects against diabetes development in multiple low-dose streptozotocin and non-obese diabetic mouse models of type I diabetes. J Endocrinol. 2008;198:581–9. doi: 10.1677/JOE-07-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mabley JG, Pacher P, Murthy KG, Williams W, Southan GJ, Salzman AL, Szabo C. The novel inosine analogue INO-2002 exerts an anti-inflammatory effect in a murine model of acute lung injury. Shock. 2009;32:258–62. doi: 10.1097/SHK.0b013e31819c3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sitkovsky MV. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- [34].Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. TRENDS in Immunology. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [35].Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–5. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–7. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shepherd RK, Linden J, Duling BR. Adenosine-induced vasoconstriction in vivo. Role of the mast cell and A3 adenosine receptor. Circ Res. 1996;78:627–34. doi: 10.1161/01.res.78.4.627. [DOI] [PubMed] [Google Scholar]
- [38].Hill RJ, Oleynek JJ, Hoth CF, Kiron MA, Weng W, Wester RT, Tracey WR, Knight DR, Buchholz RA, Kennedy SP. Cloning, expression and pharmacological characterization of rabbit adenosine A1 and A3 receptors. J Pharmacol Exp Ther. 1997;280:122–8. [PubMed] [Google Scholar]
- [39].Ham J, Rees DA. The adenosine a2b receptor: its role in inflammation. Endocr Metab Immune Disord Drug Targets. 2008;8:244–54. doi: 10.2174/187153008786848303. [DOI] [PubMed] [Google Scholar]
- [40].Merighi S, Mirandola P, Milani D, Varani K, Gessi S, Klotz KN, Leung E, Baraldi PG, Borea PA. Adenosine receptors as mediators of both cell proliferation and cell death of cultured human melanoma cells. J Invest Dermatol. 2002;119:923–33. doi: 10.1046/j.1523-1747.2002.00111.x. [DOI] [PubMed] [Google Scholar]
- [41].Mediero A, Perez-Aso M, Cronstein BN. Activation of adenosine A 2A receptor reduces osteoclast formation via PKA- and ERKl/2-mediated suppression of NFκB nuclear translocation. British Journal of Pharmacology. 2013;169:1372–1388. doi: 10.1111/bph.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]