Abstract
Gammaherpesviruses establish life-long infection in most adults and are associated with the development of B cell lymphomas. While the interaction between gammaherpesviruses and splenic B cells has been explored, very little is known about gammaherpesvirus infection of B-1 B cells, innate-like B cells that primarily reside in body cavities. This study demonstrates that B-1 B cells harbor the highest frequency of latently infected cells in the peritoneum throughout chronic infection, highlighting a previously unappreciated feature of gammaherpesvirus biology.
Keywords: Chronic infection, gammaherpesvirus, B-1 B cells
Introduction
Gammaherpesviruses, such as Epstein-Barr virus (EBV) and Kaposi's sarcoma associated Herpesvirus (KSHV) are ubiquitous pathogens that establish life-long infections and are associated with several types of cancers, including B cell lymphomas. The tropism of these viruses for B cells is well established and numerous studies over the past decades have defined the intricate interactions between gammaherpesvirus infection and biology of B cells, including in the context of viral lymphomagenesis [reviewed in (Thorley-Lawson, Hawkins et al., 2013;Cesarman, 2014)]. These studies have mostly focused on the secondary lymphoid organs (i.e. spleen and lymph nodes) where the majority of B cells are classified as B-2 B cells. In contrast, body cavities play host to B-1 B cells, a B cell population with unique development and function. Very little is known about the interaction of EBV and KSHV with primary B-1 B cells, in part due to species specificity of these human viruses and restricted availability of human body cavity-resident B-1 B cells. Importantly, the interaction of EBV and KSHV with B-1 B cells may be of relevance for viral lymphomagenesis, as KSHV infection is associated with primary effusion lymphoma, a B cell malignancy that is mostly limited to body cavities.
To overcome the species specificity of EBV and KSHV, this study utilized mouse gammaherpesvirus-68 (MHV68), a natural rodent pathogen that shares both genetic and biological features with EBV and KSHV (Efstathiou, Ho et al., 1990;Tarakanova, Suarez et al., 2005;Virgin, Latreille et al., 1997). Similar to EBV and KSHV, a majority of published MHV68 studies have focused on defining the host and viral factors involved in regulation of chronic infection and pathogenesis in the context of B-2 B cells. An early study by the Virgin group demonstrated that at 16 days following intraperitoneal inoculation, the highest frequency of MHV68 DNA positive cells was found in peritoneal macrophages, with peritoneal B cells being close second (Weck, Kim et al., 1999). In a more recent report the Tibbetts group showed that, 42 days following intraperitoneal infection, peritoneal B cells harbored the highest frequency of MHV68 DNA positive cells, with macrophages representing a second most-infected population (Li, Ikuta et al., 2008). Because bulk peritoneal B cells were examined in both studies, the type of peritoneal B cells hosting MHV68 is not known. Further, it is not clear whether route of infection alters MHV68 tropism for peritoneal cell subsets. Here we show that, independent of route of infection, MHV68 targets peritoneal B-1 B cells to establish long-term latency.
Materials and Methods
Animal studies
All experimental manipulations of mice were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. C57BL/6J were obtained from Jackson Laboratories (Bar Harbor, ME) and were housed and bred in a specific pathogen-free facility at MCW. At 6-7 weeks of age mice were intranasally inoculated with 500 PFU of MHV68 (WUMS) or intraperitoneally infected with 1000 PFU of MHV68 under light anaesthesia.
Flow Cytometry and Cell Sorting
For flow cytometry, single cell suspensions of peritoneal exudate cells were prepared in FACS buffer (PBS + 2% FCS + 0.05% sodium azide) at 1×107 nucleated cells/ml. A total of 1×106 cells were prestained with Fc block (24G2), then incubated with an optimal amount of antibody. This study used antibodies against B220 (PeCy7), CD19 (Pacific Blue), and CD11b (FITC, all from Biolegend, San Diego, CA). Data acquisition was performed on a LSR II flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR). For sorting, peritoneal exudate cells pooled from 5 mice/group were separated into specific populations defined by markers in Table 1. Sorting was performed on a BD FACS Aria III cell sorter (BD Biosciences, San Jose, CA)
Table 1. Peritoneal immune cell populations: cell surface markers and average frequency of MHV68 DNA positive cells.
| Population | Markers | 16d IP | 28d IP | 42d IP | 19d IN | 42d IN |
|---|---|---|---|---|---|---|
|
| ||||||
| B-1 B cells | B220IntCD19HiCD11b+/- | 1 in | 1 in | 1 in | 1 in | 1 in |
| 140 | 150 | 345 | 1,125 | 470 | ||
|
| ||||||
| B-2 B cells | B220HiCD19HiCD11bneg | 1 in | 1 in | 1 in | > 1 in | 1 in |
| 450 | 1,316 | 1,250 | 3,500 | 2,627 | ||
|
| ||||||
| Myeloid | B220negCD19negCD11bHi | 1 in | 1 in | 1 in | ND | >1 in |
| 115 | 410 | 2,032 | 5,000 | |||
|
| ||||||
| Triple | B220negCD19negCD11bneg | ND | ND | ND | ND | ND |
| negative | ||||||
ND=the frequency of MHV68 DNA positive cells was below the level of detection
Limiting Dilution Assays
The frequency of cells harboring viral genome in bulk or sorted populations of peritoneal cells was determined by limiting dilution PCR analysis as previously described (Tarakanova, Stanitsa et al., 2010). Similarly, limiting dilution assays were performed to determine the frequency of MHV68 reactivation from the peritoneal cells (Kulinski, Leonardo et al., 2012).
Results
Peritoneal cell populations in MHV68-infected BL6 mice
Peritoneal exudate cells in MHV68-infected animals represent a diverse group of cell types that includes CD4 and CD8 T cells, dendritic cells, granulocytes, macrophages, NK cells, and B cells (Kulinski, Darrah et al., 2015). Importantly, peritoneal B cells are comprised of distinct populations that have minimal overlap with the splenic B cells (Sindhava & Bondada, 2012). Peritoneal cavity hosts a distinct population of B-1 B cells that have intermediate expression of B220 along with high surface levels of CD19 and are further differentiated into B-1a and B-1b subpopulations based on the CD5 expression. In contrast to splenic B-2 B cells, peritoneal B-1 B cells express low levels of CD11b, a typical myeloid marker, and can phagocytose with subsequent antigen presentation to T cells (Parra, Rieger et al., 2012). Further, B-1 B cells have a limited BCR repertoire, are self-renewing, and produce “innate” antibodies in a T cell-independent manner. In addition to B-1 B cells, B220HiCD19Hi B-2 B cells are also present in the peritoneum. However, these peritoneal B-2 B cells are not simply recirculating splenic B-2 B cells. Specifically, several surface markers of the B-2 B cells in the peritoneum reflect a phenotype that is intermediate between splenic B-2 B cells and peritoneal B-1 B cells (Hastings, Tumang et al., 2006). Correspondingly, functions of peritoneal B-2 B cells are closer to B-1 B cells than splenic B-2 B cells (Hastings, Tumang et al., 2006).
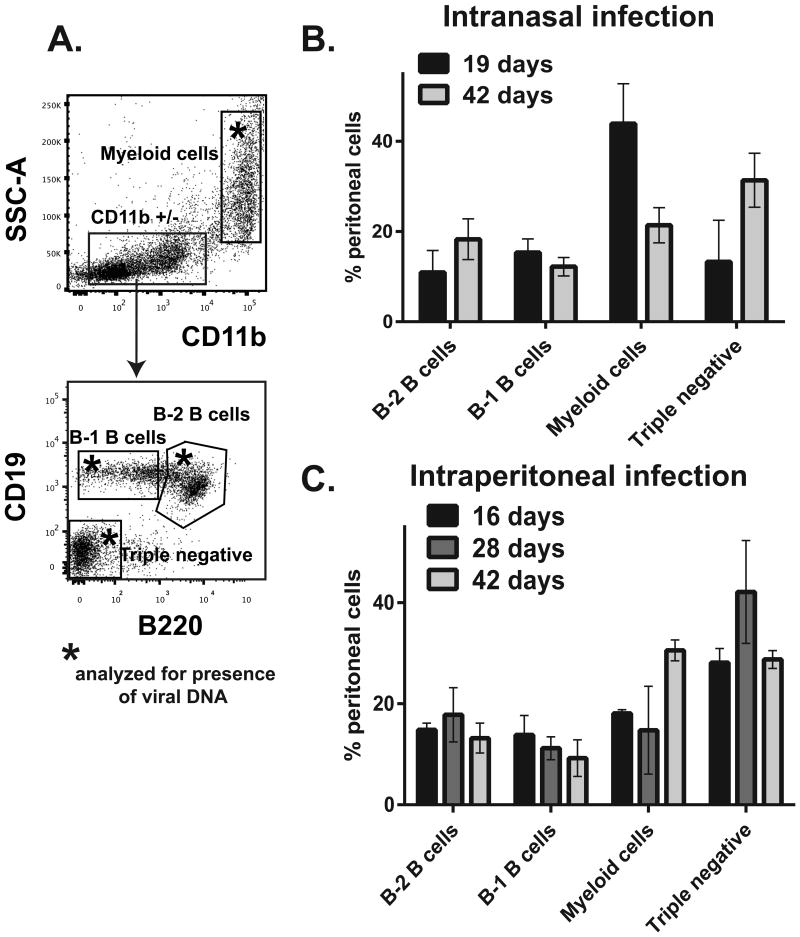
In this study, peritoneal exudate cells were divided into four broad groups to capture cells relevant for gammaherpesvirus infection: B-1 B cells, B-2 B cells, myeloid cells, and the triple negative population (Fig. 1A, specific markers used to distinguish populations are shown in Table 1). Relative abundance of these populations was analyzed at several times following intranasal or intraperitoneal MHV68 inoculation. The chosen time points represent early latency, intermediate, and long-term infection. The length or route of MHV68 infection had minimal effect on the relative abundance of B-2 and B-1 B cells in the peritoneum (Fig. 1B, C). Interestingly, intranasal infection resulted in a greater abundance of peritoneal myeloid cells at 19 days as compared to 42 days post infection (Fig. 1B). In contrast, relative abundance of peritoneal myeloid cells slightly increased in intraperitoneally-inoculated BL6 mice over the course of 42 day infection (Fig. 1C).
Figure 1. Peritoneal cell populations in MHV68-infected BL6 mice.
BL6 mice were intranasally or intraperitoneally infected with MHV68 (500 PFU/mouse and 1000 PFU/mouse, respectively); 5 mice per group were used in each experiment. At indicated times post infection, peritoneal exudate cells were harvested and pooled from all mice within the experimental group and analyzed by flow cytometry using markers in Table 1. A. Representative flow diagrams. Boxed populations with asterisk were analyzed for MHV68 DNA in subsequent analyses. B, C. Peritoneal immune cell populations at indicated times post infection. Data were pooled from 3-4 independent experiments.
Distribution of MHV68 following intraperitoneal inoculation
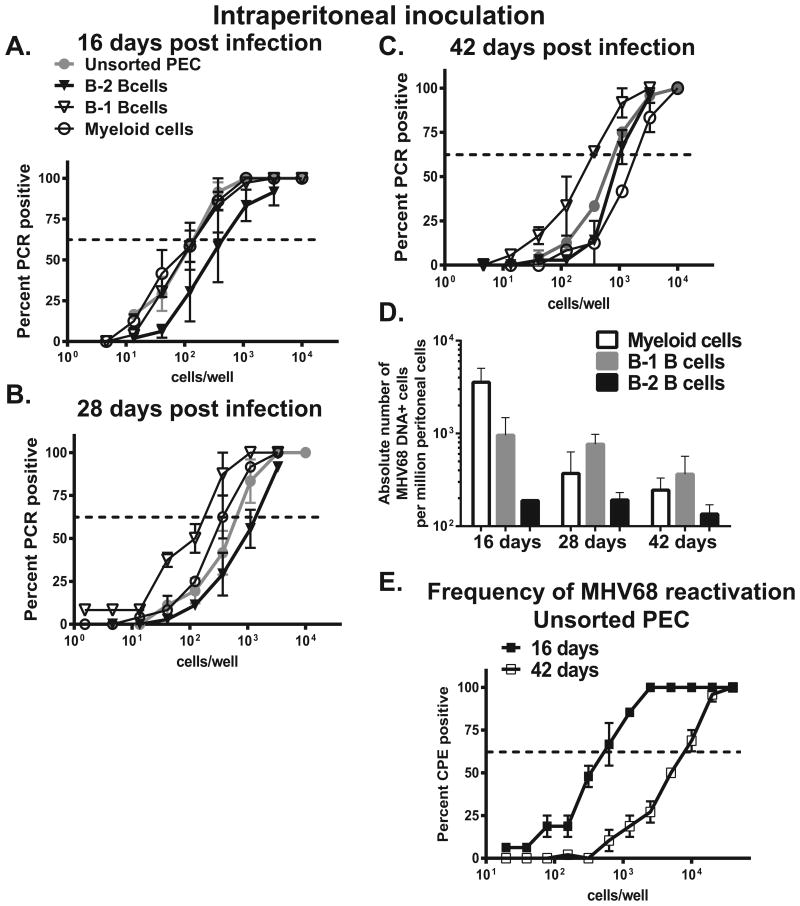
To determine the frequency of MHV68 positive cells in selected populations, peritoneal exudate cells were sorted into B-1 B cells, B-2 B cells, myeloid cells, and triple negative populations (all indicated with asterisks in Fig. 1A). Sorted populations were subjected to limiting dilution PCR assays to detect MHV68 genome. As expected, high frequency of MHV68 DNA positive cells was found in the sorted myeloid cells collected at 16 days post intraperitoneal infection (Fig. 2A, open circles, and absolute cell numbers in Fig. 2D). Intriguingly, similarly high frequency of MHV68 DNA positive cells was found in sorted B-1 B cells (Fig. 2A, open triangles). In contrast, frequency of MHV68 DNA positive cells was lower in B-2 B cells, as compared to myeloid and B-1 B cell populations (Fig. 2A, filled triangles). Of note, the frequency of MHV68 DNA positive cells in the triple negative population was below the level of detection throughout this study (data not shown).
Figure 2. Distribution of MHV68 in peritoneal cell populations following intraperitoneal infection.
BL6 mice were intraperitoneally infected with MHV68 and peritoneal exudate cells harvested and pooled as described in Fig. 1. A-C. Sorted immune cell populations were subjected to limiting dilution PCR analysis to establish the frequency of MHV68 DNA positive cells. Data were pooled from 3-4 independent experiments. Average frequency of infected cells is shown in Table 1, absolute numbers of MHV68 positive cells in each population are shown in D. E. Frequency of viral reactivation was measured in unsorted peritoneal cells harvested at indicated times post infection, data were pooled from 3 independent experiments.
As compared to 16 days post infection, the overall frequency of MHV68 DNA positive cells decreased in unsorted peritoneal cells by 28 days post infection (compare Fig. 2A and B, gray circles). Interestingly, at 28 days of infection, the frequency of MHV68 positive cells was lower in sorted myeloid cells as compared to B-1 B cells (Fig. 2B, p<0.05, absolute numbers in Fig. 2D). Peritoneal B-2 B cells continued to display the lowest frequency of infected cells at this time (28 days, Fig. 2B). Following the establishment of long-term infection (42 days, Fig. 2C), B-1 B cells continued to harbor the highest frequency of MHV68 DNA positive cells, as compared to B-2 B cells and myeloid population (see Table 1 for the summary of the frequency of infected cells, and Fig. 2D for the absolute number of infected cells in each population). Thus, B-1 B cells harbored the highest frequency of MHV68 DNA positive cells in the peritoneum of long-term infected mice.
Consistent with published studies, high frequency of MHV68 reactivation from the peritoneal cells observed at 16 days post intraperitoneal inoculation subsided over time, but was still readily measurable in 42 day-infected mice (Fig. 2E). Further, persistent MHV68 replication was below the level of detection throughout this study (data not shown), indicating that the detection of MHV68 DNA in the peritoneal cells was consistent with latent nature of virus infection.
Distribution of MHV68 following intranasal inoculation
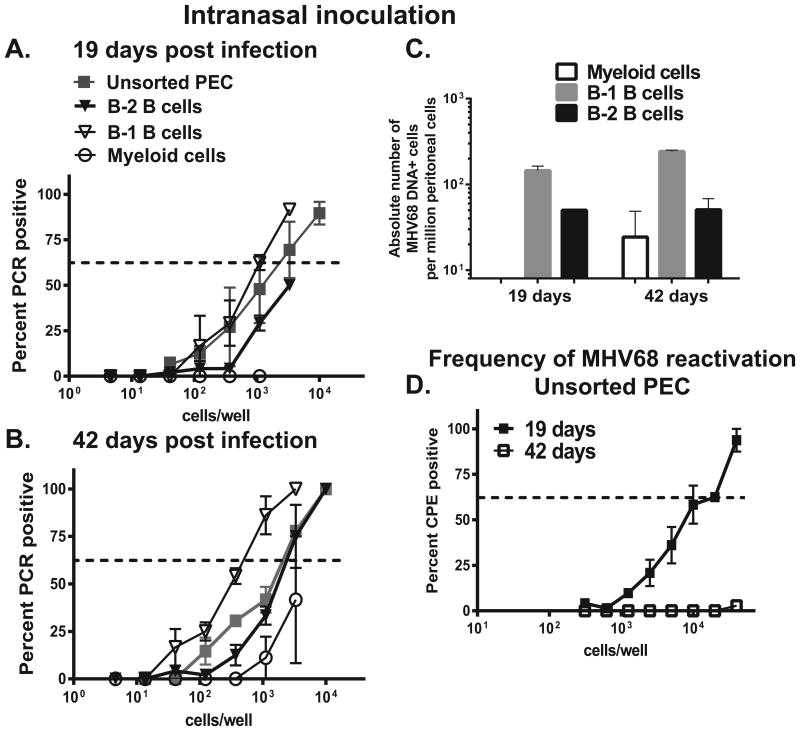
Similar analyses were performed in intranasally-infected BL6 mice to determine the extent to which the route of MHV68 inoculation alters viral tropism for peritoneal cell types. As observed in intraperitoneally-infected animals, the highest frequency of MHV68 DNA positive cells was found in sorted B-1 B cells, with B-2 B cells displaying lower frequency of infected cells (Fig. 3A, B, absolute numbers in Fig. 3C). Surprisingly, the frequency of infection was below the level of detection in peritoneal myeloid cells of intranasally-infected mice at 19 days post infection (Fig. 3A). Low level of infection was established in peritoneal myeloid cells by 42 days post infection, however, the relative frequency of infected myeloid cells was variable between the experiments and never exceeded the frequency of infection found in either B-2 or B-1 B cells (Fig. 3B, C). These route of infection-dependent differences in the latent infection of myeloid cells suggest that distinct cell types mediate MHV68 systemic spread following intranasal vs. intraperitoneal virus inoculation and, therefore, may dictate the parameters of long-term infection.
Figure 3. Distribution of MHV68 in peritoneal cell populations following intranasal infection.
BL6 mice were intranasally infected with MHV68 and peritoneal exudate cells harvested and pooled as described in Fig. 1. A, B. Sorted immune cell populations were subjected to limiting dilution PCR analysis to establish the frequency of MHV68 DNA positive cells. Data were pooled from 3-4 independent experiments. Average frequency of infected cells is shown in Table 1, absolute numbers of MHV68 positive cells in each population are shown in C. D. Frequency of viral reactivation was measured in unsorted peritoneal cells harvested at indicated times post infection, data were pooled from 3-4 independent experiments.
As previously observed, efficiency of MHV68 reactivation following intranasal infection was lower than that detected in intraperitoneally-infected mice (compare Fig. 2E and 3D), with MHV68 reactivation falling close to or below the level of detection by 42 days post infection. Considering an almost exclusive tropism of MHV68 for peritoneal B cells following intranasal inoculation, viral reactivation observed at 19 days post infection indicates that peritoneal B cells are capable of supporting MHV68 reactivation, at least during early infection.
Discussion
This study defines B-1 B cells as the preferred cell type for MHV68 infection in the peritoneal cavity, regardless of the route of initial virus inoculation or the duration of infection. Interestingly, our unpublished studies indicate that the frequency of MHV68 infection is far greater in peritoneal B-1b as compared to the B-1a B cells, at least at 42 days post intranasal inoculation (Darrah and Tarakanova, manuscript in preparation), suggesting that certain aspects of B-1a biology may be restrictive for MHV68 latency. The finding that B-1 B cells contain a high frequency of latently infected cells raises an intriguing possibility that gammaherpesvirus infection of B-1 B cells may regulate infection of B-2 B cells, a possibility to be tested in future studies. Finally, the demonstration of MHV68 tropism for B-1 B cells may have implications for virus-driven lymphomagenesis, including the development of KSHV-driven primary effusion B cell lymphoma that almost exclusively localizes to the body cavities.
Highlights.
Gammaherpesvirus targets peritoneal B-1 B cells for long-term latent infection Gammaherpesvirus tropism for B-1 B cells is independent of the route of inoculation Route of inoculation alters gammaherpesvirus tropism for peritoneal myeloid cells
Acknowledgments
We thank the Flow Core and Tamara Nelson (Children's Research Institute, Flow Cytometry Shared Resource lab, Medical College of Wisconsin) for assistance with this study. This study was supported by the ACS Research Scholar Grant RSG-12–174-01-MPC, R01CA183593, Wisconsin Breast Cancer Showhouse (V.L.T.), and AHA 15PRE22640005 (W.P.M.). We thank Gang Xin and Weiguo Cui for helpful discussions of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Cesarman E. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol. 2014;9:349–372. doi: 10.1146/annurev-pathol-012513-104656. [DOI] [PubMed] [Google Scholar]
- Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. Journal of General Virology. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur J Immunol. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]
- Kulinski JM, Darrah EJ, Broniowska KA, Mboko WP, Mounce BC, Malherbe LP, Corbett JA, Gauld SB, Tarakanova VL. ATM facilitates mouse gammaherpesvirus reactivation from myeloid cells during chronic infection. Virology. 2015;483:264–274. doi: 10.1016/j.virol.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinski JM, Leonardo SM, Mounce BC, Malherbe LP, Gauld SB, Tarakanova VL. Ataxia-Telangiectasia Mutated kinase controls chronic gammaherpesvirus infection. Journal of Virology. 2012 doi: 10.1128/JVI.00917-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ikuta K, Sixbey JW, Tibbetts SA. A replication-defective {gamma}-herpesvirus efficiently establishes long-term latency in macrophages but not B cells in vivo. Journal of Virology. 2008 doi: 10.1128/JVI.00186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, Barreda DR, Sunyer JO. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhava VJ, Bondada S. Multiple regulatory mechanisms control B-1 B cell activation. Front Immunol. 2012;3:372. doi: 10.3389/fimmu.2012.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, Gauld SB. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology. 2010;405:50–61. doi: 10.1016/j.virol.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Tarakanova VL, Suarez FS, Tibbetts SA, Jacoby M, Weck KE, Hess JH, Speck SH, Virgin HW. Murine gammaherpesvirus 68 infection induces lymphoproliferative disease and lymphoma in BALB β2 microglobulin deficient mice. J Virol. 2005;79:14668–14679. doi: 10.1128/JVI.79.23.14668-14679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Hawkins JB, Tracy SI, Shapiro M. The pathogenesis of Epstein-Barr virus persistent infection. Curr Opin Virol. 2013;3:227–232. doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. Journal of Virology. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck KE, Kim SS, Virgin HW, Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. Journal of Virology. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]