Abstract
Background
Food-mediated allergic reactions have emerged as a major health problem. The underlying mechanisms that promote uncontrolled type-2 immune response to dietary allergens in the gastrointestinal tract remain elusive.
Objective
We investigated whether altering IL-25 signaling enhances or attenuates allergic responses to food allergens.
Methods
iIL-25Tg mice, which constitutively overexpress intestinal IL-25, and Il17rb−/− mice, in which the Il17rb gene expression is disrupted, were sensitized and gavage fed with ovalbumin (OVA). We assessed symptomatic characteristics of experimental food allergy, including incidence of diarrhea, incidence of hypothermia, intestinal TH2 immune response, and serum OVA-specific IgE and mast cell protease (MCPt)-1 production.
Results
Rapid induction of Il25 expression in the intestinal epithelium preceded the onset of anaphylactic response to ingested OVA antigen. iIL-25Tg mice were more prone, and Il17rb−/− were mice more resistant, to developing experimental food allergy. Resident intestinal type-2 innate lymphoid cells (ILC2s) were identified as the major producers of IL-5 and IL-13 in response to IL-25. Reconstituting irradiated wild-type mice with Rora−/− or Il17rb−/− bone marrow (BM) resulted in a deficiency or dysfunction of the ILC2 compartment, respectively, and resistance to developing experimental food allergy. Repeated intragastric antigen challenge induced a significant increase of CD4+TH2 cells, which enhance IL-25-stimulated IL-13 production by ILC2 ex vivo and in vivo. Finally, reconstituted IL-13–deficient ILC2s had reduced capability to promote allergic inflammation, resulting in the increased resistance to experimental food allergy.
Conclusion
IL-25 and CD4+TH2 cells induced by ingested antigens enhance ILC2-derived IL-13 production, thereby promoting IgE-mediated experimental food allergy.
Keywords: IL-25, ILC2s, CD4+TH2 cells, IL-13, food allergy
Introduction
The gastrointestinal (GI) mucosa is the largest immunological site in the body and constantly encounters a myriad of commensal microbes and dietary proteins. The epithelial lumen and GI-associated lymphoid tissues function to combat invading microbes while developing immune tolerance to food antigens. The loss of mucosal tolerance to foods and the associated induction of adverse immune-mediated reactions can lead to the development of food-induced allergic disorders. Studies in both humans and animals have demonstrated that the immediate hypersensitivity response to ingested food arises from the activation of intestinal mast cells by food-specific IgE antibodies1. Although the prevalence of food-induced allergic disorders has increased significantly in industrialized countries over the past decade2, our knowledge of the underlying mechanisms that potentiate the induction of cell-mediated allergic immune responses to food allergens in the GI tract remains limited.
IL-25 (IL-17E), a distinct IL-17 inflammatory cytokine member, is a key factor that functions to promote allergic inflammation3–6. Systemic administration or overexpression of IL-25 induce elevated TH2 cytokine and eotaxin production, which result in eosinophilia, increased serum IgE, mucus hyperplasia, and pathological changes in lung and GI tissues7–10. In contrast, administration of IL-25–neutralizing antibody significantly attenuates pulmonary allergic inflammation and preventing airway hyperresponsiveness11. In addition to airway and skin IL-25, endogenous intestinal IL-25 can limit TH1 and TH17-mediated GI inflammation induced by commensal flora12, 13 and promote protective type-2 immunity to combat parasitic infection3, 14. Indeed, IL-25–deficient mice infected with Trichuris muris, the GI parasite, fail to develop a lymphocyte-dependent protective type-2 immunity to expel chronic parasitic infection14. These studies suggest that intestinal epithelium–derived IL-25 may regulate the balance of the immune response triggered by commensal microbes and dietary proteins in the GI tract.
Recent studies demonstrate that the type-2 cytokine-producing innate lymphoid cells (ILC2s) are the early cellular source of TH2 cytokine IL-5 and IL-133, 15–19. Although lacking antigen-specific receptors, ILC2s express an array of cytokine receptors; including IL-17RB, ST-2, IL-7Rα, and IL-2Rα15–18, and respond to IL-25 and IL-33 stimulation in the presence of IL-7 and/or IL-2 by producing large amounts of IL-5 and IL-1315–18. Subsequent studies reveal that ILC2s originate from common lymphoid progenitors (CLP)20, 21 and express Id2 and Rorα 15, 20, 22, the signature transcription factors for ILC lineages23–25. Similar to CD4+TH2 cells, ILC2s require transcription factor Gata3 for differentiation and maintenance26. Parallel to their critical role in protective immunity against helminthic infection, ILC2s can promote allergic asthma19, 27, 28. Whether ILC2s promote the induction of experimental food allergy has not been determined. Herein, we show that mice overexpressing intestinal IL-25 or lacking a component of IL-25 receptor, IL-17RB, were prone or resistant to developing experimental food allergy, respectively. Our studies suggest that IL-25 and ingested antigen-induced CD4+TH2 cells can enhance ILC2-derived IL-13 production that promotes the development of experimental food allergy.
Materials and Methods
Further information can be found in the Methods section in this article’s Online Repository at www.jacionline.org
IgE-mediated experimental food allergy
Mice were sensitized twice within a two-week interval by intraperitoneal injection with 100 μg OVA and 1 mg alum. Two weeks after the second sensitization, mice were orally gavaged with 50 mg OVA in 250 μl saline for a total of six times within two weeks and subsequently examined for the symptomatic features in experimental food allergy2, 3. The manifestations of systemic symptoms begin with diarrhea (profuse liquid stool), airway hyperreactivity, and then hyperthermia (rectal temperature drop > 2°C)4, 5, 30 to 45 minutes after the last challenge. Blood samples and intestine tissues were collected from mice euthanized immediately after the measurement of rectal temperature.
Measuring parameters of food allergy
To measure intestinal mast cell number and levels of goblet cell hyperplasia, duodenal tissue was fixed in 10% formalin and processed by standard histological techniques. 5–8–μm tissue sections were stained with Leder stain for chloroacetate esterase (CAE) activity in intestinal mast cells or periodic acid-Schiff (PAS) for mucins in goblet cells. Stained cells were quantified as previously described3. To measure secreted mediators, serum samples were analyzed using ELISA kits of OVA-specific IgE (MD Bioproducts), MCPt-1 (eBioscience), and OVA-specific IgG1 (Alpha Diagnostic International). Diarrhea assessments (profuse liquid stool) and hyperthermia measurements (rectal temperature drop > 2°C) are performed as previously described4.
Statistical analysis
For comparisons between experimental groups, statistical significance was determined using unpaired Student’s t test. For the measurement of food allergy parameters, 3 independent experiments (n=4, total 12 mice per group) were performed in blinded fashion for Figure 1B–1E, Figure 3, 4, 5, and 6A–6B. 2 independent experiments were performed for Figure 1A and Figure 6C–6D (n=4, total 8 mice per group). Results were considered significant at P ≤ 0.05. Error bars denote mean ± S.D. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. ns, not significant. ND, not detected. All data were analyzed using Prism (Graphpad Software).
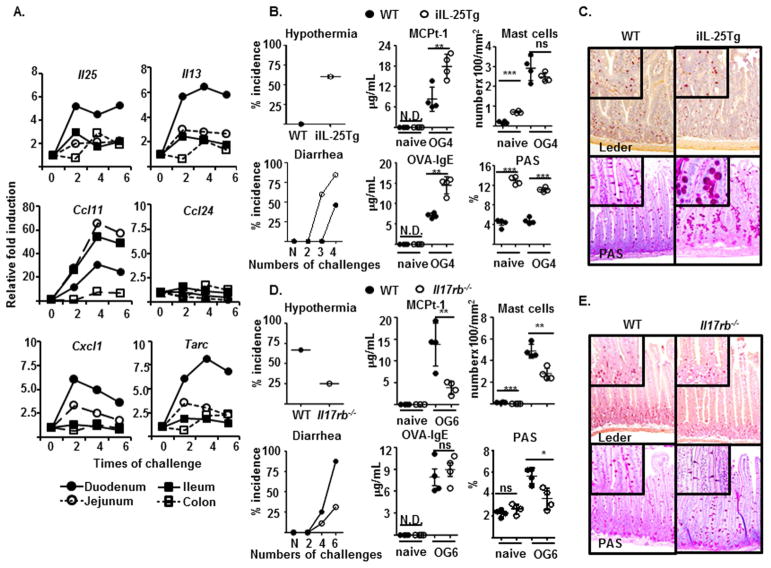
FIG 1.
(A) Expression levels of indicated genes by indicated tissues of sensitized BALB/c mice after indicated times of intragastric OVA challenge were examined and compared as described in the methods. (B–E) Indicated murine strains were sensitized and orally gavaged (OG) with OVA for four (B and C), six (D and E), or the indicated times (B and D) before measuring the indicated features of experimental food allergy and staining of intestinal mastocytosis and GC hyperplasia (C and E).
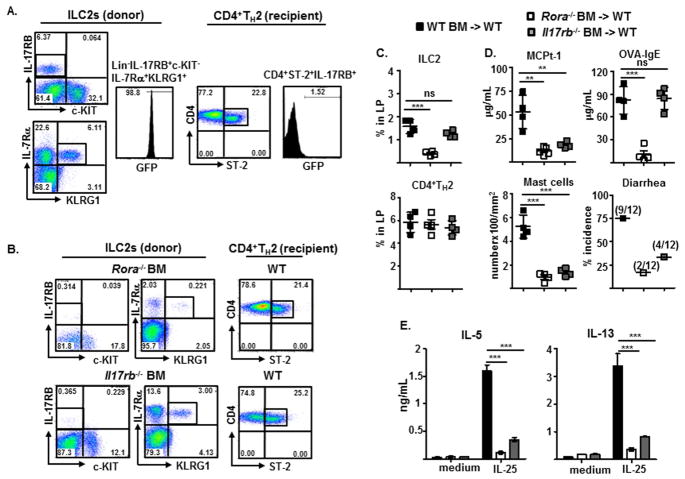
FIG 3.
Detection (A and B) and frequency (C) of donor-derived ILC2s (Lin−CD3−CD4−IL-17RB+c-KIT−IL-7Rα+KLRG1+) and recipient-derived CD4+TH2 cells (Lin−CD3+CD4+IL-17RB+ST-2+), and measurements of indicated parameters of experimental food allergy (D) and indicated cytokine production by IL-25-stimulated LP cells (E), from irradiated WT BALB/c recipients reconstituted with BM progenitors from 4GET mice (A), or WT BALB/c, Rora−/−, or Il17rb−/− mice (B–E).
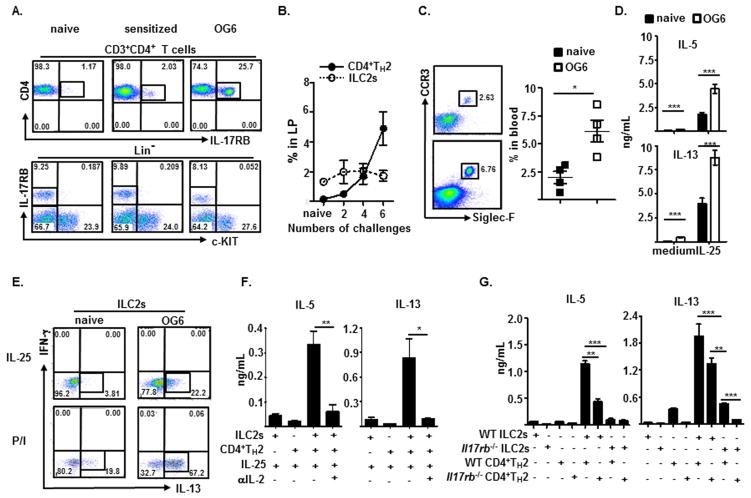
FIG 4.
Detection and frequency of intestinal CD4+IL-17RB+TH2 cells and Lin−c-KIT−IL-17RB+ILC2s (A and B), blood CCR3+Siglec-F+CD11b+ eosinophils (C), and intracellular IL-13-producing ILC2s (E) from naïve or sensitized mice orally gavaged (OG) with OVA for 6 (A, C, and E) or indicated times (B). IL-5 and IL-13 production by medium- or IL-25-stimulated LP cells (D) or by indicated cells from WT (F and G) or Il17rb−/− mice (G) OG with OVA for 6 times after 3 day co-culture with IL-25 only (F and G) or plus anti-IL-2 or control antibodies (F).
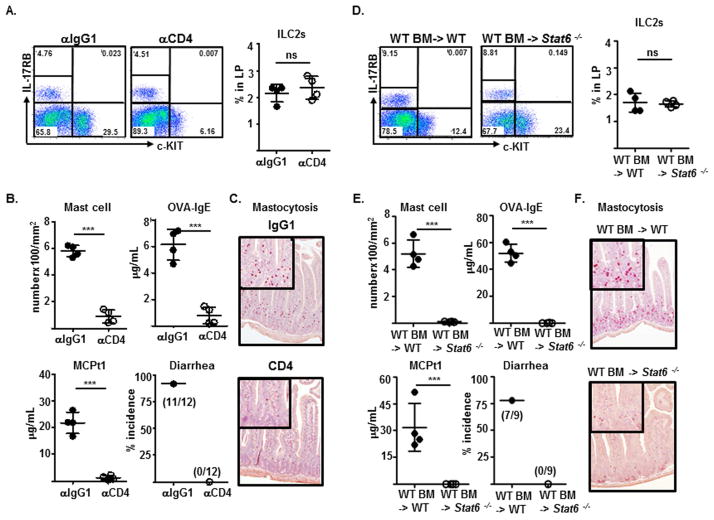
FIG 5.
Detection and frequency of ILC2s (A and D), measurement of indicated features of experimental food allergy (B and E), and staining of intestinal mastocytosis (C and F), in sensitized WT BALB/c mice treated with indicated antibodies one day before the first and fourth intragastric OVA challenges (A–C) or in irradiated recipients reconstituted with BM progenitors from WT or Stat6−/− mice (D–F).
FIG 6.
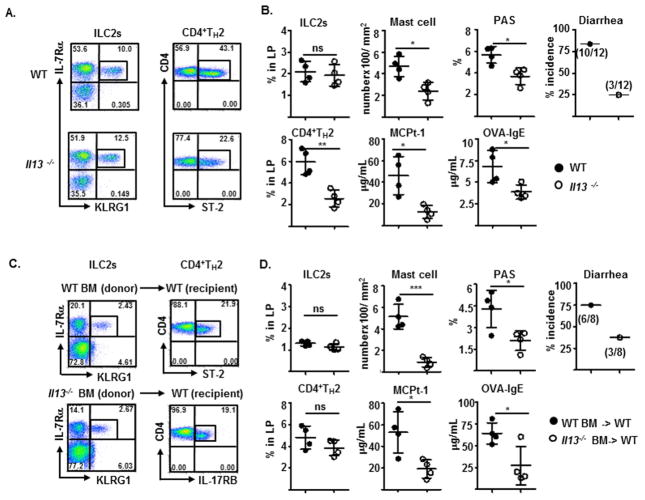
Detection (A and C) and frequency of Lin−IL-7Rα+KLRG1+ILC2s and CD4+ST-2+TH2 cells in, and measurements of indicated parameters of experimental food allergy of (B and D), sensitized WT or Il13−/− mice (A and B) or irradiated WT BALB/c recipients reconstituted with BM progenitors from WT or Il13−/− mice after six intragastric OVA challenge (C and D).
Results
Early induction of intestinal IL-25 promotes susceptibility to experimental food allergy
To determine the involvements of IL-25 in regulating food allergy, intestinal Il25 expression was examined. Compared to naïve mice, sensitized mice received only two intragastric OVA challenges rapidly upregulated Il25 expression (> 5 fold) in the duodenal epithelium; this Il25 expression remained elevated until the onset of anaphylactic response to ingested OVA (Fig. 1A). Concomitantly, the expression of Il13 (> 5 fold) and chemokine genes, including Tarc (> 7 fold), Cxcl1 (> 7 fold), and Ccl11 (> 20 fold) (eotaxin 1), but not Ccl24 (eotaxin 2), were also upregulated, primarily in the small intestinal epithelium prior to the onset of experimental food allergy. To address whether dysregulated IL-25 signaling contributes to susceptibility to developing experimental food allergy, we generated IL-25 transgenic mice lines (iIL-25Tg) that constitutively overexpress murine IL-25 driven by the promoter of intestinal fatty acid–binding protein (iFABP) in the small intestinal epithelium and Il17rb−/− mice that had disrupted Il17rb gene expression. Although intestinal IL-25 overexpression induced increases of intestinal Il5 (> 20,000 fold) and Il13 (> 75 fold) gene expression, and mast cell (MC) numbers and levels of goblet cell hyperplasia in naïve or sensitized iIL-25Tg mice (Fig. 1B and data not shown), these mice did not develop symptomatic features of IgE-mediated experimental food allergy, including allergic diarrhea, hypothermia, intestinal mastocytosis, or increased serum OVA-specific IgE and/or mast cell protease 1 (MCPt-1), the latter of which indicates MC degranulation (Fig. 1B). Notably, a regimen of only four intragastric OVA antigen challenges was sufficient to induce sensitized iIL-25Tg mice, but not their littermate controls, to manifest systemic symptoms beginning with diarrhea (>84%, profuse liquid stool) and then hyperthermia (>60%) within 45 min (Fig. 1B). These iIL-25Tg mice also produced higher titers of serum MCPt-1 and OVA-specific IgE and exhibited more pronounced goblet cell (GC) hyperplasia, but not intestinal mastocytosis, compared to those of littermate controls (Fig. 1B and C). In contrast, sensitized mice lacking IL-17RB were more resistant to manifest allergic diarrhea and hypothermia than wild-type BALB/c mice after six times of intragastric OVA challenge (Fig. 1D). These Il17rb−/− mice produced less MCPt-1 and displayed less intestinal mastocytosis and GC hyperplasia, while producing comparable amounts of OVA-specific IgE (Fig. 1D and E). These results suggest that intestinal epithelium Il25 expression increases at the early phase of experimental food allergy and the alterations in IL-25 signaling can positively or negatively regulate the susceptibility to developing experimental food allergy.
Intestinal ILC2s produce IL-5 and IL-13 in response to IL-25
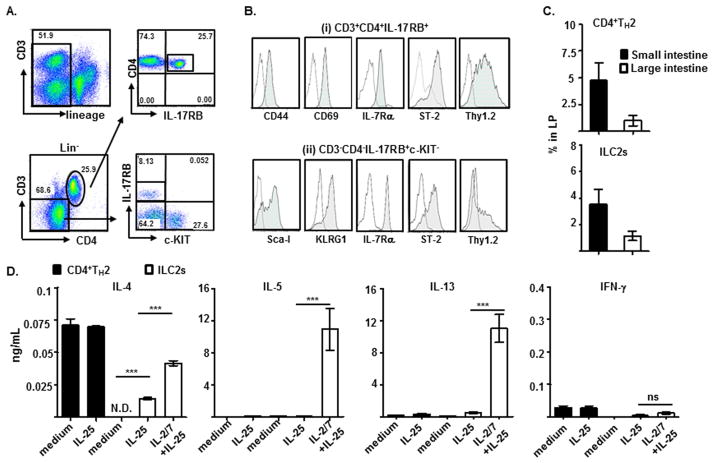
Next, we search for intestinal IL-17RB-expressing immune cells during the development of experimental food allergy. Two dominant IL-17RB–expressing cell populations were identified in the laminar propria (LP) of the small intestine of mice with active allergic diarrhea: (i) CD3+CD4+IL-17RB+ cells and (ii) CD3−CD4−IL-17RB+c-KIT− cells, both of which expressed hematopoietic cell lineage marker CD45, but not other known cell lineage markers (Lin−) (Fig. 2A and data not shown). CD3+CD4+IL-17RB+ cells expressed surface markers of TH2 effector/memory cells, including; CD44, CD69, IL-7Rα, ST-2, and Thy1.2, and produced significant amounts of TH2 cytokines, IL-4, IL-5, and IL-13, not IL-17 or IFN-γ upon CD3/CD28 re-stimulation (Fig. 2B and see Fig. E1A and E1B in this article’s Online Repository at www.jacionline.org). Notably, the Lin−CD3−CD4−IL-17RB+c-KIT− cells expressed Sca-I, KLRG1, IL-7Rα (CD127), ST-2, Thy1.2, ICOS, IL-2Rα (CD25), and MHCII, the signature markers of recently described ILC2s30, but not FcεR, IL-3R (CD123), and Siglec-F (Fig. 2B and see Fig. E2A in this article’s Online Repository at www.jacionline.org). On the contrary, Lin−CD3−CD4−IL-17RB−c-KIT+ cells expressed surface FcεRαI, not IL-2Rα (CD25), IL-3R (CD123), or ICOS, suggesting that these cells are of MC lineage (see Fig. E2A in this article’s Online Repository at www.jacionline.org). Thus, systemic administrations of IL-25 proteins in mice resulted in the selective expansion of Lin−CD3−CD4−IL-17RB+c-KIT− cells, not Lin−CD3−CD4−IL-17RB−c-KIT+ cells in their mesenteric lymph nodes (MLN) (see Fig. E2B in this article’s Online Repository at www.jacionline.org). Comparative gene expression analyses among examined hematopoietic cell lineages revealed that both IL-17RB-expressing cell subsets expressed high levels of Gata3 (>5 ×103 fold), and TH2 cytokine genes Il4 (>5 ×103 fold), Il5 (>1 ×104 fold), and Il13 (>5 ×104 fold); however, only CD3−CD4−IL-17RB+c-KIT− cells expressed ILC2 signature transcripts; Id2 (>50 fold), and Rora (>3×103 fold) (see Fig. E1C in this article’s Online Repository at www.jacionline.org). Thus, Lin−CD3+CD4+IL-17RB+ and Lin−CD3−CD4−IL-17RB+c-KIT− cell subsets represent the intestinal CD4+TH2 cells and ILC2s, respectively.
FIG 2.
Detection (A), phenotypic analysis (B), and frequencies (C) of Lin−CD3+CD4+IL-17RB+TH2 cells and Lin−CD3−CD4−IL-17RB+c-KIT−ILC2s in mice developed food allergy. (D) Indicated cytokines produced by purified Lin−CD3+CD4+IL-17RB+ TH2 cells and Lin−CD3−CD4−IL-17RB+c-KIT−ILC2s from mice developed food allergy after stimulation with IL-25 alone or IL-25 plus IL-2 and IL-7 (IL-2/7) were measured and compared.
Both IL-17RB–expressing CD4+TH2 cells (~5.0%) and ILC2s (~3.0%) accumulated preferentially in the small intestine of mice developed experimental food allergy (Fig. 2C), the primary anatomical site that expressed induced Il25 transcript (Fig. 1A). IL-25 alone induced very few TH2 cytokine production by purified intestinal CD4+TH2 cells and ILC2s; however, the addition of IL-2 and/or IL-7 potentiated ILC2s, but not CD4+TH2 cells, to respond to IL-25 stimulation by producing large amounts of IL-5 (>10 ng/ml per 104 cells) and IL-13 (>10 ng/ml per 104 cells), and very little IL-4 (<0.05 ng/ml per 104 cells) and IFN-γ (<0.1 ng/ml per 104 cells) (Fig. 2D). These results suggest that both CD4+TH2 cells and ILCs are intestinal IL-17RB–expressing cells and that ILC2s are the primary IL-5 and IL-13 producers in response to IL-25 stimulation in experimental food allergy.
IL-25-responsive ILC2s promote susceptibility to experimental food allergy
To determine the contributions of ILC2s to the development of food allergy, we develop a reconstitution model of experimental food allergy, adoptively transferring BM cells from 4GET (Il4 expression–driven GFP reporter) BALB/c mice into OVA-sensitized BALB/c mice one day after sub-lethal irradiation (protocol diagramed in Fig. E3A in this article’s Online Repository at www.jacionline.org). After the second sensitization and repeated intragastric OVA challenge, transferred BM cells replenished the IL-17RB+c-KIT−IL-7Rα+KLRG+ ILC2 compartment with GFP-marked cells in the irradiated recipients, whereas most of the CD4+ST-2+IL-17RB+ TH2 cells lacked GFP expression and were therefore derived from the sensitized recipients (Fig. 3A). Consistently, most of replenished ILC2s were Thy1.1high/low (>96%) in the irradiated Thy1.2+ recipients that generated primarily Thy1.2-expressing CD4+ T cell compartment (>96%) after reconstitution with congenic Thy1.1+BM cells. Furthermore, ILC2 frequencies were comparable (~2.0%) between reconstituted wild type (WT) and STAT6−/− Thy1.2+ recipients (see Fig. E3B and E3C in this article’s Online Repository at www.jacionline.org). Thus, BM transplants will reconstitute innate ILC2 cell lineage within 2 weeks, whereas de novo T cell generation after thymus engraftment from donor-derived hematopoietic progenitors require >2 months31. Having established a reconstitution model of experimental food allergy, we showed that irradiated mice reconstituted with BM cells that lacked transcription factor ROR-α, a transcription factor for ILC2 development20, failed to develop ILC2s, while CD4+TH2 cells developed normally (Fig. 3B and C). Consequently, irradiated mice reconstituted with WT, not ROR-α-deficient, BM cells exhibited pronounced intestinal mastocytosis, produced increased MCPt-1 and OVA-specific IgE, and developed allergic diarrhea after repeated antigen challenge (Fig. 3D). While IL-17RB–deficient BM cells developed into ILC2s normally, these reconstituted IL-17RB–deficient ILC2s did not promote experimental food allergy in the irradiated recipients that produced OVA-specific IgE normally (Fig. 3B–D). Notably, LP cells from the small intestine of WT BM reconstituted mice produced significant higher amounts of IL-5 and IL-13 than those by LP cells from Rora−/− or Il17rb−/− BM reconstituted recipients which lacked ILC2s or generated dysfunctional ILC2s, respectively, in response to IL-25 stimulation ex vivo (Fig. 3E). These results demonstrate that intestinal ILC2s are the primary IL-5 and IL-13 producers in response to IL-25 stimulation and play a key role in promoting experimental food allergy in vivo.
Antigen-induced CD4+TH2 cells enhance ILC2 function in response to IL-25
Recent reports suggest an interplay relationship between innate ILC2s and adaptive CD4+ T cells in the protective response against helminthic infection32, 33. To address whether ingested antigen-induced CD4+TH2 immune response is involved in ILC2 function, the frequencies of intestinal ILC2s and CD4+TH2 cells during the development of experimental food allergy were examined. While very few CD4+TH2 cells (<0.1%) could be detected, a considerable pool of ILC2s resided in the small intestine of naïve or sensitized mice (1.5–3.0%) constantly (Fig. 4A and 4B). Notably, repeated intragastric OVA antigen challenge induced a significant accumulation of intestinal CD4+TH2 cells (<0.1% to >5%), not ILC2s and an increase of peripheral eosinophils (Fig. 4A–C). Correspondingly, intestinal LP cells from re-challenged mice produced significant higher amounts of IL-4, IL-5, and IL-13 than those by LP cells from naïve mice after OVA peptide stimulation ex vivo (see Fig. E4A in this article’s Online Repository at www.jacionline.org). Although ILC2 frequencies were comparable among naïve, sensitized, and re-challenged mice, the capability of ILC2s to produce IL-5 and IL-13 after IL-25 or PMA/ionomycin stimulation was significantly enhanced only in re-challenged mice that developed a considerable pool of CD4+TH2 cells (Fig. 4A, 4D, and 4E). To directly assess whether CD4+TH2 cells would enhance ILC2 responsiveness to IL-25 stimulation, we measured their TH2 cytokine-producing capability in the presence or absence of CD4+TH2 cells ex vivo. Indeed, co-culture of in vitro-derived CD4+TH2 cells significantly enhanced the responsiveness of ILC2s to IL-25 stimulation, as these stimulated, co-culture ILC2s produced large amounts of IL-5 and IL-13 (Fig. 4F and see Fig. E4B in this article’s Online Repository at www.jacionline.org). Intriguingly, the presence of OVA peptides further enhanced IL-5 production by co-cultured OVA antigen-specific CD4+TH2 cells and ILC2s in response to IL-25 stimulation (see Fig. E4B in this article’s Online Repository at www.jacionline.org). Treatments with antibodies against IL-2 greatly diminished the ILC2s’ enhanced responsiveness to IL-25 stimulation in CD4+TH2 cell co-culture (Fig. 4F). Furthermore, ILC2s purified from the small intestine of re-challenged WT or Il17rb−/− mice failed to respond to IL-25 when cultured alone; however, purified WT CD4+TH2 cells from mice developed active allergic diarrhea greatly enhanced the IL-25–stimulated IL-5 and IL-13 production by co-cultured WT, but not IL-17RB-deficient, ILC2s (Fig. 4G). Notably, IL-17RB-deficient CD4+ST-2+TH2 cells purified from Il17rb−/− mice, which were resistant to developing experimental food allergy, were less capable of potentiating the IL-5 and IL-13 production by WT ILC2s in response to IL-25 (Fig. 4G). Collectively, these intriguing findings suggest that IL-17RB–expressing CD4+TH2 cells induced by ingested antigens enhance the capability of intestinal ILC2 residents to respond to epithelial-derived IL-25 by producing prodigious amounts of IL-5 and IL-13, and thereby promote experimental food allergy.
ILC2s fail to promote experimental food allergy in mice that lack CD4+TH2 cells
Next, we address whether the induction of CD4+TH2 cells by ingested antigens is essential for ILC2 function in promoting experimental food allergy. Compared to mice received isotype-matched antibodies, mice ablated of CD4+T cells after anti-CD4 antibody treatments failed to develop symptomatic features of experimental food allergy, despite their intestinal ILC2 compartment remaining intact (Fig. 5A–C). Although replenished ILC2s restored the susceptibility of irradiated wild-type recipients to develop experimental food allergy after BM reconstitution, these donor-derived ILC2s failed to restore the capability of irradiated Stat6−/− recipients, which lacked the CD4+TH2 cells, to develop intestinal mastocytosis, produce MCPt-1 or OVA-specific IgE, or allergic diarrhea (Fig. 5D–F). These results suggest that ILC2s alone are insufficient to drive the development of experimental food allergy and that the induction of intestinal CD4+TH2 cells by ingested antigens is a prerequisite for ILC2 function to promote experimental food allergy.
IL-13 production by ILC2s promotes experimental food allergy
To understand the mechanisms underlying the function of ILC2s in promoting the susceptibility to experimental food allergy, we examine the role of IL-13, the major cytokine produced by ILC2s and shown to be involved in the development of IgE-mediated experimental food allergy34. Although ILC2s developed normally in Il13−/− mice, much fewer CD4+TH2 cells were induced in Il13−/− mice than in wild-type mice after repeated intragastric OVA antigen challenge (Fig. 6A). Consequently, Il13−/− mice exhibited reduced levels of intestinal mastocytosis, goblet cell hyperplasia, produced less MCPT-1 and OVA-specific IgE, and were more resistant to developing experimental food allergy, compared to those in wild-type mice (Fig. 6B). Consistently, the capabilities of WT and IL-13-deficient BM cells were comparable in replenishing ILC2s in irradiated recipients that developed CD4+TH2 cell normally (Fig. 6C and 6D). However, the recipients reconstituted with Il13−/− BM cells exhibited less pronounced intestinal mastocytosis and goblet cell hyperplasia, produced fewer MCPt-1 and OVA-IgE, and were thereby more resistant to developing an anaphylactic response to ingested OVA antigen, compared to those received WT BM cells (Fig. 6D). These intriguing results suggest that IL-13 elicited by ILC2s may play a key role in promoting intestinal allergic inflammation that promote the development of IgE-mediated experimental food allergy.
Discussion
The immune response to allergenic dietary proteins depends on the balance of a complex interplay between immune and non-immune cell interactions in the GI tract. Mechanistically, it remains unclear how an adverse allergic reaction to ingested food can be initiated and amplified, leading to the loss of oral tolerance. TSLP, IL-33, and IL-25, the epithelial-derived cytokines, have been shown to play an important role in initiating and amplifying type-2 immune responses in allergic asthma and against parasitic infection6, 35. TSLP can potentiate dendritic cells to promote naïve CD4+ T cells to differentiate into CD4+TH2 cells and maintain CD4+TH2 memory/effector cells36–39, and enhance allergic sensitization in the cutaneous sensitization model of food allergy40. While IL-33 is responsible for the development of allergic lung diseases41–43, this cytokine appears to be dispensable for cutaneous allergic sensitization, but essential for inducing IgE-dependent anaphylaxis40. Whether IL-25 is involved in regulating the dysregulated type-2 immune response to ingested food antigens has not been established. Herein, we demonstrate that repeated intragastric antigen challenge induce an increase in Il25 gene expression by intestinal epithelium before the onset of anaphylactic response to ingested food. Studies using genetically modified mice further demonstrate that IL-25 signaling promotes the susceptibility to developing experimental food allergy. Notably, CD4+TH2 cell induction by ingested antigens appears to be a prerequisite for intestinal ILC2 residents to produce large amounts of IL-13 in response to IL-25 stimulation and to promote the susceptibility to experimental food allergy. Our studies further suggest that intestinal IL-25 signaling promotes the interplay between CD4+TH2 cells and ILC2s to elicit allergic reactions to ingested food.
The findings that IL-25 mediates protective immunity against GI helminthic infection, such as Trichuris muris and Nippostrongylus braziliensis, and Trichinella spiralis by promoting a TH2 cytokine–dependent immune response3, 14, 44, 45 suggest a potential involvement of IL-25 in the allergic reactions to ingested food antigens. Indeed, analyses of iIL-25Tg and Il17rb−/− mice reveal their susceptibility and resistance to developing IgE-mediated experimental food allergy, respectively, substantiating the role of IL-25 in promoting allergic reaction to ingested antigens. While intestinal ILC2s are identified to be the primary IL-25-responding cells, ILC2s alone are insufficient to drive intestinal anaphylaxis in naïve or sensitized transgenic mice overexpressing IL-25. Because their capability to produce IL-5 and IL-13 in response to IL-25 and to promote experimental food allergy requires CD4+IL-17RB+TH2 cells that are induced after repeated intragastric OVA antigen challenge. In agreement with recent studies32, 33, anti–IL-2 antibody treatments result in a significant reduction of IL-5 and IL-13 production by IL-25–stimulated ILC2s co-cultured with CD4+TH2 cells. Notably, IL-17RB–deficient CD4+ST-2+TH2 cells are less capable of enhancing ILC2s to produce TH2 cytokine production in response to IL-25, possibly that IL-17RB–deficient CD4+ST-2+TH2 cells produce less IL-2 after IL-25 stimulation. Our studies support a view that IL-25 enhances the concerted interactions between intestinal ILC2s and antigen-induced CD4+TH2 cells to amplify allergic reactions to ingested food antigen.
A role of ILC2s in promoting IgE-mediated experimental food allergy has been further substantiated in a reconstitution model of experimental food allergy. Donor-derived ILC2s promote irradiated recipients mice to develop experimental food allergy after wild-type BM reconstitution. However, irradiated recipients reconstituted with Rora−/− or Il17rb−/− BM that are defective in ILC2 development or function, respectively, are resistant to developing experimental food allergy. It appears that IL-13 produced primarily by ILC2s plays a key role in promoting the susceptibility to experimental food allergy, possibly by inducing goblet cell hyperplasia, increase intestinal permeability, and modulate gut barrier function46–48. In contrast to our findings, a recent study suggest that mice ablated of ILC2 and deficient of IL-25 or IL-13 remain capable of developing splenic TH2 immune response and systemic anaphylactic response to antigen administered intraperitoneally after sensitization by orally gavaged cholera toxin (CT)49, 50. The discrepancies may be attributable to differences in the experimental approaches, including; sensitization adjuvant (alum vs. CT), murine strains (BALB/c vs C3H/HeJ or C57/B6)34, 50, 51, and the anatomic locations where the anaphylaxis is induced (intestine vs. peritoneal)34, 49, 50. Importantly, previous studies demonstrate that anaphylactic response to ingested antigens administered by intragastric inoculations following alum sensitization is dependent on the classic mast cell/IgE/FcεR pathway52, 53, whereas the anaphylaxis induced by intraperitoneal antigens following CT sensitization can be mediated by the alternative IgG/FcγR pathway54, 55. It remains to be determined whether the differences in the genetic predisposition to allergic sensitization between BALB/c and C57/B6 strains and/or the antigen administration routes results in this perceived discrepancy in the necessity of IL-25 signaling in experimental food allergy.
In our murine food allergy model, mice eventually develop systemic manifestations pertaining to some characteristics of food allergy–induced life-threatening anaphylaxis in humans, including; cutaneous and mesenteric vascular leak, airway hyperresponsiveness, and hypothermia52, 56, despite that “food allergic” mice do not develop skin allergic disorders that are often comorbid in some of food allergy patients57. Although the initial triggers and genetically predisposing factors that initiate allergic sensitization in the GI tract remain unclear, our findings suggest that IL-25-mediated collaborations between ILC2s and CD4+TH2 cells can be a key step in amplifying the cascade of allergic reactions to ingested antigens at the effector phase of IgE-mediated food allergy via the mast cell/IgE pathway52, 58. These studies underscore the importance of understanding the mechanisms that underlie intestinal allergic response to ingested food and the knowledge gained from the basic studies may eventually serve as the rationale to design innovative approaches for the care of food allergy in humans.
Supplementary Material
Key messages.
Alteration of IL-25 signals can positively or negatively regulate the susceptibility to experimental food allergy.
Intestinal ILC2s are the primary IL-5 and IL-13 producers in response to IL-25 stimulation and play a key role in promoting experimental food allergy.
Ingested antigen-induced CD4+TH2 cells potentiate ILC2s’ capability to respond to IL-25 by producing prodigious amounts of IL-13, that promotes experimental food allergy.
Acknowledgments
We thank S. Hottinger for editorial assistance. This work is supported by the National Institutes of Health (AI090129-1 to Y.H.W., A1073553 to S.P.H), the Digestive Health Center (P30 DK078392, Pilot and Feasibility Award to Y.H.W.), American Partnership For Eosinophilic Disorders (APFED) (HOPE Pilot Grant to Y.H.W.), Campaign Urging Research For Eosinophilic Diseases (CURED) Foundation, the Buckeye Foundation, and the Food Allergy Research Education Fund to M.E.R., and VA Merit Award to F.D.F.
Abbreviations used
- ILC2s
type-2 innate lymphoid cells
- TH2
T helper type 2
- MCPt-1
mast cell protease-1
- OVA
ovalbumin
- BM
bone marrow
- GI
gastrointestinal
- LP
laminar propria
- Lin
lineage
- CT
cholera toxin
- OG
oral gavage
- GC
goblet cell
- 4GET
Il4 expression–driven GFP reporter
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finkelman FD. Anaphylaxis: lessons from mouse models. The Journal of allergy and clinical immunology. 2007;120:506–15. doi: 10.1016/j.jaci.2007.07.033. quiz 16–7. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 3.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of experimental medicine. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. The Journal of experimental medicine. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y-H, Liu Y-J. TSLP, OX40L, and IL-25 in allergic responses. Clin Exp Allergy. 2009 doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 8.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 9.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–40. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 10.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–67. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 11.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. The Journal of allergy and clinical immunology. 2007;120:1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–8. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso R, Sarra M, Stolfi C, Rizzo A, Fina D, Fantini MC, et al. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136:2270–9. doi: 10.1053/j.gastro.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 14.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–9. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 16.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature immunology. 2011;12:1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nature immunology. 2012;13:229–36. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. Journal of immunology. 2011;187:5505–9. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–6. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 23.Mjosberg J, Bernink J, Peters C, Spits H. Transcriptional control of innate lymphoid cells. European journal of immunology. 2012;42:1916–23. doi: 10.1002/eji.201242639. [DOI] [PubMed] [Google Scholar]
- 24.Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 2012;12:445–57. doi: 10.1016/j.chom.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells - how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 26.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The Transcription Factor GATA-3 Controls Cell Fate and Maintenance of Type 2 Innate Lymphoid Cells. Immunity. 2012;37:634–48. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. The Journal of allergy and clinical immunology. 2012;129:191–8. e1–4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature immunology. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol. 2008;20:697–702. doi: 10.1016/j.coi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annual review of immunology. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 31.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–8. [PubMed] [Google Scholar]
- 32.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-Mediated Dialog between Group 2 Innate Lymphoid Cells and CD4(+) T Cells Potentiates Type 2 Immunity and Promotes Parasitic Helminth Expulsion. Immunity. 2014;41:283–95. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–8. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 34.Brandt EB, Munitz A, Orekov T, Mingler MK, McBride M, Finkelman FD, et al. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. The Journal of allergy and clinical immunology. 2009;123:53–8. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 37.Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt RW, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Du J, Zhu J, Yang X, Zhou B. Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol. 2015;135:781–91. e3. doi: 10.1016/j.jaci.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 2014;26:539–49. doi: 10.1093/intimm/dxu058. [DOI] [PubMed] [Google Scholar]
- 41.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, et al. IL-33 and Thymic Stromal Lymphopoietin Mediate Immune Pathology in Response to Chronic Airborne Allergen Exposure. J Immunol. 2014;193:1549–59. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110:13921–6. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao A, Urban JF, Jr, Sun R, Stiltz J, Morimoto M, Notari L, et al. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol. 2010;185:6921–9. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angkasekwinai P, Srimanote P, Wang YH, Pootong A, Sakolvaree Y, Pattanapanyasat K, et al. Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infection and immunity. 2013;81:3731–41. doi: 10.1128/IAI.00646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 47.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 48.Wu D, Ahrens R, Osterfeld H, Noah TK, Groschwitz K, Foster PS, et al. Interleukin-13 (IL-13)/IL-13 receptor alpha1 (IL-13Ralpha1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl- secretion. The Journal of biological chemistry. 2011;286:13357–69. doi: 10.1074/jbc.M110.214965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu DK, Mohammed-Ali Z, Jimenez-Saiz R, Walker TD, Goncharova S, Llop-Guevara A, et al. T helper cell IL-4 drives intestinal Th2 priming to oral peanut antigen, under the control of OX40L and independent of innate-like lymphocytes. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.29. [DOI] [PubMed] [Google Scholar]
- 50.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e1–8. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow’s milk hypersensitivity. The Journal of allergy and clinical immunology. 1999;103:206–14. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 52.Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, et al. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. The American journal of pathology. 2012;180:1535–46. doi: 10.1016/j.ajpath.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. The Journal of allergy and clinical immunology. 2010;125:469–76. e2. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011;6:e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. The Journal of allergy and clinical immunology. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. quiz 58. [DOI] [PubMed] [Google Scholar]
- 56.Brown SG, Stone SF. Laboratory diagnosis of acute anaphylaxis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011;41:1660–2. doi: 10.1111/j.1365-2222.2011.03893.x. [DOI] [PubMed] [Google Scholar]
- 57.Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2009. The Journal of allergy and clinical immunology. 2010;125:85–97. doi: 10.1016/j.jaci.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 58.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of clinical investigation. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.