Abstract
Background and Aims
Prior studies by our group demonstrated the efficacy of a brief but intensive behavioral intervention for producing initial smoking abstinence among opioid-dependent patients. In the present study, our aim was to promote longer-duration abstinence in this population. Following an initial 2-week incentive intervention for smoking abstinence, we examined whether a 10-week maintenance arm involving continuation of contingent reinforcement will produce greater smoking abstinence than a similar duration of noncontingent reinforcement.
Design
Randomized, 12-week, parallel-group study.
Setting
Outpatient research clinic in Burlington, Vermont, USA.
Participants
Opioid-maintained smokers (n = 88) who provided breath carbon monoxide and urinary cotinine specimens and received contingent reinforcement for smoking abstinence during Weeks 1-2 (Phase 1), with 63 randomized on Day 14 to an Extended Contingent (EC; n = 31) or Extended Noncontingent (EN; n = 32) experimental condition for Weeks 3-12 (Phase 2).
Intervention and control
The EC condition consisted of voucher values that escalated across consecutive negative samples until they reached $30, after which they remained at $30 per negative sample. A positive or a missing sample resulted in no vouchers for that day and resent the value of the next negative same to $9. Two consecutive negatives returned the schedule to the pre-reset value. The EN control condition consisted of vouchers delivered for providing scheduled samples but independent of smoking status.
Measurements
The primary outcome was percentage of biochemically-abstinent samples during Phase 2. Secondary measures included abstinence status at final study visit, complete abstinence, participants' longest duration of continuous abstinence, cotinine and CO levels and self-reported cigarettes per day.
Findings
EC participants achieved greater smoking abstinence during Phase 2 than EN participants (46.7% vs. 23.5% negative samples, respectively; OR=2.98, 95% CI: 1.16-7.65, X21=5.0, p=0.02). When longest duration of continuous abstinence was compared between experimental groups, EC participants achieved twice the mean duration of continuous abstinence compared with EN participants (3.31 vs. 1.68 weeks; t61=1.83, p=0.07). An effect of experimental condition was also seen on mean cotinine levels (42.5 vs. 210.6 ng/ml, respectively; F1,61=5.9, p=0.02).
Conclusions
Among opioid-maintained smokers receiving an initial period of daily contingent incentives, a contingent reinforcement intervention appears to be more effective at extending smoking abstinence than non-contingent reinforcement over 10 weeks.
Keywords: Smoking abstinence, opioid dependence, methadone, buprenorphine, incentive, behavioral, contingency management
INTRODUCTION
Maintenance therapy with opioid agonists represents the most widely used and efficacious treatment for opioid dependence (1–3). Despite the efficacy of methadone and buprenorphine treatment in reducing illicit opioid use, concurrent use of other substances remains a significant problem among some patients (4). One of the most virulent forms of other substance abuse is cigarette smoking, perhaps due to a pharmacological interaction between opioids and nicotine whereby opioids may increase the reinforcing effects of cigarettes (5–10). Indeed, 80–90% of opioid-dependent patients smoke cigarettes (11–14), in striking contrast to 18% in the general U.S. adult population (15). Finally, as with the general population, smoking in opioid-dependent individuals is associated with substantial morbidity and mortality (16, 17).
Most opioid-maintained patients are aware of the serious health risks associated with smoking, express interest in quitting and desire assistance with smoking cessation (11, 14, 18–22). In one survey, for example, methadone-maintained patients rated smoking cessation as one of the most important services needed during their methadone treatment (18). Despite this, there have been relatively few scientific efforts to develop smoking interventions for opioid-maintained smokers. In the limited studies to date investigating the efficacy of pharmacotherapies in this population, outcomes have been modest (9, 23–29). The feasibility of using behavioral interventions, wherein patients earn tangible incentives contingent on smoking abstinence, has also been investigated (30–33). However, while some of those studies suggested that opioid-maintained smokers may be sensitive to incentive-based treatments, sustained abstinence was generally low and the overall modest outcomes led researchers to suggest that opioid patients may not respond to incentive interventions for smoking cessation (31, 34).
We have been programmatically developing and evaluating the efficacy of a behavioral intervention designed to maximize patients' success in smoking cessation. This treatment includes more rigorous biochemical monitoring, frequent clinical contact and greater magnitudes of abstinence-contingent financial incentives than were used in prior studies (35). These parameters are crucial determinants of the efficacy of behavioral-economic interventions and thus we sought to leverage them here with this group of recalcitrant smokers (36–40).
In our initial studies, our aim was to maximize the percentage of smokers who achieved abstinence during the first two weeks following their quit date, as abstinence during this period is associated with longer-term outcomes (41–45). We developed a brief but intensive 2-week intervention wherein participants reviewed with staff a National Cancer Institute booklet on quitting smoking (46), set a quit date, visited the clinic daily thereafter and earned voucher-based incentives contingent upon biochemically-verified smoking abstinence. In two randomized trials, methadone- or buprenorphine-maintained patients were randomized to receive a Contingent incentive condition or a Noncontingent control condition wherein they received incentives independent of smoking status (47, 48). In both studies, Contingent participants achieved significantly more smoking abstinence than Noncontingent (55% vs. 5–17%, respectively), as well as longer durations of continuous abstinence (6.3–7.7 vs. 0.6–2.4 days, respectively).
Our next step in this line of research is to learn more about how to maintain the smoking abstinence achieved in the initial two weeks of the intervention. There is tremendous need for interventions that will produce sustained smoking abstinence among opioid-maintained patients. While having procedures to promote initial abstinence is a necessary step in developing an efficacious intervention to promote longer-term abstinence, we do not consider initial abstinence to be sufficient in and of itself. Thus, in the present randomized trial, smokers received an initial 2-week incentive intervention for smoking abstinence, after which we examined whether a 10-week maintenance arm involving continuation of contingent reinforcement will produce greater smoking abstinence than a similar duration of noncontingent reinforcement.
METHODS
Participants
This randomized, parallel-group study was conducted in an outpatient research clinic in Burlington, Vermont. Opioid-maintained adults were recruited via flyers distributed to treatment providers between April 2009 and March 2013. Eligible participants had to be 18–65 years of age, report smoking ≥10 cigarettes per day for ≥ 1 year, be interested in cessation and be stable in methadone or buprenorphine treatment (i.e., no dose change or significant illicit drug use in the past month). Confirmation of participants' dose and urinalysis results were obtained from their treatment provider. Those who were pregnant or nursing or those with severe mental illness were not eligible and were referred to other smoking cessation resources in the community. The study was approved by the University of Vermont Institutional Review Board and participants provided informed consent to participate.
Intake
Participants completed self- and experimenter-administered instruments at intake, including a Smoking History and Demographic Questionnaire, the Addiction Severity Index (ASI; 49), Fagerström Test for Nicotine Dependence (FTND; 50, 51), Minnesota Nicotine Withdrawal Questionnaire (MNWQ; 52), Nicotine Dependence Syndrome Scale (53) and the Questionnaire of Smoking Urges (QSU; 54), and Michigan Alcoholism Screening Test (MAST; 55). A modified version of the Time-Line Followback (56) was used to evaluate past 30-day smoking, alcohol use, any use of smoking pharmacotherapies, and any changes in opioid maintenance dose. Psychological symptoms were assessed via the Beck Depression Inventory (BDI-II; 57), Beck Anxiety Inventory (BAI; 58) and the Brief Symptom Inventory (BSI; 59). Participants provided breath carbon monoxide (CO) and urine cotinine samples and were compensated $35 for completing the intake.
Pharmacotherapy
All participants were offered bupropion (Zyban®) as an optional pharmacotherapy for use during the study. Bupropion is a non-nicotinic, first-line medication for smoking cessation (60, 61). At intake, staff reviewed bupropion information with the participant and assessed their interest using a single-item question: “Are you interested in receiving bupropion (Zyban®) to help you quit smoking during this study?” (Yes/No). Interested participants completed a medical history and met with the study physician. Upon approval, they began bupropion therapy according to manufacturer guidelines (GlaxoSmithKline Zyban®). A one-week lead-in period was used for stabilization on the medication before participants' quit date, during which they ingested one 150 mg tablet for the first 3 days and two 150 mg tablets daily thereafter. Observation of ingestion took place thrice-weekly during this initial week (Monday, Wednesday, Friday) and at each study visit thereafter, with the morning dose observed and evening dose taken at home (and both doses taken at home on non-visit days). Participants were provided with sufficient doses to last until their next visit. Research staff monitored medication adherence as well as any side effects.
Phase 1 study procedures
Procedures during Weeks 1-2 (Phase 1) were identical for all participants and are consistent with our prior randomized studies (47, 48). After reviewing the National Cancer Institute's smoking cessation pamphlet (46), participants were assisted in identifying either the upcoming or following Monday as their “quit date”, which served as the first day of the abstinence-monitoring period. Beginning on the quit date, participants visited the clinic daily for two weeks and provided a CO and cotinine sample at each visit. CO was assessed via handheld monitors (Bedfont EC50 Smokerlyzer, Bedfont Scientific Ltd., Kent, England) and cotinine via an on-site enzyme multiplied immunoassay test with a semi-quantitative cotinine assay on an MGC240 machine (Microgenics, Fremont CA). Abstinence on Days 1-5 was defined as CO ≤ 6ppm; on Days 6–14, as cotinine ≤ 80ng/ml. CO was used early in the intervention to allow us to reinforce initial smoking abstinence, and the cotinine measure was used later to provide a more sensitive test likely to detect even low levels of ongoing smoking. This method of transitioning from CO to cotinine for monitoring of smoking status has been shown to be effective for promoting smoking abstinence in prior studies (47, 48). All participants earned voucher-based incentives contingent upon smoking abstinence, using the schedule of reinforcement described previously (47, 48). Briefly, the first negative sample earned $9.00, and values escalated by $1.50 with each subsequent negative sample for a maximum possible of $362.50. A positive or a missing sample resulted in no vouchers for that day and reset the value of the next negative sample to the initial $9.00. However, to encourage abstinence following a relapse, two consecutive negatives returned the schedule to the pre-reset value.
At each visit, self-report measures of withdrawal and craving were completed using the MNWQ and the Questionnaire of Smoking Urges-Brief Version (QSU-B; 62). Staff also monitored any recent smoking, use of nicotine replacement, bupropion or other smoking pharmacotherapies, any alcohol use, as well as any changes in methadone or buprenorphine dose using a Time-Line Followback questionnaire.
Phase 2 randomization
On Day 14, participants were randomly assigned by research staff using a minimization allocation procedure to one of two experimental conditions: (1) an Extended Contingent (EC) condition or (2) an Extended Noncontingent (EN) condition (63). Participant characteristics used to balance the randomization included opioid maintenance drug (methadone, buprenorphine), maintenance dose (≤ 100mg methadone/ ≤8mg buprenorphine, > 100mg methadone/ > 8mg buprenorphine), abstinence during Phase 1 (< 50%, ≥ 50% negative samples), use of bupropion (yes, no) and whether the participant had participated in a prior smoking study (yes, no).
Phase 2 study procedures
During Weeks 3-12 (Phase 2), abstinence was defined as cotinine ≤ 80ng/ml. The schedule of visits during Phase 2 was identical for both groups (i.e., daily during Weeks 1-2, thrice-weekly in Weeks 3–5, twice-weekly in Weeks 6–8, once-weekly in Weeks 9–12).
Extended Contingent
EC participants received 10 additional weeks of abstinence reinforcement during Weeks 3-12. Voucher values continued to escalate across consecutive negative samples until they reached $30, after which they remained at $30 per negative sample. The reset component remained in place throughout Phase 2. EC participants could earn a maximum of $570 during Phase 2 if they remained completely abstinent.
Extended Noncontingent
EN participants received 10 weeks of noncontingent reinforcement during Weeks 3-12, with vouchers delivered for providing scheduled samples but independent of smoking status. Earnings were yoked to the mean earnings of the EC group ($353.40), with the aim of producing a comparable amount of clinical contact and resources between groups. Earnings at each study visit ranged from $0 to $50 and were randomly determined by a computer program developed by us. Participants were told that they would receive vouchers independent of their smoking results and were not informed of their yoked status. To further emphasize that voucher delivery was not linked to smoking status, vouchers were provided prior to collection of biochemical samples. All other aspects of the study were identical across the two groups (e.g., frequency of visits, monitoring of smoking, data collection, protocol for spending vouchers).
Outcome measures and data analysis
The primary outcome measure was the percentage of biochemically-confirmed abstinent samples during Phase 2. Secondary measures included abstinence status at final visit, complete abstinence during Phase 2, participants' longest duration of continuous abstinence during Phase 2, cotinine and CO levels, and self-reported cigarettes per day. Experimental groups were compared on baseline demographic, opioid and smoking characteristics using two-sample t-tests, Wilcoxon rank sum tests, and chi-square tests. Primary analyses included all subjects randomized independent of early dropout and noncompliance, consistent with an intent-to-treat approach to clinical trials. Missing samples were considered positive. Logistic regression based on generalized estimating equations was used to compare the percentage of abstinent samples during Phase 2 between groups. The model included variables representing group, visit and their interaction, which were tested with a Wald chi-square. If the interaction was not significant, a main effect model was used to obtain an estimated odds ratio for group independent of visit. Participants' longest duration of continuous abstinence was compared across groups using a two-sample t-test, and dichotomous outcomes were compared using chi-square tests. Repeated measures analyses of variance were used to compare groups across study visits on cotinine and CO levels and self-reported cigarette use. Because the distribution of cotinine values was heavily skewed, data were log transformed prior to analysis and corresponding means represent geometric means. F-tests corresponding to simple effects were used to compare groups at each study visit and chi-square tests were used to compare groups on dichotomous outcomes. Statistical significance was determined based on α=.05 with all analyses performed using SAS statistical software Version 9.3 (SAS Institute, Cary, NC). The study had estimated power of 0.80 to detect an odds ratio of approximately 3.75 when comparing the EC and EN conditions in overall abstinence in Phase 2.
RESULTS
Participants
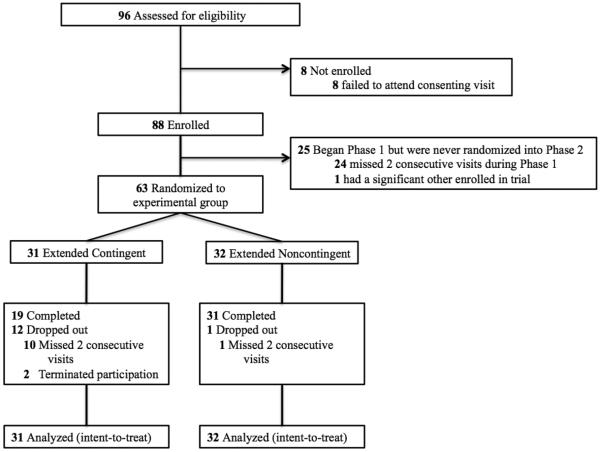
Eighty-eight participants were enrolled into Phase 1 of the study, with 63 randomized to EC (n=31) or EN (n=32) experimental groups in Phase 2 (Figure 1). There were no significant differences between groups on baseline demographic, opioid or smoking characteristics (Table 1).
Figure 1.
CONSORT profile of the randomized 12-week trial of Extended Contingent vs. Extended Noncontingent experimental conditions in promoting smoking abstinence among opioid-maintained patients.
Table 1.
Baseline Demographic and Smoking Characteristics
| Total Sample (n=63) | Extended Contingent (n=31) | Extended Noncontingent (n=32) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 34.4 ± 10.3 | 36.2 ± 10.4 | 32.7 ± 10.1 |
| Education (years) | 12.8 ± 1.5 | 12.6 ± 1.2 | 13.0 ± 1.6 |
| Male (%) | 41 | 42 | 41 |
| Caucasian (%) | 97 | 100 | 94 |
| Never married (%) | 62 | 65 | 59 |
| Employed (%) | 35 | 32 | 38 |
| Opioid Treatment Characteristics | |||
| Methadone maintained (%) | 71 | 68 | 75 |
| Opioid maintenance dose > 100mg methadone or 8mg buprenorphine (%) | 52% | 52% | 53% |
| Length of time in treatment (months) | 10.5 (4.3–28) | 12.2 (4.0–36.5) | 10 (5.0–24.3) |
| Smoking Characteristics | |||
| Number of cigarettes smoked/day (past 7 days) | 18.2 ± 9.5 | 17.7 ± 7.2 | 18.7 ± 11.3 |
| Age at 1st cigarette (years) | 13.0 ± 2.9 | 13.5 ± 3.0 | 12.6 ± 2.7 |
| Mean number of years smoked regularly | 16.7 ± 10.9 | 17.5 ± 10.6 | 15.8 ± 11.3 |
| Fagerstrom Test for Nicotine Dependence (0–10) | 4.8 ± 2.1 | 4.9 ± 2.1 | 4.7 ± 2.0 |
| Expired breath CO at study intake (ppm) | 10.3 ± 5.6 | 10.2 ± 6.0 | 10.4 ± 5.4 |
| Urinary cotinine at study intake (ng/ml) | 1343.3 ± 631.8 | 1223.6 ± 696.2 | 1459.3 ± 548.7 |
| Planning to quit next 30 days (%) | 98 | 100 | 97 |
| Smoking Quit History | |||
| Ever tried to quit (%) | 94 | 90 | 97 |
| Previous quit attempts (no.) | 8.6 ± 9.2 | 7.6 ± 8.3 | 9.6 ± 10.1 |
| Longest duration quit (days) | 105.0 (14.0–365.0) | 127.5 (14.0–365.0) | 90.0 (14.0–365.0) |
| ASI Subscale Scores b | |||
| Medical | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.4 |
| Employment | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| Alcohol | 0.0 ± 0.1 | 0.0 ± 0.2 | 0.0 ± 0.1 |
| Drug | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Opiate | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Cocaine | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.1 |
| Legal | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| Family/Social | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.1 ± 0.2 |
| Psychiatric | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 |
p-values based on independent sample t-tests for continuous variables and chi-square measures for categorical variables
Addiction Severity Index (Range 0–1)
Note. Values represent M ± SD unless otherwise indicated. There was no statistical evidence of chance imbalances on any of the characteristics shown between randomized groups (all p>0.05).
Smoking abstinence
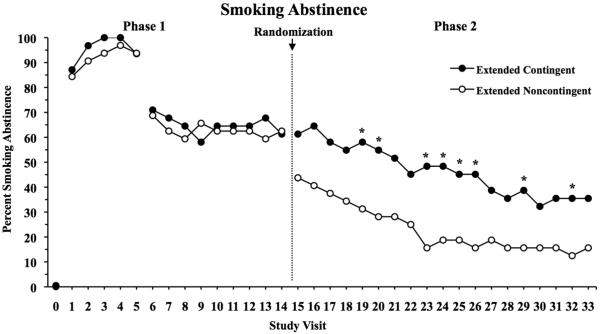
During Phase 1 (Visits 1–14) when all participants received incentives contingent upon abstinence, 74.5% of samples were smoking negative and 61.9% of patients were smoking-negative at Visit 14 (Figure 2). There were no differences between groups on any abstinence measure during this pre-randomization phase, including percent of participants abstinent at the last Phase 1 visit (p=0.92), overall amount of abstinence during Phase 1 (p=0.77) or longest duration of continuous abstinence (p=0.95).
Figure 2.
Percent of participants abstinent as a function of intake (Visit 0) and subsequent study visits (Visits 1–33). During Phase 1, participants provided breath CO and urinary cotinine specimens daily and earned voucher-based incentives contingent on smoking abstinence. During Phase 2, participants were randomized to either a group that continued to receive abstinent-contingent incentives (Extended Contingent) or a group that received incentives independent of smoking status (Extended Noncontingent). Abstinence at Study Visits 1-5 was defined as a breath CO ≤ 6ppm; starting at Visit 6, it was defined using the more sensitive measure of urine cotinine ≤ 80ng/ml and this remained in place for the remainder of the study. Data are presented for Extended Contingent (filled symbols) and Extended Noncontingent (open symbols) groups, with asterisks indicating a significant difference between experimental conditions at that study visit.
During Phase 2 (Visits 15–33), there was a significant difference between groups on total abstinence, with 46.7% vs. 23.5% smoking-negative samples in the EC and EN groups, respectively (OR=2.98, 95% CI: 1.16-7.65, X21=5.0, p=0.02). Percent of participants abstinent at each visit is shown in Figure 2. There was no evidence that the difference between groups was dependent on study visit (X218=16.3, p=0.57). At the final study visit (Visit 33), 35.5% and 15.6% of EC and EN participants were abstinent, respectively (X21=3.27, p=.07). When longest duration of continuous abstinence was compared between experimental groups, EC participants achieved twice the mean duration of continuous abstinence compared to EN participants (3.31 vs. 1.68 weeks; t61=1.83, p=0.07). Finally, complete abstinence across all 19 visits in Phase 2 was relatively rare and did not differ between groups (22.6% and 12.5% for EC vs. EN groups, respectively; X21=1.11, p=0.29).
For the subgroup of participants who were abstinent at the end of Phase 1 (EC n=19; EN n=20), mean longest duration of continuous abstinence during Phase 2 was 5.47 and 2.85 weeks for EC and EN participants, respectively (t37=2.0, p=0.05), with 37% (EC) and 20% (EN) achieving complete abstinence throughout the entire 10 weeks (X21=1.36, p=0.24).
Biochemical and self-report measures of smoking
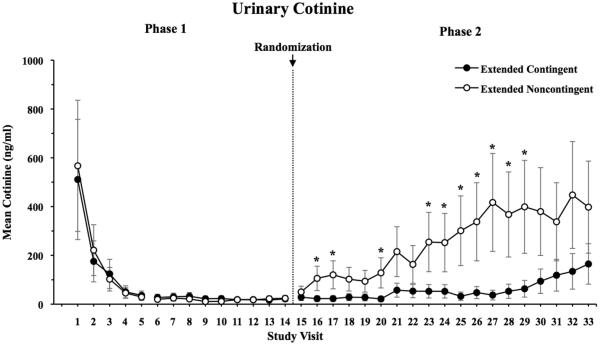
During Phase 1, mean urinary cotinine levels were 38.6 ng/ml for the overall group. Mean cotinine values across visits are shown in Figure 3. During Phase 2, there was a significant difference between groups (F1,61=5.9, p=0.02), with mean cotinine levels of 42.5 vs. 210.6 ng/ml in the EC and EN groups, respectively. There was no statistical evidence that the group difference was dependent on study day (F18,916=1.09, p=0.36), though it did take several days for cotinine values in the groups to diverge.
Figure 3.
Mean urine cotinine values (ng/ml) as a function of study visit. Data are presented for Extended Contingent (filled symbols) and Extended Noncontingent (open symbols) groups, with asterisks indicating a significant difference between experimental conditions at that study visit. Error bars represent SEM.
During Phase 1, mean CO levels were 1.62 ppm for the overall group. During Phase 2, COs (collapsed across study visits) were 3.53 and 5.51 ppm in the EC and EN groups, respectively. The difference between groups was not significant (F1,61=2.9, p=0.10) and there was no evidence of a group by day interaction (F18,916=0.54, p=0.94). However, there was a significant difference at end of study (Visit 33), with COs significantly lower in the EC vs. EN group (5.30 vs. 8.45 ppm, respectively; F1,916=5.09, p=0.02).
During Phase 1, participants reported smoking 1.18±0.37 cigarettes per day. During Phase 2, differences between experimental groups in self-reported number of cigarettes smoked per day varied across study visits (F65,3513=1.35, p=0.03 for group by visit interaction). EC participants reported smoking significantly fewer cigarettes per day than EN participants on 26 of the 66 study days in Phase 2, generally during the first month following randomization (p's<.05).
Effect of bupropion
At the time of randomization, there were no differences between EC and EN groups in the percent of individuals using bupropion (29% vs. 34%, respectively, X21=0.21, p=0.65). Furthermore, there was no significant difference in smoking abstinence between subjects using versus not using bupropion during Phase 1 (X21=0.16, p=0.69) or Phase 2 (X21=2.52, p=0.11) with estimated odds ratios of 0.82 (95% CI: 0.32 –2.14) and 0.41 (95% CI: 0.14–1.43), respectively.
DISCUSSION
We sought to evaluate whether continued reinforcement is necessary for promoting extended smoking abstinence following an initial brief but efficacious behavioral intervention with opioid-dependent smokers. In Phase 1, participants achieved high levels of abstinence, with 61.9% smoking abstinent at the end of Week 2. These findings replicate the amounts of initial abstinence (approximately 55%) seen in our prior incentive studies with opioid-dependent smokers and provide additional evidence that methadone- and buprenorphine-maintained patients are sensitive to incentive interventions for smoking (47, 48).
In Phase 2, the experimental groups quickly diverged following randomization, with participants assigned to the EC condition achieving significantly more smoking abstinence during Weeks 3-12 than those in the EN condition (i.e., 46.7% and 23.5% abstinent samples, respectively). These data support the use of ongoing, intermittent reinforcement to promote extended smoking abstinence. They also compare favorably to prior studies using abstinence-contingent incentives to promote smoking cessation among opioid-maintained patients which have generally shown modest treatment effects (30–33). Several methodological details may account for these differences. First, we used a rigorous monitoring procedure, involving frequent collection of biochemical samples and a CO-to-cotinine transition, that permitted detection of ongoing smoking and minimized delay in reinforcing recent abstinence when it occurred. In contrast, prior incentive interventions have generally relied on a lower frequency of testing (2–3 times per week) or high CO cutoffs (8–10 ppm), both of which can permit low levels of smoking to go undetected and undermine future abstinence (31–33). Second, participants in the present study could earn an average of $77.70 in incentives per week, compared to the relatively low magnitude of voucher earnings in prior studies ($10.00 – $37.29 per week; 31–33). Magnitude of reinforcement is a crucial determinant of incentive intervention efficacy, with larger magnitudes associated with more favorable outcomes (36–40). Finally, participants were required to be stable in their current opioid treatment, with no significant changes in opioid dose or illicit drug use in the past month. While one prior study did limit enrollment to patients who were abstinent from illicit drug use (31), none required participants to be stable on their opioid dose and one even involved concurrent initiation of opioid-maintenance treatment and the smoking cessation attempt (30). Clinical stability is likely important to consider when undertaking smoking cessation with a patient in opioid treatment, as changes in opioid dose may influence the number of cigarettes smoked (5, 7, 64, 65), and ongoing illicit opiate or cocaine use can increase smoking rates (5–7, 66–68). As a result, 61.9% of our participants were abstinent at the end of Week 2, compared to 6–12% in a prior 12-week incentive intervention (33).
It is also worth noting that participants randomized to the EN control condition provided 23.5% smoking-negative samples during Phase 2, and 16% were biochemically-verified as abstinent at the final study visit. This exceeds the near-zero abstinence rates typically seen in noncontingent or other control conditions. These data suggest that some beneficial effects of an initial, albeit brief, exposure to abstinence-contingent incentives may persist, even when followed by 10 weeks of noncontingent control condition. This is consistent with prior studies suggesting a dose-dependent relationship between duration of abstinence and later outcomes (76).
There was no significant effect of bupropion in the present study, consistent with prior studies showing no robust contribution of bupropion or other pharmacotherapies in opioid-maintained smokers (23, 27, 29, 30, 48). In the only randomized trial to date of bupropion for smoking cessation among opioid-maintained smokers, for example, there were no differences in abstinence, with only 13.7% and 11.4% of patients receiving bupropion and placebo smoking-abstinent during the 10-week study, respectively (30). However, the primary aim of this study was to evaluate the efficacy of the behavioral intervention; thus, we did not include random assignment to medication group, pharmacotherapy was optional, and there was no double-blind medication administration or placebo comparison group. As prior studies have suggested that a self-selection bias may exist among smokers who elect to take a smoking pharmacotherapy (69, 70), caution must be taken when interpreting bupropion outcomes in this trial. However, at least under the present experimental conditions, we found no significant benefit of adding bupropion to an intensive behavioral intervention for smoking cessation in opioid-maintained smokers.
Several potential limitations of this study should be noted. First, participants were required to be stable in their opioid treatment in order to participate. While this may limit the generality of our findings, we do not believe it is excessively limiting as many opioid-maintained patients achieve prolonged stability and it is this subset of patients who are likely the best candidates for a smoking intervention during treatment. Second, the costs associated with offering financial incentives of a sufficient magnitude and duration to produce lasting behavior change could limit the large-scale dissemination. However, several observations are worth noting on this point. Whereas monetary incentives are among the most highly valued rewards, opioid clinics are uniquely positioned to leverage other naturally occurring, relatively low-cost clinic privileges to promote positive behavior change (e.g., medication take-home doses, scheduling flexibility, fee rebates; 71, 72). Considering the high smoking rates, poor treatment response and public health costs associated with smoking among opioid-dependent patients, there is potential for using Medicaid or other funds as incentives for cessation (73, 74). Also important to remember is that the costs of incentive interventions are proportional to the degree of behavior change they produce, with higher costs when the intervention produces the desired results (which are accompanied by health-related improvements and net savings) and reduced costs when the individual fails to respond. Finally, the differences between experimental conditions in complete abstinence were less robust than was seen with overall abstinence. That is, while the EC intervention doubled amounts of continuous abstinence achieved during Phase 2, the majority of participants did not achieve complete abstinence during this period. While increasing overall amounts of abstinence is an important step towards achieving longer-term success, prolonged durations of complete abstinence are ideal and should be the aim of future research efforts.
The present study demonstrates the efficacy of incentive interventions in promoting extended periods of smoking abstinence among opioid-dependent smokers. While future efforts should further explore innovative ways to implement incentive treatments over extended durations, results from this randomized trial support the potential of extended-duration incentives as a tool to promote clinically relevant durations of smoking abstinence in this challenging population.
ACKNOWLEDGEMENTS
This research was supported by National Institute of Drug Abuse grant R01 DA019550 (Sigmon), with additional support by National Institute of Drug Abuse grant T32 DA007242 (Higgins) and National Institute of General Medical Sciences center grant P20GM103644 (Higgins). We thank John Brooklyn MD, Nick Donovan, Alexisa Haire and Edward Reimann for their assistance in conducting this study.
ClinicalTrials.gov Identifier: NCT00718835
Footnotes
Conflict of interest declaration: None.
REFERENCES
- 1.Johnson RE, Chutaupe MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 2.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stotts AL, Dodrill DC, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–40. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stitzer ML, Sigmon SC. Other substance use disorders: prevalence, consequences, detection and management. In: Strain EC, Stitzer ML, editors. The Treatment of Opioid Dependence. The Johns Hopkins University Press; Baltimore, MD: 2006. pp. 365–97. [Google Scholar]
- 5.Chait LD, Griffiths RR. Effects of methadone on human cigarette smoking and subjective ratings. J Pharmacol Exp Ther. 1984;229:636–40. [PubMed] [Google Scholar]
- 6.Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- 7.Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86:417–25. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- 8.Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependence on cocaine and opioids. Nicotine Tob Res. 2002;4:223–8. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- 9.Story J, Stark MJ. Treating cigarette smoking in methadone maintenance clients. J Psychoactive drugs. 1991;23:203–15. doi: 10.1080/02791072.1991.10472237. [DOI] [PubMed] [Google Scholar]
- 10.Talka R, Salminen O, Tuominen RK. Methadone is a non-competitive antagonist at the α4β2 and α3* Nicotinic Acetylcholine Receptors and an Agonist at the α7 Nicotinic Acetylcholine Receptor. Basic Clin Pharmacol Toxicol. 2014;116:321–8. doi: 10.1111/bcpt.12317. [DOI] [PubMed] [Google Scholar]
- 11.Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug Alcohol Depend. 1997;4:123–32. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- 12.Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: a review. Nicotine Tob Res. 2011;13:401–11. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addict Behav. 2006;31:2127–34. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. Am J Public Health. 2001;91:296–9. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Current Cigarette Smoking Among Adults— United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 16.Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Prev Med. 1994;23:61–9. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- 17.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 18.Abbott PJ. Ongoing evaluation of patients' needs in an opioid treatment program. Prof Case Manag. 2010;15:145–52. doi: 10.1097/NCM.0b013e3181c8c72c. [DOI] [PubMed] [Google Scholar]
- 19.Clark JG, Stein MD, McGarry KA, Gogineni A. Interest in smoking cessation among injection drug users. Am J Addict. 2001;10:159–66. doi: 10.1080/105504901750227804. [DOI] [PubMed] [Google Scholar]
- 20.Frosch DL, Shoptaw S, Jarvik ME, Rawson RA, Ling W. Interest in smoking cessation among methadone maintained outpatients. J Addict Dis. 1998;17:9–19. doi: 10.1300/J069v17n02_02. [DOI] [PubMed] [Google Scholar]
- 21.Olsen Y, Alford DP, Horton NJ, Saitz R. Addressing smoking cessation in methadone programs. J Addict Dis. 2005;24:33–48. doi: 10.1300/J069v24n02_04. [DOI] [PubMed] [Google Scholar]
- 22.Sees KL, Clark HW. When to begin smoking cessation in substance abusers. J Subst Abuse Treat. 1993;10:189–95. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- 23.Miller ME, Sigmon SC. Are pharmacotherapies ineffective in opioid-dependent smokers? Reflections on the scientific literature and future directions. Nicotine Tob Res. doi: 10.1093/ntr/ntv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahvi S, Ning Y, Segal KS, Richter KP, Arnsten JH. Varenicline efficacy and safety among methadone maintained smokers: A randomized placebo-controlled trial. Addiction. 2014;109:1554–63. doi: 10.1111/add.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict. 2010;19:401–8. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, et al. Smoking cessation treatment in community-based substance-abuse rehabilitation programs. J Subst Abuse Treat. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Richter KP, McCool RM, Catley D, Hall M, Ahluwalia JS. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment facilities. J Addict Dis. 2005;24:79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- 28.Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- 29.Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone-maintained smokers: A randomized clinical trial. Drug Alcohol Depend. 2013;133:486–93. doi: 10.1016/j.drugalcdep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooney ME, Poling J, Gonzalez G, Gonsai K, Kosten T, Sofuoglu M. Preliminary study of buprenorphine and bupropion for opioid dependent smokers. Am J Addict. 2008;17:287–92. doi: 10.1080/10550490802138814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz JM, Rhoades H, Grabowski J. Contingent reinforcement for reduced carbon monoxide levels in methadone maintenance patients. Addict Behav. 1995;20:171–9. doi: 10.1016/0306-4603(94)00059-x. [DOI] [PubMed] [Google Scholar]
- 32.Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav. 1996;21:409–12. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 33.Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–28. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 34.Okoli CT, Khara M, Procyshyn RM, Johnson JL, Barr AM. Smoking cessation interventions among individuals in methadone maintenance: A brief review. J Subst Abuse Treat. 2010;38:191–9. doi: 10.1016/j.jsat.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Prev Med. 2012;55:S24–32. doi: 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 37.Lussier JP, Heil SH, Mongeon JA, Badger GA, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 38.Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. J Appl Behav Anal. 1996;29:495–504. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend. 2000;58:103–9. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 40.Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–38. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- 41.Frosch DL, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. J Subst Abuse Treat. 2002;23:425–30. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- 42.Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ. 1994;309:842–6. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend. 2006;85:138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271:589–94. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- 45.Yudkin PL, Jones L, Lancaster T, Fowler GH. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. Br J Gen Pract. 1996;46:145–8. [PMC free article] [PubMed] [Google Scholar]
- 46.National Cancer Institute . Clearing the Air: Quit Smoking Today. National Institutes of Health, U.S. Department of Health and Human Services; Bethesda, MD: 2003. Publication No. P133. [Google Scholar]
- 47.Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: a pilot study. J Appl Behav Anal. 2008;41:527–38. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp Clin Psychopharmacol. 2010;18:37–50. doi: 10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index, Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–23. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Fagerström KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire. J Behav Med. 1989;12:159–82. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 51.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 52.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 53.Shiffman S, Waters A. Hickcox M. The nicotine dependence syndrome scale: A multidimensional measure of nicotine dependence. Nicotine Tob Res. 2004;6:327–48. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- 54.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–76. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 55.Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 56.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 57.Beck AT, Beck RW. Screening depressed patients in family practice: a rapid technique. Postgrad Med. 1972;52:81–5. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- 58.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 59.Derogatis LR. Brief Symptom inventory. National Computer Systems, Inc; Minneapolis, MN: 1993. [Google Scholar]
- 60.Fiore MC, Bailey WC, Cohen SJ. Treating Tobacco Use and Dependence: Clinical Practice Guideline. US Dept of Health and Human Services, US Public Health Service; Rockville, MD: 2000. [Google Scholar]
- 61.Fiore MC, Jaén C R, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. US Dept of Health and Human Services, US Public Health Service; Rockville, MD: 2008. [Google Scholar]
- 62.Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-B) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 63.Altman DG, Bland JM. Treatment allocation by minimization. BMJ. 2005;330:843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bigelow GE, Stitzer ML, Griffiths RR, Liebson IA. Contingency management approached to drug self-administration and drug abuse: efficacy and limitations. Addict Behav. 1981;6:241–52. doi: 10.1016/0306-4603(81)90022-8. [DOI] [PubMed] [Google Scholar]
- 65.Patrick ME, Dunn KE, Badger GJ, Heil SH, Higgins ST, Sigmon SC. Spontaneous reductions in smoking during double-blind buprenorphine detoxification. Addict Behav. 2014;39:1353–6. doi: 10.1016/j.addbeh.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins ST, Budney AJ, Hughes JR, Bickel WK, Lynn M, Mortensen A. Influence of cocaine use on cigarette smoking. JAMA. 1994;272:1724. [PubMed] [Google Scholar]
- 67.Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol. 1997;5:263–8. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- 68.Schmitz JM, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug Alcohol Depend. 1994;34:237–42. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 69.Kotz D, Fideler J, West R. Factors associated with the use of aids to cessation in English smokers. Addiction. 2009;104:1403–10. doi: 10.1111/j.1360-0443.2009.02639.x. [DOI] [PubMed] [Google Scholar]
- 70.Shiffman S, Di Marino ME, Sweeney CT. Characteristics of selectors of nicotine replacement therapy. Tob Control. 2005;14:346–55. doi: 10.1136/tc.2004.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaper J, Wagena EJ, Willemsen MC, van Schayck CP. Reimbursement for smoking cessation treatment may double the abstinence rate: results of a randomized trial. Addiction. 2005;100:1012–20. doi: 10.1111/j.1360-0443.2005.01097.x. [DOI] [PubMed] [Google Scholar]
- 72.Stitzer M, Bigelow G, Lawrence C, Cohen J, D'Lugoff B, Hawthorne J. Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav. 1977;2:9–14. doi: 10.1016/0306-4603(77)90003-x. [DOI] [PubMed] [Google Scholar]
- 73.Higgins ST. Comments on contingency management and conditional cash transfers. Health Econ. 2010;19:1255–1258. doi: 10.1002/hec.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pear R. Congress plans incentives for healthy habits. New York Times. http://www.nytimes.com/2009/05/10/ health/policy/10health.html?_r 5 1; 2009.
- 75.Rosenberg T. A payoff out of poverty? NY Times Magazine. http://www.nytimes.com/2008/12/21/magazine/ 21cash-t.html?_r 5 1.; 2008.
- 76.Ashare RL, Wileyto EP, Perkins KA, Schnoll RA. The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. J Addict Med. 2013;7:249–254. doi: 10.1097/ADM.0b013e31829363e1. [DOI] [PMC free article] [PubMed] [Google Scholar]