Abstract
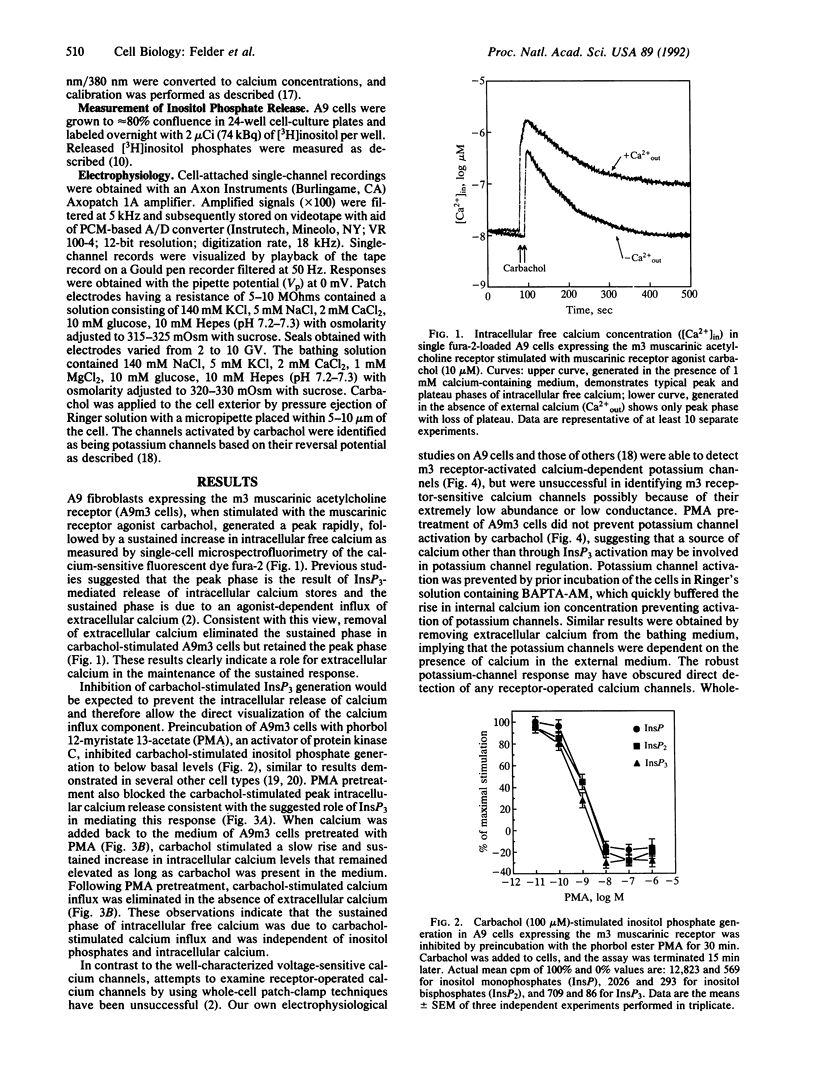
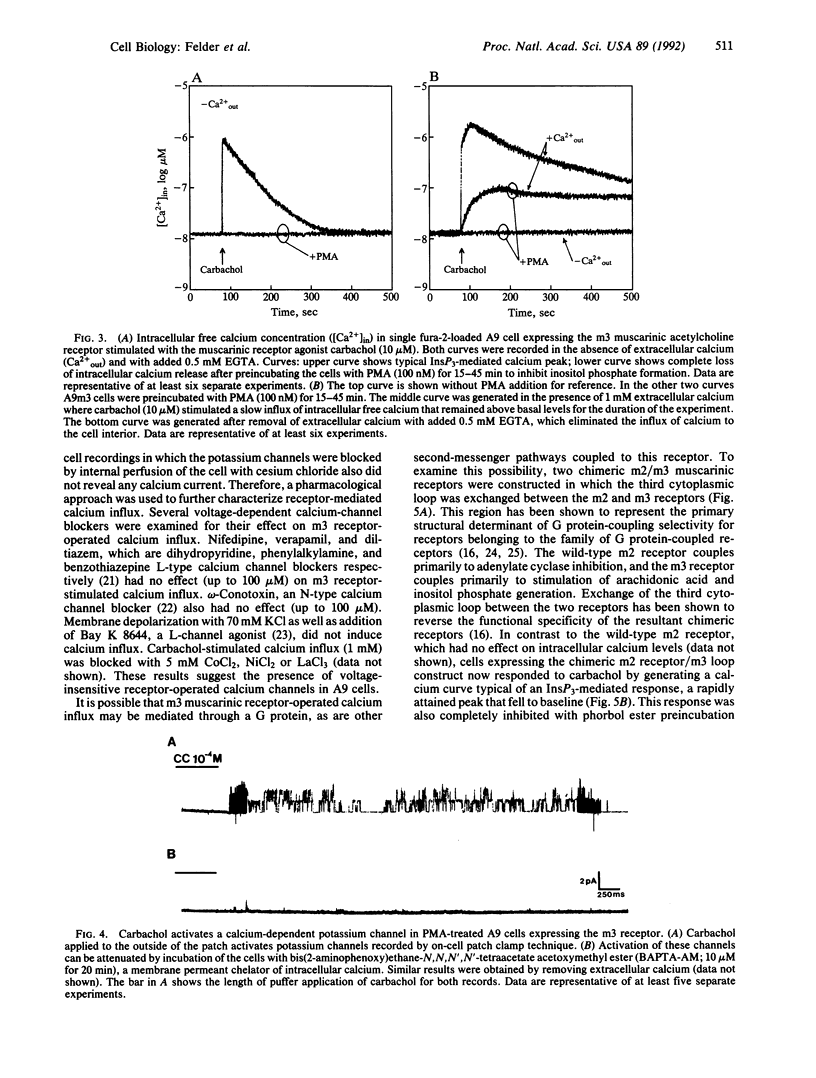
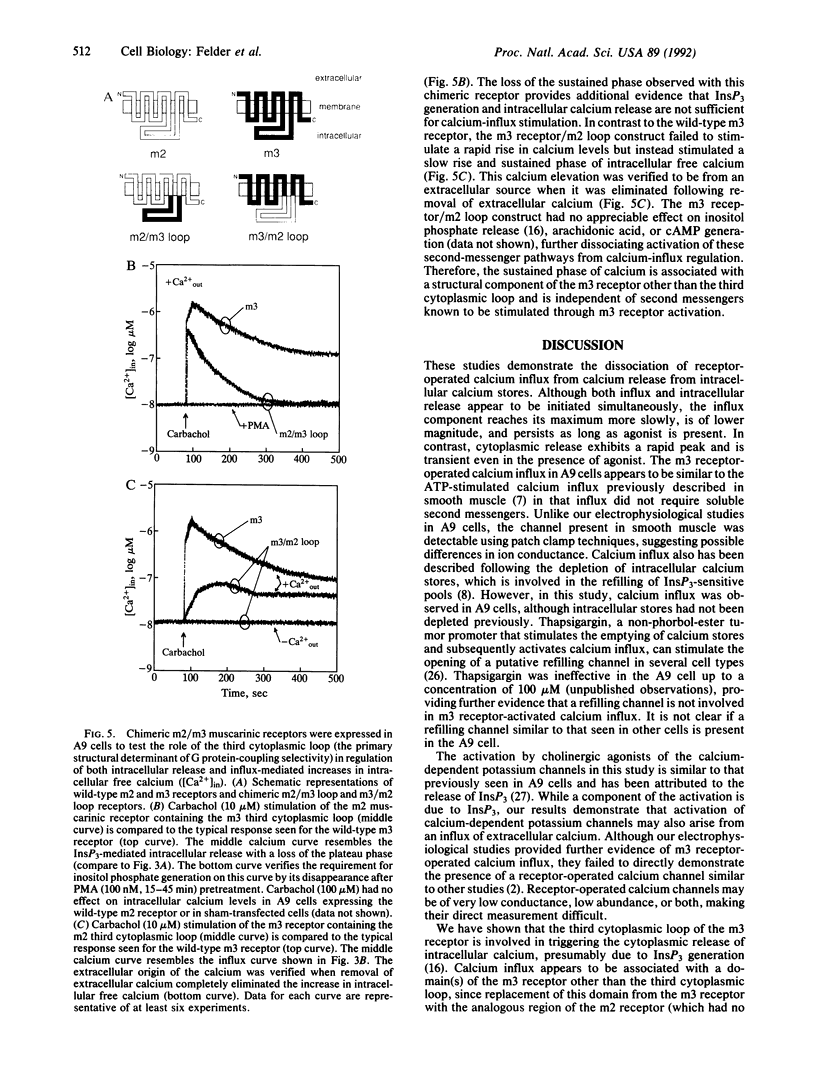
Receptor-mediated changes in cytoplasmic calcium concentrations occur either through release from intracellular calcium stores or by the opening of channels in the plasma membrane, allowing influx of calcium from the extracellular fluid. Carbachol, a muscarinic receptor agonist, stimulated both calcium influx and inositol 1,4,5-trisphosphate (InsP3)-mediated intracellular calcium release in A9 fibroblast cells expressing a m3 muscarinic receptor clone. The calcium influx persisted even after pretreatment of cells with phorbol 12-myristate 13-acetate, which completely prevented the rise in inositol phosphates and intracellular calcium levels. The calcium influx was blocked by divalent cations but was not affected by inhibitors of voltage-dependent calcium channels or high potassium depolarization, indicating the presence of a receptor-operated and voltage-insensitive calcium channel in these cells. Calcium influx was not stimulated by the addition of cAMP analogs or arachidonic acid. To examine the possible involvement of G proteins in m3 receptor-activated calcium influx, two chimeric m2 and m3 muscarinic receptors were expressed in A9 cells in which the third cytoplasmic loop (the primary structural determinant in G protein coupling selectivity of muscarinic receptors) had been exchanged between the m2 receptor, which has no effect on calcium influx, and the m3 receptor. Calcium influx was found to be associated with a structural component of the m3 muscarinic receptor other than the third cytoplasmic loop.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Dieter P., Kinsella J., Tamura K., Kanterman R. Y., Axelrod J. A transfected m5 muscarinic acetylcholine receptor stimulates phospholipase A2 by inducing both calcium influx and activation of protein kinase C. J Pharmacol Exp Ther. 1990 Dec;255(3):1140–1147. [PubMed] [Google Scholar]
- Felder C. C., Kanterman R. Y., Ma A. L., Axelrod J. A transfected m1 muscarinic acetylcholine receptor stimulates adenylate cyclase via phosphatidylinositol hydrolysis. J Biol Chem. 1989 Dec 5;264(34):20356–20362. [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Horn V. J., Baum B. J., Ambudkar I. S. Beta-adrenergic receptor stimulation induces inositol trisphosphate production and Ca2+ mobilization in rat parotid acinar cells. J Biol Chem. 1988 Sep 5;263(25):12454–12460. [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. V., Barker J. L., Bonner T. I., Buckley N. J., Brann M. R. Electrophysiological characterization of cloned m1 muscarinic receptors expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4056–4060. doi: 10.1073/pnas.85.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. V., Barker J. L., Goodman M. B., Brann M. R. Inositol trisphosphate mediates cloned muscarinic receptor-activated conductances in transfected mouse fibroblast A9 L cells. J Physiol. 1990 Feb;421:499–519. doi: 10.1113/jphysiol.1990.sp017958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Aosaki T., Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987 Feb;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Koshiyama H., Tashjian A. H., Jr Evidence for multiple intracellular calcium pools in GH4C1 cells: investigations using thapsigargin. Biochem Biophys Res Commun. 1991 May 31;177(1):551–558. doi: 10.1016/0006-291x(91)92019-g. [DOI] [PubMed] [Google Scholar]
- Kubo T., Bujo H., Akiba I., Nakai J., Mishina M., Numa S. Location of a region of the muscarinic acetylcholine receptor involved in selective effector coupling. FEBS Lett. 1988 Dec 5;241(1-2):119–125. doi: 10.1016/0014-5793(88)81043-3. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Wess J., Bonner T. I., Dörje F., Brann M. R. Delineation of muscarinic receptor domains conferring selectivity of coupling to guanine nucleotide-binding proteins and second messengers. Mol Pharmacol. 1990 Oct;38(4):517–523. [PubMed] [Google Scholar]
- Wess J., Brann M. R., Bonner T. I. Identification of a small intracellular region of the muscarinic m3 receptor as a determinant of selective coupling to PI turnover. FEBS Lett. 1989 Nov 20;258(1):133–136. doi: 10.1016/0014-5793(89)81633-3. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986 Mar 15;261(8):3501–3511. [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]




