Abstract
Infection-induced autoimmunity is thought to be a contributing factor in antibiotic-refractory Lyme arthritis, but studies of autoimmunity have been hindered by difficulty in identifying relevant auto-antigens. We developed a novel approach that begins with the identification of T cell epitopes in synovial tissue using tandem mass spectrometry. Herein, we identified an immunogenic HLA-DR-presented peptide (T cell epitope) derived from the source protein matrix metalloproteinase-10 (MMP-10) from the synovium of a patient with antibiotic-refractory arthritis. This finding provided a bridge for the identification of autoantibody responses to MMP-10, the “first autoimmune hit” in a subgroup of patients with erythema migrans, the initial skin lesion of the infection. Months later, after priming of the immune response to MMP-10 in early infection, a subset of patients with antibiotic-responsive or antibiotic-refractory arthritis had MMP-10 autoantibodies, but only patients with antibiotic-refractory arthritis had both T and B cell responses to the protein, providing evidence for a “second autoimmune hit”. Further support for a biologically relevant autoimmune event was observed by the positive correlation of anti-MMP-10 autoantibodies with distinct synovial pathology. This experience demonstrates the power of new, discovery-based methods to identify relevant autoimmune responses in chronic inflammatory forms of arthritis.
Keywords: Lyme arthritis, Autoimmunity, MMP-10, HLA-DR-presented peptides, Autoimmunity, Erythema migrans
1. Introduction
Lyme disease, a tick-transmitted infection caused by Borrelia burgdorferi, occurs in temperate regions on North America, Europe, and Asia [1–3]. According to estimates from the Centers for Disease Control and Prevention, approximately 300,000 new cases of the infection occur yearly in the United States, primarily in the northeastern U.S. [4,5]. The disease usually begins with an expanding skin lesion, erythema migrans (EM), which occurs at the site of the tick bite [1]. Months to several years later, untreated patients in the northeastern U.S. often develop Lyme arthritis (LA). Although it is no longer possible to study the natural history of the disease in the same patient longitudinally because of antibiotic therapy for early infection, many patients, who often lack signs or symptoms of early disease, still develop LA months to several years after the initial tick bite and exposure to B. burgdorferi.
In most patients, LA resolves with appropriate antibiotic therapy, called antibiotic-responsive arthritis. However, a small percentage of patients develop persistent, proliferative synovitis despite 2–3 months of oral and intravenous antibiotic therapy, called antibiotic-refractory arthritis [6]. In these patients, the synovial lesion, which shows marked synovial hypertrophy, vascular proliferation, infiltrating mononuclear cells, and intense expression of HLA-DR molecules, is similar to that seen in other forms of chronic inflammatory arthritis, including rheumatoid arthritis (RA) [7]. After antibiotic therapy, we treat antibiotic-refractory LA patients with disease modifying anti-rheumatic drugs (DMARDs), the treatment used for other forms of chronic inflammatory arthritis [6,8].
It has been proposed that persistent infection, retained spirochetal antigens, or infection-induced autoimmunity may play a role in antibiotic-refractory LA [9]. B. burgdorferi can establish persistent infections in multiple immunocompetent animal models [10–13]. In C3H/HeN mice, in which the goal was to assess the long-term fate of persisting non-cultivable spirochetes, a dosage regimen of ceftriaxone for 25 days, a regimen that does not to mimic human treatment, was utilized to analyze this population of bacteria [14,15]. In this model, the mice remained infected following antibiotic treatment, and persistent, non-cultivatable spirochetes re-emerged, as shown by PCR, histology, xenodiagnosis, and cytokine analysis [15]. Although patients with refractory arthritis might have persistent infection in a protected site, such as tendons [16], culture and PCR results for B. burgdorferi from synovial tissue during the post-antibiotic period have been uniformly negative [17], and reactivation of infection has not been observed even during treatment with immunosuppressive DMARDs after antibiotic therapy [6].
Regarding the retained spirochetal antigen hypothesis, animal models show that highly cationic outer-surface proteins of B. burgdorferi may bind to cartilage [18]. Moreover, in a myeloid differentiation factor 88-deficit (MyD88−/−) mouse model, which have high pathogen loads, B. burgdorferi antigens are retained near cartilage surfaces after antibiotic therapy, and patellae homogenates from these mice induce macrophages to secrete TNF-α [19]. However, in human LA, in which patients with antibiotic-responsive or antibiotic-refractory arthritis have low pathogen loads [17], it is not yet clear whether retained spirochetal antigens may be a factor in persistent synovitis after antibiotic treatment.
The first observation suggesting that autoimmunity may contribute to the pathogenesis of antibiotic-refractory LA was that specific HLA-DR alleles, such as the DRB1*0401 allele, were increased in frequency in these patients [20] and these DRB1 molecules bound an immunodominant epitope of B. burgdorferi outer-surface protein A (OspA) [21]. In an initial search for molecular mimicry between spirochetal and host proteins, partial sequence homology was found between this epitope of B. burgdorferi OspA and an epitope of human LFA-1 [22] or MAWD-BP [23], but these self-proteins stimulated only low-level T cell responses and did not induce autoantibody responses. Later, human cytokeratin-10 was identified as having a cross-reactive target ligand recognized by anti-OspA antibodies in a small group of patients with antibiotic-refractory arthritis, but T cell reactivity was not explored [24]. Finally, several nonprotein antigens and neural proteins have been reported to induce T or B cell responses in patients with neuroborreliosis [25–29] or post-Lyme disease syndrome [30]. However, it was unclear whether any of these human self-antigens were the targets of pathogenic autoantibody responses in patients with antibiotic-refractory arthritis.
In general, studies of autoimmune diseases have been hindered by difficulty in identifying relevant autoantigens. However, new discovery-based methods offer innovative approaches. Microarrays that express most human proteins or sequencing of plasmablast receptors may give a broad view of autoantibody specificities [31–34], but it is often difficult to assess and validate the pathogenic potential of these autoantibodies. We have developed a novel approach that combines discovery-based proteomics to identify HLA-DR-presented T cell epitopes in synovial tissue in individual patients, followed by translational research to determine T and B cell responses to implicated peptides and source proteins in many patients. Finally, histologic findings in synovial tissue are correlated with autoimmune responses to gain clues to the pathologic potential of these responses. In our experience, a principle advantage of starting with the identification of T cell epitopes is that this approach often provides a focused result leading to the identification of immunogenic T and B cell responses that can be correlated with disease-specific synovial pathology.
To date, we have observed specific trends in our discovery-based identification of autoantigens. Usually 1–3 immunogenic HLA-DR-presented self-peptides, or candidate autoantigens, have been identified from the 100–150 non-redundant self-peptides isolated from each patient. In Lyme disease, these peptides were derived from the source protein endothelial cell growth factor (ECGF) [35] or apolipoprotein B-100 (apoB-100) [36], from annexin A2 in both RA and LA [37], and from N-acetylglucosamine-6-sulfatase and filamin A in RA. In Lyme disease, autoantibodies to each of these self-antigens (but minimal, if any, T cell responses) were found in a subset of patients with EM early in the infection. Months later, after the initial infection and priming of the early autoantibody response, subgroups of patients with antibiotic-responsive or antibiotic-refractory LA had T and B cell responses to these autoantigens. Other authors recently confirmed the presence of ECGF autoantibodies in patients with EM, neuroborreliosis, or LA [38]. Although annexin A2 has been recognized as an autoantigen in several rheumatic diseases [39–41], the other proteins have not been previously described as autoantigens in any disease.
We report herein the identification of a newly recognized autoantigen in antibiotic-refractory LA, matrix metalloproteinase-10 (MMP-10), which serves as a target of T and B cell responses, and these responses correlate with distinct pathologic findings in synovial tissue. Taken together with previous findings, a clearer conceptual framework is emerging of how autoimmunity to one or more autoantigens may develop in a substantial number of patients early in the infection and become pathogenic in the small percentage who develop antibiotic-refractory arthritis later in the illness.
2. Materials and methods
2.1. Study patients
All patients with Lyme disease met the Centers for Disease Control and Prevention criteria for that infection [42]. LA patients, who were seen from 1998 to 2014, were categorized as having antibiotic-responsive or antibiotic-refractory arthritis, as previously defined [6]. In these patients, serum samples and PBMC were obtained, and if available, synovial fluid and synovial tissue. For each patient, the first sample collected was used in this study. In patients with acute-phase EM, all of whom had positive cultures for B. burgdorferi from lesional skin, serum samples and peripheral blood mononuclear cells (PBMC) were assayed. B. burgdorferi OspC subtypes were determined from these cultures in a previous study [43]. Using a previously described definition of disease severity, EM patients were stratified into 3 groups according to the number of symptoms [44]. Serum and synovial fluid samples were stored at –80 °C; PBMC and synovial tissue were stored in liquid nitrogen.
For comparison, PBMC were assayed from patients with new-onset rheumatoid arthritis (RA) and serum samples were analyzed from both new-onset and chronic RA patients and from patients with spondyloarthropathy (SpA) or systemic lupus erythematosus (SLE). All RA patients met the 2010 American College of Rheumatology/European League against Rheumatism criteria for that disease [37,45]. For healthy control subjects, serum samples and PBMC were collected from healthy hospital personnel who did not have a history of LA, and serum samples were obtained from healthy blood bank donors.
The studies conducted from 1988 to 2002 were approved by the Human Investigations Committee at Tufts Medical Center, and those conducted after 2002 were approved by the Institutional Review Board at Massachusetts General Hospital. All patients and control subjects gave written informed consent.
2.2. Enzyme-linked immunospot T cell assay
The detailed method for isolation and identification of in vivo HLA-DR-presented peptides from patients' synovial tissue was described previously [46]. For this study, all non-redundant HLA-DR-presented peptides identified from the synovial tissue of patient LA4 were synthesized by Mimotopes (Victoria, Australia). Individual peptides (1 μM) were first pooled into 41 peptide sets (3 peptides per set with the last pool containing 4 peptides) and tested in duplicate wells (2 × 105 cells/well) for reactivity with that patient's PBMC using a human IFN-γ ELISpotplus kit (Mabtech); the peptides in immunoreactive pools were then retested individually. The dominant response was to MMP-10 (stromelysin 2), which was studied in detail here. For validation of the antigenicity of MMP-10 peptides, HPLC-purified peptides obtained from the MGH Peptide/ Protein Core Facility were tested with samples from multiple case and control patients, using previously published procedures [35–37]. Since they would be expected to have different HLA-DR genotypes, a computer algorithm TEPITOPE 2000 [47,48] was used to identify 2 additional predicted promiscuous peptides (those predicted to bind >20 HLA-DR molecules). A positive response was defined as >3 standard deviations (SD) above the mean value in 12 healthy control subjects.
2.3. Enzyme-linked immunosorbent assay (ELISA) for anti-MMP-10 IgG, anti-MMP-3 IgG, or two-tier IgM or IgG testing for B. burgdorferi
Recombinant human MMP-10 (R&D Systems) was diluted (0.1 μg/ml) in carbonate coating buffer, added to Immulon 1B ELISA plates (Thermo Scientific), and incubated overnight at 4 °C. All remaining incubations were conducted on a platform shaker set at 200 revolutions per minute at room temperature. The plates were incubated with a 3% bovine serum albumin (BSA; Equitech-Bio, Inc.) in PBS–0.05% Tween 20 (PBST) blocking buffer. Plates were incubated with patient serum or joint fluid samples (1:200) or the positive control anti-MMP-10 MAb910 (1:200; R&D Systems). Following washes with PBST, horseradish peroxidase–conjugated goat anti-human IgG (Santa Cruz Biotechnology) or horseradish peroxidase–conjugated donkey anti-mouse IgG (Santa Cruz Biotechnology) was added, followed by TMB substrate (BD Biosciences). For interplate standardization, the positive control MAb910 was included on each plate. A positive antibody response was defined as >3 SD above the mean in 58 healthy subjects; this mean + 3 SD value characteristically corresponded to >0.51 OD450.
To examine specificity of the antibody response to MMP-10, serum samples were also tested with MMP-3 (R&D Systems), using the same methods detailed for MMP-10. Seropositivity responses to the B. burgdorferi were analyzed by ELISA and Western blot as previously described [49–51].
2.4. Multiplex assays for measurement of MMP-10 and MMP-3
The levels of MMP-10 and MMP-3 in paired serum and synovial fluid were assessed using bead-based multiplex assays (R&D Systems) coupled with the Luminex-200 System Analyzer (Luminex) following the manufacturer's instructions. Measurements were determined only in the subset of patients in whom serum and synovial fluid were available. To insure consistency, all samples were tested in the same assay.
2.5. Immunohistochemistry
To determine the expression and distribution of MMP-10 in synovial tissue, cryostat sections, which were available from 4 patients, were stained according to our protocol used previously [52]. Briefly, after blocking, the slides were immunostained with mouse monoclonal anti-MMP-10 MAb910 (2.5 μg/mL). For negative controls, sections were incubated with affinity-purified mouse isotype control antibodies (Sigma). The concentration of isotype controls was matched to the conditions of MAB910. After washing, sections were incubated with the corresponding biotinylated secondary antibody (Biogenex) for 20 min at room temperature, rinsed in PBS, and incubated with peroxidase-streptavidin (Biogenex) for 10 min. The substrate was diaminobenzidine (Biogenex), and the counter stain was Mayer's hematoxylin. Images of MMP-10 staining from one representative patient were obtained with a Nikon Eclipse ME6000 microscope using a Nikon digital camera DXM1200C and processed with NISelements AR2-30 imaging software. Cells implicated in MMP-10 expression were assessed further by re-examining serial sections from the same fields of slides stained previously with vimentin to identify synovial fibroblasts or with CD31 to identify endothelial cells [52].
Previously, synovial tissue samples from 14 patients who underwent arthroscopic synovectomies for antibiotic-refractory LA were examined for histologic features and specific cell types [52]. Sufficient samples were available here from 13 patients for MMP-10 correlations. Each histologic finding of the 13 patients was ranked from 1 to 13, with 13 being the highest ranked patient and 1 being the lowest ranked patient. These histologic data were correlated with the ranked anti-MMP-10 absorbance values (ranked from the highest to the lowest absorbance value). The researchers who ranked the histologic findings and determined MMP-10 antibody levels were blinded to each other's findings.
2.6. Data analysis and statistics
Quantitative data with a normal distribution of values were analyzed using unpaired t-test with Welch's correction, whereas quantitative data with large variation in values were analyzed using the Mann–Whitney test, a non-parametric test. Categorical data were analyzed using Fisher's exact test or Chi-square test. Correlations were analyzed using Pearson correlation test. All analyses were performed using GraphPad Prism 6.
3. Results
3.1. Identification of HLA-DR-presented peptides (T cell epitopes) in patient LA4
In an effort to understand the spectrum of self and foreign antigens that are presented in vivo by HLA-DR molecules during LA, tissue obtained during therapeutic synovectomies from antibiotic-refractory LA patients were processed and analyzed. Patient LA4, a 38-year-old man with the HLA-DRB1*1101/1302 alleles, had persistent, proliferative synovitis in a knee after the completion of oral and IV antibiotic therapy for LA. He was strongly seropositive for antibody to B. burgdorferi with expansion of the response to 7 of 10 spirochetal proteins tested by Western blot. Sixteen months after the completion of antibiotic therapy, he underwent a therapeutic synovectomy. The synovial tissue had negative PCR and culture results for B. burgdorferi. Using tandem mass spectrometry [46], 124 non-redundant, HLA-DR-presented self-peptide sequences were identified from this patient's synovial tissue, which were derived from 103 source proteins (Fig. 1). The overall approach leading to the identification of MMP-10 as a candidate T cell autoantigen is outlined in Fig. 1.
Fig. 1.
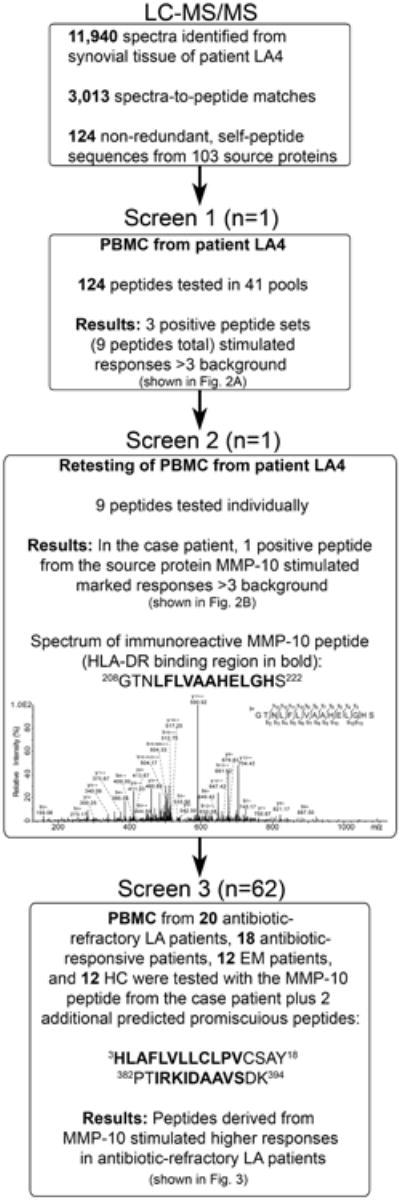
Screening process for identification of candidate autoantigens. The approach for identification of HLA-DR-presented peptides in synovial tissue. The mass spectrum of the most immunoreactive peptide, which was derived from MMP-10, is shown in Screen 2.
For initial testing, the 124 synthesized peptides were pooled in sets with 3 peptides per well, and peptide pools were used to stimulate patient LA4's PBMC (Fig. 1). Three peptide sets, consisting of 9 peptides, induced T cell responses that were >3 times background in human IFN-γ ELISpot assays (Fig. 2A). When the 9 peptides were retested individually using the patient's PBMC, 2 peptides induced a response that was >3 times background (Fig. 2B). However, only 1 peptide induced a marked T cell response of 58 SFU/106 PBMC (Fig. 2B). The peptide, 208GTNLFLVAA-HELGHS222 (the predicted HLA-DR binding sequence in bold), was isolated from the patient's synovial tissue and was derived from part of the highly conserved catalytic domain of MMP-10 [53,54]. Because patient LA4 had a marked T cell response to MMP-10, we examined the immune response to this protein in large numbers of patients and control subjects.
Fig. 2.
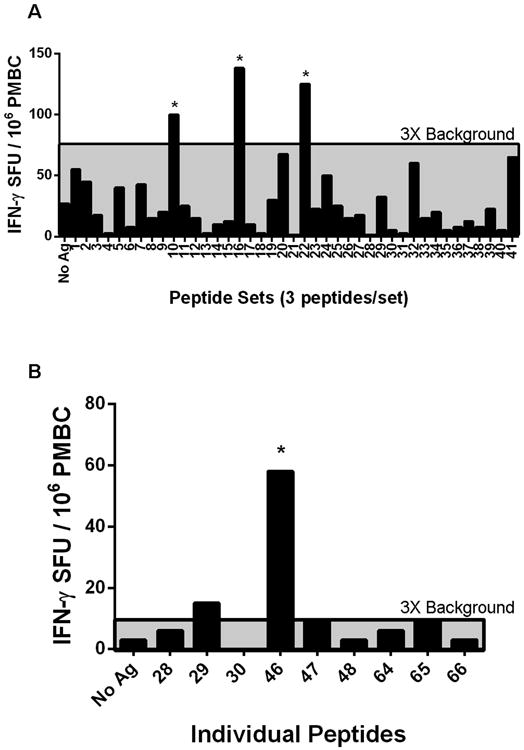
T cell autoreactivity with peptides isolated from synovial tissue of patient LA4. Screening of 124 HLA-DR-presented peptides identified from the synovial tissue of one patient (LA4) for T cell reactivity using the patient's own PBMC, as measured with the human IFN-γ ELISpot assay. A. Peptides isolated from the synovial tissue of patient LA4 were synthesized and tested in pools with the patient's PMBC. A positive result was defined as >3× background of unstimulated cells (area above the gray shaded region). Stars indicate the 3 peptide pools that were >3× background. B. Individual peptides from the 3 positive peptide pools were retested with PBMC from patient LA4. A positive result was defined as >3× background of untreated cells (area above the gray shaded region). The star indicates the peptide with the greatest reactivity, which was derived from the source protein MMP-10.
3.2. T cell responses to MMP-10
Since patients and control subjects would be predicted to have many different HLA-DR types and because cell numbers for ELISpot assays are limited in human samples, our approach for the identification of T cell responses in multiple individuals involves pooling the initial peptide along with peptides from the same source protein that are predicted to be promiscuous HLA binders (>20 HLA-DR molecules) [35–37,47,48]. Therefore, in addition to the HLA-DR-presented MMP-10 peptide identified in the case patient, two other peptides were synthesized that were predicted to be promiscuous HLA-DR binders [47,48]. These peptides (with the predicted HLA-DR binding sequence in bold) included 3HLAFLVLLCLPVCSAY18, which was derived from the signal/pro-peptide domain of the protein, and 382PTIRKIDAAVSDK394, which was found in the hemopexin repeat domain [53,54]. None of the 3 T cell epitope sequences tested was found in another human or B. burgdorferi protein.
To assess T cell reactivity to these peptides, PMBC from 50 patients with early or late manifestations of Lyme disease were tested using the IFN-γ ELISpot assay. In all patients with EM, PBMC were obtained in the acute phase of the infection when B. burgdorferi was cultured from lesional skin. Although it is nearly impossible to culture B. burgdorferi from joints in patients with LA, PBMC in those with antibiotic-responsive arthritis were collected while they were still infected prior to or soon after the start of antibiotic therapy, whereas cells from patients with antibiotic-refractory arthritis were usually obtained at or after the conclusion of antibiotic therapy when few, if any, live spirochetes remained.
Of the 12 B. burgdorferi culture-positive patients with EM, 1 (8%) had a low-level, positive T cell response to MMP-10 that was ≥3 standard deviations (SD) above the mean value of 12 healthy control subjects (Fig. 3). Similarly, only 1 of 18 patients with antibiotic-responsive arthritis (6%) had low-level T cell reactivity to MMP-10. In contrast, 5 of 20 (25%) patients with antibiotic-refractory arthritis, robust T cell responses to MMP-10 peptides. The magnitude of the T cell responses in patients with refractory arthritis was significantly greater than in healthy controls (P = 0.03), and tended to be greater than in patients with EM (P = 0.07) or in patients with antibiotic-responsive arthritis (P = 0.09) (Fig. 3). Similar to the values in healthy control subjects, PBMC from 10 patients with RA did not react with the MMP-10 peptides, and the mean T cell response of this group was not significantly different from that of healthy controls or EM patients. Thus, robust T cell reactivity with MMP-10 peptides was found only in patients with antibiotic-refractory LA. Full-length recombinant MMP-10 was not tested in the human IFN-γ ELISpot assay because the protein disrupted the read-out of the assay, as was the case with full-length ECGF [35].
Fig. 3.
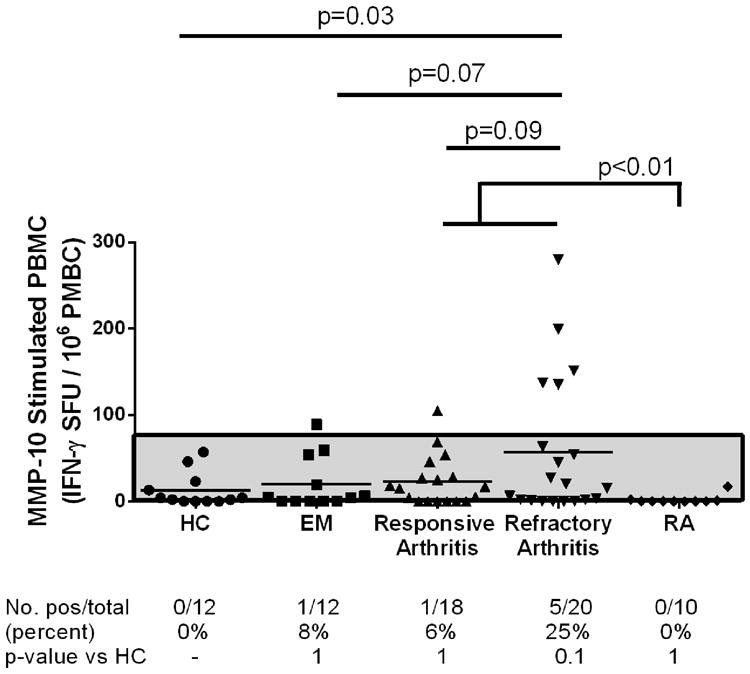
T cell autoreactivity with MMP-10 peptides in Lyme disease patients and control subjects. PBMC from healthy controls (HC), rheumatoid arthritis patients (RA), and Lyme disease patients with erythema migrans (EM), or antibiotic-responsive or antibiotic-refractory arthritis were stimulated with a pool of 3 peptides, including the single MMP-10 peptide isolated from the synovial tissue of patient LA4 and 2 predicted promiscuous HLA-DR binding peptides from MMP-10. The results were measured using the human IFN-γ ELISpot assay. A positive result was defined as >3 SD above the mean SFU/106 cells of HC (area above the gray shaded region). The distribution of values is shown in the graph, and the number and percentage of patients with positive responses in each patient group are reported below the graph. The distribution of values between groups was compared using an unpaired t-test with Welch's correction, and the identity of groups is compared using Fisher's exact test.
3.3. B cell responses to MMP-10
Because serum samples were more readily available than PBMC, we were able to analyze B cell responses to MMP-10 in 297 patients with early or late manifestations of Lyme disease and in 208 individuals in comparison groups. Of 92 culture-positive patients with EM, 23 (25%) had anti-MMP-10 autoantibodies compared with none of 58 healthy control subjects (P < 0.0001), and the mean MMP-10 antibody level in these patients was significantly higher than that in healthy control subjects (P < 0.0001) (Fig. 4). Similarly, 13 of 91 patients (14%) with antibiotic-responsive LA had antibody responses to MMP-10, and 25 of 114 patients (22%) with antibiotic-refractory arthritis had antibody responses to the protein that were significantly greater than in healthy controls (P = 0.002 and <0.0001, respectively). Also, the mean levels of MMP-10 antibodies were significantly higher in antibiotic-responsive and antibiotic-refractory patient groups than in healthy controls (in both instances, P < 0.0001). Of 97 patients with RA, 6 (6%) also had antibody responses to MMP-10, but the frequency and levels of such antibodies were significantly less than that in patients with EM or in those with antibiotic-responsive or antibiotic-refractory arthritis. Fifty of the 97 RA patients had new-onset disease, and 47 had chronic RA. All 50 patients with new-onset disease were seen prior to therapy with DMARDs, and they had active synovitis. Disease activity was not assessed in the 47 patients with chronic RA. However, MMP-10 absorbance values were similar in both RA groups, suggesting that inactive disease was not the explanation for negative MMP-10 antibody levels. Similarly, none of 28 patients with spondyloarthropathy (SpA) and none of the 25 patients with systemic lupus erythematosus (SLE) had MMP-10 autoantibodies, and the magnitude of the absorbance values in these patients was not different from that in RA patients or healthy control subjects. Thus, MMP-10 immunogenicity was prominent in Lyme disease, but not in other autoimmune rheumatic diseases.
Fig. 4.
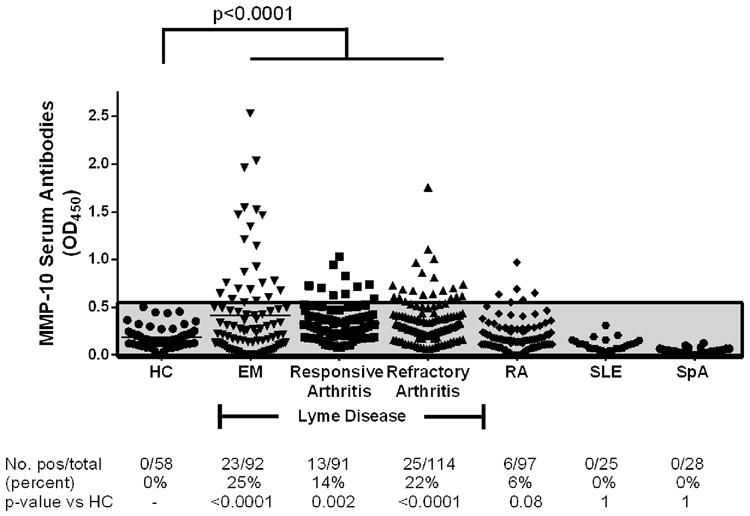
Anti-MMP-10 IgG autoantibody responses in Lyme disease patients and control subjects. IgG anti-MMP-10 autoantibody responses in sera of healthy control (HC) subjects, and patients with erythema migrans (EM), antibiotic-responsive Lyme arthritis, antibiotic-refractory Lyme arthritis, or rheumatoid arthritis (RA), 28 patients with spondyloarthropathy (SpA), and 25 patients with systemic lupus erythematosus, (SLE) measured by ELISA. The shaded gray area, which was defined as the normal range, corresponds to 3 SD above the mean value of healthy control subjects. The distribution of values is shown in the graph, and number and percentage of patients with positive responses in each group are also reported below the graph. The distribution of values between groups was compared using an unpaired t-test with Welch's correction, and the identity of groups is compared using Fisher's exact test.
3.4. Contributing factors to MMP-10 autoantibody responses in EM patients
Although B. burgdorferi can be cultured readily from skin biopsy samples of EM skin lesions, only a minority of patients have detectable antibody responses to the spirochete during the acute phase of the infection [49,55]. Yet, 23 of 92 EM patients (25%) had anti-MMP-10 autoantibodies at that time, a median of 4 days after onset of the skin lesion (range 1–21 days) (Fig. 4). In an effort to identify factors that may contribute to the development of auto-antibody responses in early Lyme disease, we analyzed clinical and laboratory findings in the 92 culture-positive EM patients, including the genotype of B. burgdorferi isolates cultured from the patient, the frequency of antibody reactivity to B. burgdorferi by standard two-tier testing [51], and the number of reported symptoms, a measure of disease severity [43,44]. The results were stratified according to the presence or absence of MMP-10 auto-antibody responses.
The OspC subtype of the infecting strain of B. burgdorferi was determined in a previous study in 90 of the 92 patients with EM [43]. In the northeastern United States, OspC types A and K, which are the two most common types, each typically constitute about 30% of the strains identified [43,44,55,56]. OspC type A strains are associated with the greatest inflammatory potential [44,57] and with the most severe disease [44]. Of 68 MMP-10 antibody-negative patients, 17 (25%) were infected with OspC type A strains and 22 (32%) were infected OspC type K strains, the typical distribution of these strains (Fig. 5A). In contrast, 10 of the 22 MMP-10 antibody-positive patients (45%) were infected with OspC type A strains, and only 3 (14%) were infected with OspC type K strains. These differences in the distribution of OspC types were of possible significance (P = 0.1, Chi-square test). The other OspC types were similar in distribution between MMP-10-positive and MMP-10-negative patients.
Fig. 5.
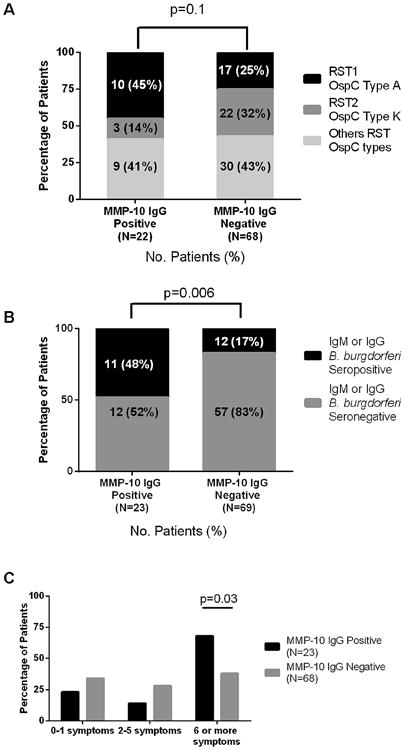
Correlation of clinical factors with MMP-10 autoantibody responses in erythema migrans patients. Determinations of B. burgdorferi subtypes, B. burgdorferi IgM or IgG seropositivity, and disease severity were stratified according to the presence or absence of MMP-10 autoantibody responses in patients with erythema migrans (EM). A. The number and percentage of EM patients infected with OspC type A, OspC type K or other OspC type strains are stratified according to positive or negative antibody responses for MMP-10. Differences between groups were calculated using Chi-square test. B. Patients with positive or negative IgM or IgG antibody responses to B. burgdorferi were stratified according to MMP-10 antibody positivity. P-values comparing the frequency of MMP-10 antibody-positive or antibody-negative patients for IgM or IgG seropositivity were calculated using Fisher's exact test. C. The number of symptoms was stratified according to MMP-10 positive or negative antibodies. P-values comparing the frequency of MMP-10 antibody-positive or antibody-negative patients for the range of symptoms were calculated using Fisher's exact test.
By standard two-tier testing (ELISA and Western blot) [51], 20 of the 92 patients (22%) had positive IgM antibody responses to B. burgdorferi, 4 (4%) had positive IgG responses, and 4 (4%) had both positive IgM and IgG responses, which is typical of patients with EM during the acute-phase of the infection. Of the 23 patients with IgG autoantibody responses to MMP-10, 11 (48%) had IgM and/ or IgG antibody responses to B. burgdorferi by two-tier testing. In contrast, only 12 of 69 (17%) MMP-10 autoantibody-negative patients had antibody responses to B. burgdorferi (P = 0.006, Fisher's exact test) (Fig. 5B). Therefore, patients who had MMP-10 IgG autoantibodies were more likely to be seropositive (IgM or IgG) for B. burgdorferi.
We have previously analyzed the severity of early infection according to the number of symptoms associated with EM, most commonly headache, neck stiffness, fever, arthralgias, myalgias, malaise and fatigue; and stratified the results into 3 groups. Patients with 0–1 symptoms were defined as having mild disease, 2–5 symptoms as moderate disease, and ≥6 symptoms as severe disease [43,44]. Of the 23 patients with positive MMP-10 autoantibody responses, 15 (68%) had severe disease, whereas only 3 (14%) had moderate disease, and 5 (23%) had mild disease (Fig. 5C). In comparison, among the 68 patients who lacked MMP-10 antibody responses, 26 (38%) had severe disease, 19 (28%) had moderate disease, and 23 (34%) had mild disease. Thus, the majority of patients with MMP-10 antibody responses (68%) had severe disease compared with 38% of those with negative MMP-10 antibody responses (P = 0.03, Fisher's exact test). The duration of EM did not correlate with MMP-10 antibody reactivity. The median duration of the EM skin lesion prior to study entry and antibiotic treatment was 4 days in both MMP-10-positive and MMP-10-negative groups.
Taken together, EM patients with MMP-10 autoantibodies tended to have more frequent infection with the potentially more inflammatory OspC type A strains; they were more commonly seropositive for IgM or IgG antibody responses to B. burgdorferi, and they more often had severe disease. These findings indicate that multiple factors associated with early infection may contribute to the development of autoimmune responses observed later in the disease. Analysis of these same factors is difficult in LA patients because of the inability to culture B. burgdorferi from joints, the finding of B. burgdorferi seropositivity in all patients, and the usual lack of systemic symptoms. However, MMP-10 antibody titers did not correlate with the degree of knee swelling, the joint usually affected in antibiotic-refractory LA.
3.5. MMP-10 protein in serum, synovial fluid, and synovial tissue
For MMP-10 to become the target of an autoimmune response in joints, one would predict that the protein would be present in high concentrations in LA patients' inflamed synovial fluid and peripheral blood. Therefore, we measured MMP-10 protein concentrations in a subset of LA patients in whom concomitant serum and synovial fluid samples were available and in randomly selected serum samples from patients with EM, RA, or healthy controls.
In the serum of 10 healthy control subjects, the mean concentration of MMP-10, 986 pg/mL, was consistent with published data [58,59] (Fig. 6A). The mean concentration of MMP-10 in EM patients was similar to that in healthy controls; only 1 of 10 patients (10%) had a level that was slightly >3 SD above the mean value in healthy controls. In comparison, 4 of 10 patients (40%) with antibiotic-responsive arthritis and 4 of 13 patients (31%) with antibiotic-refractory arthritis had elevated serum levels of MMP-10, and the mean value (∼1413 pg/mL) in both groups was significantly higher than that in EM patients or in healthy control subjects (Mann–Whitney test). In RA patients, the concentration of MMP-10 in serum was not significantly different than that in healthy controls, which is consistent with the fact that the 10 RA patients usually lacked antibody responses to MMP-10. Both antibiotic-refractory and antibiotic-responsive arthritis patients had significantly greater levels of MMP-10 protein in synovial fluid than in serum (P < 0.0001, Mann–Whitney test), and the mean levels (∼7000 pg/mL) were similar in both responsive and refractory groups (Fig. 6A).
Fig. 6.
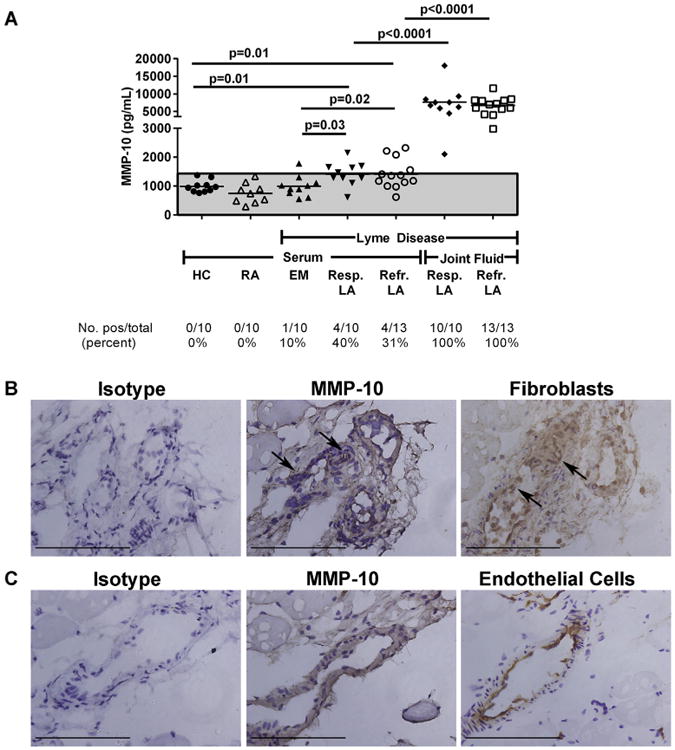
MMP-10 protein in Lyme disease patients and control groups. A. MMP-10 protein concentrations were measured in matched serum and synovial fluid samples in patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis (LA), and in serum samples from patients with erythema migrans (EM) or rheumatoid arthritis (RA), and in healthy controls (HC) subjects. MMP-10 protein concentrations are shown, as measured by Luminex assay. The area above the shaded gray area is >3 standard deviations above the mean value of HC. P-values were calculated using Mann–Whitney test. (B + C) The panels in parts B and C are serial sections from the same area of the slides. Bars = 100 μm. B. MMP-10 staining is observed in the synovial tissue and is associated with staining for synovial fibroblasts (arrows). No staining is observed in the mouse isotype control. C. MMP-10 staining is observed in the synovial tissue and is associated with staining for endothelial cells. No staining is observed with the mouse isotype control.
To determine where MMP-10 protein was expressed in joints, synovial samples from 4 patients were stained for MMP-10 protein. A light background of diffuse MMP-10 staining was observed in superficial regions of the tissue near the synovial lining layer. More intense staining was seen in association with apparent synovial fibroblasts, identified by antibody staining to vimentin, and around endothelial cells, identified by CD31 antibody staining. Representative images of MMP-10 staining in association with fibroblasts and endothelial cells are shown in Fig. 6B and C. Thus, MMP-10 protein concentrations were elevated in LA patients in synovial fluid and synovial tissue, providing ample substrate for processing and presentation of HLA-DR peptides.
3.6. Comparison of MMP-10 (stromelysin 2) and MMP-3(stromelysin 1) protein concentrations and immunogenicity
MMP-10 (stromelysin 2, NP_002416.1) and MMP-3 (stromelysin 1, NP_002413.1) have considerable sequence homology; they are 78% identical and 88% similar; and they have similar function and substrate specificities [53,60]. Therefore, to assess the specificity of the autoimmune response to MMP-10, protein concentrations and antibody levels of MMP-3 were measured in the same patient and control groups in whom MMP-10 protein concentrations were determined.
The mean concentration of MMP-3 in serum from healthy control subjects was 21,000 pg/mL, which was similar to published values [61,62], and much higher than the serum levels of MMP-10. In 10 patients with EM, the concentrations of MMP-3 in serum were similar to those in 10 healthy control subjects and in 10 patients with RA (Fig. 7). In contrast, 8 of 10 patients (80%) with antibiotic-responsive LA and 8 of 13 patients (62%) with antibiotic-refractory arthritis had serum levels of MMP-3 (mean value, ∼250,000 pg/mL) that were >3 SD above the mean value in healthy controls. The differences between the values of responsive and refractory arthritis patients and those in healthy control subjects were statistically significant (P = 0.0007 and P = 0.0001, respectively, Mann–Whitney test) (Fig. 7). Moreover, both in the refractory and responsive groups, the mean levels of MMP-3 (∼3,000,000 pg/mL) were approximately 10 times higher in synovial fluid than in serum (P < 0.0001). Thus, the protein concentrations of MMP-3 (stromelysin-1) were much greater than those for MMP-10 (stromelysin-2) in both serum and synovial fluid.
Fig. 7.
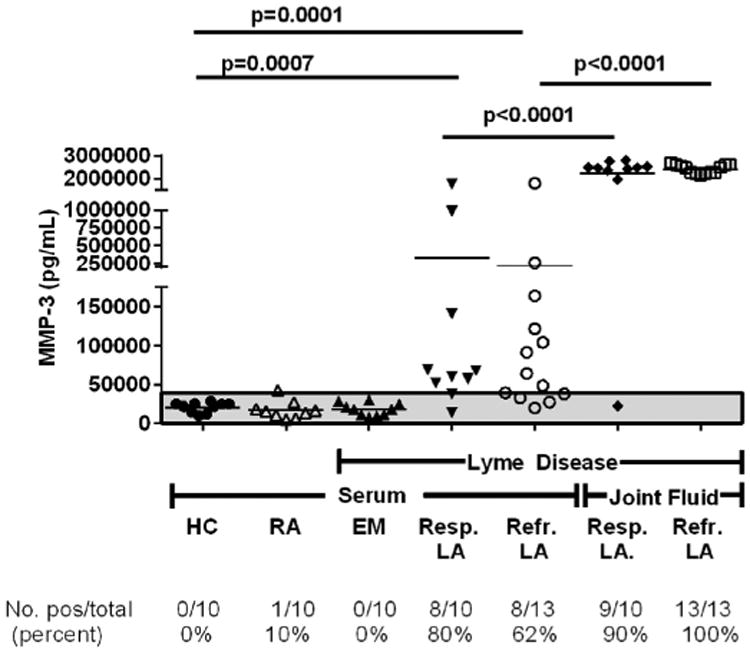
MMP-3 protein concentrations in Lyme disease patients and control groups. MMP-3 protein concentrations were measured in matched serum and synovial fluid samples in patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis (LA), and in serum samples from patients with erythema migrans (EM) or rheumatoid arthritis (RA), and in healthy controls (HC) subjects. MMP-3 protein concentrations are shown, as measured by Luminex assay. The area above the shaded gray area is >3 standard deviations above the mean value of HC. P-values were calculated using Mann–Whitney test.
Despite the greater amounts of MMP-3 protein, the autoimmune response was directed primarily against MMP-10. In a randomly selected subset of 30 patients with EM, 5 (17%) had antibody responses to MMP-10 compared with 1 (3%) who had reactivity with MMP-3, and the mean MMP-10 antibody level was higher than the mean MMP-3 antibody level (P = 0.05) (Fig. 8). Similarly, of the 64 patients with antibiotic-refractory arthritis, 6 (9%) had antibody responses to MMP-10 compared with only 1 (2%) who had reactivity with MMP-3 (P = 0.05). Moreover, the mean MMP-10 antibody level was significantly higher than the mean MMP-3 antibody level (P = 0.005). There was a similar trend in patients with antibiotic-responsive arthritis, but the differences were not statistically significant. All patients with MMP-3 antibody responses also had MMP-10 antibody reactivity. In contrast, the subset of RA patients tested here did not have either MMP-10 or MMP-3 autoantibodies. Thus, in patients with antibiotic-refractory LA, MMP-10 autoantibodies were significantly more common than autoantibody responses to MMP-3, even though the protein concentrations of MMP-3 were significantly higher than those of MMP-10.
Fig. 8.
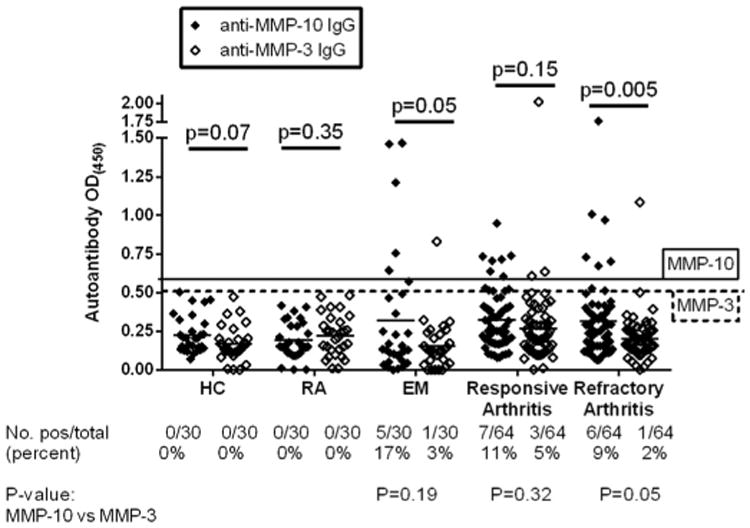
IgG MMP-10 and MMP-3 autoantibodies in Lyme disease patients and control groups. MMP-10 and MMP-3 autoantibodies were measured in serum samples from patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis (LA), erythema migrans (EM), rheumatoid arthritis (RA), and in healthy controls (HC) subjects, using an ELISA. The solid line corresponds to 3 standard deviations above the mean value of healthy control subjects for MMP-10 while the dashed line corresponds to 3 standard deviations above the mean value of healthy control subjects for MMP-3. The distribution of MMP-10 and MMP-3 autoantibody values were compared for each group using unpaired t-test with Welch's correction, and the identity of groups were compared using Fisher's exact test.
3.7. Histologic findings in synovial tissue according to MMP-10 antibody responses
In a previous study [52], synovial tissue samples from 14 patients with antibiotic-refractory LA who underwent synovectomies were examined for histologic features and specific cell types. Sufficient samples were available here from 13 patients for MMP-10 correlations. Each finding was ranked from 1 to 13, with 13 being the highest ranked patient. Synovial tissue was not available in the responsive group because these patients do not undergo synovectomies. Among these 13 patients, none had a positive result for MMP-10 autoantibodies that was >3 SD above the mean value in healthy controls. Nevertheless, in the current study, the ranks for histologic findings were correlated with anti-MMP-10 antibody values ranked from the highest to the lowest absorbance value.
When the ranks of MMP-10 antibody absorbances and histologic findings were analyzed, there were numerous significant correlations. First, the magnitude of MMP-10 autoantibodies correlated directly with the amount of tissue staining for the B cell chemoattractant CXCL13 (P = 0.02) and with the number of plasma cells in the tissue (P = 0.02), suggesting that patients with MMP-10 auto-antibody responses may have local antibody production to the protein in synovial tissue (Fig. 9). In addition, higher MMP-10 antibody levels correlated with measures of cell proliferation, including greater synovial lining layer thickness (P = 0.02) and greater numbers of synovial fibroblasts (P = 0.01). Finally, there was a trend toward correlation with markers for cell activation, VCAM (P = 0.04) and ICAM (P = 0.06). Thus, the higher the absorbance value, the greater the staining for CXCL13, plasma cell numbers, synovial lining layer thickness and synovial fibroblast proliferation. Representative examples of these histologic findings from one patient each with high or low MMP-10 antibody absorbance values are compared in Fig. 10.
Fig. 9.
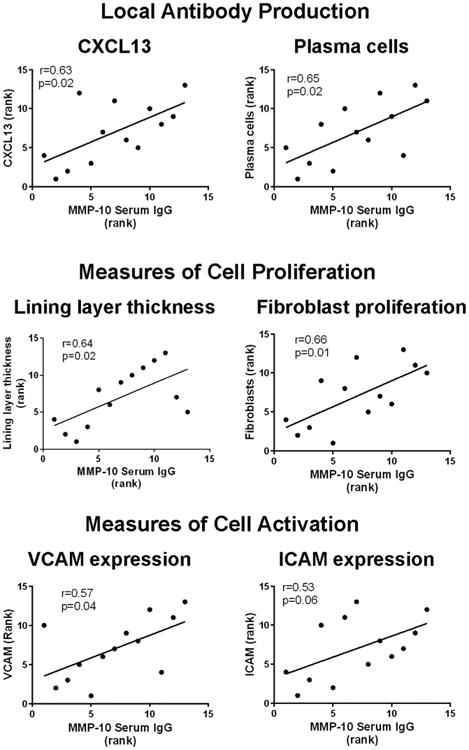
Correlation between synovial tissue histologic rankings and IgG MMP-10 antibody rankings. Correlations between IgG anti-MMP-10 antibody absorbance rankings and histologic rankings for CXCL13 staining, plasma cells, lining layer thickness, fibroblast proliferation, VCAM, and ICAM are shown in patients with antibiotic-refractory Lyme arthritis (LA) who underwent synovectomies. Only significant correlations are shown. The correlations were determined by Pearson's correlation test.
Fig. 10.
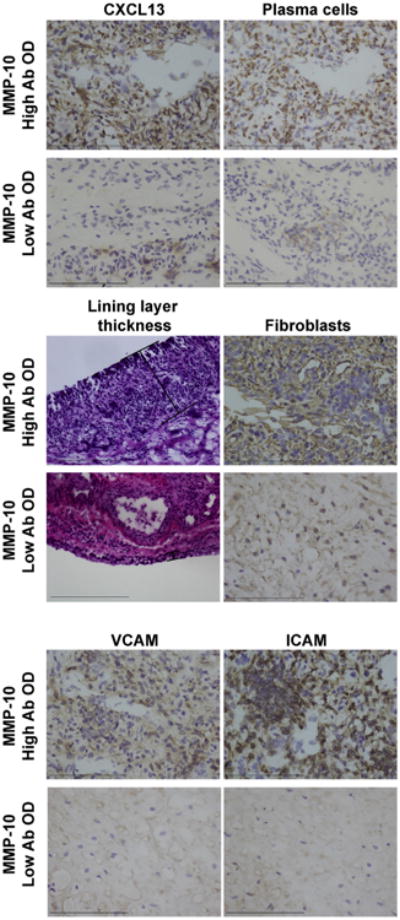
Immunohistochemical staining of synovial tissue from two representative patients with antibiotic-refractory LA who had high or low rankings for serum MMP-10 IgG antibodies. The patient with the highest ranking for MMP-10 antibodies had marked staining for CXCL13, plasma cells, lining layer thickness, fibroblast proliferation, and expression of the cellular activation markers VCAM and ICAM. In contrast, the patient with the lowest ranking had minimal staining for these histologic findings. Brown indicates specific staining of the immune cells and purple is the counter stain (hematoxylin). Lining layer thickness was assessed by hematoxylin and eosin (H&E) stain. Bars = 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In contrast, no relationship was observed between MMP-10 antibody levels and fibrosis, cellular infiltration of the sublining layer, lymphoid aggregates, obliterative vascular lesions, or with the numbers of T or B cells, macrophages, myeloid dendritic cells, or follicular dendritic cells. Moreover, when the ranked absorbance values of autoantibodies for the closely related MMP-3 protein were correlated with histologic rankings, no significant correlations were found (Fig. S1), attesting to the specificity of the histologic correlations with MMP-10 antibody values.
4. Discussion
Using our previously described approach of discovery-based proteomics to identify relevant T cell epitopes in synovial tissue, and translational research to determine the antigenicity of the epitopes [35–37,46], we identified MMP-10 as a novel T cell auto-antigen in an initial patient with antibiotic-refractory LA. This observation provided a bridge to determine that peptides of MMP-10 or its source protein were targets of T and B cell responses in subgroups of patients with early or late manifestations of Lyme disease. Moreover, the magnitude of anti-MMP-10 autoantibodies was associated with distinct synovial pathology. Using this approach, we have now identified 4 immunogenic peptides out of 573 HLA-DR-presented peptides (0.07%) identified from the synovial tissue of 5 patients with antibiotic-refractory Lyme arthritis. The peptides were derived from 4 source proteins, ECGF, apoB-100, annexin A2, and MMP-10. Altogether, 23 patients (25%) with EM, the initial skin lesion of the disorder, had autoantibody responses to MMP-10 early in the illness, with minimal, if any, T cell responses. Of the 23 patients,12 (52%) also had reactivity with one or more of the other known autoantigens, but these responses were not pathogenic. Late in the illness, 34 of the 159 LA patients (21%) who were tested for all 4 known autoantigens had MMP-10 autoantibodies, and 13 of the 34 patients (38%) had reactivity with one of the other autoantigens. The complete number of possible auto-antigens in Lyme disease is not yet known, but more than 4 probably serve this function. Therefore, these percentages are likely to increase as more autoantigens are identified.
In this study, we identified for the first time, clinical factors associated with early MMP-10 autoantibody responses. Such patients were more commonly infected with highly inflammatory OspC type A strains; they were more frequently seropositive for B. burgdorferi, and they more often had severe disease. In a previous study, serum samples from patients with EM who were infected with OspC type A strains had significantly higher levels of a range of cytokines and chemokines than patients infected with other strains (44). Thus, spirochetal and host factors that lead to marked inflammation would appear to set the stage for the development of autoimmune phenomena.
In addition to cytokine stimulation, B. burgdorferi induces host cells to secrete a number of MMPs. Chondrocyte tissue cultures have shown that B. burgdorferi stimulates the production of numerous MMPs, including MMP-1 [63–66], MMP-3 [63–66], MMP-13 [63], and MMP-19 [63], while spirochete-stimulated PMBC induce expression of MMP-9 [67,68] and MMP-10 [63,69]. Mouse models of infection have demonstrated that only MMP-3 and MMP-9 are made in response to B. burgdorferi [63], whereas in EM lesions of human patients only MMP-9 appears to be selectively upregulated [70]. Moreover, B. burgdorferi binds several host proteases, particularly plasmin [71–76]. Plasmin can activate proMMP forms of MMP-1, -3, -10, and 13, and this has been shown to be a relevant pathway of MMP activation in vivo [60,77–80]. These enzymes degrade extracellular matrix components [71–73,75,81,82], and presumably help the host in cellular recruitment, tissue repair [83], and in modulation of cytokine and chemokine activity [84]. Conversely, degradation of the extracellular matrix may aid spirochetes in dissemination [63,67,71,85].
We postulate that early in the infection, close interactions between certain spirochetal and host proteins, within the context of marked production of pro-inflammatory cytokines and MMPs, may lead to the development of autoantibody responses to a few specific human proteins. In EM skin lesions, proMMP-10 may be upregulated in response to B. burgdorferi; and spirochete-bound plasmin may cleave proMMP-10 into an active form. Immune processing of spirochete-plasmin-MMP-10 complexes or MMP-10 immune complexes may play a permissive role in the development of autoreactive, MMP-10-specific B cells. Although MMP-10 does not have sequence homology with any Borrelia-derived protein, it is possible that there is structural mimicry between a B cell epitope of MMP-10 and a spirochetal protein. Alternately, a pool of naturally occurring autoantibodies may be upregulated as part of the host's immune response in early infection. However, this initial autoantibody response, “the first autoimmune hit”, does not appear to be pathogenic.
LA usually begins months after dissemination of B. burgdorferi to joints accompanied by a marked immune response to the spirochete [1,7,49]. Although it has been difficult to culture B. burgdorferi from joints, it has sometimes been possible to determine the infecting strain by PCR testing of synovial fluid, primarily in patients seen before or early in the course of antibiotic treatment. This work has shown that most patients who develop antibiotic-refractory arthritis had been infected with OspC type A strains [86], the strain implicated here in MMP-10-antibody-positive EM patients. Moreover, these antibiotic-refractory LA patients infected with OspC type A strains also have especially high levels of the IFN-γ-inducible chemokines, CXCL9 and CXCL10, which are chemoattractants for CD4+ and CD8+ T effector cells [44,57].
As part of this expanded immune response, the highly inflammatory milieu in the joint includes increased expression of many MMPs [63,65,87]. In an initial study, joint fluid of patients with antibiotic-responsive arthritis, seen during infection, had increased expression of MMP-1 and MMP-3 [63,65]. In contrast, patients with antibiotic-refractory arthritis, seen after presumed spirochetal killing with antibiotic therapy, had a different MMP expression profile. They had increased levels of MMP-8 and -9, which are produced by neutrophils, the most abundant cell type in synovial fluid; and B. burgdorferi-infected chondrocyte cultures from healthy donors showed no induction of these MMPs [63,65,84]. In a previous report [63], as in our study, antibiotic-refractory and antibiotic-responsive patients had similarly high concentrations of MMP-10 in synovial fluid, and the protein was seen near the lining layer in association with fibroblasts and endothelial cells. Although we did find elevated MMP-10 protein levels in serum of RA patients, MMP-10 production has been reported in RA synovial tissue in association with synovial fibroblasts and with endothelial cells in areas of neovascularization [88], as observed in Lyme synovia. Why then is MMP-10 only immunogenic Lyme disease? The key difference is presumably priming of the immune response to MMP-10 in early Lyme disease because of close interactions between the spirochete and host. Later, abundant amounts of MMP-10 are available for HLA-DR presentation in joints, thereby contributing to a “second autoimmune hit”.
Despite the significantly higher concentrations of MMP-3 than MMP-10 in serum and synovial fluid, which are similar proteins in amino acid sequence, structure and function, autoantibodies to MMP-3 were found in only a few antibiotic-refractory LA patients. The fact that this may happen with MMP-10, but rarely with the more abundant closely related protein, MMP-3, suggests a molecular specificity of these interactions. Presumably, epitope recognition in the part of MMP-10 protein that is not shared with MMP-3 (∼20% of amino acids) is important for B cell reactivity. However, a few patients with EM or with responsive or refractory arthritis, each of whom had antibody reactivity with MMP-10, also had elevated antibody responses to MMP-3. In these patients, we propose that the antibody response was initially directed against a specific MMP-10 antibody epitope, but epitope spreading, accompanied by CD4+ T cell help, led to reactivity with a shared portion of these two proteins, resulting in reactivity against MMP-3 in a few patients.
Why then does an autoantibody response to MMP-10 apparently become pathogenic in patients with antibiotic-refractory LA? First, compared with patients with antibiotic-responsive arthritis, those with antibiotic-refractory arthritis have significantly higher levels of inflammatory cytokines in synovial fluid, including exceptionally high levels of CXCL9 and CXCL10, the major chemoattractants of CD4+ and CD8+ T effector cells [89]. High levels of IFN-γ and TNF-α [57,90] lower the immunoregulatory set point of these T cells and can lead to immune dysregulation [89,91]. To date, increased ratio of CD4+ Teff/Treg cells is the only factor identified that correlates directly with the post-antibiotic duration of arthritis [89]. Furthermore, the significantly higher levels of pro-inflammatory cytokines can stimulate other cells in the joint to express MMP-10, including synovial fibroblasts and articular chondrocytes [88]. Thus, we postulate that the combination of excessive inflammation, immune dysregulation of CD4+ T cells, and recruitment and activation of MMP-10-specific T cells, presumably contributes to an altered immune state, “a second autoimmune hit”. During this stage, the IgG antibody response to the protein is changed to an immunoreactive state, perhaps by affinity maturation, epitope spreading, or IgG subclass changes. Finally, large amounts of MMP-10 protein and anti-MMP-10 antibodies in joints may cause immune complex formation, and increased uptake of these complexes may contribute further to joint inflammation and autoimmunity.
We do not think that a single autoantibody response accounts solely for this outcome. Other factors associated with the infection or host immune response are surely involved and influence the development of chronic, proliferative synovitis. However, as shown here, the strong positive correlations between the magnitude of MMP-10 autoantibody responses and distinct synovial pathology suggests that this autoantibody response may be a contributing factor in disease pathogenesis. Moreover, higher MMP-10 absorbance values were associated with apparent local antibody production, greater degrees of cell proliferation, and more intense expression of cell activation markers. Even though none of the 13 patients in whom synovial tissue was available had what we defined as a positive antibody response to MMP-10 (≥3 standard deviations above the mean of HC), the magnitude of the anti-MMP-10 antibody absorbance in these patients correlated directly with specific pathologic findings in their synovial tissue. Thus, the percentage of patients with biologically relevant MMP-10 antibody responses is probably higher than that shown here by ELISA with the stringent cutoff of 3 standard deviations above the mean of healthy controls.
Antibiotic-refractory LA does not persist indefinitely. In most patients, synovitis resolves within months to several years after antibiotic treatment, assisted by DMARDs such as methotrexate [6], which are thought to inhibit T cell activation and cell proliferation [92]. In these patients, we postulate that the innate immune “danger” signals provided by live spirochetes or spirochetal remnants are no longer present, and without these signals, the adaptive immune response to autoantigens eventually regains homeostasis, and the arthritis resolves.
Finally, we think that our study has two areas of importance beyond Lyme disease. There is a growing interest in RA in the possibility that certain infectious agents stimulate autoimmune responses that appear to contribute to disease pathogenesis [93–95]. Lyme disease is the latest example of this phenomenon. LA, which is definitely caused by an infectious agent, provides a unique human arthritis model to study infection-induced autoimmunity. In contrast with RA in which it is difficult to identify patients in the pre-clinical, autoimmune phase of the disease, patients with EM can be readily identified, which allows for characterization of early immune events. Moreover, in patients with LA, one can compare advantageous (responsive) or disadvantageous (refractory) immune responses that lead to a “second autoimmune hit”. Thus, lessons learned in this Lyme disease model may provide clues for investigation in other forms of chronic inflammatory arthritis. Second, we think that our novel approach to autoantigen discovery will be valuable in other forms of chronic inflammatory arthritis, including RA [37], and in other immune-mediated diseases in which specific targets are not yet known. In our experience, when a given autoimmune T cell response is amplified enough to give a robust response from PBMC, it hones the search to an autoantigen that is likely to be a target of T and B cell responses with pathologic potential.
Supplementary Material
Acknowledgments
We thank Dr. John M. Aversa for help in obtaining synovial tissue at the time of the patient's arthroscopic synovectomy, Dr. Diana Londono and Dr. Diego Cadavid for help with histology analyses of synovial tissue, and Gail McHugh for laboratory support.
This work was supported by funding from the NIH [R01 AI101175 to A.C.S., K01-AR062098 to K.S., and P41 GM104603 and S10 OD010724 to C.E.C], the Mathers Foundation; English, Bonter, Mitchell Foundation; Littauer Foundation; Eshe Fund; and the Lillian B. Davey Foundation to [A.C.S.], and Proteomics Center Contract [HHSN268201000031C to C.E.C.].
Footnotes
The authors have no conflicts of interest to disclose. There is a pending patent pertaining to the results in the paper.
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2016.02.005.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 4.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis. 2015;21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 8.Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29:269–280. doi: 10.1016/j.idc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 10.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One. 2012;7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 12.Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio. 2012;3:e00434–e00512. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moody KD, Barthold SW, Terwilliger GA, Beck DS, Hansen GM, Jacoby RO. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg. 1990;42:165–174. doi: 10.4269/ajtmh.1990.42.165. [DOI] [PubMed] [Google Scholar]
- 14.Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother. 2008;52:1728–1736. doi: 10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One. 2014;9:e86907. doi: 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bockenstedt LK, Wormser GP. Review: unraveling Lyme disease. Arthritis Rheumatol. 2014;66:2313–2323. doi: 10.1002/art.38756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63:2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gondolf KB, Mihatsch M, Curschellas E, Dunn JJ, Batsford SR. Induction of experimental allergic arthritis with outer surface proteins of Borrelia burgdorferi. Arthritis Rheum. 1994;37:1070–1077. doi: 10.1002/art.1780370713. [DOI] [PubMed] [Google Scholar]
- 19.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Investig. 2012;122:2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 21.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–971. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 23.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Human homologues of a Borrelia T cell epitope associated with antibiotic-refractory Lyme arthritis. Mol Immunol. 2008;45:180–189. doi: 10.1016/j.molimm.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh S, Seward R, Costello CE, Stollar BD, Huber BT. Autoantibodies from synovial lesions in chronic, antibiotic treatment-resistant Lyme arthritis bind cytokeratin-10. J Immunol. 2006;177:2486–2494. doi: 10.4049/jimmunol.177.4.2486. [DOI] [PubMed] [Google Scholar]
- 25.Kuenzle S, von Budingen HC, Meier M, Harrer MD, Urich E, Becher B, et al. Pathogen specificity and autoimmunity are distinct features of antigen-driven immune responses in neuroborreliosis. Infect Immun. 2007;75:3842–3847. doi: 10.1128/IAI.00260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunemann JD, Gelderblom H, Sospedra M, Quandt JA, Pinilla C, Marques A, et al. Cerebrospinal fluid-infiltrating CD4+ T cells recognize Borrelia burgdorferi lysine-enriched protein domains and central nervous system auto-antigens in early Lyme encephalitis. Infect Immun. 2007;75:243–251. doi: 10.1128/IAI.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin R, Ortlauf J, Sticht-Groh V, Bogdahn U, Goldmann SF, Mertens HG. Borrelia burgdorferi–specific and autoreactive T-cell lines from cerebrospinal fluid in Lyme radiculomyelitis. Ann Neurol. 1988;24:509–516. doi: 10.1002/ana.410240406. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Monco JC, Seidman RJ, Benach JL. Experimental immunization with Borrelia burgdorferi induces development of antibodies to gangliosides. Infect Immun. 1995;63:4130–4137. doi: 10.1128/iai.63.10.4130-4137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler CM, Garcia Monco JC, Benach JL, Golightly MG, Habicht GS, Steere AC. Nonprotein antigens of Borrelia burgdorferi. J Infect Dis. 1993;167:665–674. doi: 10.1093/infdis/167.3.665. [DOI] [PubMed] [Google Scholar]
- 30.Chandra A, Wormser GP, Klempner MS, Trevino RP, Crow MK, Latov N, et al. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun. 2010;24:1018–1024. doi: 10.1016/j.bbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52:2645–2655. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 32.Sokolove J, Lindstrom TM, Robinson WH. Development and deployment of antigen arrays for investigation of B-cell fine specificity in autoimmune disease. Front Biosci (Elite Ed) 2012;4:320–330. doi: 10.2741/379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan YC, Kongpachith S, Blum LK, Ju CH, Lahey LJ, Lu DR, et al. Barcode-enabled sequencing of plasmablast antibody repertoires in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2706–2715. doi: 10.1002/art.38754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 35.Drouin EE, Seward RJ, Strle K, McHugh G, Katchar K, Londono D, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013;65:186–196. doi: 10.1002/art.37732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowley JT, Drouin EE, Pianta A, Strle K, Wang Q, Costello CE, et al. A highly expressed human protein, apolipoprotein B-100, serves as an auto-antigen in a subgroup of patients with Lyme disease. J Infect Dis. 2015;212:1841–1850. doi: 10.1093/infdis/jiv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pianta A, Drouin EE, Crowley JT, Arvikar S, Strle K, Costello CE, et al. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin Immunol. 2015;160:336–341. doi: 10.1016/j.clim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang KS, Klempner MS, Wormser GP, Marques AR, Alaedini A. Association of immune response to endothelial cell growth factor with early disseminated and late manifestations of Lyme disease but not posttreatment Lyme disease syndrome. Clin Infect Dis. 2015;61(11):1703–1706. doi: 10.1093/cid/civ638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ao W, Zheng H, Chen XW, Shen Y, Yang CD. Anti-annexin II antibody is associated with thrombosis and/or pregnancy morbidity in antiphospholipid syndrome and systemic lupus erythematosus with thrombosis. Rheumatol Int. 2011;31:865–869. doi: 10.1007/s00296-010-1379-4. [DOI] [PubMed] [Google Scholar]
- 40.Cockrell E, Espinola RG, McCrae KR. Annexin A2: biology and relevance to the antiphospholipid syndrome. Lupus. 2008;17:943–951. doi: 10.1177/0961203308095329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salle V, Maziere JC, Smail A, Cevallos R, Maziere C, Fuentes V, et al. Anti-annexin II antibodies in systemic autoimmune diseases and antiphospholipid syndrome. J Clin Immunol. 2008;28:291–297. doi: 10.1007/s10875-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 42.Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. Case definitions for public health surveillance. MMWR Recomm Rep. 1990;39:1–43. [PubMed] [Google Scholar]
- 43.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol. 2006;44:4407–4413. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 46.Seward RJ, Drouin EE, Steere AC, Costello CE. Peptides presented by HLA-DR molecules in synovia of patients with rheumatoid arthritis or antibiotic-refractory Lyme arthritis. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.002477. M110 002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sturniolo T, Bono E, Ding J, Raddrizzani L, Tuereci O, Sahin U, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555–561. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 48.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. 2008;4:e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis. 2008;47:188–195. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis. 2013;75:9–15. doi: 10.1016/j.diagmicrobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control, Prevention A. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 52.Londono D, Cadavid D, Drouin EE, Strle K, McHugh G, Aversa JM, et al. Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheumatol. 2014;66:2124–2133. doi: 10.1002/art.38618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagase H, Visse R, Murphy G. Structure and function of matrix metal-loproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- 55.Wormser GP, Nowakowski J, Nadelman RB, Visintainer P, Levin A, Aguero-Rosenfeld ME. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol. 2008;15:1519–1522. doi: 10.1128/CVI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, et al. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du X, Lin BC, Wang QR, Li H, Ingalla E, Tien J, et al. MMP-1 and Pro-MMP-10 as potential urinary pharmacodynamic biomarkers of FGFR3-targeted therapy in patients with bladder cancer. Clin Cancer Res. 2014;20:6324–6335. doi: 10.1158/1078-0432.CCR-13-3336. [DOI] [PubMed] [Google Scholar]
- 59.Coll B, Rodriguez JA, Craver L, Orbe J, Martinez-Alonso M, Ortiz A, et al. Serum levels of matrix metalloproteinase-10 are associated with the severity of atherosclerosis in patients with chronic kidney disease. Kidney Int. 2010;78:1275–1280. doi: 10.1038/ki.2010.329. [DOI] [PubMed] [Google Scholar]
- 60.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of met-alloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 61.Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–698. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CH, Lin KC, Yu DT, Yang C, Huang F, Chen HA, et al. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology (Oxford) 2006;45:414–420. doi: 10.1093/rheumatology/kei208. [DOI] [PubMed] [Google Scholar]
- 63.Behera AK, Hildebrand E, Scagliotti J, Steere AC, Hu LT. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine Lyme arthritis. Infect Immun. 2005;73:126–134. doi: 10.1128/IAI.73.1.126-134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Behera AK, Thorpe CM, Kidder JM, Smith W, Hildebrand E, Hu LT. Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways. Infect Immun. 2004;72:2864–2871. doi: 10.1128/IAI.72.5.2864-2871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin B, Kidder JM, Noring R, Steere AC, Klempner MS, Hu LT. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J Infect Dis. 2001;184:174–180. doi: 10.1086/322000. [DOI] [PubMed] [Google Scholar]
- 66.Behera AK, Hildebrand E, Uematsu S, Akira S, Coburn J, Hu LT. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin alpha 3 beta 1. J Immunol. 2006;177:657–664. doi: 10.4049/jimmunol.177.1.657. [DOI] [PubMed] [Google Scholar]
- 67.Gebbia JA, Coleman JL, Benach JL. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect Immun. 2001;69:456–462. doi: 10.1128/IAI.69.1.456-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gebbia JA, Coleman JL, Benach JL. Selective induction of matrix metal-loproteinases by Borrelia burgdorferi via toll-like receptor 2 in monocytes. J Infect Dis. 2004;189:113–119. doi: 10.1086/380414. [DOI] [PubMed] [Google Scholar]
- 69.Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ, et al. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog. 2009;5:e1000444. doi: 10.1371/journal.ppat.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Z, Chang H, Trevino RP, Whren K, Bhawan J, Klempner MS. Selective up-regulation of matrix metalloproteinase-9 expression in human erythema migrans skin lesions of acute Lyme disease. J Infect Dis. 2003;188:1098–1104. doi: 10.1086/379039. [DOI] [PubMed] [Google Scholar]
- 71.Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, Benach JL. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 72.Coleman JL, Roemer EJ, Benach JL. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect Immun. 1999;67:3929–3936. doi: 10.1128/iai.67.8.3929-3936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coleman JL, Sellati TJ, Testa JE, Kew RR, Furie MB, Benach JL. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman JL, Benach JL. Use of the plasminogen activation system by microorganisms. J Lab Clin Med. 1999;134:567–576. doi: 10.1016/s0022-2143(99)90095-1. [DOI] [PubMed] [Google Scholar]
- 75.Klempner MS, Noring R, Epstein MP, McCloud B, Rogers RA. Binding of human urokinase type plasminogen activator and plasminogen to Borrelia species. J Infect Dis. 1996;174:97–104. doi: 10.1093/infdis/174.1.97. [DOI] [PubMed] [Google Scholar]
- 76.Toledo A, Coleman JL, Kuhlow CJ, Crowley JT, Benach JL. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect Immun. 2012;80:359–368. doi: 10.1128/IAI.05836-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eeckhout Y, Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977;166:21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He CS, Wilhelm SM, Pentland AP, Marmer BL, Grant GA, Eisen AZ, et al. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989;86:2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okada Y, Gonoji Y, Naka K, Tomita K, Nakanishi I, Iwata K, et al. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- 80.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29:10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- 81.Hu LT, Pratt SD, Perides G, Katz L, Rogers RA, Klempner MS. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect Immun. 1997;65:4989–4995. doi: 10.1128/iai.65.12.4989-4995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu LT, Perides G, Noring R, Klempner MS. Binding of human plasminogen to Borrelia burgdorferi. Infect Immun. 1995;63:3491–3496. doi: 10.1128/iai.63.9.3491-3496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 84.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 85.Heilpern AJ, Wertheim W, He J, Perides G, Bronson RT, Hu LT. Matrix metalloproteinase 9 plays a key role in Lyme arthritis but not in dissemination of Borrelia burgdorferi. Infect Immun. 2009;77:2643–2649. doi: 10.1128/IAI.00214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones KL, McHugh GA, Glickstein LJ, Steere AC. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of RST 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60:2174–2182. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu LT, Eskildsen MA, Masgala C, Steere AC, Arner EC, Pratta MA, et al. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 2001;44:1401–1410. doi: 10.1002/1529-0131(200106)44:6<1401::AID-ART234>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 88.Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54:3244–3253. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- 89.Vudattu NK, Strle K, Steere AC, Drouin EE. Dysregulation of CD4þCD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2013;65:1643–1653. doi: 10.1002/art.37910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 91.Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, et al. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum. 2010;62:2127–2137. doi: 10.1002/art.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005;114:154–163. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Smit M, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer E, van Winkelhoff AJ. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012;14:R222. doi: 10.1186/ar4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


