Abstract
Enhanced perception may allow for visual search superiority by individuals with Autism Spectrum Disorder (ASD), but does it occur over time? We tested high-functioning children with ASD, typically developing (TD) children, and TD adults in two tasks at three presentation rates (50, 83.3, & 116.7 ms/item) using rapid serial visual presentation (RSVP). In the Color task, participants detected a purple target letter amongst black letter distractors. In the Category task, participants detected a letter amongst number distractors. Slower rates resulted in higher accuracy. Children with ASD were more accurate than TD children and similar to adults at the fastest rate when detecting color-marked targets, indicating atypical neurodevelopment in ASD may cause generalized perceptual enhancement relative to typically developing peers.
Keywords: Autism, RSVP, visual search, attention, perception, cognition, development
Introduction
Autism spectrum disorder (ASD) is a relatively common neurodevelopmental condition characterized by atypical social communication and repetitive or stereotyped interests and patterns of behavior (APA, 2014). Although not part of the nosology, studies of individuals with ASD consistently demonstrate the presence of atypical perception, in which individuals with ASD are found to detect and discriminate between sensory inputs more efficiently than IQ and age-matched typically developing (TD) individuals. It is not clear, however, exactly what is different about the way that individuals with ASD process visual information, though there has been much speculation (O’Riordan, 2004; Simmons et al., 2009). Originally, general findings of impaired holistic processing were considered to lead individuals with ASD to be better at focusing on parts as opposed to whole gestalts (Happe & Frith, 2006). Later, this enhanced local processing was found to be related to both detection and discrimination abilities (Mottron, Burack, Dawson, Soulières, & Hubert, 2006). If enhancements in perceptual processing related to detection or discrimination are generally enhanced in ASD, then performance on visual search tasks by individuals with ASD should be consistently superior across experimental paradigms that make similar processing demands and that require participants to detect or discriminate between features. Alternatively, and in line with both the enhanced perceptual functioning model and the temporal binding hypothesis of ASD, enhanced perception might be domain specific. Specifically, theoretical accounts of enhanced perception in ASD have suggested that individuals with ASD demonstrate domain-specific strengths in the extraction of psychophysical dimensions (Mottron et al., 2013), such as color, shape, and orientation. They are also better at determining how things are different than how they are the same, leading to difficulties in categorization and generalization (Soulières, Mottron, Giguère, & Larochelle, 2011; Soulières, Mottron, Saumier, & Larochelle, 2007). Here, participants complete two versions of a task in which they were asked to detect a target letter in a rapidly presented stream of distractors. One of the tasks required low-level feature binding and the other required that participants discriminate between two categories of stimuli, letters and numbers. Contrasting the performance of individuals with ASD on these tasks not only allows us to examine the specificity of enhanced perception in ASD, but also to examine whether findings of enhanced perception, generally noted in static tasks, extend to the temporal domain.
Many of the studies in which enhanced perceptual processing is noted in ASD require participants to discriminate between low-level features of a stimulus (Bonnel, Mottron, Peretz, Trudel, & Gallun, 2003; O’Riordan & Passetti, 2006). For example, Bonnel et al. found that high-functioning individuals with ASD were better able to detect pitch differences than IQ- and age-matched TD adults. In the visual domain, individuals with ASD are better at disembedding figures (Jarrold, Gilchrist, & Bender, 2005; Shah & Frith, 1983), detecting the local level of a compound stimulus, and are better at detecting a target (e.g. a blue ‘S’) in an array of distractors that share common features (e.g. blue T’s and red S’s) (Frith & Happe, 1999, 2005; Meilleur, Berthiaume, Bertone, & Mottron, 2014; Mottron et al., 2013; Plaisted, O’Riordan, & Baron-Cohen, 1998; Shah & Frith, 1993). O’Riordan, Plaisted, Driver, and Baron-Cohen (2001) examined two types of static visual search in participants with ASD and TD participants between the ages of 6 years 5 months and 10 years 9 months of age. Participants were matched according to their performance on Raven’s Progressive Matrices, a measure of general fluid intelligence (Raven, Court, & Raven, 1990). O’Riordan et al. showed participants displays containing set sizes of 5, 15 or 25 elements. The elements were red or green in color, and their form was the letters S, T, or X. In the feature task, targets differed from non-targets in form (e.g., red S among green T and red X distractors). In the conjunctive task, the target shared one feature each with the two types of distractors (e.g., red X among red T and green X distractors). Reaction times to both types of displays increased with increasing display size, but to a smaller degree in ASD participants than in TD participants in the conjunctive task, and accuracy did not differ between groups. The authors made the task harder in a second experiment by presenting arrays of lines, one of which was tilted. In this case, they found faster RTs in both feature and conjunctive tasks for the participants with ASD. This finding of enhanced featural and conjunctive discrimination abilities has been replicated on multiple occasions (Gonzalez, Martin, Minshew, & Behrmann, 2013; Jarrold et al., 2005) and appears to be predictive of ASD in infants (Gliga, Bedford, Charman, & Johnson, 2015). It is present throughout development, ranging from toddlerhood (Kaldy, Kraper, Carter, & Blaser, 2011) to adolescence (O’Riordan, 2004) and through adulthood (e.g., Plaisted et al., 1998), and is found in audition (Bonnel, et al., 2003) and touch (O’Riordan & Passetti, 2006).
While these findings are important to advancing our understanding of how individuals with ASD process the sensory world around them, they generally rely on static displays in which all of the to-be-examined stimuli are present on the screen and are unchanging over the course of a trial. However, the continuous nature of conscious experience necessitates that veridical information integration over both space and time is coordinated across attention, perception, and working memory. How the brain can accomplish this feat with dynamic stimuli is of increasing theoretical interest as researchers develop a better understanding of how attention, perception, and working memory interact over time (Faw, 2003; Hagmann & Cook, 2013; Spivey & Dale, 2006; Tononi & Koch, 2008). In addition, given current theories suggesting that temporal processing is atypical in ASD (Martin, Poirier, & Bowler, 2010; Wallace & Happe, 2008), studying visual strengths in dynamic contexts allows us to determine whether enhanced perception extends to the temporal domain, or whether temporal processing difficulties undermine the expression of this enhanced perception.
Dynamic Search
To our knowledge, only two studies of individuals with autism (Chen et al., 2012; Joseph, Keehn, Connolly, Wolfe, & Horowitz, 2009) have examined dynamic visual search by presenting sequentially changing displays. Joseph et al. presented displays in which the locations of targets and distractors in the display were replotted every 500 ms. Participants looked for a T among L’s and pressed one button to indicate the target was present (half the trials) and another button to indicate the target was absent, while eye-tracking recorded pupil position and movement. Joseph et al. found that, despite similarities in eye fixations, ASD participants produced faster reaction times and superior performance in comparison to TD participants in the dynamic condition. This could be due to enhanced discriminative perception, allowing the ASD participants to reject a display faster and more confidently than TD participants.
More recently, Chen et al. (2012) used coherent motion random dot patterns to examine enhanced local processing of dynamic visual stimuli. They found enhanced performance among participants with ASD relative to TD participants on a speed discrimination task with long but not short intervals. They attributed these findings to superior encoding given longer processing time, a claim which is backed by a study that demonstrate people with ASD require less inspection time than TD participants in order to make accurate line-length comparisons (Barbeau, Soulières, Dawson, Zeffiro, & Mottron, 2013). Since previous studies have found reduced working memory in ASD (Koshino et al., 2005, 2008; Russell, Jarrold, & Henry, 1996), Chen et al. suggest that ASD participants exhibit enhanced perception across space and time. To test the temporal contribution of visual processing, it is important to test the impact of sequential, rather than simultaneous, presentation.
Temporal Feature-Binding
One method for testing visual processing speed and efficiency over time is rapid serial visual presentation (RSVP) (Potter, Wyble, Hagmann, & McCourt, 2014; Potter, 1976). RSVP experiments present a series of figures or images sequentially, generally with no time between one image’s offset and the onset of the next. Embedding a target image among distractor images allows researchers to assess participants’ ability to detect the target under increasingly difficult perceptual conditions as presentation rate increases. This approach is relevant to autism because of recent findings that the binding window for multisensory stimulation may be extended in ASD compared to TD participants (Foss-Feig, Kwakye, Cascio, Burnette, Kadivar, Stone, & Wallace, 2010). If the binding of auditory and visual stimuli can occur over an extended period, it is possible that the binding of multiple visual features is similarly extended over time.
The goal of the present study was to test whether previous findings of enhanced perception in visual search in individuals with ASD were supported in the temporal domain by using RSVP. Based on the previous literature, we expected high-functioning children with ASD to detect targets at fast rates more easily than TD children matched on cognitive ability and age due to enhanced visual processing speed. We employed two types of target/distractor combinations that altered one aspect of the targets. The Color task presented letters, with color as the target’s anomalous feature. That is, all letters were black, except the target, which was purple. In this task, participants would have to detect the target feature and then bind that feature to the co-occurring stimulus. Thus, this task emphasized the ability to rapidly bind distinct aspects of a stimulus together. The Category task presented numbers, except for the target, which was a letter. This task tested the ability to rapidly extract the visual form of each stimulus in the RSVP and compare it against a target category, and does not require binding of features. If visual search enhancements are domain-general, then we would expect individuals with an ASD to outperform their TD peers on both tasks. However, if enhancements in visual search are domain-specific, then we would expect to see a dissociation in performance enhancements between the two tasks, with accuracy advantages being specific to the Color task. For each task, we compared performance between pairs of groups (TD adults and children; children with and without ASD). In addition, to assess the relationships between task performance, individual differences and diagnostic characterizations, we correlated task performance with age, IQ, and scores on two diagnostic measures.
Methods
Participants
Three groups of participants were tested: 1) TD adults, 2) TD children, and 3) children with ASD. Overall, there were 69 participants: 37 adults, 16 TD children, and 16 children with ASD. Child participants were matched on the basis of age and scaled scores on the Perceptual Reasoning Index (PRI) of the Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI-II; Wechsler & Hsiao-Pin, 2011). Perceptual reasoning was selected as the matching variable because it was most relevant to this task (Burack, Iarocci, Flanagan, & Bowler, 2004).
For recruitment of adult participants, the Psychology Research Participation Pool at Syracuse University was utilized. These participants were completing research studies for credit for entry-level psychology courses. For child participants, recruitment was based primarily on word of mouth, school listserv emails, and flyers placed throughout the community. Exclusionary criteria for all participants included a history of seizure disorders, academic or psychiatric problems, and non-corrected vision problems.
Participants ranged from 7 years, 1 month to 25 years, 6 months in age. The mean age of the adult group (15 F) was 19.3 years (SD=1.33), the mean age of the TD children (8 F) was 11.4 years (SD=2.5), and the mean age of the children with ASD (5 F) was 11.6 (SD=2.5). Information pertaining to IQ scores and diagnosis as a function of group can be found in Table 1. TD children (or their parents) completed the Behavior Assessment Scale for Children (BASC; Reynolds & Kamphaus, 2004), and the Sensory Profile (Brown & Dunn, 2002). All TD children were in the non-clinical range on these tests.
Table 1.
Descriptive statistics for ASD and TD children groups. F and p-values are given for comparisons between the two groups of children.
| Group | Adults | ASD | TD | F | p |
|---|---|---|---|---|---|
| N | 37 | 16 | 16 | - | - |
| Mean Age | 19.3 | 11.6 (2.5) | 11.4 (2.5) | 0.47 | 0.830 |
| Age Range | 18–25.6 | 7.6–17 | 7.1–16.6 | - | - |
| VIQ | - | 99.4 (12.0) | 113.8 (10.2) | 13.34 | 0.001 |
| PIQ | - | 103.8 (8.5) | 102.6 (8.9) | 0.148 | 0.703 |
| FSIQ | - | 101.8 (6.7) | 109.6 (7.1) | 10.12 | 0.003 |
Additional enrollment criteria for ASD group participants included scores above the diagnostic cut-off on the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2012) and the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) administered by research reliable clinicians. Fifteen of the sixteen ASD participants were administered the ADOS-2, Module 3, for fluent speech in children and adolescents, and one was administered Module 4, for fluent speech in adolescents and adults. The ADOS-2 and ADI-R means were calculated across three and five domains respectively. For the ADOS, the mean score in the Social Affect (SA) domain was 10.5 (SE=0.98), the mean in the Restricted and Repetitive Behavior (RRB) domain was 5.5 (SE=0.39), and the mean Overall was 16.1 (SE=1.03). For the ADI-R, we obtained ratings for 14 of the 16 ASD participants. The mean for the Qualitative Abnormalities in Reciprocal Social Interaction domain was 17.14 (SE=1.76), the means for Qualitative Abnormalities in Communication for verbal and nonverbal subdomains were 15.28 (SE=1.49) and 7.64 (SE=1.14) respectively, while the mean for the Restricted, Repetitive and Stereotyped Patterns of Behavior domain was 5.64 (SE=0.56).
We obtained medical history for the child participants. At the time of participation, 9 of the 32 child participants were prescribed medication. Seven of the children with ASD and none of the TD children were prescribed psychiatric medication. Three ASD participants were prescribed medications to treat ADHD (Intuniv & Focalin), two were prescribed antipsychotic medication (Clomipramine & Risperdal), and two were prescribed anti-depressant medication (Celexa). The remaining two ASD participants were prescribed medications to treat non-psychological conditions like acne and asthma. For all the participants with an ASD, including those on ADHD medication, BASC scores on the externalizing domain, which would be indicative of ADHD behaviors and symptomology, were below clinical cutoff (T-scores less than 65).
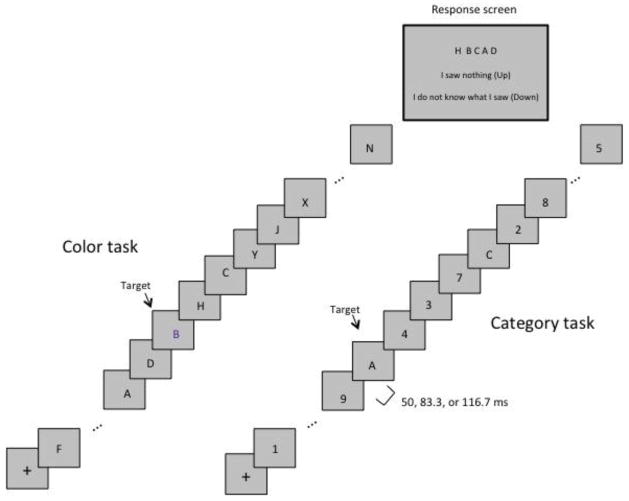
Experimental Task
Two visual search tasks, Color and Category, were used in RSVP. Schematics of example trials of each task are shown in Figure 1. In both tasks, participants were asked to detect a target element among distractor elements presented sequentially. In the Color task, participants were told that they would see a rapid stream of letters, one of which would be purple. They were asked to report which letter was purple. In the Category task, they were informed they would see a sequence of black numbers, but among them would be one black letter. They were asked to report the letter. After each sequence, five letters were presented simultaneously in a randomly shuffled order, one of which had been presented as the target in the RSVP sequence. For the Color task, the five letters included the two letters presented before and after the target. Adult participants were asked to recognize the letter they saw using the keyboard, or to push the up arrow or down arrow, which respectively represented “I do not know what I saw” or “I saw nothing.” These two responses occurred less than 0.01% of the time across all participant groups. Child participants responded verbally and an experimenter pressed the keys for them.
Figure 1.
Schematic of experimental methods used in the Color and Category RSVP tasks.
Three different rates of presentation were used: 50, 83.3, and 116.7 ms/item (fast, medium, and slow, respectively). There was no inter-stimulus interval between items such that each stimulus stayed on screen for the entire duration and was then replaced by the next item in the sequence. There were a total of six blocks of trials, three each for the Category and Color tasks. Each of the three blocks in each task presented the sequences at one of the three rates. The order of tasks (Color, Category) was counterbalanced according to the participants’ subject number (even/odd). There were six rate orders, and participant number determined which of the orders participants received across blocks for both tasks.
Stimuli were presented on a grey background (RGB=127,127,127) at the center of a screen in 48-point Arial font. Stimulus timing was confirmed with timestamps collected for each stimulus. Letter stimuli included the 16 uppercase letters A, B, C, D, F, H, J, K, L, N, P, R, T, V, X and Y, while number stimuli included the eight numbers 2, 3, 4, 5, 6, 7, 8 and 9. Each trial consisted of a fixation cross, followed by distractors and the target. All distractors were presented in black color. The fixation cross was presented for 200 ms followed by the RSVP stream (see Figure 1). The target, which appeared in every trial, could occur in positions 6 through 12 in the stream, which was 16 items long in the Color task and 20 items long in the Category task. The range of target positions was set to be in the middle of the stream to avoid primacy and recency effects (Neath & Crowder, 1996). The target was randomly selected on each trial, and the selection of distractors was randomized in each trial. There were no repetitions of letters within a trial in the Color task. The numbers in the Category task could be repeated, but a repeated number was required to be at least two positions from its previous appearance.
There were 42 trials in each block for the first adult participant, and 70 trials per block for the remaining 36 participants. The first three TD child participants received 21 trials per block, and the remaining 13 received 42 trials per block. The first two ASD child participants received 21 trials per block, and the remaining 14 received 42 trials per block. One-way ANOVA of accuracy between groups of participants with different numbers of trials showed no performance differences between populations.
The experiments were programmed and executed with Stream (Wyble, 2013), a programming interface that uses Psychophysics toolbox (Brainard, 1997) in Matlab. Stimuli were presented on either a Dell Optiplex 960 with a Dell P2210 monitor (1680 × 1000, 60 Hz) or a Macintosh Mini with a Dell U2412M monitor (1680 × 1050, 60 Hz). The visual angle of all the stimuli was similar, measuring 20 and 24 inches from the PC and Macintosh computers’ monitors, respectively, and subtending approximately 1.2 degrees vertically and 1.3 degrees horizontally from the center of the screen.
Procedure
The experiment took place in the Center for Autism Research in Electrophysiology lab (CARE Lab) in the Central New York Medical Center. The Syracuse University Institutional Review Board approved all consent and testing procedures. Prior to the experiment, the different groups completed appropriate informed consent and assent procedures. Adult participants completed informed consents. For child participants their legal guardians completed informed consents and the children completed informed assents. Following consent procedures, adult participants were brought to the testing computer and child participants were administered IQ testing. ASD participants received diagnostic testing at this time as well. Participants were then seated in front of a computer monitor. Participants were asked to look for one target among distractors and report it at the end of an RSVP stream. They completed the experimental task while seated a comfortable distance from the screen with the lights in the room turned off.
Results
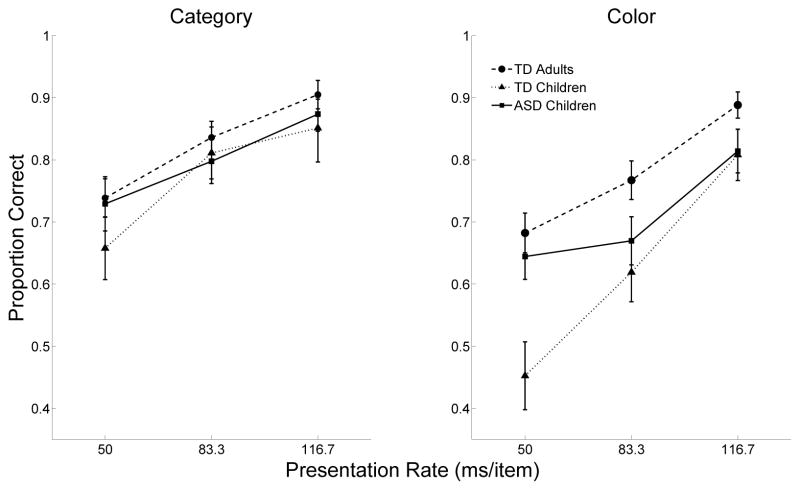
Figure 2 shows the accuracy of each of the three groups in the two tasks across the three rates. To analyze our results, we conducted mixed model repeated measures ANOVA with type 3 sums of squares to compare the accuracy of all three groups at all three rates in the Category and Color tasks separately. In each task, each pair of groups was compared (e.g., TD adults vs. TD children; TD children vs. children with ASD) to better evaluate developmental and ASD specific effects. A small number of participants who were tested early in the experiment received fewer numbers of trials than later participants. Regardless, all analyses produced the same results with and without these participants.
Figure 2.
Proportion correct responses in the Category and Color tasks by TD adults, TD children, and children with ASD at three rates of presentation. Error bars are standard error of the mean, within groups.
Category Task
A group × rate ANOVA of accuracy in the Category task resulted in a main effect of rate, F(2,132)=39.0, p=0.001, ηG2=0.132. As expected, accuracy at the slow rate (M=0.89) was better than the medium rate (M=0.82), t(68)=5.65, p<0.001, r=.56, which was in turn better than the fast rate (M=0.72), t(68)=4.66, p<0.001, r=.49. There were no main effects or interactions with the factor of group (Fs<1), suggesting a similar pattern of performance across the three groups of participants. Linear trends best described performance in the Category task in all groups.
Effect of age
A two-way ANOVA of accuracy in the Category task produced only a main effect of speed, F(2,102)=35.98, p<0.001, ηG2=0.153, suggesting that accuracy patterns were similar between the TD children and adults.
Effect of ASD
In the Category task (left panel of Fig. 1), overall accuracy was very similar between the two groups: Children with ASD had numerically, but not significantly, higher overall accuracy (M=0.800, SE=0.032) than TD children (M=0.773, SE=0.044). A group × rate ANOVA produced only an effect of rate, F(2,60)=27.23, p=0.001, ηG2=0.15, with slower rates producing higher accuracy. There was no effect of group (F(1,30)=0.24) or interaction (F(2,60)=1.68). From fast to slow, mean accuracy increased monotonically (M=0.54, 0.64, and 0.81). The differences in accuracy between the slow and medium as well as between the medium and fast rates were significant, ts(62)>1.98, ps<0.05, r=.24.
Color Task
A group × rate ANOVA of accuracy in the Color task revealed main effects of Group, F(2,66)=6.96, p=0.001, ηG2=0.12, and Rate, F(2,132)=51.96, p=0.001, ηG2=0.23, as well as an interaction, F(4,132)=2.6, p=0.037, ηG2=0.029. Among the groups, adults (M=0.78, SE=0.023), performed significantly better than TD children (M=0.63, SE=0.041), t(51)=3.49, p=0.001, r=.44, but not better than children with ASD (M=0.71, SE=0.027), t(51)=1.79, p=0.08, r=.24. Also, across all groups, the slow rate (M=0.85, SE=0.018) produced better accuracy than the medium rate (M=0.71, SE=0.023), t(68)=4.88, p<0.001, r=.51, which in turn was better than the fast rate (M=0.62, SE=0.026), t(68)=2.60, p=0.01, r=.30. Planned two-sample t-tests revealed significant differences in accuracy at the fast rate between the TD children (M=0.43) and both the children with ASD (M=0.65), t(30)=2.85, p=0.008, r=.46, and the TD adults (M=0.68), t(51)=3.7, p=0.0004, r=.46. At the medium rate, TD children (M=0.62) were no different from children with ASD (M=0.67) but were significantly less accurate than the adults (M=0.77), t(51)=2.56, p=0.013, r=.34. There were no significant differences between groups at the slow rate. Children with ASD and TD adults did not differ in their performance on the Color task at any of the rates, ts(51)<1.86, ps>0.099, r=.25.
Visual inspection of the lines in Figure 2 revealed that differences between the tasks changed in a linear fashion with regard to rate, except for the ASD participants in the Color task, suggesting that performance decrements at the fastest rate were not as severe for children with ASD. This was confirmed with a post-hoc analysis of trends. For the Color task, ASD accuracy was better fit by a quadratic (R2=1) than a linear function (R2=0.84, p=0.25), but the TD children (R2=1, p=0.026) and adults’ (R2=0.99, p=0.06) accuracy levels were fit extremely well by a linear function.
Effects of age
Among TD children and adults, a group × rate ANOVA of accuracy in the Color task resulted in main effects of group, F(1,51)=12.24, p<0.001, ηG2=0.132, and rate, F(2,102)=46.43, p<0.001, ηG2=0.247, as well as a significant interaction, F(2,102)=3.47, p=0.034, ηG2=0.0240, indicating that the groups performed at different levels depending on the rate of presentation. At the slow rate, there was a marginally significant difference between children and adults, t(51)=1.9, p=0.067, r=.23, but at the medium and fast rates there were highly significant differences, ts(51)>2.57, ps<0.013, r=.34.
Effects of ASD
In the Color task (right panel of Fig. 1), children with ASD had slightly, but not significantly, higher overall accuracy (M=0.709, SE=0.028) than TD children (M=0.626, SE=0.041). A group × rate ANOVA of accuracy in the Color task resulted in a group × rate interaction, F(2,60)=4.5, p=0.015, ηG2=0.052. Planned paired t-tests confirmed that children with ASD showed significant performance differences between the fast and slow and the medium and slow rates, ts(15)>3.49, ps<0.0033, r=.67, but not between the fast and medium rates, t(15)=0.54. TD children showed performance differences between all rates, ts(15)>3.84, ps<0.0016, r=.70.
Correlations
There were no significant correlations between Full Scale IQ (FSIQ) or the Verbal Comprehension Index (VCI) and mean accuracy in either group of children. There was, however, a significant correlation between accuracy and PRI among those with ASD in the Category, r=0.62, t(14)=2.92, p=0.01, but not the Color task, r=0.43, t(14)=1.79, p=0.09. TD children did not exhibit a correlation between PRI and accuracy in either task, rs<0.4, ts(14)<1.6, ps>0.12. There were no significant correlations between any of the ADOS-2 measures and accuracy among the ASD participants. Age (in months) positively correlated with mean accuracy among ASD participants in the Category, r=0.50, t(14)=2.16, p=0.048, and the Color task, r=0.71, t(14)=3.81, p=0.002. TD children’s age significantly correlated with accuracy in the Color task, r=0.64, t(14)=3.11, p=0.007, and marginally correlated with Category accuracy, r=0.46, t(14)=1.96, p=0.06, suggesting developmental improvements in accuracy in both groups of children.
Discussion
Using RSVP techniques, we found that children with ASD were overall better than TD children and, at the fastest rate, no different than adults at finding a target stimulus embedded in a sequence of distractors in a color target detection task. The difference between TD children and children with ASD was especially prominent at fast rates of presentation (50 ms/item). Among the children with ASD, there was a plateau with respect to the performance decrements noted with increasing speed at the fastest rate of presentation on the Color task. Whereas for the TD adults and TD children, performance decreased linearly between rates of increasing speed (12% for the adults and approximately 27% for the children), the performance accuracy of the participants with ASD decreased only 3% between the medium and fastest speed of presentation.
Importantly, the accuracy advantage noted among children with ASD was not generalized, but rather was evident only on the Color task. This implies that the perceptual advantage of individuals with ASD is most clearly expressed in a task that requires very rapid temporal feature binding, rather than the letter-number detection and categorization required by the Category task. In the Category task, determining which letter was presented and thus should be reported utilized the same aspect of a stimulus that was used to determine whether a target was a letter vs. a number distractor. Participants were required to categorize the ongoing stream into letters and numbers and report the letters at the end, an area of processing in which individuals with an ASD have shown difficulty (Soulières et al., 2011, 2007). In the Color task, the letter that coincided with the different color was the letter that had to be reported. The difference between these two cases is that the Color task requires binding of information from one low-level stimulus dimension with another, while the Category task did not. These results extend findings of enhanced perceptual processing from the spatial to the dynamic, temporal domain. With a larger sample size, the Category task may show results that support enhanced perception as well, and so this distinction between Color and Category remains an open question.
Theories of ASD
There are two competing theories of mechanisms underlying enhanced perception in ASD. The enhanced perceptual functioning model and its expansions (Mottron, Dawson, Soulières, Hubert, & Burack, 2006; Mottron et al., 2013) purports that ASD perception involves enhanced low-level and mid-level cognitive processing caused by the mechanism of veridical mapping. The enhanced performance we found in the Color task corresponds with this model’s proposed superior coding properties of local neural networks.
Previous research has also shown that atypical temporal binding may contribute to atypical perception in ASD. According to the temporal binding deficit hypothesis of autism (Brock, Brown, Boucher, & Rippon, 2002), weak central coherence forces persons with ASD to rely on a combination of coding. Thus, the bias for local rather than global perception may extend from the spatial to the temporal domain. The temporal binding deficit appears to exist between, not within, local networks (in fact, binding may be enhanced within networks). Our results do not allow us to rule out the impact of temporal binding on our tasks, as it is unclear whether color target perception does or does not require temporal binding. It could be that this task requires binding between distinct networks (this is often assumed), but it could also be that color information is locally bound to shape information. Thus, further research should attempt to disentangle the predictions made from the enhanced perceptual processing model and the temporal binding hypothesis in the temporal domain.
Biological underpinning
Neural development in autism differs from typical development at several key time points and affects some neural regions more than others. For example, cortical minicolumns are more densely packed in ASD than in TD (Casanova et al., 2006), perhaps leading to reduced connections with frontal areas (Courchesne et al., 2007). A preponderance of evidence now suggests that autism is characterized by neural overgrowth in early childhood (Courchesne, Campbell, & Solso, 2011; Courchesne & Pierce, 2005). Recent analysis of brain network organization in autism suggests they have a less modular organization and a tendency for greater interaction between neural subsystems, as well as shorter average distances between functional networks (Rudie et al., 2013). Such developmental effects could allow for enhanced visual perception by way of increased global efficiency between networks. These factors as well as the results of the current study support the Enhanced Perceptual functioning theory, which posits that overfunctioning in the brain regions involved in perception can help explain superior performance in visual and auditory tasks (Mottron et al, 2006). In future research, it will be critical to determine if findings of enhanced perception in ASD can be generalized to other sensory modalities in dynamic contexts.
Another aspect of vision central to our result is that of lateral, feedforward, and feedback processing. A number of findings indicate a bias for local details over global gestalts in ASD (Happé & Frith, 2006). Local bias may be due to atypical contributions from bottom-up (feedforward), top-down (feedback), and horizontal (lateral inhibition) neural processes (Gustafsson, 1997). Event Related Potentials (ERPs) measured during a texture segregation task (Vandenbroucke, Scholte, Van Engeland, Lamme, & Kemner, 2008) revealed diminished activity in low-level central occipital sites in ASD compared to TD participants from about 120 ms after stimulus presentation. Later processing in lateral occipital sites, from 223–243 ms, was enhanced in the ASD group. These results indicate that atypical horizontal connections affect early visual processing in ASD. Whether our results can be explained by the same mechanistic imbalance could be determined with ERP measurement during the RSVP task. Furthermore, Potter, et al. (2014) suggest that ultrafast presentation rates (<50 ms/item) can prevent recurrent feedback from informing participants about stimuli with top-down information. Thus, with faster rates, we could potentially eliminate the role of feedback in assessing how ASD impacts the neurodevelopment of rapid visual perception.
Conclusions
The ASD participants did not show the same decrease in accuracy with faster rates as the other two groups in the Color task. Instead, their performance plateaued between the two fastest rates. It will be important to see if this performance floor holds up for even faster rates, or if the rates we chose are somehow in the ASD participants’ “sweet spot” of rapid perceptual encoding relative to the other groups. Furthermore, the fact that this effect difference occurred for the Color targets and not the Category targets suggests that the types of stimuli being presented differentially alter this effect. In addition, only high-functioning individuals with ASD were tested here, and as such, generalization to the entire spectrum cannot be made. Further research on the attentional blink, in which there are multiple targets to be detected in close proximity, could also help to reveal the time course of enhanced visual perception in ASD.
One alternative explanation for our results is that participants with ASD more effectively memorized which elements were distractors as the sequence unfolded, allowing them to eliminate those elements from the pool of possible targets. This notion contradicts previous findings of reduced working memory in ASD (Koshino et al., 2005, 2008; Russell et al., 1996). Additionally, such a memory-based explanation does not explain why children with ASD performed better than TD children on the Color rather than the Category task, while superior color-binding does help explain this difference. Chen et al. (2012) and Joseph et al. (2009) believed that perceptual encoding was most critical to success on their changing spatial display tasks, as opposed to remembering which elements were distractors. This interpretation of the previous experiments as well as the present research is supported by what we know about visual search and perception in ASD from experiments that required spatial search of static displays (e.g., O’Riordan et al., 2001; Plaisted et al., 1998). Since the present research eliminated spatial variability, focusing on change over time, it is likely that enhanced discrimination in autism affects encoding across the combination of space and time, and in each dimension independently. However, we tentatively assert that such superior encoding is not generalized, given our divergent results in the Category and Color tasks. Instead, enhanced perception may stem from the efficiency with which features are bound to targets. Therefore, our findings suggest that perceptual advantages in feature-binding in ASD extend to the temporal domain.
Additional support for enhanced perception in ASD comes from the significant correlation between PRI and accuracy in our ASD participants. The PRI is a non-verbal, perceptual measure of intelligence, and was used to match participants in the ASD and TD groups. By matching on PRI we made it as difficult as possible to find differences between the groups based on perceptual abilities (Burack, et al., 2004). It is no surprise that an explicitly perceptual task would benefit from improved perceptual intelligence, but the fact that we did not find a correlation in the TD group indicates that perceptual encoding in the form of color-binding contributes more to rapid visual perception in ASD than TD. Further research is required to assess how rapid visual processing in autism affects information integration over time, since such low-level sensory processing, requiring continuous updating over time, may be a root cause of social and communication difficulties (Dawson, Meltzoff, Osterling, & Rinaldi, 1998; Rapin, 1997; Stevenson et al., 2014).
In sum, our results strongly indicate that visual perception is enhanced in children with ASD during temporal search when feature-binding is required. In concert with previous results on spatially relevant enhanced perception (Chen et al., 2012; Joseph et al., 2009), it seems likely that enhanced visual perceptual abilities in autism are generalized across space and time. Our findings were not consistent across both tasks. Accuracy did not differ between groups in the Category task, suggesting that a task without feature-binding was not difficult enough to elicit developmental or group differences, that enhanced perception is not notable on categorization tasks, or that our relatively small sample size masked potential differences. Thus, future experiments on visual search over time in ASD should employ a range of rates and tasks and a larger sample to pinpoint the circumstances that allow for findings of enhanced perception.
Research Highlights.
The present study tested whether enhanced perception occurs in the temporal domain in ASD using rapid serial visual presentation (RSVP).
We tested high-functioning children with ASD, typically developing (TD) children, and TD adults in two tasks at three presentation rates (50, 83.3, 116.7ms).
Slower rates resulted in higher accuracy across groups.
Children with ASD were significantly more accurate than TD children and similar to adults at the fastest rate when detecting color-marked targets.
Acknowledgments
Grant sponsor: NIH; Grant number: 1R01MH101536–01 to N.R. and 5R01MH064824-13 to W.R.K.; Hill Collaboration on Environmental Medicine Diseases of the Nervous System Focus to N.R., W.R.K., & B.W
We would like to thank all of our undergraduate volunteers in the CARE lab for running participants. We would also like to thank the families and children who graciously gave their time for this research. This project was made possible by grant support from a pilot grant from the Hill Collaboration in Environmental Medicine to NR, BW, and WRK, as well as by the NIH (1R01MH101536–01 to NR, and 5R01MH064824-13 to WRK).
Footnotes
The authors declare no conflict of interest.
References
- Barbeau EB, Soulières I, Dawson M, Zeffiro TA, Mottron L. The level and nature of autistic intelligence III: Inspection time. Journal of Abnormal Psychology. 2013;122(1):295. doi: 10.1037/a0029984. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. Cognitive Neuroscience, Journal of. 2003;15(2):226–235. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Development and Psychopathology. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. http://doi.org/10.1017/S0954579402002018. [DOI] [PubMed] [Google Scholar]
- Brown C, Dunn W. Adolescent-Adult Sensory Profile: User’s Manual. Therapy Skill Builders; 2002. [Google Scholar]
- Burack JA, Iarocci G, Flanagan TD, Bowler DM. On Mosaics and Melting Pots: Conceptual Considerations of Comparison and Matching Strategies. Journal of Autism and Developmental Disorders. 2004 doi: 10.1023/b:jadd.0000018076.90715.00. http://doi.org/10.1023/B:JADD.0000018076.90715.00. [DOI] [PubMed]
- Casanova MF, van Kooten IAJ, Switala AE, van Engeland H, Heinsen H, Steinbusch HWM, … Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathologica. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. http://doi.org/10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Norton DJ, McBain R, Gold J, Frazier JA, Coyle JT. Enhanced local processing of dynamic visual information in autism: evidence from speed discrimination. Neuropsychologia. 2012;50(5):733–739. doi: 10.1016/j.neuropsychologia.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. http://doi.org/10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005 doi: 10.1016/j.conb.2005.03.001. http://doi.org/10.1016/j.conb.2005.03.001. [DOI] [PubMed]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi: 10.1016/j.neuron.2007.10.016. http://doi.org/10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Development. 1998;69(5):1276–1285. http://doi.org/10.1111/j.1467-8624.1998.tb06211.x. [PMC free article] [PubMed] [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Consciousness and Cognition. 2003;12:83–139. doi: 10.1016/s1053-8100(02)00030-2. http://doi.org/10.1016/S1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multisensory temporal binding window in autism spectrum disorders. Experimental Brain Research. 2010;203(2):381–9. doi: 10.1007/s00221-010-2240-4. http://doi.org/10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Happe F. Theory of Mind and Self-Consciousness : What Is It Like to Be Autistic? 1999;14(1):1–22. [Google Scholar]
- Frith U, Happe F. Autism spectrum disorder. Current Biology. 2005;15(19):R786–90. doi: 10.1016/j.cub.2005.09.033. http://doi.org/10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Gliga T, Bedford R, Charman T, Johnson M. Enhanced Visual Search in Infancy Predicts Emerging Autism Symptoms. Current Biology. 2015;25:1–4. doi: 10.1016/j.cub.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Martin JM, Minshew NJ, Behrmann M. Practice makes improvement: How adults with autism out-perform others in a naturalistic visual search task. Journal of Autism and Developmental Disorders. 2013;43(10):2259–2268. doi: 10.1007/s10803-013-1772-4. http://doi.org/10.1007/s10803-013-1772-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. Inadequate cortical feature maps: A neural circuit theory of autism. Biological Psychiatry. 1997;42(12):1138–1147. doi: 10.1016/s0006-3223(97)00141-8. [DOI] [PubMed] [Google Scholar]
- Hagmann CE, Cook RG. Active change detection by pigeons and humans. Journal of Experimental Psychology. Animal Behavior Processes. 2013;39(4):383–9. doi: 10.1037/a0033313. http://doi.org/10.1037/a0033313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Gilchrist ID, Bender A. Embedded figures detection in autism and typical development: Preliminary evidence of a double dissociation in relationships with visual search. Developmental Science. 2005 doi: 10.1111/j.1467-7687.2005.00422.x. http://doi.org/10.1111/j.1467-7687.2005.00422.x. [DOI] [PubMed]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Developmental Science. 2009;12:1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. http://doi.org/10.1111/j.1467-7687.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Developmental Science. 2011;14:980–988. doi: 10.1111/j.1467-7687.2011.01053.x. http://doi.org/10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cerebral Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule (ADOS-2) Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- Martin JS, Poirier M, Bowler DM. Brief report: Impaired temporal reproduction performance in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2010;40(5):640–646. doi: 10.1007/s10803-009-0904-3. http://doi.org/10.1007/s10803-009-0904-3. [DOI] [PubMed] [Google Scholar]
- Meilleur AAS, Berthiaume C, Bertone A, Mottron L. Autism-specific covariation in perceptual performances: “g” or “p” Factor? PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0103781. http://doi.org/10.1371/journal.pone.0103781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Bouvet L, Bonnel A, Samson F, Burack JA, Dawson M, Heaton P. Veridical mapping in the development of exceptional autistic abilities. Neuroscience and Biobehavioral Reviews. 2013 doi: 10.1016/j.neubiorev.2012.11.016. http://doi.org/10.1016/j.neubiorev.2012.11.016. [DOI] [PubMed]
- Mottron L, Burack JAJ, Dawson M, Soulières I, Hubert B. Enhanced perceptual functioning in the development of autism. The Development of Autism: Perspectives from Theory and Research. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. http://doi.org/10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006 doi: 10.1007/s10803-005-0040-7. http://doi.org/10.1007/s10803-005-0040-7. [DOI] [PubMed]
- Neath I, Crowder RG. Distinctiveness and very short-term serial position effects. Memory. 1996;4:225–242. doi: 10.1080/09658211.1996.9753032. http://doi.org/10.1080/096582196388933. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology Human Perception and Performance. 2001;27:719–730. doi: 10.1037//0096-1523.27.3.719. http://doi.org/10.1037/0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Passetti F. Discrimination in autism within different sensory modalities. Journal of Autism and Developmental Disorders. 2006;36:665–675. doi: 10.1007/s10803-006-0106-1. http://doi.org/10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: a research note. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39:777–783. http://doi.org/10.1111/1469-7610.00376. [PubMed] [Google Scholar]
- Potter MC. Short-term conceptual memory for pictures. Journal of Experimental Psychology. Human Learning and Memory. 1976;2(5):509–22. [PubMed] [Google Scholar]
- Potter MC, Wyble B, Hagmann CE, McCourt E. Detecting meaning in RSVP at 13 ms per picture. Attention, Perception & Psychophysics. 2014 doi: 10.3758/s13414-013-0605-z. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autism. The New England Journal of Medicine. 1997;337(2):97–104. doi: 10.1056/NEJM199707103370206. http://doi.org/10.1056/NEJM199707103370206. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Raven’s coloured progressive matrices. Oxford: Oxfords Psychologists Press; 1990. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment scale for children. Bloomington, MN: Pearson Assessments; 2004. [Google Scholar]
- Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, Brookheimer SY, Dapretto M. Altered functional and structural brain network organization in autism. NeuroImage: Clinical. 2013;2:79–94. doi: 10.1016/j.nicl.2012.11.006. http://doi.org/10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Jarrold C, Henry L. Working Memory in Children with Autism and with Moderate Learning Difficulties. Journal of Child Psychology and Psychiatry. 1996;37:673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. http://doi.org/10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism diagnostic interview-Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. http://doi.org/10.1111/1469-7610.ep11422546. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic children show superior performance in block design tasks? Journal of Child Psychology and Psychiatry. 1993;14:331–340. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Research. 2009;49(22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Soulières I, Mottron L, Giguère G, Larochelle S. Category induction in autism: slower, perhaps different, but certainly possible. Quarterly Journal of Experimental Psychology (2006) 2011;64(2):311–327. doi: 10.1080/17470218.2010.492994. http://doi.org/10.1080/17470218.2010.492994. [DOI] [PubMed] [Google Scholar]
- Soulières I, Mottron L, Saumier D, Larochelle S. Atypical categorical perception in autism: Autonomy of discrimination? Journal of Autism and Developmental Disorders. 2007;37(3):481–490. doi: 10.1007/s10803-006-0172-4. http://doi.org/10.1007/s10803-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Spivey MJ, Dale R. Continuous dynamics in real-time cognition. Current Directions in Psychological Science. 2006;15:207–211. [Google Scholar]
- Stevenson Ra, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, Wallace MT. Multisensory temporal integration in autism spectrum disorders. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2014;34(3):691–7. doi: 10.1523/JNEUROSCI.3615-13.2014. http://doi.org/10.1523/JNEUROSCI.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Koch C. The neural correlates of consciousness. Annals of the New York Academy of Sciences. 2008;1124(1):239–261. doi: 10.1196/annals.1440.004. http://doi.org/10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke MWG, Scholte HS, Van Engeland H, Lamme VAF, Kemner C. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain. 2008;131:1013–1024. doi: 10.1093/brain/awm321. http://doi.org/10.1093/brain/awm321. [DOI] [PubMed] [Google Scholar]
- Wallace G, Happe F. Time perception in autism spectrum disorders. Research in Autism Spectrum Disorders. 2008;2:447–455. http://doi.org/10.1016/j.rasd.2007.09.005. [Google Scholar]
- Wechsler D, Hsiao-pin C. WASI-II: Wechsler abbreviated scale of intelligence. Pearson; 2011. [Google Scholar]