Abstract
Using adult identified bone mineral density (BMD) loci, we calculated genetic risk scores (GRS) to determine if they were associated with changes in BMD during childhood. Longitudinal data from the Bone Mineral Density in Childhood Study were analyzed (N = 798, 54% female, all European ancestry). Participants had up to 6 annual dual energy X-ray scans, from which areal BMD (aBMD) Z-scores for the spine, total hip, and femoral neck were estimated, as well as total body less head bone mineral content (TBLH-BMC) Z-scores. Sixty-three single-nucleotide polymorphisms (SNPs) were genotyped, and the percentage of BMD-lowering alleles carried was calculated (overall adult GRS). Subtype GRS that include SNPs associated with fracture risk, pediatric BMD, WNT signaling, RANK-RANKL-OPG, and mesenchymal stem cell differentiation were also calculated. Linear mixed effects models were used to test associations between each GRS and bone Z-scores, and if any association differed by sex and/or chronological age. The overall adult, fracture, and WNT signaling GRS were associated with lower Z-scores (eg, spine aBMD Z-score: βadult = −0.04, p = 3.4 × 10−7; βfracture = −0.02, p = 8.9 × 10−6; βWNT = −0.01, p = 3.9 × 10−4). The overall adult GRS was more strongly associated with lower Z-scores in females (p-interaction ≤ 0.05 for all sites). The fracture GRS was more strongly associated with lower Z-scores with increasing age (p-interaction ≤ 0.05 for all sites). The WNT GRS associations remained consistent for both sexes and all ages (p-interaction > 0.05 for all sites). The RANK-RANKL-OPG GRS was more strongly associated in females with increasing age (p-interaction < 0.05 for all sites). The mesenchymal stem cell GRS was associated with lower total hip and femoral neck Z-scores, in both boys and girls, across all ages. No associations were observed between the pediatric GRS and bone Z-scores. In conclusion, adult identified BMD loci associated with BMD and BMC in the pediatric setting, especially in females and in loci involved in fracture risk and WNT signaling.
Keywords: DXA, GENETIC RESEARCH, GENERAL POPULATION STUDIES, CHILDHOOD, PUBERTY
Introduction
More than 10% of US adults aged 55 years and older have osteoporosis (>15% of women),(1) and more than 3 million incident fractures require medical treatment in the United States each year.(2) Enhancing bone accrual to maximize peak bone mass (PBM) in early life could help offset the risk of bone fragility in adulthood. PBM and bone mineral density (BMD) are heritable traits,(3,4) and genome-wide associations studies (GWAS) have already established 63 BMD loci in adult populations.(5–9) A proportion of these loci increase the risk of fracture,(5) and some are in proximity to genes that encode proteins that are involved in biological pathways essential for bone health (these pathways include WNT signaling, RANK-RANKL-OPG, and mesenchymal stem cell differentiation).(5) Furthermore, some of these loci have been shown to operate in early life and may contribute to attainment of PBM.(10–12)
The greatest rate of bone accrual occurs between ages 11 to 13 years in females and ages 13 to 15 years in males,(13) and males tend to achieve a higher PBM in early adulthood compared with females.(13) Given that females are more likely to experience bone fragility in adulthood, it is important to know if these sex and maturation PBM-related differences in early life are partly explained by genetics. Interestingly, a recent longitudinal study involving a sample of UK children investigated the reported GWAS-implicated BMD variants and found that the overall genetic risk score (number of BMD-lowering alleles carried at all known adult GWAS-implicated loci) was negatively associated with BMD and bone mineral content (BMC) at age 13 years and was associated with a slower rate of bone accrual between ages 13 and 17 years.(14) These findings are indicative of a stronger genetic association with increasing chronological age. Similar findings were observed with genetic risk scores restricted to subsets of these loci specifically associated with pediatric BMD, fracture, WNT signaling, and mesenchymal stem cell differentiation.(14) Importantly, sex interactions with the genetic risk scores were not investigated and puberty-stage data were not available to allow for the investigation of genetic risk score interactions by biological age.(14) Moreover, in this study, bone phenotypes were limited to total body less head (TBLH), offering little information about the sites of greatest clinical interest in assessment of adult bone fragility.(14)
We previously reported sex and/or puberty-stage-specific associations between GWAS-implicated adult BMD loci and pediatric bone Z-scores in a longitudinal study of US children.(15) In that study, we analyzed each locus individually and did not investigate genetic risk scores or genetic interactions with chronological age. Building on our past work, we calculated overall adult, pediatric, fracture, WNT, RANK-RANKL-OPG, and mesenchymal stem cell genetic risk scores to determine if they associated with longitudinal bone Z-scores for the spine, total hip, femoral neck, and TBLH in childhood and adolescence, and if the strength of any association varied by sex, chronological age, or puberty stage.
Materials and Methods
Sample
We analyzed data from the Bone Mineral Density in Childhood Study (BMDCS). This was a prospective, longitudinal study established to develop reference BMD and BMC growth charts in healthy US children.(16,17) The participants were recruited in 2002–2003 at five sites across the US (Children’s Hospital of Los Angeles, Cincinnati Children’s Hospital Medical Center, Creighton University, Children’s Hospital of Philadelphia, and Columbia University Medical Center). Females were aged 6 to 15 years and males were aged 6 to 16 years at enrollment, and they were followed up annually for 6 years. In 2006–2007, 5- and 19-year-olds were additionally enrolled to extend the reference percentiles; these participants were followed up annually for 2 years. To be enrolled, the participants had to meet the following criteria: term birth (≥37 weeks gestation), birth weight >2.3 kg, no evidence of precocious or delayed puberty, and height, weight, or BMI within the 3rd to the 97th percentiles for age and sex. Participants were excluded at baseline if they had experienced more than two fractures by age 10 years or more than three fractures after age 10 years. Current or previous users of bone health medications or children who had a medical condition known to affect bone health were also excluded, as were children who had experienced extended bed rest. At the final study visit, the participants were invited to provide a blood or saliva sample to allow for the extraction of DNA. For our present genetic study, we included only one of each member of a sibling pair and excluded participants of non-European ancestry; ancestry was determined using whole genome genotyping data by principal components analyses and ADMIXTURE. Written informed consent was obtained for all participants 18 years and older. For participants younger than 18 years, written informed consent was obtained from parents/guardians and the participant provided their assent. Institutional Review Boards at each study site approved the study.
Bone outcomes
We used dual-energy X-ray absorptiometry (DXA) to derive estimates of areal BMD (aBMD) of the spine, total hip, and femoral neck. Both hip sites were included because total hip has a larger amount of cortical bone than the femoral neck, whereas, conversely, the femoral neck has a greater proportion of trabecular bone. We also included an estimate of TBLH bone mineral content (TBLH-BMC); BMC was used over aBMD for TBLH because it is the preferred measure of bone status for the total body when adjusted for body size.(18) The DXA scanners used were Hologic, Inc. models (Bedford, MA, USA; QDR4500A, QDR4500W, Delphi A, and Apex). All scans were centrally analyzed using Hologic software (Discovery 12.3 at baseline and Apex 2.1 at follow-up using the “compare” feature) at the University of California, San Francisco’s DXA Core Laboratory and adjusted for machine differences and longitudinal drift. Z-scores for each aBMD measure and TBLH-BMC were calculated using the BMDCS reference values to account for known nonlinear increases, increasing variability, and sex differences in aBMD/BMC during growth.(16) Bone Z-scores were adjusted for height-for-age Z-score to account for known effects of size on DXA outcomes. These bone Z-scores were the outcome of interest because declines in bone Z-score over time reflect failure to maintain stability in bone “status” relative to one’s peers in the reference population.
Genetic risk scores
We extracted DNA from blood or saliva and genome-wide single-nucleotide polymorphism (SNP) genotyped the DNA at CHOP’s Center for Applied Genomics, using the Illumina Infinium II OMNI Express plus Exome BeadChip technology (Illumina, San Diego, CA, USA).(19) The 63 SNPs previously associated with adult BMD(5) were either directly genotyped or imputed. The SNPs were leveraged to calculate genetic risk scores (GRS), with all being biallelic (X = “non-risk” allele; x = “BMD-lowering” allele; genotypes: XX, Xx, or xx), allowing us to calculate the percentage of BMD-lowering alleles carried for each participant. The 63 SNPs are listed in Supplemental Table S1, along with subset GRS assignment: fracture (16 loci), pediatric (8 loci), WNT (8 loci), RANK-RANKL-OPG (3 loci), and mesenchymal stem cell (3 loci).(14)
Maturation
Age in years was determined from date of birth to the nearest year. Trained physicians or nurses, with expertise in pediatric endocrinology, estimated puberty stage based on testicular volume in males and breast development in females; we categorized the participants as prepubertal, pubertal, and postpubertal (Tanner stages I, II to IV, and V, respectively).(20,21)
Statistical analyses
We first used linear mixed-effects models to test for main GRS associations with bone Z-scores, adjusting for sex and the time-varying covariates (Z) age, puberty stage, dietary calcium, physical activity,(22) and BMI Z-score(23) [M1].
with and independently
We then tested if the GRS associations were the same for males and females by introducing statistical interaction terms between the GRS and sex [M2]. This was followed by a series of models that tested if the GRS associations changed with increasing chronological and biological age. For the former, we introduced statistical interaction terms between the GRS and age [M3]; for the latter, we introduced statistical interaction terms between the GRS and puberty stage [M4]; both sets of analyses adjusted for sex and the time-varying covariates (Z), dietary calcium, physical activity, and BMI Z-score. Note that higher-order age interactions (age2 = age × age) were considered when testing if the GRS associations were influenced by chronological age; this was to determine if any GRS-age interactions were nonlinear.
with and independently
Finally, we tested if the GRS associations differed by sex and age (chronological and biological) [M5] by introducing three-way interactions terms (GRS-age/puberty stage-sex), adjusting for the time-varying covariates (Z) dietary calcium, physical activity, and BMI Z-score.
with and independently
For all models, the between-subject variability was modeled as a random effect and, we used the method of maximum likelihood (ML) estimation. Robust standard errors were calculated using the Huber-White approach. Bone Z-scores account for expected age- and sex-specific increases in BMC and BMD during growth; therefore, negative GRS associations reflect failure to maintain bone status relative to peers. All analyses were conducted using Stata version 12.0 (StataCorp LP, College Station, TX, USA).
Results
At baseline, the average age of the participants was 11 years and more than half were prepubertal (Table 1). On average, the overall adult GRS was 50% and ranged from 38% to 60%. The averages (and ranges) for the subtype GRS were: 49% (37% to 62%) for the fracture GRS; 48% (19% to 81%) for the WNT signaling GRS; 42% (0% to 100%) for the RANK-RANKL-OPG GRS; 53% (0% to 100%) for the mesenchymal stem cell GRS; and 42% (13% to 75%) for the pediatric GRS.
Table 1.
Characteristics of the Study Sample at Baseline
| All (N = 784) |
Males (n = 361) |
Females (n = 423) |
|
|---|---|---|---|
| Age (years), mean (SD) | 11.0 (4.60) | 11.0 (4.56) | 11.0 (4.64) |
| Tanner I, n (%) | 408 (52.0) | 195 (54.0) | 213 (50.4) |
| Tanner II–IV, n (%) | 192 (24.5) | 76 (21.1) | 116 (27.4) |
| Tanner V, n (%) | 184 (23.5) | 90 (24.9) | 94 (22.2) |
| Genetic risk score, mean (SD), % | |||
| Overall adult | 50.1 (4.04) | 49.9 (4.13) | 50.3 (3.95) |
| Fracture | 49.4 (7.92) | 48.9 (8.24) | 49.9 (7.61) |
| WNT | 48.4 (11.30) | 47.9 (11.56) | 48.8 (11.06) |
| Mesenchymal | 53.2 (19.64) | 52.7 (19.63) | 53.6 (19.66) |
| RANK-RANKL-OPG | 42.0 (19.21) | 42.8 (19.33) | 41.3 (19.09) |
| Pediatric | 41.5 (11.4) | 41.4 (11.38) | 41.6 (11.48) |
The descriptive data are for the participants with total body less head bone mineral content data (TBLH-BMC) at baseline. The overall adult and subtype GRS did not differ between males and females (p > 0.05).
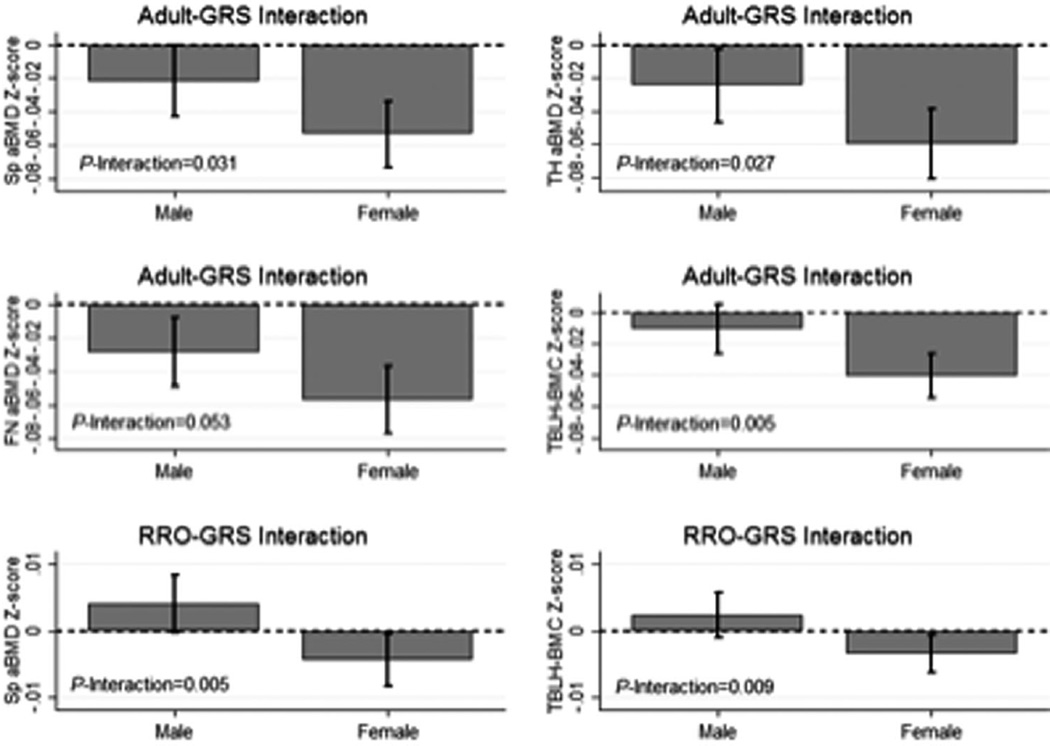
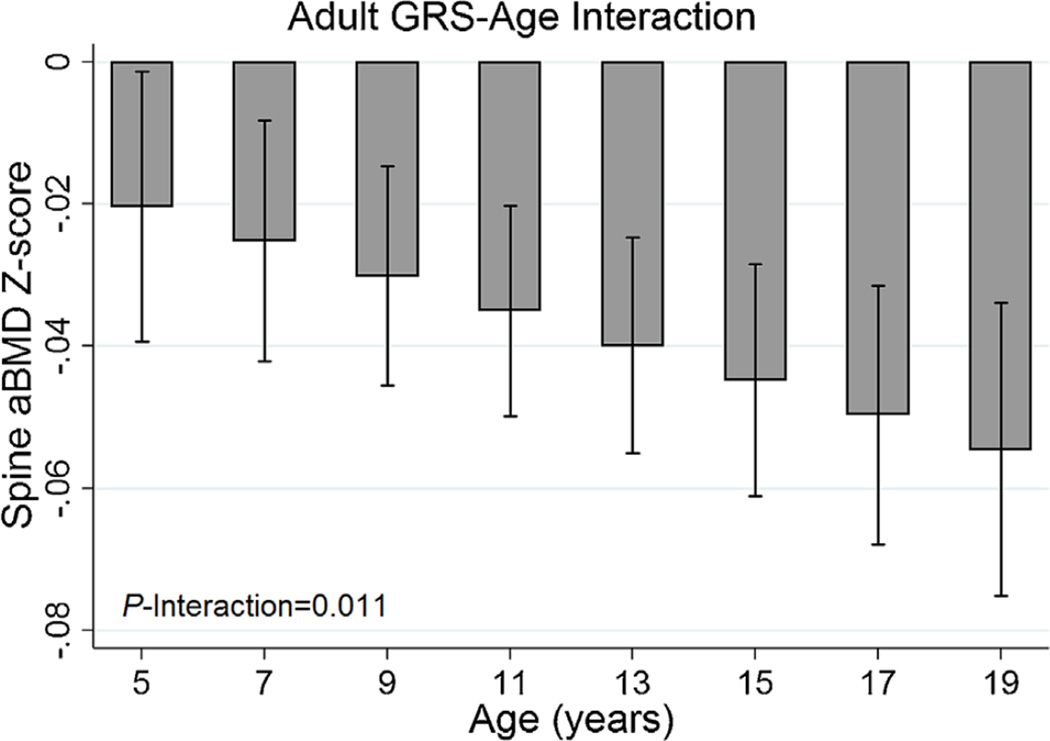
The adult GRS was associated with lower bone Z-scores at all skeletal sites, with the association strongest for femoral neck aBMD Z-score (beta = −0.04, p = 5.7 × 10−9, Table 2). The associations between the adult GRS and all bone Z-scores were stronger among females (Fig. 1). We found statistical evidence that the negative association between the adult GRS and spine aBMD Z-score was stronger with increasing chronological age (Fig. 2) and during Tanner stage V (Supplemental Fig. S1). There were no such age or puberty stage interactions with the adult GRS for other bone Z-scores (Supplemental Table S2).
Table 2.
Main Genetic Risk Score Associations With Bone Z-Scores
| Bone Z-score | n | Genetic risk score |
Beta (SE)a | p Value |
|---|---|---|---|---|
| Spine | 798 | Adult | −0.038 (0.007) | 3.4 × 10−7 |
| Total hip | 798 | Adult | −0.043 (0.008) | 1.0 × 10−7 |
| Femoral neck | 798 | Adult | −0.043 (0.007) | 5.7 × 10−9 |
| TBLH-BMC | 794 | Adult | −0.026 (0.005) | 1.3 × 10−6 |
| Spine | 819 | Fracture | −0.018 (0.004) | 8.9 × 10−6 |
| Total hip | 819 | Fracture | −0.016 (0.004) | 9.5 × 10−5 |
| Femoral neck | 819 | Fracture | −0.016 (0.004) | 4.5 × 10−5 |
| TBLH-BMC | 816 | Fracture | −0.012 (0.003) | 4.8 × 10−5 |
| Spine | 819 | WNT | −0.010 (0.003) | 3.9 × 10−4 |
| Total hip | 819 | WNT | −0.008 (0.003) | 0.007 |
| Femoral neck | 819 | WNT | −0.009 (0.003) | 0.001 |
| TBLH-BMC | 816 | WNT | −0.008 (0.002) | 1.4 × 10−4 |
| Spine | 819 | RANK-RANKL-OPG | 0.000 (0.002) | 0.791 |
| Total hip | 819 | RANK-RANKL-OPG | −0.003 (0.002) | 0.064 |
| Femoral neck | 819 | RANK-RANKL-OPG | −0.001 (0.002) | 0.362 |
| TBLH-BMC | 816 | RANK-RANKL-OPG | −0.001 (0.001) | 0.538 |
| Spine | 818 | Mesenchymal | −0.001 (0.002) | 0.490 |
| Total hip | 818 | Mesenchymal | −0.004 (0.002) | 0.016 |
| Femoral neck | 818 | Mesenchymal | −0.003 (0.001) | 0.026 |
| TBLH-BMC | 815 | Mesenchymal | −0.001 (0.001) | 0.328 |
| Spine | 819 | Pediatric | −0.004 (0.003) | 0.155 |
| Total hip | 819 | Pediatric | −0.005 (0.003) | 0.083 |
| Femoral neck | 819 | Pediatric | −0.003 (0.003) | 0.206 |
| TBLH-BMC | 816 | Pediatric | −0.002 (0.002) | 0.325 |
Beta coefficients represent the change in bone Z-score per additional 1% increase in genetic risk score.
Fig. 1.
Sex interactions with overall adult and RANKL-RANK-OPG (RRO) genetic risk scores (GRS). Sex interactions with the overall adult GRS were observed for spine (Sp), total hip (TH), and femoral neck (FN) areal bone mineral density (aBMD) and total body less head bone mineral content (TBLH-BMC) (top and middle rows). Sex interactions with the RRO GRS were observed for Sp aBMD and TBLH-BMC (bottom row).
Fig. 2.
Adult genetic risk score (GRS) by age interactions with spine areal bone mineral density (Sp aBMD).
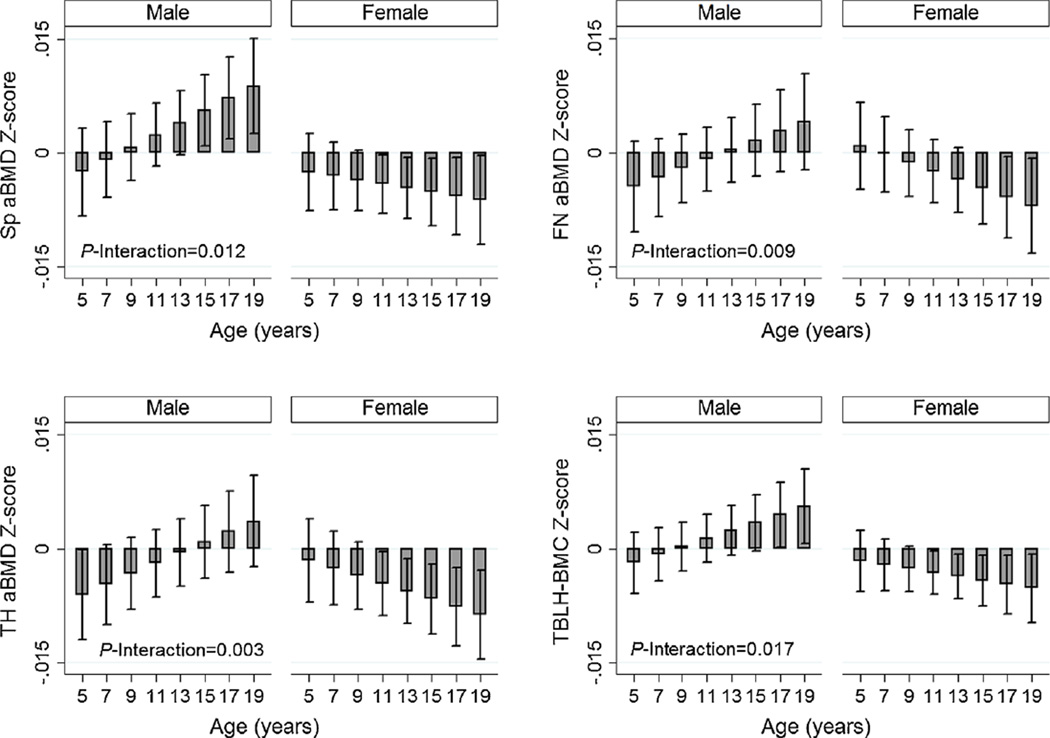
The fracture GRS was associated with lower bone Z-scores, with the association strongest for spine aBMD Z-score (beta = − 0.02, p = 8.9 × 10−6, Table 2). We found no evidence that the associations between fracture GRS and bone Z-score differed by sex (Supplemental Table S2). However, we observed that the association between fracture GRS became progressively stronger with increasing chronological age for spine, total hip, and femoral neck aBMD Z-scores and TBLH-BMC Z-score (Fig. 3). The age interactions were nonlinear for spine, total hip, and TBLH-BMC; the associations were weakest between ages 5 to 11 years and then increased in strength thereafter (Fig. 3). Similarly, the fracture GRS was most strongly associated with lower total hip aBMD and TBLH-BMC Z-scores during Tanner stage V (Supplemental Fig. S1).
Fig. 3.
Fracture genetic risk score (GRS) by age interactions for spine (Sp), total hip (TH), and femoral neck (FN) areal bone mineral density (aBMD) and total body less head bone mineral content (TBLH-BMC). The p-interactions with an asterix (*) are for the nonlinear interactions (fracture GRS-age2 interactions).
The WNT GRS was associated with lower bone Z-scores, with the association strongest for spine aBMD (beta = −0.01, p = 3.9 × 10−4, Table 2). There was no evidence that these associations differed by sex, chronological age, or puberty stage (Supplemental Table S2).
We observed no associations between the RANK-RANKL-OPG GRS and the bone Z-scores overall (Table 2). However, the RANK-RANKL-OPG GRS was associated with lower spine aBMD and TBLH-BMC Z-scores among females (Fig. 4). Further, as females aged, the negative association between the RANK-RANKL-OPG GRS and bone Z-scores was progressively stronger (Fig. 4). In contrast, there was no association between the RANK-RANKL-OPG GRS and bone Z-scores in males, except for males aged 15 years or older for spine aBMD and TBLH-BMC where positive associations were observed (Fig. 4).
Fig. 4.
Age and sex interactions with RANKL-RANK-OPG genetic risk score (GRS) for spine (Sp), total hip (TH), and femoral neck (FN) areal bone mineral density (aBMD) and total body less head bone mineral content (TBLH-BMC).
The mesenchymal GRS was associated with lower total hip and femoral neck aBMD Z-scores (Table 2). There was no evidence of sex or chronological age differences with regard to the association between the mesenchymal GRS and bone Z-scores (Supplemental Table S2). However, mesenchymal GRS was negatively associated with total hip aBMD and TBLH-BMC Z-scores during Tanner stage I (Supplemental Fig. S1).
We found no associations between the pediatric GRS and bone Z-scores (Table 2). There was no evidence of sex or chronological age differences with regard to the association between the pediatric GRS and bone Z-scores (Supplemental Table S2). However, the pediatric GRS was negatively associated with total hip aBMD Z-score during Tanner stage V (Supplemental Fig. S1).
Discussion
Adult bone fragility has its origins in early life, but the role of GWAS-implicated BMD loci, first identified in adults,(5–9) is not well characterized in the pediatric setting. Moreover, the life stage at which these loci associate with bone outcomes is particularly important for understanding relationships between PBM development and bone fragility later in life. We demonstrated that carrying a higher percentage of known adult BMD-lowering alleles (overall adult GRS) was associated with lower bone Z-scores at multiple skeletal sites in childhood. The fracture GRS and the WNT signaling GRS showed particularly strong negative associations. Interestingly, the overall adult GRS was more strongly associated with lower bone Z-scores in females, and the negative effect of the fracture GRS on bone Z-scores was stronger as children aged (chronological and biological). In contrast, the negative association of the WNT signaling GRS with bone Z-scores remained consistently strong for both sexes and all ages. For comparison, the same GRS were associated with bone phenotypes (BMD, BMC, and bone area of TBLH only) in a study by Warrington and colleagues that assessed longitudinal data from the Avon Longitudinal Study of Parents and Children (ALSPAC).(14) Sex interactions were not investigated in that study and only TBLH was measured. However, age interactions were observed where the overall adult, fracture, and WNT signaling GRS were more strongly associated with bone outcomes with increasing chronological age from age 9 to 17 years; there was also some evidence of the same chronological age association pattern for the mesenchymal GRS and TBLH-BMC. Overall, the findings from both studies indicate that adult GWAS-implicated BMD loci operate in the pediatric setting, especially loci that are involved in fracture risk and WNT signaling. However, in contrast to the results reported by Warrington and colleagues, our findings suggest that the overall adult GRS (especially in females) and the WNT signaling GRS associations occur earlier and are more persistent throughout childhood. Therefore, early intervention to help increase PBM may be particularly important for those at genetic predisposition to lower BMD.
A unique contribution of our study is that we tested for sex interactions with each GRS. This approach revealed that the overall adult GRS was more strongly associated with bone Z-scores in females. Furthermore, the RANK-RANKL-OPG GRS was negatively associated with bone Z-score in females, and this association increased in strength with increasing chronological age. These results extend our previously reported sex differences regarding individual adult BMD genetic loci.(15) Understanding musculoskeletal sex differences is a major area of emphasis for the NIH(24) and is of clinical and public health significance given that osteoporosis and fracture are more common in females.(1) Although it is not known why the GRS associated with bone Z-scores differentially by sex, our results strongly suggest that the processes leading to sex differences in adult bone fragility begin during childhood. It is possible that differences in sex hormones, levels and distribution of fat and lean mass, and/or levels of weight-bearing physical activity could influence how male and female skeletons are affected by known BMD-lowering genetic variants. Interestingly, a study involving adults investigated if the individual GWAS-implicated BMD loci interacted with sex to influence spine and femoral neck aBMD.(25) In contrast to our findings in the present study, and in our earlier work,(15) there was no evidence of sex differences. It would be of interest to conduct a longer follow-up study in the future to determine at which point in the life span these genetic sex differences no longer exist, especially because there is evidence that associations between serum OPG and aBMD in adults have been shown to differ for men and women and in women taking estrogen.(26)
It is notable that associations between the pediatric GRS and bone Z-scores were not among the strongest that we observed. We only found that the pediatric GRS was associated with lower total hip and femoral neck aBMD Z-scores during Tanner stage V. This latter observation is consistent with the findings reported by Warrington and colleagues, who found that the pediatric GRS was more strongly associated with bone phenotypes with increasing chronological age.(14) Importantly, the 8 SNPs comprising the pediatric GRS were identified in a GWAS using data from the ALSPAC(11) and our GWAS using BMDCS data only identified the CPED1 locus.(12) Additional research is needed to determine if there are genetic loci beyond those first discovered in adults that are primarily pediatric BMD susceptibility loci.
Finally, we found that the mesenchymal stem cell GRS was associated with lower total hip and femoral neck aBMD Z-scores, and these associations did not differ by sex or chronological age. Warrington and colleagues also reported associations between the mesenchymal stem cell GRS and TBLH bone phenotypes and only found evidence of an age interaction for TBLH-BMC.(14) However, we did find that the mesenchymal stem cell GRS was more strongly associated with lower total hip and TBLH-BMC Z-scores during Tanner stage I. This biological age association underscores that some known adult BMD loci operate in the pediatric setting, even before the onset of puberty.
Major strengths of our study include the large sample of males and females, with up to 6 repeated bone Z-score measurements at multiple skeletal sites. The age range of our sample allowed us to test for chronological and biological age interactions. Also, trained endocrinologists or nurses performed the puberty stage assessments at each study visit. Our DXA methods allowed for TBLH and site-specific bone Z-scores to be calculated, and bone Z-scores account for the expected sex- and age-related increases in bone outcomes during growth. However, our study does have limitations. Our results can only be generalized to children of European ancestry living in the US. The SNPs used to calculate the bone signaling pathway GRS are in proximity to genes encoding proteins that are important for bone physiology, but these SNPs may not necessarily regulate the function of the gene closest in proximity. We used unweighted GRS, as did Warrington and colleagues, but it is known that the individual SNP associations with BMD are not equal in strength. Estrada and colleagues calculated a weighted GRS, specific to the femoral neck, in adults. Using the same weights, we reevaluated our associations with femoral neck aBMD Z-score; the weighted GRS yielded very similar results to our unweighted GRS analyses (Supplemental Table S3). We found evidence of nonlinear age interactions with the fracture GRS for some bone Z-score outcomes (Fig. 3). These nonlinear interactions indicated weaker associations at younger ages. Alternatively, these nonlinear interactions could be indicative of a threshold effect, with the fracture GRS associated with bone Z-scores after the onset of puberty. Although this is not supported by our fracture GRS interactions with Tanner stage (Supplemental Fig. S1), additional age interaction analyses in a large sample of prepubertal children are warranted.
We conclude that the adult identified BMD loci operate in the pediatric setting, especially among females, and particularly with loci involved in fracture risk and WNT signaling. Our findings provide insight into the development of disparities between males and females in bone fragility later in life and potential mechanisms whereby attainment of PBM is influenced by genetic risk.
Supplementary Material
Acknowledgments
The study was funded by R01 HD58886; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contracts (N01-HD-1-3228, -3329, -3330, -3331, -3332, -3333); and the CTSA program Grant 8 UL1 TR000077. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We appreciate the dedication of the study participants and their families, and the support of Dr Karen Winer, Scientific Director of the Bone Mineral Density in Childhood Study.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Study conception and design: JAM, SFAG, and BSZ. Acquisition of data: SFAG, BSZ, HJK, JML, VG, SEO, and JAS. Data analysis: JAM, BSZ, and OE. Interpretation of data: JAM, AC, OE, SEM, SMR, HJK, JML, VG, SEO, JAS, AK, BSZ, and SFAG. Drafting manuscript: JAM, BSZ, and SFAG. Revising manuscript content: AC, OE, SEM, SMR, HJK, JML, VG, SEO, JAS, and AK. Approving final version of manuscript: JAM, AC, OE, SEM, SMR, HJK, JML, VG, SEO, JAS, AK, BSZ, and SFAG. JAM takes full responsibility for the integrity of the data analysis.
References
- 1.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Gueguen R, Jouanny P, Guillemin F, Kuntz C, Pourel J, Siest G. Segregation analyses and variance components analyses of bone mineral density in healthy families. J Bone Miner Res. 1995;10(12):2017–2022. doi: 10.1002/jbmr.5650101223. [DOI] [PubMed] [Google Scholar]
- 4.Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8(1):1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- 5.Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards JB, Rivadeneira F, Inouye M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371(9623):1505–1512. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivadeneira F, Styrkarsdottir U, Estrada K, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan EL, Danoy P, Kemp JP, et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet. 2011;7(4):e1001372. doi: 10.1371/journal.pgen.1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, et al. New sequence variants associated with bone mineral density. Nat Genet. 2009;41(1):15–17. doi: 10.1038/ng.284. [DOI] [PubMed] [Google Scholar]
- 10.Medina-Gomez C, Kemp JP, Estrada K, et al. Meta-analysis of genome-wide scans for total body BMD in children and adults reveals allelic heterogeneity and age-specific effects at the WNT16 locus. PLoS Genet. 2012;8(7):e1002718. doi: 10.1371/journal.pgen.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemp JP, Medina-Gomez C, Estrada K, et al. Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. PLoS Genet. 2014;10(6):e1004423. doi: 10.1371/journal.pgen.1004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesi A, Mitchell JA, Kalkwarf HJ, et al. A trans-ethnic genome-wide association study identifies gender-specific loci influencing pediatric aBMD and BMC at the distal radius. Hum Mol Genet. 2015;24(17):5053–5059. doi: 10.1093/hmg/ddv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14(10):1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 14.Warrington NM, Kemp JP, Tilling K, Tobias JH, Evans DM. Genetic variants in adult bone mineral density and fracture risk genes are associated with the rate of bone mineral density acquisition in adolescence. Hum Mol Genet. 2015;24(14):4158–4166. doi: 10.1093/hmg/ddv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JA, Chesi A, Elci O, et al. Genetics of bone mass in childhood and adolescence: effects of sex and maturation interactions. J Bone Miner Res. 2015;30(9):1676–1683. doi: 10.1002/jbmr.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 18.Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Hakonarson H, Grant SF, Bradfield JP, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448(7153):591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 20.Tanner J. Growth at adolescence. Oxford: Blackwell Scientific Publisher; 1962. [Google Scholar]
- 21.Zachmann M, Prader A, Kind HP, Hafliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29(1):61–72. [PubMed] [Google Scholar]
- 22.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC., Jr Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6(11):1227–1233. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 24.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu CT, Estrada K, Yerges-Armstrong LM, et al. Assessment of gene-by-sex interaction effect on bone mineral density. J Bone Miner Res. 2012;27(10):2051–2064. doi: 10.1002/jbmr.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern A, Laughlin GA, Bergstrom J, Barrett-Connor E. The sex-specific association of serum osteoprotegerin and receptor activator of nuclear factor kappaB legend with bone mineral density in older adults: the Rancho Bernardo study. Eur J Endocrinol. 2007;156(5):555–562. doi: 10.1530/EJE-06-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.