Abstract
Impulsive choice is a diagnostic feature and/or complicating factor for several psychological disorders and may be examined in the laboratory using delay-discounting procedures. Recent investigators have proposed using quantitative measures of analysis to examine the behavioral processes contributing to impulsive choice. The purpose of this study was to examine the effects of physical activity (i.e., wheel running) on impulsive choice in a single-response, discrete-trial procedure using two quantitative methods of analysis. To this end, rats were assigned to physical activity or sedentary groups and trained to respond in a delay-discounting procedure. In this procedure, one lever always produced one food pellet immediately, whereas a second lever produced three food pellets after a 0, 10, 20, 40, or 80-second delay. Estimates of sensitivity to reinforcement amount and sensitivity to reinforcement delay were determined using (1) a simple linear analysis and (2) an analysis of logarithmically transformed response ratios. Both analyses revealed that physical activity decreased sensitivity to reinforcement amount and sensitivity to reinforcement delay. These findings indicate that (1) physical activity has significant but functionally opposing effects on the behavioral processes that contribute to impulsive choice and (2) both quantitative methods of analysis are appropriate for use in single-response, discrete-trial procedures.
Keywords: delay discounting, exercise, female, impulsive choice, physical activity
1. Introduction
Impulsive choice, operationally defined as choosing a smaller, immediate reinforcer over a larger, delayed reinforcer, is a diagnostic feature of attention deficit hyperactivity disorder (ADHD) and is a complicating factor in other disorders (e.g., substance use disorder, binge-eating disorder; Bickel et al., 2012; Patros et al., 2016). Impulsive choice is typically examined experimentally using delay-discounting procedures, in which the delay and magnitude of a reinforcer are systemically varied across two alternatives. In these procedures, subjects reliably choose the larger of two reinforcers when both are available at equivalent delays, but reallocate their behavior to the alternative with the smaller reinforcer when the delay to the larger reinforcer increases. This shift in preference is presumed to a reflect the subject’s “discounting” of the larger reinforcer, and can be quantified by plotting the percentage choice of the large reinforcer as a function of its delay. Using this analysis, interventions to decrease impulsive choice may then be examined (see reviews by Bickel et al., 2014; Koffarnus et al., 2013).
Exercise, defined as engagement in physical activity to increase health and fitness, has been touted as a potential treatment for many types of mental disorders, including those in which impulsive choice is a diagnostic feature or complicating factor (e.g., Smith and Lynch, 2012; Vancampfort et al., 2013; Wigal et al., 2013). Exercise is often modeled in laboratory animals by giving subjects free access to activity wheels in the home cage. To our knowledge, no published studies have examined the effects of physical activity on delay discounting.
Many investigators have noted that data generated in delay-discounting procedures reflect two independent behavioral phenomena that collectively determine how an organism will allocate its behavior across two alternatives (e.g., Locey and Dallery, 2009; Maguire et al., 2009; Mobini et al., 2002; Pitts and Febbo, 2004; Ta et al., 2008). Sensitivity to delay reflects the degree to which choice is determined by the delay to the reinforcer. Subjects with greater sensitivity to delay will allocate fewer of their responses to the larger reinforcer if the larger reinforcer is delayed relative to the smaller reinforcer. Sensitivity to amount reflects the degree to which choice is determined by the magnitude of the reinforcer. Subjects with greater sensitivity to amount will allocate more responses to the larger reinforcer if delay is held constant between the two alternatives. Because these factors are independent, they could work together to (1) increase impulsive choice if sensitivity to delay is high and sensitivity to amount is low or (2) decrease impulsive choice if sensitivity to delay is low and sensitivity to amount is high. Alternatively, these factors could work in opposition to one another to (3) cancel out the effects of the other if (a) sensitivity to delay is high and sensitivity to amount is high or (b) sensitivity to delay is low and sensitivity to amount is low. In such cases, null effects in delay-discounting procedures may actually reflect significant effects on two opposing behavioral processes.
Recently, Pitts (2014) described a quantitative method of analysis to elucidate the effects of sensitivity to amount and sensitivity to delay in delay-discounting procedures. The method uses a logarithmically transformed equation of response ratios that is based on both the general matching law and hyperbolic discounting (Mazur, 1987). Previously, the model was applied to data generated in pigeons responding under a free-operant, concurrent-chains procedure and treated with the psychomotor stimulant methamphetamine (Pitts and Febbo, 2004). The model revealed that methamphetamine decreased sensitivity to reinforcement delay, suggesting that this may be a behavioral mechanism by which stimulants influence impulsive choice. Pitts (2014) further suggests that the model may also be applicable to single-response, discrete-trial procedures, but cautions that the use of the model in these procedures has not been validated.
The aims of the present study were to (1) examine the effects of physical activity on delay discounting and (2) compare two quantitative methods of analysis to determine if they would yield similar estimates of sensitivity to amount and sensitivity to delay. In regard to the latter aim, we compared the analysis of logarithmically transformed response ratios described by Pitts (2014) to a simple linear analysis previously used for single-response, discrete trials data (Koffarnus and Woods, 2013). Rats were assigned to sedentary or physical activity conditions and trained in a delay-discounting task using two response alternatives. On one response alternative, one food pellet was always immediately available following a single lever press. On a second response alternative, three food pellets were available at increasing delays ranging from 0 to 80 seconds. Delay discounting was determined by plotting the percentage of responses allocated to the larger reinforcer as a function of its delay. Estimates of sensitivity to delay and sensitivity to amount were then calculated using the two methods described above. If the two methods yielded similar estimates, we took this as converging evidence for their utility in examining the behavioral mechanisms of impulsive choice in single-response, discrete-trial procedures.
2. Material and Methods
2.1 Animals and Apparatus
Sixteen, female, Long-Evans rats were obtained at weaning (~21 days) from Charles River Laboratories (Raleigh, NC, USA) and assigned randomly to two groups. Sedentary rats (n = 8) were housed individually in polycarbonate cages (interior dimensions: 50 × 28 × 20 cm) that permitted no activity beyond normal cage ambulation. Physical activity rats (n = 8) were housed individually in polycarbonate cages of equal dimensions but with an activity wheel (interior diameter: 35 cm) affixed to the interior of the cage. All subjects remained in their respective groups for the duration of the study, which lasted approximately 11 weeks. Home cages were kept in a temperature- and humidity-controlled colony room maintained on a 12-h light/dark cycle. Rats remained undisturbed in their home cages for the first six weeks of the study until the beginning of behavioral training and testing (see below). After six weeks in the colony, rats were food restricted to no less than 85% of their free-feeding body weight and began behavioral training (body weights did not significantly differ between groups; mean physical activity = 215 g; mean sedentary = 201 g). Wheel revolutions were counted with mechanical switches and were recorded weekly for the first six weeks of the study, and then daily with the initiation of behavioral training. Estrous phase was not monitored. All subjects were treated in accordance with the guidelines of the Animal Care and Use Committee of Davidson College and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 2011).
Behavioral training and testing took place in polycarbonate and aluminum operant conditioning chambers (interior dimensions: 31 × 24 × 21 cm) from Med Associates, Inc. (St Albans, VT). Each chamber was equipped with two retractable response levers located 10 cm above the chamber floor and a single houselight located on the rear wall. Levers could be depressed with a force of ~0.25 N. A white stimulus light located above the response lever signaled the availability of a food pellet from a pellet dispenser located behind the front wall. Experimental events were programmed and data were collected through software and interfacing supplied by Med Associates, Inc.
2.2 Lever-Press Training
Six weeks after arrival, all rats were food restricted to no less than 85% of their free feeding body weight and trained to lever press during daily experimental sessions. All experimental sessions were conducted during the light phase of the light/dark cycle so as not to interfere with nocturnal running. During the initial training sessions, responses on the left and right response levers were reinforced on alternating days. Each training session began with illumination of the house light, extension of either the left or right response lever into the chamber, and illumination of the stimulus light above the extended lever. Each response on the lever produced a single 45-mg grain pellet on a fixed ratio (FR1) schedule of reinforcement, followed by a 5-s blackout during which responding had no programmed consequences. Each session continued for 2 h or until 40 reinforcers were delivered, whichever occurred first. Training continued in this manner until 40 reinforcers were obtained on both the left and right response levers in at least two consecutive sessions for each lever. All rats met the acquisition requirement within 7 days.
2.3 Delay-Discounting Procedure
Once rats acquired the lever-press response, the delay-discounting procedure was introduced and remained in effect for the duration of the study (Anderson and Diller, 2010; Evenden and Ryan, 1996). During these sessions, the magnitude of the reinforcer on one of the two response alternatives was increased from one to three pellets. Also, a delay was inserted between each response and the delivery of the larger reinforcer. All sessions consisted of five components during which the delay to the larger reinforcer systematically increased across components: 1st component = 0 s delay, 2nd component = 10 s delay, 3rd component = 20 s delay, 4th component = 40 s delay, 5th component = 80 s delay. Each component consisted of two forced-choice trials in which only one lever was extended into the chamber, followed by six free-choice trials in which both levers were extended into the chamber. Consequently, each session contained a total of 40 trials, 10 forced choice trials and 30 free choice trials. The beginning of each component was signaled by flashing the houselight for 5 s (0.5 s on/off).
Each forced-choice trial began with illumination of the house light, the insertion of one response lever into the chamber, and illumination of the white stimulus light above the lever. A single response on the extended lever produced one 45 mg grain pellet immediately (SSR: smaller, sooner reinforcer) or three 45 mg grain pellets after the specified delay (LLR: larger, later reinforcer). No time limit was placed on a given trial, meaning that a trial did not terminate until a response was emitted. On the following forced choice trial, the opposite lever extended into the chamber. These stipulations insured that each rat sampled both the SSR and LLR lever (and hence the delay imposed on the LLR lever) at the beginning of each component. During the delay interval, the lever retracted and the stimulus light above the lever turned off. Each reinforcer delivery was followed by a 100-s blackout period during which both levers remained retracted and the stimulus lights remained off. SSR and LLR lever assignments were counterbalanced across rats and remained consistent throughout the study.
Each free-choice trial began with illumination of the house light, the insertion of both response levers into the chamber, and illumination of the white stimulus light above each lever. A single response on the SSR lever produced one 45 mg grain pellet immediately and a single response on the LLR produced three 45 mg grain pellets after the specified delay. During the delay interval, both levers retracted and the stimulus lights above both levers turned off. Each reinforcer delivery was followed by a blackout period during which both levers remained retracted and the stimulus lights remained off. The duration of the blackout period varied as a function of both delay and latency to respond according to the following formula: blackout duration = 100 − (delay + latency to respond), in seconds. This stipulation insured that the overall time between trials was always consistent across components and between the two response alternatives. If no response was recorded within 20 s, both levers retracted, both stimulus lights turned off, and an omission was recorded. The next trial began automatically after 80 s elapsed (100 s total).
Testing continued in the delay-discounting procedure for 37 consecutive days. At this point, responding (as measured by the primary outcome variables) had exhibited stability over 12 consecutive sessions.
2.4 Data Analysis
Delay discounting data were first analyzed as percent choice for the LRR (LLR choices/total choices × 100) averaged over the final 12 sessions. A 2 × 5 mixed ANOVA was used to evaluate the effect of group (between subjects measure: physical activity and sedentary), delay (repeated measure: 0, 10, 20, 40, 80 s), and the group × delay interaction (Anderson and Diller, 2010; Evenden and Ryan, 1996). Post hoc analyses were conducted comparing between-group differences in percent choice for the LRR at each delay.
To evaluate potential differences in sensitivity to reinforcement amount and sensitivity to reinforcement delay as a function of physical activity we used two analytic approaches. First, a simple regression equation with percent LLR choice as the criterion and delay as the predictor variable was used to determine slopes (i.e., sensitivity to reinforcement delay) and y-intercepts (i.e., sensitivity to reinforcement amount) for each subject (Koffarnus and Woods, 2013). The negation of the slope was used to place slope values into positive space. In this analysis, a larger y-intercept value indicates greater sensitivity to reinforcement amount and a greater probability of choosing the larger, later reinforcer (i.e., less impulsive choice); a larger slope indicates greater sensitivity to reinforcement delay and a greater probability of choosing the smaller, sooner reinforcer (i.e., greater impulsive choice). Regression intercepts and slopes for individual rats were calculated and compared between the two groups via independent-samples t-tests.
Slope and y-intercept data were then compared to data derived from an equation previously used by Pitts and colleagues to evaluate delay-discounting data (Pitts and Febbo, 2004; Pitts, 2014):
where RL is the number of responses on the LLR lever for a set delay, RS is the number of responses on the SSR lever for a set delay, DL is the delay for the LLR reinforcer, DS is the delay for the SSR reinforcer, AL is the amount of the LLR reinforcer, and AS is the amount of the SSR reinforcer. When calculating SA (sensitivity to amount) and SD (sensitivity to delay), log(RL/RS) was graphed as a function of log(1 + DL/1 + DS), and the y-intercept was divided by log(AL/AS) to determine SA, and the negation of the slope was used to determine SD. In this analysis, a larger SA value indicates greater sensitivity to reinforcement amount and a greater probability of choosing the larger, later reinforcer (i.e., less impulsive choice); a larger SD indicates greater sensitivity to reinforcement delay and a greater probability of choosing the smaller, sooner reinforcer (i.e., greater impulsive choice). Thus, SA should measure the same behavioral process as that estimated by the y-intercept from the linear analysis, and SD should measure the same behavioral process as that estimated by the slope from the linear analysis.
SA and SD values for individual rats were calculated by averaging RL and RS values for each delay and compared between the two groups via independent-samples t-tests. Pearson product-moment correlations for indices of reinforcement amount (i.e., y-intercept and SA) and reinforcement delay (i.e., slope and SD) were conducted to evaluate correspondence between index measures. To determine the reliability of these values over time, average values over the final 2, 6, and 12 days were calculated and compared between groups. The correlation between average daily wheel running and study outcomes (slope, y-intercept, SD, and SA) was examined using Pearson bivariate correlations.
Any rat that (1) averaged greater than 2 omissions per session, (2) exhibited a delay discounting curve with a positive slope, or (3) failed to choose the LLR lever over the SSR lever on greater than 50% of trials at a 0-s delay was removed from the study and did not contribute to the statistical analysis. The later two criteria were used to exclude rats that failed to discriminate the different contingencies regarding delay and amount between the two response alternatives. This resulted in the removal of one physical activity rat and two sedentary rats from the study. An additional sensitivity analysis was conducted using a more stringent exclusion criterion (i.e., failure to choose the LLR lever over the SSR lever on greater than 75% of trials at a 0 s delay) that resulted in the additional removal of two physical activity and one sedentary rat.
3. Results
Wheel running increased weekly in individual rats until reaching a peak during the 4th, 5th, or 6th week of wheel exposure (Figure 1). Maximal rates of wheel running varied considerably across rats, ranging from 8,513 to 15,422 revolutions/day. Wheel running declined in most rats during the 7th week of wheel exposure with the initiation of behavioral training. The decline in daily wheel running continued for approximately three weeks before plateauing during the final two weeks of the study (i.e., during the 10th and 11th week of wheel exposure).
Figure 1.
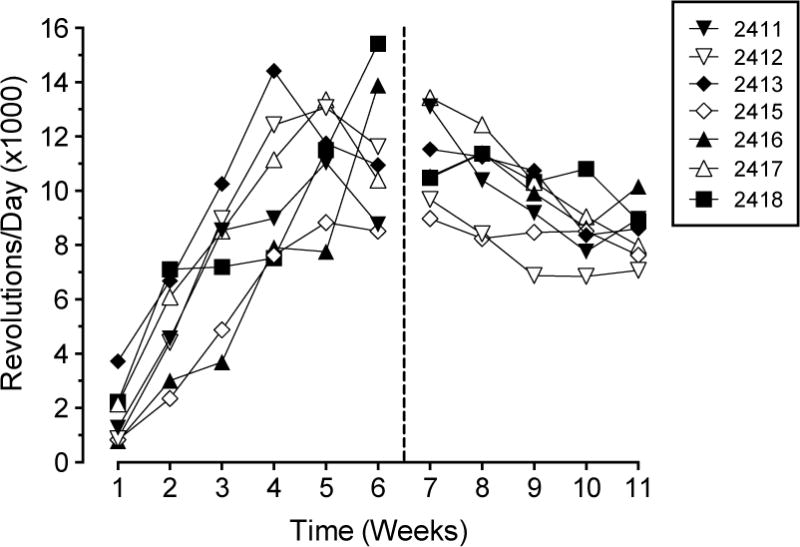
Wheel running in seven physical activity rats. Data depict wheel revolutions/day (×1000) plotted as a function of time (expressed in “weeks” of 5- to 10-day intervals). Reference line after week 6 (vertical broken line extending from abscissa) indicates the beginning of behavioral training.
The percentage of responses allocated to the larger, later reinforcer lever (%LLR) decreased as a function of delay, and this was consistent in both sedentary (Figure 2) and physical activity (Figure 3) rats. The overall ANOVA indicated a significant main effect of group, F4,44 = 5.106, p = .045, delay, F4,44 = 61.986, p < .001, and a group × delay interaction, F4,44 = 3.234, p = .021. The significant interaction indicated an effect of physical activity on impulsive choice that varied as a function of delay. Post-hoc comparisons revealed significant differences at the 0-s and 10-s delays, with a greater percentage LLR responses in the sedentary than physical activity subjects, t11 values > 2.506, p < .05 (Figure 4). Performance at other delays did not significantly differ by group.
Figure 2.
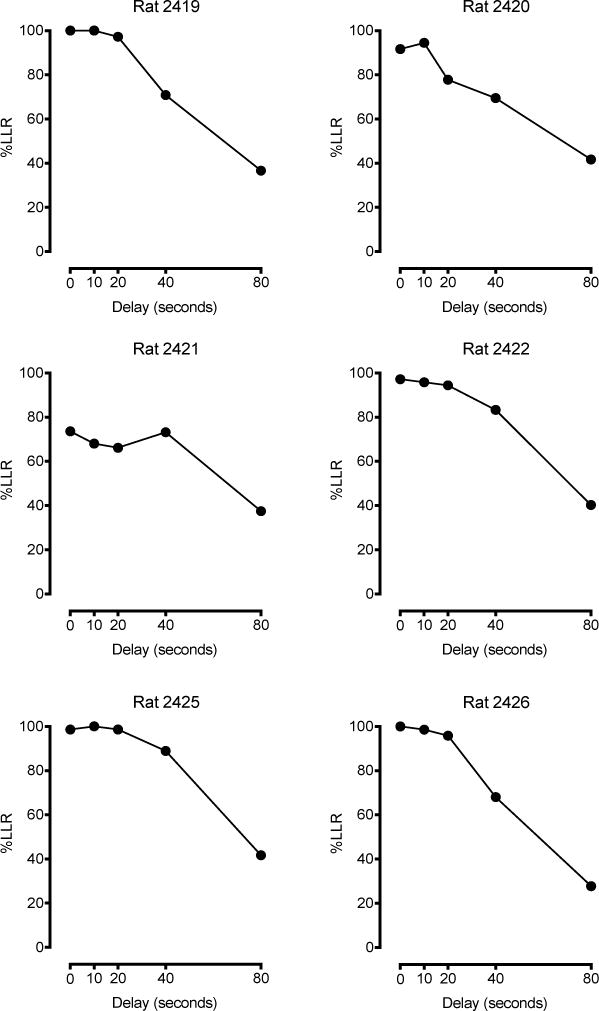
Delay discounting in six sedentary rats. The percentage of responses allocated to the larger, later reinforcer lever (%LLR) are plotted as a function of delay interval (s). All plots depict data averaged across the last 12 consecutive days of testing.
Figure 3.

Delay discounting in seven physical activity rats. The percentage of responses allocated to the larger, later reinforcer lever (%LLR) are plotted as a function of delay interval (s). All plots depict data averaged across the last 12 consecutive days of testing.
Figure 4.
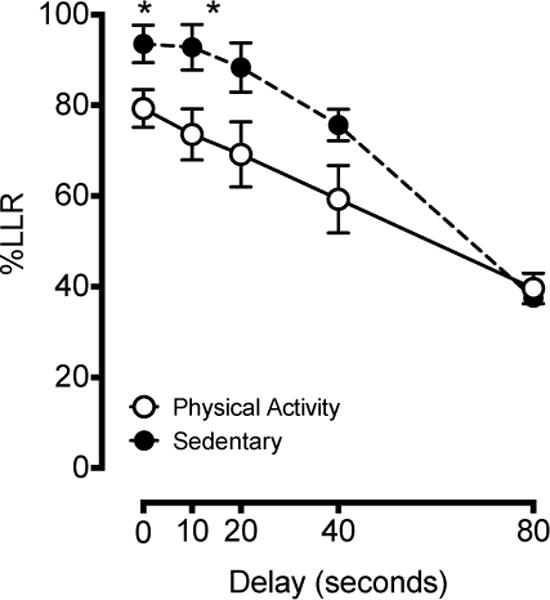
Group delay discounting performance. The percentage of responses allocated to the larger, later reinforcer lever (%LLR) are plotted as a function of delay interval (s). All plots depict data averaged across the last 12 consecutive days of testing. Physical activity rats are represented by closed circles and the dotted line and sedentary rats by open circles and the solid line. Asterisks (*) indicate significant differences between groups as determined by independent-samples t-tests.
Large and consistent differences between the two groups emerged when sensitivity to amount (y-intercept and SA) and sensitivity to delay (slope and SD) were considered separately using a simple regression equation and the logarithmically transformed equation (logarithmically transformed plots in Figures 5 and 6; group data in Table 1). Individual slope, y-intercept, SA, and SD values derived from these data were consistently greater in sedentary than physical activity rats (Figure 7), and these differences were statistically significant, p < .05. A similar effect of slope was observed when the delay data were expressed as a proportion of the 0-s delay (delay performance/0-s delay performance), t11 = 2.23, p = .048. Average daily wheel running was not significantly correlated with intercepts, slopes, SA, or SD values in the physical activity rats, r = .07 to .55, p values > .05. Robust and significant correlations were observed between the two method of analysis on estimates of sensitivity to amount (r2 = .80, p < .001) and sensitivity to delay (r2 = .80 p < .001; Figure 8). Comparison of model fits indicated that the linear regression equation was a good fit for the data (mean R2 = .83). Model fits did not significantly differ between groups for either function (Table 1). Regardless of the method of analysis, physical activity decreased both sensitivity to reinforcement amount and sensitivity to reinforcement delay, which have opposing effects on measures of impulsive choice.
Figure 5.
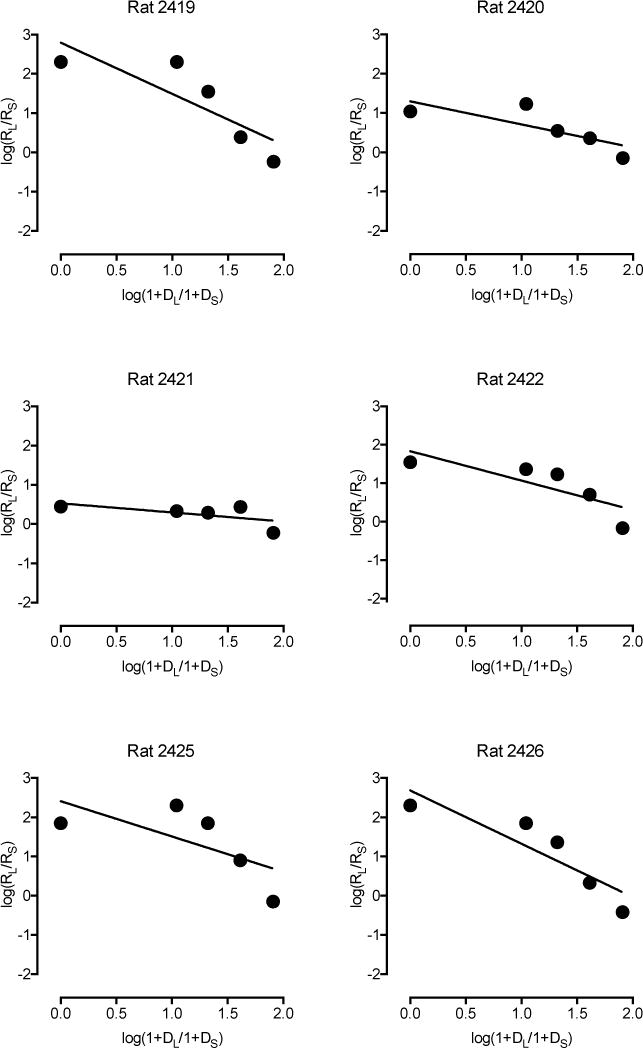
Sensitivity to reinforcement amount and delay in six sedentary rats. Log response ratios are plotted as a function of log delay ratios using a linear equation. All plots depict data averaged across the last 12 consecutive days of testing.
Figure 6.
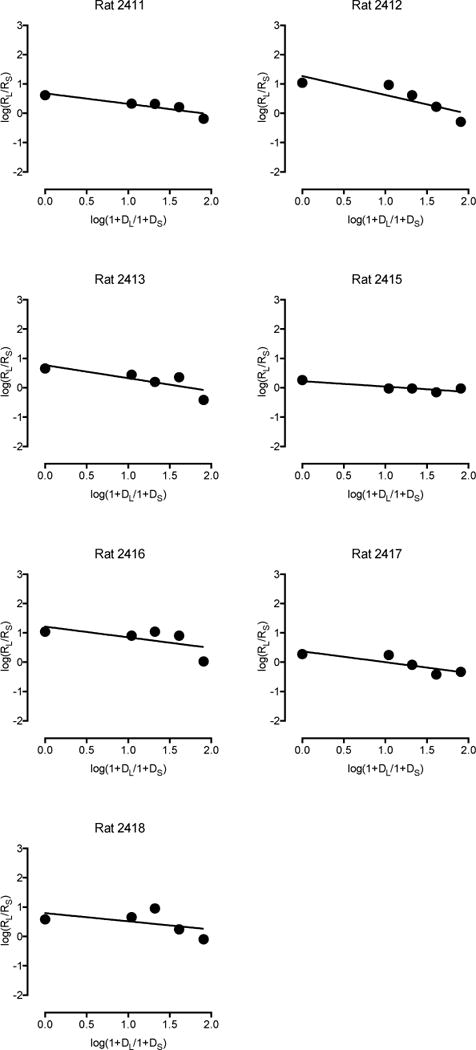
Sensitivity to reinforcement amount and delay in seven physical activity rats. Log response ratios are plotted as a function of log delay ratios using a linear equation. All plots depict data averaged across the last 12 consecutive days of testing.
Table 1.
Sensitivity to Amount and Delay in Physical Activity and Sedentary Subjects
| Physical Activity (n = 7) | Sedentary (n = 6) | |
|---|---|---|
| Linear Function | ||
| Amount (Y-Intercept) | 78.95 (6.19) | 99.41 (5.10)* |
| Delay (-Slope) | 0.49 (0.07) | 0.73 (0.08)* |
| Fit (R2) | 0.76 (0.09) | 0.91 (0.04) |
| Logarithmic Transformation | ||
| Amount (SA) | 1.59 (0.31) | 4.03 (0.76)* |
| Delay (SD) | 0.38 (0.05) | 0.89 (0.18)* |
| Fit (R2) | 0.62 (0.08) | 0.60 (0.06) |
Note. All values represent mean (standard error of the mean).
Group difference p < .05.
Figure 7.
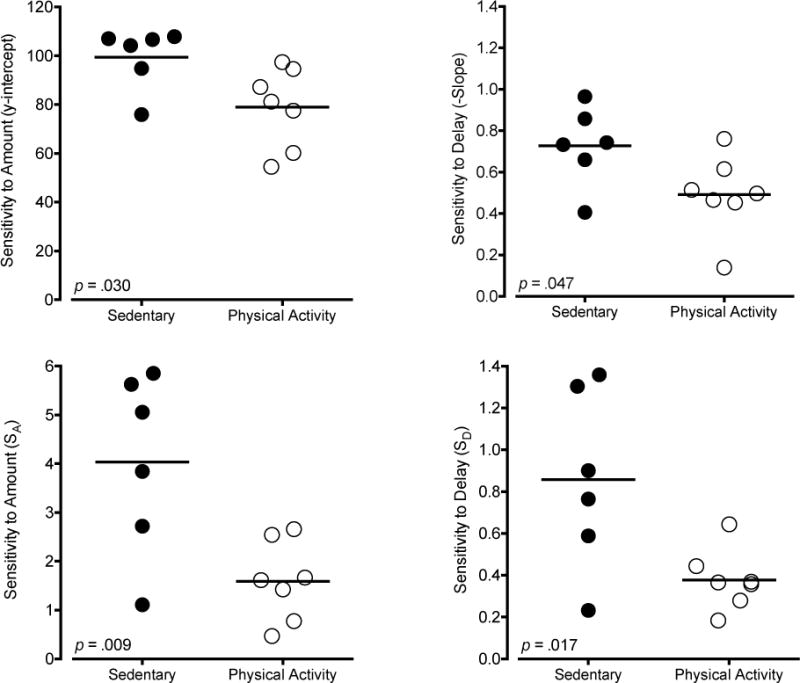
Sensitivity to reinforcement amount (y-intercept: top left panel; SA: bottom left panel) and sensitivity to reinforcement delay (slope: top right panel; SD: bottom right panel). Data are shown for six sedentary rats (filled symbols) and seven physical activity rats (open symbols). Solid horizontal lines indicate group averages. Slope and y-intercept were derived from simple regression equation with %LLR as the criterion and delay interval (s) as the predictor. SA and SD values were derived from logarithmically transformed values as depicted in Figures 5 and 6. All values reflect the average from the last 12 consecutive days of testing.
Figure 8.
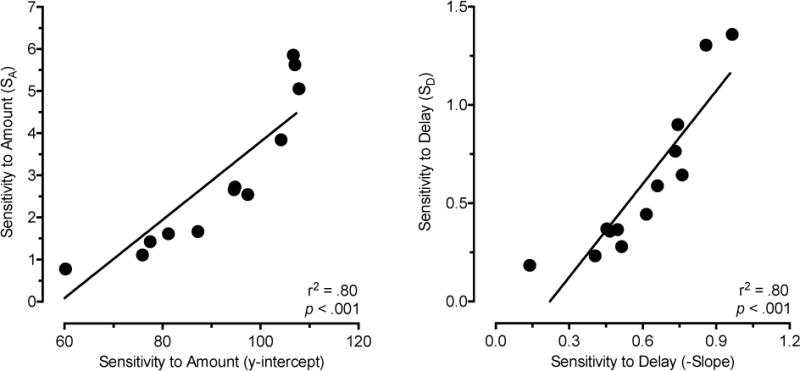
Correlation between measures of sensitivity to reinforcement amount and between measures of sensitivity to reinforcement delay. Correlations represent comparisons of simple linear regression (x-axis) and logarithmically transformed equation (y-axis) estimates. The left panel plots the correlation between simple regression y-intercept and logarithmically transformed equation SA values (sensitivity to reinforcement amount). The right panel indicates correlation between simple regression slope and logarithmically transformed equation SD values (sensitivity to reinforcement delay).
Measures of sensitivity to amount and sensitivity to delay were highly reliable across sessions. The direction and magnitude of the effects of physical activity on y-intercept, slope, SA values, and SD values were similar whether the data were analyzed over the last two, six, or twelve consecutive days of testing (Figure 9). Analyses conducted using a more stringent exclusion criteria (i.e., failure to choose the LLR lever over the SSR lever on greater than 75% of trials at a 0-s delay) did not qualitatively change study outcomes (data not shown).
Figure 9.
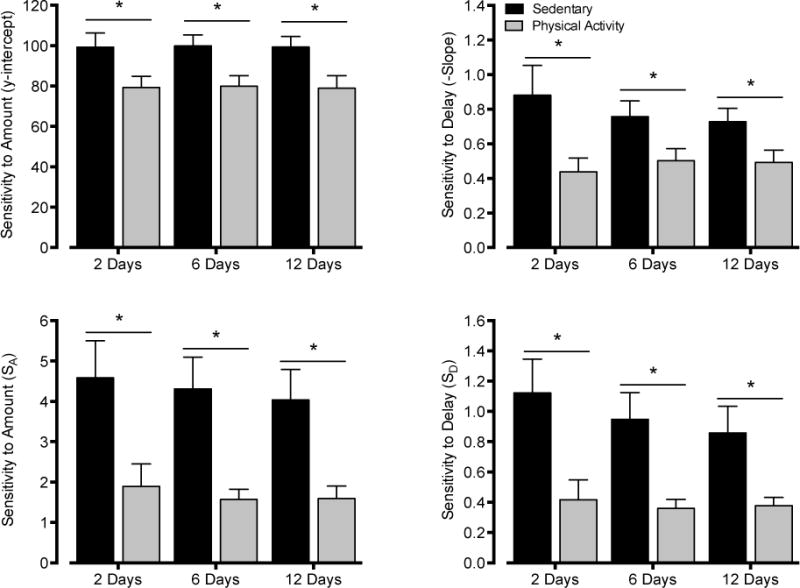
Sensitivity to reinforcement amount (y-intercept: top left panel; SA: bottom left panel) and sensitivity to reinforcement delay (slope: top right panel; SD: bottom right panel) in sedentary (black bars) and physical activity (gray bars) rats. Data reflect averages from the final two, six, and twelve consecutive days of testing. Asterisks (*) indicate significant differences between groups as determined by independent-samples t-tests.
4. Discussion
The first aim of this study was to examine the effects of physical activity on impulsive choice using a delay-discounting procedure. Our primary analysis revealed a significant group (physical activity vs. sedentary) by delay interaction. In other words, physical activity significantly influenced impulsive choice, but this effect varied across the delays tested. The nature of this interaction was revealed by further quantitative analysis, showing that physical activity reduced both sensitivity to reinforcement amount and sensitivity to reinforcement delay. These effects were robust, statistically significant, and consistent across (at least) 12 consecutive days of testing. These findings cannot be attributed to the effects of physical activity on the rate or probability of responding, given that rats in the present study received an average of 99% of the available reinforcers, regardless of group assignment.
Physical activity reduced sensitivity to reinforcement amount (y-intercept, SA), meaning that the difference in magnitude between one and three food pellets had less influence on the response allocation of physical activity rats than sedentary control rats. Importantly, differences on this measure do not reflect differences in sensitivity to reinforcement in general, differences in sensitivity to any establishing operation, or differences in the motor ability to perform a response. Indeed, most studies have reported physical activity increases responding maintained by food. For instance, free access to running wheels for 21 hours/day in the home cage (Smith and Witte, 2012) or for 1 hour/day immediately prior to each experimental session (Belke, 2006) increased responding maintained by sucrose on progressive ratio and fixed interval schedules of reinforcement (but see McMaster and Carney, 1985). In delay-discounting procedures, reductions in sensitivity to amount shift responding away from the larger reinforcer to the smaller reinforcer in the absence of a delay, thereby decreasing the y-intercept of the delay-discounting function and functionally increasing impulsive choice.
Physical activity reduced sensitivity to reinforcement delay (slope, SD), meaning that the delay to reinforcement imposed on the LLR lever had less influence on the response allocation of physical activity rats than of sedentary control rats. A number of controlling variables may mediate the effects of physical activity on sensitivity to delay, including effects on conditioned reinforcement and timing. In the present study, all delay intervals were associated with a consistent set of visual stimuli (houselight on; stimulus light off; lever retracted), and physical activity may have increased the reinforcing strength of these stimuli because of their association with the larger, later reinforcer. However, these same visual stimuli were also present during the blackout period that separated each reinforcer delivery from the next trial, which lasted as long as 100 seconds under the 0-second delay condition. Consequently, it is unlikely that considerable reinforcing strength was conferred to these stimuli given their weak association with reinforcement delivery. Alternatively, physical activity may influence timing, which may be defined in this context as a temporal discrimination between the delay intervals. Physical activity may have caused an underestimation of the elapsed time between the response and reinforcer, resulting in a response allocation at longer delays that are more typical of shorter delays (i.e., greater response allocation to the LLR lever and a decrease in impulsive choice). Arguing against this possibility, human participants overestimated the length of temporal intervals during a moderate exercise condition compared to a resting control condition (Lambourne, 2012); however, that effect was only apparent during an acute bout of exercise and involved a time scale on the order of milliseconds. We know of no other studies that have explicitly examined the effects of physical activity on timing, so possible mechanisms by which physical activity may alter temporal discriminations remain speculative.
The second aim of this study was to compare two quantitative methods of analysis to determine if they would yield similar estimates of sensitivity to amount and sensitivity to delay. Koffarnus and Woods (2013) used a simple linear analysis to determine these estimates in a single-response, discrete-trial procedure of delay discounting. They reported that a linear analysis was as predictive as a hyperbolic equation (first described by Mazur, 1987) at predicting demand for cocaine, and that statistical conclusions from the two methods were not appreciatively different. We compared this linear analysis to the analysis of logarithmically transformed response ratios described by Pitts (2014). Variations of this equation have been used in a number of studies using concurrent-chains choice procedures to separate sensitivity to reinforcement amount from sensitivity to reinforcement delay (e.g., Maguire et al., 2009; Pope et al., 2015; Ta et al., 2008). This equation is based on the general matching law and hyperbolic discounting, and thus has inherent advantages when examining determinants of choice behavior. The caveat of this equation is that raw data must be converted to response ratios, which is commonly done in concurrent-chains procedures because behavior is free to oscillate between multiple response alternatives. In contrast, behavior in a discrete-trial procedure does not have the same degree of flexibility; consequently, the mechanisms underlying response allocation in discrete-trial procedures may not be identical to those mediating response allocation in free-operant procedures. Because this equation had not previously been applied to discrete-trial data, we chose to compare it to the simple linear regression equation to determine if it would yield similar estimates of sensitivity to amount and sensitivity to delay.
The two quantitative methods of analysis yielded remarkably consistent estimates of sensitivity to reinforcement amount (y-intercept, SA) and sensitivity to reinforcement delay (slope, SD). A correlational analysis comparing these methods revealed relationships that were robust, positive, linear, and statistically significant. Moreover, the effects of physical activity on sensitivity to amount and sensitivity to delay were the same regardless of the method of analysis used. Both methods revealed that physical activity reduced sensitivity to amount and sensitivity to delay, and that these effects were consistent whether data from the final 2, 6, or 12 days were considered. These data suggest that the two methods of analysis are measuring the same underlying processes in discounting procedures, and that both methods of analysis are appropriate for use in single-response, discrete-trial procedures.
The procedures and analyses used in this investigation were selected because of their ability to provide insight on the mechanisms mediating the effects of physical activity on impulsive choice. Despite their utility, several limitations must be acknowledged. First, only females were examined and we do not know whether similar findings would be obtained in males. Females were chosen because they run more than males (Eikelboom and Mills, 1988; Smith et al., 2011; 2012) and because of their general underrepresentation in preclinical research (Clayton and Collins, 2014; Klein et al., 2015). Preclinical studies examining sex differences in impulsive choice have produced equivocal results, with studies reporting greater impulsive choice in males (Bayless et al., 2013), greater impulsive choice in females (Koot et al., 2009; Perry et al., 2007), and no sex differences (Perry et al., 2008a). We do not know of any data that speak directly to sex differences in sensitivity to reinforcer amount and delay. Second, we used an ascending delay interval, progressively increasing the interval from 0 to 80 s, and we do not know if our findings extend to other variations of delay. Previous studies have reported that the effects of many pharmacological interventions (e.g., amphetamine, methylphenidate, yohimbine) depend on the order of delay presentation (Maguire et al., 2014; Schwager et al., 2014; Tanno et al., 2014). Third, we cannot rule out the possible role of environmental enrichment. Environmental enrichment, which typically includes social contact, novelty, and physical activity, decreases measures of impulsive choice (Perry et al., 2008b; Kirkpatrick et al., 2013; 2014, but see Hellemans et al., 2005). It is possible that the effects of physical activity in the present study are due to an enrichment-related effect; however, it is also possible that the effects of environmental enrichment reported in previous studies may be due to the physical activity component of the manipulation. Finally, although several outcomes from the current study demonstrate the effects of amount and delay in isolation (e.g., standardizing by the 0 s delay), a potential limitation is that we cannot unequivocally rule out the potential for an interaction between amount and delay (whether simple multiplicative or more complex). Several investigators have explored such interactions between amount and delay, and their conclusions have varied from study to study (e.g., Beeby and White, 2013; Green and Snyderman, 1980).
Despite these limitations, the methods employed in this investigation do offer a number of distinct advantages. For instance, delay-discounting procedures offer an “apples-to-apples” comparison for relevant experimental manipulations by allowing one factor to be held constant (e.g., reinforcement amount) as another factor is systematically varied (e.g., reinforcement delay). Discrete-trial procedures also allow choice behavior to be examined in the absence of rate-dependent effects, which can often confound measures of choice in free-operant procedures (Pitts and Febbo, 2004). Finally, these procedures are relatively insensitive to manipulations that have direct effects on motor behavior, which can also confound measures collected under free-operant conditions (Ho et al., 1999).
Impulsive choice is a diagnostic feature of ADHD and is a complicating factor in substance use and binge eating disorders (Bickel et al., 2012; Patros et al., 2016). The question remains whether exercise and other forms of physical activity are beneficial in clinical populations, at least in regard to their effects on impulsive choice (the effects of exercise on other features of psychological disorders – such as depression, anxiety, and cognitive impairment – are less equivocal and are clearly beneficial). The present data offer mixed support for this possibility – although exercise would limit the discounting of a larger, later reinforcer, it would also limit the value placed on the larger reinforcer. Perhaps more importantly, the present findings emphasize that psychological constructs such as impulsivitiy rarely reflect unitary behavioral phenomena. Impulsivity and other behavioral “traits” are often used as substitutes for explanatory descriptions of empirical observations, a practice that ultimately limits our ability to understand and predict behavior. Fortunately, quantitative methods of analysis are now available that allow investigators to examine the behavioral processes that give rise to these constructs. In this study, we present evidence that two of these quantitative methods provide reliable and complementary descriptions of the behavioral processes contributing to impulsive choice. As such, these data may be taken as converging evidence for their utility in examining the determinants of choice in single-response, discrete-trial procedures.
Highlights.
We examined the effects of physical activity on impulsive choice
Sensitivity to amount and sensitivity to delay were determined using two methods
Physical activity decreased sensitivity to amount and sensitivity to delay
Both quantitative methods revealed consistent results for discrete-trial data
Acknowledgments
Role of Funding Sources
This work was supported by the National Institutes of Health (NIDA Grants R01DA031725 and R01DA027485 to MAS) and the Duke Endowment. These funding sources had no role in study design, data collection or analysis, or preparation and submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no financial conflicts of interest in regard to this research.
References
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2010;21:754–64. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Darling JS, Daniel JM. Mechanisms by which neonatal testosterone exposure mediates sex differences in impulsivity in prepubertal rats. Horm Behav. 2013;64:764–9. doi: 10.1016/j.yhbeh.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Beeby E, White KG. Preference reversal between impulsive and self-control choice. J Exp Anal Behav. 2013;99:260–76. doi: 10.1002/jeab.23. [DOI] [PubMed] [Google Scholar]
- Belke TW. Responding for sucrose and wheel-running reinforcement: effect of pre-running. Behav Processes. 2006;71:1–7. doi: 10.1016/j.beproc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–97. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuroeconomic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–27. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–30. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Green L, Snyderman M. Choice between rewards differing in amount and delay: toward a choice model of self control. J Exp Anal Behav. 1980;34:135–47. doi: 10.1901/jeab.1980.34-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–20. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Ho MY, Mobini S, Chiang TJ, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology (Berl) 1999;146:362–72. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research. Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Kirkpatrick K, Marshall AT, Clarke J, Cain ME. Environmental rearing effects of impulsivity and reward sensitivity. Behav Neurosci. 2013;127:712–24. doi: 10.1037/a0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Marshall AT, Smith AP, Koci J, Park Y. Individual differences in impulsive and risky choice: effects of environmental rearing conditions. Behav Brain Res. 2014;269:115–27. doi: 10.1016/j.bbr.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Scheibinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I. Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acac Sci. 2015;112:5257–8. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99:32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addict Biol. 2013;18:8–18. doi: 10.1111/j.1369-1600.2011.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot S, van den Bos R, Adriani W, Laviola G. Gender differences in delay discounting under mild food restriction. Behav Brain Res. 2009;200:134–43. doi: 10.1016/j.bbr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Lambourne K. The effects of acute exercise on temporal generalization. Q J Exp Psychol. 2012;65:526–40. doi: 10.1080/17470218.2011.605959. [DOI] [PubMed] [Google Scholar]
- Locey ML, Dallery J. Isolating behavioral mechanisms of intertemporal choice: nicotine effects on delay discounting and amount sensitivity. J Exp Anal Behav. 2009;91:213–23. doi: 10.1901/jeab.2009.91-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology. 2014;87:173–9. doi: 10.1016/j.neuropharm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Rodewald AM, Hughes CE, Pitts RC. Rapid acquisition of preference in concurrent schedules: effects of d-amphetamine on sensitivity to reinforcement amount. Behav Processes. 2009;81:238–43. doi: 10.1016/j.beproc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior: The Effect of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McMaster SB, Carney JM. Exercise-induced changes in schedule controlled behavior. Physiol Behav. 1985;35:337–41. doi: 10.1016/0031-9384(85)90305-1. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160:290–8. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev. 2016;43:162–74. doi: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–37. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008a;16:165–77. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008b;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RC. Reconsidering the concept of behavioral mechanisms of drug action. J Exp Anal Behav. 2014;101:422–41. doi: 10.1002/jeab.80. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Febbo SM. Quantitative analyses of methamphetamine’s effects on self-control choices: implications for elucidating behavioral mechanisms of drug action. Behav Processes. 2004;66:213–33. doi: 10.1016/j.beproc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Pope DA, Newland MC, Hutsell BA. Delay-specific stimuli and genotype interact to determine temporal discounting in a rapid-acquisition procedure. J Exp Anal Behav. 2015;103:450–71. doi: 10.1002/jeab.148. [DOI] [PubMed] [Google Scholar]
- Schwager AL, Haack AK, Taha SA. Impaired flexibility in decision making in rats after administration of the pharmacological stressor yohimbine. Psychopharmacology. 2014;231:3941–52. doi: 10.1007/s00213-014-3529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology. 2011;218:357–69. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: evidence from preclinical studies. Front Psychiatry. 2012;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20:437–46. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta WM, Pitts RC, Hughes CE, McLean AP, Grace RC. Rapid acquisition of preference in concurrent chains: effects of d-amphetamine on sensitivity to reinforcement delay. J Exp Anal Behav. 2008;89:71–91. doi: 10.1901/jeab.2008.89-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology. 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Vanderlinden J, De Hert M, Adamkova M, Skjaerven LH, Catalan-Matamoros D, Lundvik-Gyllensten A, Gomez-Conesa A, Ijntema R, Probst M. A systematic review on physical therapy interventions for patients with binge eating disorder. Disabil Rehabil. 2013;35:2191–6. doi: 10.3109/09638288.2013.771707. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Emmerson N, Gehricke JG, Galassetti P. Exercise: applications to childhood ADHD. J Atten Disord. 2013;17:279–90. doi: 10.1177/1087054712454192. [DOI] [PubMed] [Google Scholar]


