Abstract
Regulatory T cells (Treg) play an important role in modulating the immune response and has attracted increasing attention in diverse fields such as cancer treatment, transplantation and autoimmune diseases. CC chemokine receptor 4 (CCR4) is expressed on the majority of Tregs, especially on effector Tregs. Recently we have developed a diphtheria‐toxin based anti‐human CCR4 immunotoxin for depleting CCR4+ cells in vivo. In this study, we demonstrated that the anti‐human CCR4 immunotoxin bound and depleted monkey CCR4+ cells in vitro. We also demonstrated that the immunotoxin bound to the CCR4+Foxp3+ monkey Tregs in vitro. In vivo studies performed in two naive cynomolgus monkeys revealed 78–89% CCR4+Foxp3+ Treg depletion in peripheral blood lasting approximately 10 days. In lymph nodes, 89–96% CCR4+Foxp3+ Tregs were depleted. No effect was observed in other cell populations including CD8+ T cells, other CD4+ T cells, B cells and NK cells. To our knowledge, this is the first agent that effectively depleted non‐human primate (NHP) Tregs. This immunotoxin has potential to deplete effector Tregs for combined cancer treatment.
Keywords: NHP Treg, CCR4, Immunotoxin, Diphtheria toxin
Highlights
CCR4 immunotoxin bound and depleted monkey CCR4+ cells in vitro.
CCR4 immunotoxin bound to monkey CCR4+Foxp3+ Tregs in vitro.
CCR4 immunotoxin effectively depleted monkey CCR4+Foxp3+ Tregs in vivo.
CCR4 immunotoxin has potential to deplete effector Tregs for cancer treatment.
1. Introduction
Treg constitute a major inhibitory cell population capable of suppressing the immune responses. Scientists and clinicians are seeking an effective in vivo Treg depleting agent to facilitate cancer treatment and vaccination (Li et al., 2010; Klages et al., 2010; Teng et al., 2010; Bos et al., 2013). CCR4 was found to be highly expressed on FoxP3hi CD45RA− effector‐type Treg, whereas naïve Foxp3lo CD45RA+ Treg, CD8+ T cells, NK cells, CD14+ monocytes/macrophages, dendritic cells and B cells had barely detectable levels of CCR4 expression at both the mRNA and protein level (Sugiyama et al., 2013). We hypothesized that targeting Treg through CCR4 would result in depletion of effector Tregs. Recently, we have developed a novel diphtheria toxin‐based anti‐human CCR4 immunotoxin using unique diphtheria toxin‐resistant yeast Pichia Pastoris expression system (Wang et al., 2015). In vitro potency characterization using human CCR4+ CCRF‐CEM leukemia cell line and in vivo efficacy study using human CCR4+ tumor bearing NSG mouse model identified the foldback diabody anti‐human CCR4 immunotoxin as the most potent isoform (Wang et al., 2015). In this study, we used non‐human primate to assess the Treg depletion function of the anti‐human CCR4 immunotoxin. We first demonstrated that the CCR4 immunotoxins bound and depleted monkey CCR4+ cells in vitro. We also demonstrated that the CCR4 immunotoxin bound to monkey CCR4+Foxp3+ Tregs in vitro. We then demonstrated that the immunotoxin effectively depleted monkey CCR4+Foxp3+ Tregs in vivo.
2. Materials and methods
2.1. Antibodies and immunotoxins
FITC‐anti‐human CD3ɛ mAb (clone# SP34‐2, cat# 556611), PE‐anti‐human CD3ɛ mAb (clone#SP‐34‐2, cat# 556612), PerCp‐anti‐human CD4 mAb (clone# L200, cat# 550631), PerCp‐mouse IgG1 κ (clone# MOPC‐21, cat# 559425), APC‐anti‐human CD4 mAb (clone# L200, cat# 551980), APC‐mouse IgG1 κ (clone# MOPC‐21, cat# 550854), APC‐anti‐human CD25 mAb (clone# M‐A251, cat# 561399) and FITC‐anti‐human CD45RA (clone# 5H9, cat# 556626) were purchased from BD. APC‐anti‐human CD8 mAb (clone# RPA‐T8, cat# 301014), PE‐CD20 (clone# 2H7, cat#302306), Biotin‐anti‐human CD16 mAb (clone# 3G8, cat# 302004), PE‐CD16 mAb (clone# 3G8, cat# 302008), FITC‐anti‐human CD14 mAb (clone# M5E2, cat# 301803), PerCp‐Cy5.5‐anti‐human CD11b (clone# M1/70, cat# 101228), PE‐anti‐human CD194 (CCR4) mAb (Clone# L291H4, cat# 359412), PE‐mouse IgG1, κ (clone# MOPC‐21, cat# 400139), Alexa Fluor® 647 anti‐human Foxp3 mAb (clone# 150D, cat# 320014), Alex Fluor 647 Mouse IgG1 κ (clone# MOPC‐21, cat# 400136), PE‐streptavidin (cat# 405204) and APC‐streptavidin (cat# 405207) were purchased from Biolegend. Human/rat CCR4 fluorescein mAb (clone 205410, cat# FAB1567F), human/rat CCR4 PE mAb (clone 205410, cat# FAB1567P) and mouse IgG2B fluorescein isotype control (clone# 133303, cat# IC0041F) were purchased from R&D Systems. Propidium Iodide (cat# 81845) was purchased from Sigma. The monovalent, bivalent and single‐chain foldback diabody anti‐human CCR4 immunotoxins were produced in our lab using unique diphtheria toxin resistant yeast Pichia Pastoris expression system (Wang et al., 2015) and biotinylated as previously described (Wang et al., 2015).
2.2. In vivo monkey Treg depletion
Two male cynomolgus monkeys (M1815: 5.1 kg, M1915: 5.3 kg) were maintained in Massachusetts General Hospital (MGH) non‐human primate facility. MGH is an AAALAC accredited institute. All experiments were conducted with the approved MGH IACUC protocol (2012N000134). The foldback diabody anti‐human CCR4 immunotoxin was IV bolus injected at 25 μg/kg, BID for four consecutive days, 6 h apart. 2–3 mL of saline was injected before and after the immunotoxin injection. Sedation was performed for the immunotoxin injection and blood collection. The blood was collected daily for flow cytometry analysis in the first week and twice weekly thereafter. The animals were closely monitored twice daily during the immunotoxin injection and once daily after the immunotoxin treatment for any adverse effects. Clinical assessments for adverse events include daily clinical observation, complete blood counts and serum chemistries. The animals were weighed weekly.
Treg and other cell populations in peripheral blood were measured 3 times before the immunotoxin administration to obtain an accurate baseline. Following immunotoxin administration, peripheral blood flow cytometry were performed daily for the first week and twice weekly thereafter to monitor the effect of the immunotoxin treatment on all peripheral blood cell populations including T cells, B cells, NK cells, and monocytes. A combination of CD4, CCR4, CD45RA, Foxp3 were used to monitor the Treg populations (CCR4+ cell: CD4+CCR4+; CCR4+ Treg: CCR4+Foxp3+ among the gated CD4+ cells, Effector‐type Treg: CD45RA−Foxp3+ among the gated CD4+ cells). The off‐target deletion on other cell lineages was monitored by flow cytometry using antibodies against CD3, CD4, CD8, CD20, CD16, CD14 and CD11b (CD4+ T cell: CD3+CD4+; CD8+ T cell: CD3+CD8+; CD20+ B cell: CD3−CD20+; NK cell: CD16+CD8+; Monocyte: CD14+CD11b+).
Lymph node biopsies were performed prior to the immunotoxin injection on day ‐7 and after the immunotoxin administration on day 4. Treg depletion in the lymph node was monitored by flow cytometry using antibodies against CD4, CCR4, CD45RA and Foxp3 (CCR4+ cell: CD4+CCR4+, CCR4+ Treg: CCR4+Foxp3+ among the gated CD4+ cells, Effector‐type Treg: CD45RA−Foxp3+ among the gated CD4+ cells). The off‐target depletion in the lymph node was monitored by flow cytometry using antibodies against CD3, CD4, CD8 and CD20 (CD4 T cell: CD3+CD4+, CD8 T cell: CD3+CD8+, B cell: CD3−CD20+).
2.3. Monkey PBMC isolation (small volume blood collection maximal of 2 mL)
Monkey PBMC isolation was performed following BL‐2 rules. 7 mL of washing buffer (1% FBS in PBS, sterile with 0.22 μM filter) was added to a 15 mL conical tube. 1–2 mL of monkey blood was added to the prepared 7 mL of washing buffer to a total of 9 mL and mixed by inverting the tube gently. 5 mL of Histopaque‐1077 (Sigma, cat# H8889) was added to the bottom of a new 15 mL conical tube. The blood/washing buffer mixture was slowly overlaid on Histopaque‐1077. The tube was centrifuged at 2600 rpm for 30 min with the brake off. The buffy layer was transferred to a new 15 mL conical tube and washing buffer was added to a total of 15 mL. The tube was centrifuged at 1500 rpm for 10 min with the brake on. The supernatant was decanted and the cells were loosened gently by taping the tube wall. 4.5 mL of pure water (HyClone cell culture grade pure water, Thermo, cat# SH30529.03) was added to the cells and mixed by pipetting up and down. 0.5 mL of 10× DPBS (Cellgro, cat# 20‐031‐CV) was immediately (within 10 s) added and mixed by inverting. The tube was centrifuged at 1500 rpm for 10 min with the brake on. The supernatant was decanted and the remaining supernatant was carefully removed with pipette. The pellet was loosened by tapping the tube wall. 3–5 mL of the washing buffer was added and filtered with a 40 μM cell strainer (Corning, cat# 431750). The cells were counted with trypan blue. Monkey PBMC isolation from big volume blood, in vitro binding and depletion analysis of the anti‐human CCR4 immunotoxins to human CCR4 on monkey PBMC using flow cytometry was performed as previously described (Wang et al., 2015).
2.4. Monkey whole blood flow cytometry analysis
The monkey whole blood staining for flow cytometry analysis was performed following BL‐2 rules. 100 μL of the heparinized monkey blood was added into each flow cytometry tube. 2 mL of the FACS buffer (1× Hanks Balanced Salt Solution with Ca+ and Mg+, 0.1% Bovine serum albumin and 0.1% sodium azide) was added into the tube and mixed by vortex. The tube was spun down at 1200 rpm for 5 min in room temperature. The supernatant was discarded gently and washed once more using the FACS buffer as above. 10 μL of the conjugated antibody was added to the tube and mixed by gentle vortex. The tube was covered with tin foil and incubated at 4 °C for 30 min. The cells were washed twice with 2 mL of the FACS buffer at 1200 rpm for 5 min in room temperature. The supernatant was discarded and suspended by ratcheting. 2 mL of 1xBD FACS lysing buffer (BD BioSciences, cat# 349202) was added into the tube and capped. The tube was mixed by vortex and incubated for 15 min. The tube was mixed by vortex and spun down at 1200 rpm for 5 min in room temperature. The cells were washed twice again with the FACS buffer. 400 μL of the FACS buffer was added into the tube and stored at 4 °C in the dark until running on the flow cytometry machine.
2.5. Foxp3 flow cytometry analysis for monkey PBMC
Monkey PBMC was re‐suspended at 1 × 107 cells/mL in cold FACS buffer (1× Hanks Balanced Salt Solution with Ca+ and Mg+, 0.1% Bovine serum albumin and 0.1% sodium azide). 100 μL of the cell suspension was aliquoted into each tube (1 × 106 cells). The surface staining was performed as normal surface staining procedure. The cells was washed twice using cold FACS buffer. The cells were spun down and the supernatant was poured off. The pellet was loosened with pulse vortex. 1 mL of fresh 1× fixation/permeabilization working solution [1 volume of 4× fixation/permeabilization concentrate (cat# 5123‐43, eBioscience) with 3 volumes of the fixation/permeabilization diluents (cat# 5223–56, eBioscience)] was added and mixed by pulse vortex. The tube was incubated for 30 min–45 min at 4 °C in the dark. 2 mL of the fresh 1× permeabilization buffer (10× permeabilization buffer, eBioscience, cat# 8333–56) was added into the tube and spun down. The supernatant was poured off and washed once more. The conjugated anti‐Foxp3 mAb or isotype control was added into the tube and incubated at 4 °C in the dark for at least 30 min. The cells were washed twice with fresh 1× permeabilization buffer. 300 μL of FACS buffer was added and stored at 4 °C until running the flow cytometry machine.
3. Results and discussion
Since our immunotoxin was constructed using anti‐human CCR4 scFv (1567) (Parent mAb clone# 205410) (Wang et al., 2015), PE‐anti‐human CD194 (CCR4) mAb (Clone# L291H4, Biolegend) was used for all of the in vitro and in vivo depletion analysis by flow cytometry. In order to assume the in vitro and in vivo depletion data, we have performed the in vitro competition analysis with increasing concentration of the unlabeled foldback diabody anti‐human CCR4 immunotoxin to block the binding of the two anti‐human CCR4 mAbs (clone#205410 and L291H4) to monkey CCR4+ PBMC (Figure 1A). The data demonstrated that the competition of the CCR4 immunotoxin did not affect the frequency detection of the saturating concentration of the two anti‐human CCR4 mAbs (clone#205410 and L291H4) to the monkey CCR4+ PBMC. However the competition affected the binding intensity (mean fluorescence intensity) of the two antibodies (Figure 1A and data not shown). Clone# L291H4 was chosen for the following experiments as it is less competing than clone# 205410. To further confirm the competition data, we have repeated the competition assay using the biotinylated foldback diabody anti‐human CCR4 immunotoxin. As shown in Figure 1B, both biotinylated foldback diabody anti‐human CCR4 immunotoxin and anti‐human CCR4 mAb (clone#205410 or L291H4) double stained to the CCR4+ monkey PBMC, which further confirmed that the competition of the CCR4 immunotoxin did not affect the frequency detection of the saturating concentration of the anti‐human CCR4 mAbs (clone# 205410 or L291H4) to the monkey CCR4+ PBMC.
Figure 1.
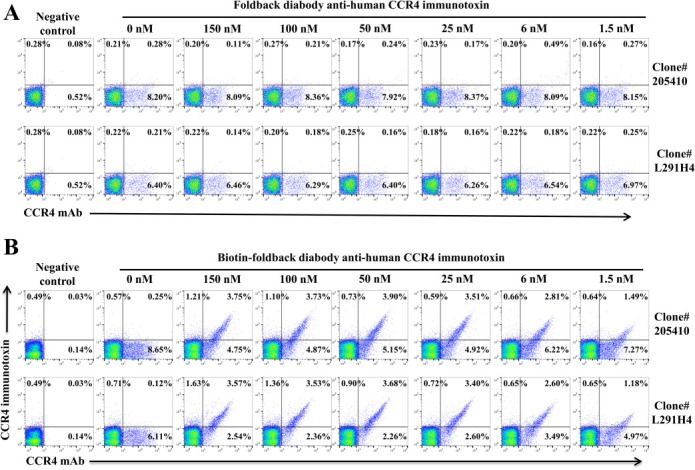
Flow cytometry competition analysis with increasing concentration of the unlabeled (A) or biotinylated (B) foldback diabody anti‐human CCR4 immunotoxins to block the binding of the saturating concentration of the two anti‐human CCR4 mAbs (clone#205410 and L291H4) to the monkey CCR4+ PBMC. Monkey PBMC was incubated with the unlabeled (A) or biotinylated (B) foldback diabody anti‐human immunotoxin at 4 °C for 1 h and washed twice, then stained with the anti‐human CCR4 mAb (A) or the anti‐human CCR4 mAb and APC‐streptavidin (B) at 4 °C for 30 min. The data are representative of two individual experiments.
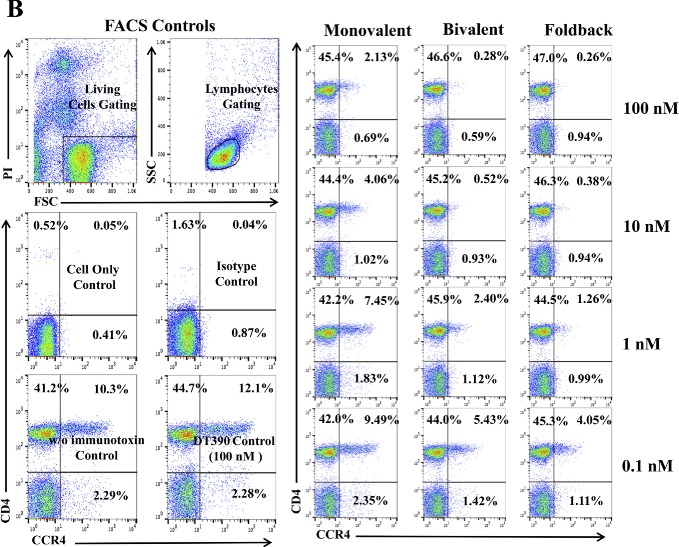
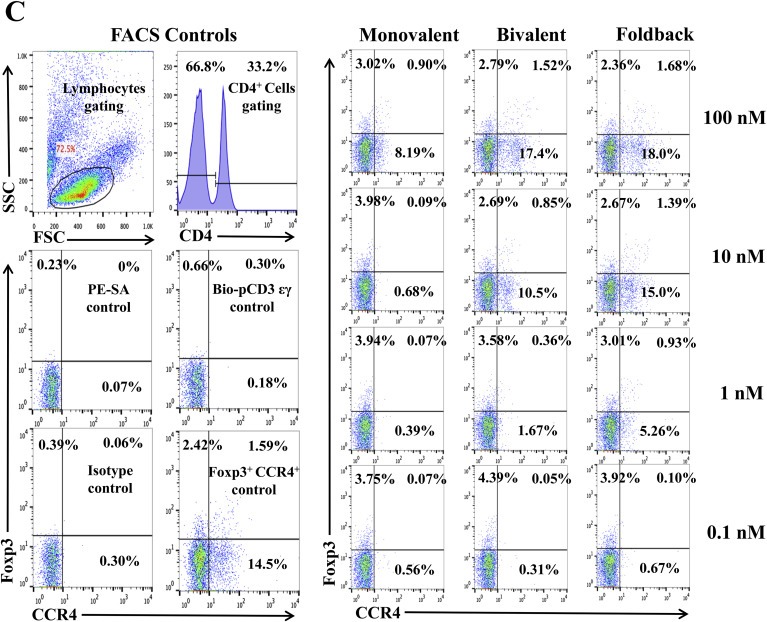
We hypothesized that the anti‐human CCR4 immunotoxin would effectively deplete CCR4+ Tregs in vivo for cancer treatment. Non‐human primate is the most reliable preclinical model to assess the Treg depletion function. To investigate whether these immunotoxins can react cross‐species with NHP CCR4+ PBMC, we first performed in vitro binding and depletion assays. All three versions of the biotinylated anti‐human CCR4 immunotoxins, monovalent, bivalent and foldback diabody, bound to CCR4+ monkey PBMC in a dose‐dependent fashion with the foldback diabody isoform demonstrating the strongest binding affinity, followed by the bivalent isoform and then the monovalent isoform (Figure 2A). These results are consistent with the previous binding analysis performed with human CCR4+ CCRF‐CEM leukemia cell line and human PBMC (Wang et al., 2015). In vitro depletion assays also demonstrated a dose dependent depletion of CCR4+ monkey PBMC with the foldback diabody version showing the most efficacy (Figure 2B). Next, the binding affinity of the immunotoxins to CCR4+Foxp3+ Tregs in monkey PBMC was assessed. The immunotoxins bound to the CCR4+Foxp3+ Tregs within monkey PBMC in a dose dependent manner, and again with the foldback diabody isoform showing stronger binding compared to the other two isoforms (Figure 2C). These in vitro data prompted us to test in vivo Treg depletion function of the immunotoxins in nonhuman primates.
Figure 2.
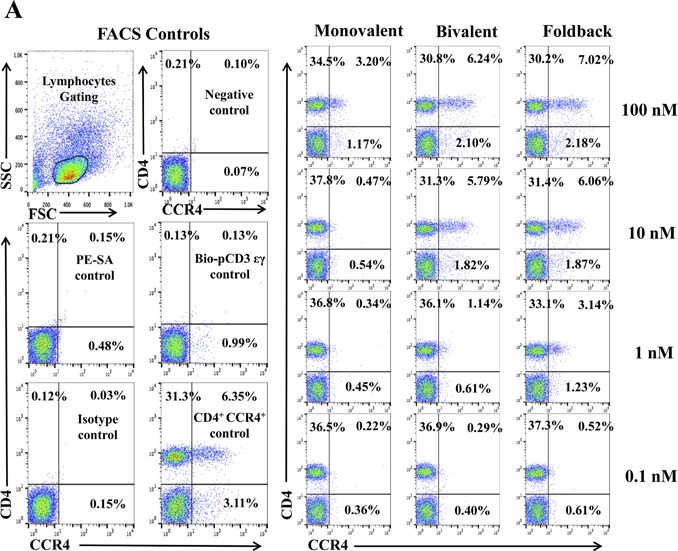
In vitro binding and depletion analysis of the anti‐human CCR4 immunotoxins to monkey CCR4+ PBMC. (A) Flow cytometry binding analysis of the anti‐human CCR4 immunotoxins to CCR4+ cells within monkey PBMC. Monkey PBMC was stained with the biotinylated anti‐human CCR4 immunotoxin as primary staining and PE‐conjugated streptavidin as secondary staining. First panel: monovalent anti‐human CCR4 immunotoxin [DT390‐scFv (1567)]; second panel: bivalent anti‐human CCR4 immunotoxin [DT390‐BiscFv (1567)]; third panel: single‐chain foldback diabody anti‐human CCR4 immunotoxin. Monkey PBMC with only the secondary staining (PE‐conjugated streptavidin) served as the negative control and human CCR4 fluorescein mAb (clone#205410) as the CCR4 positive control, mouse IgG2B fluorescein for the isotype control of human CCR4 fluorescein mAb (clone#205410), PerCp anti‐human CD4 mAb (clone# L200) as CD4 positive control, PerCp mouse IgG1 κ (clone# MOPC‐21) for the isotype control of PerCp anti‐human CD4 mAb (clone# L200). Biotin‐labeled porcine CD3‐εγ (Peraino et al., 2012) was included as a negative control for background due to protein biotinylation. (B) In vitro depletion of the CCR4+ cells within monkey PBMC using the anti‐human CCR4 immunotoxins. Monkey PBMC was incubated with the unlabeled anti‐human CCR4 immunotoxin at 37 °C for 48 h and analyzed by flow cytometry using PE anti‐human CCR4 mAb (clone# L291H4). First panel: monovalent anti‐human CCR4 immunotoxin [DT390‐scFv (1567)]; second panel: bivalent anti‐human CCR4 immunotoxin [DT390‐BiscFv (1567)]; third panel: single‐chain foldback diabody anti‐human CCR4 immunotoxin. PE anti‐human CCR4 antibody (clone #L291H4) for the CCR4 positive control, PE‐mouse IgG1, κ as isotype control of PE‐anti‐human CCR4 mAb (clone# L291H4), APC anti‐human CD4 mAb (clone# L200) for the CD4 positive control, APC mouse IgG1 κ (clone# MOPC‐21) for the isotype control of APC anti‐human CD4 mAb (clone# L200). DT390 (100 nM) was included as negative depleter control. Propidium iodide was added before running the FACS machine for gating the living cells. All control cells in Figure 2B were also incubated at 37 °C for 48 h. (C) Flow cytometry binding analysis of the anti‐human CCR4 immunotoxins to the Foxp3+CCR4+ monkey PBMC. Monkey PBMC was stained with Alexa Fluor© 647 anti‐human Foxp3 mAb (clone# 150D) and biotinylated anti‐human CCR4 immunotoxin. First panel: monovalent anti‐human CCR4 immunotoxin [DT390‐scFv(1567)]; second panel: bivalent anti‐human CCR4 immunotoxin [DT390‐BiscFv(1567)]; third panel: single‐chain foldback diabody anti‐human CCR4 immunotoxin. Monkey PBMC with only the secondary staining (PE‐conjugated streptavidin) served as the negative control, human CCR4 fluorescein mAb (clone#205410) as the CCR4 positive control, mouse IgG2B fluorescein for the isotype control of human CCR4 fluorescein mAb (clone#205410), Alexa Fluor© 647 anti‐human Foxp3 mAb (clone# 150D) for the Foxp3 positive control, Alex Fluor 647 Mouse IgG1 κ (clone# MOPC‐21) for the isotype control of Alexa Fluor© 647 anti‐human Foxp3 mAb (clone# 150D). Biotin‐labeled porcine CD3‐εγ (Peraino et al., 2012) was included as a negative control for background due to protein biotinylation. All Figure 2C analysis was CD4 gated. All of the data (A‐C) are representative of three individual experiments.
Figure 2.
(Continued).
Figure 2.
(Continued).
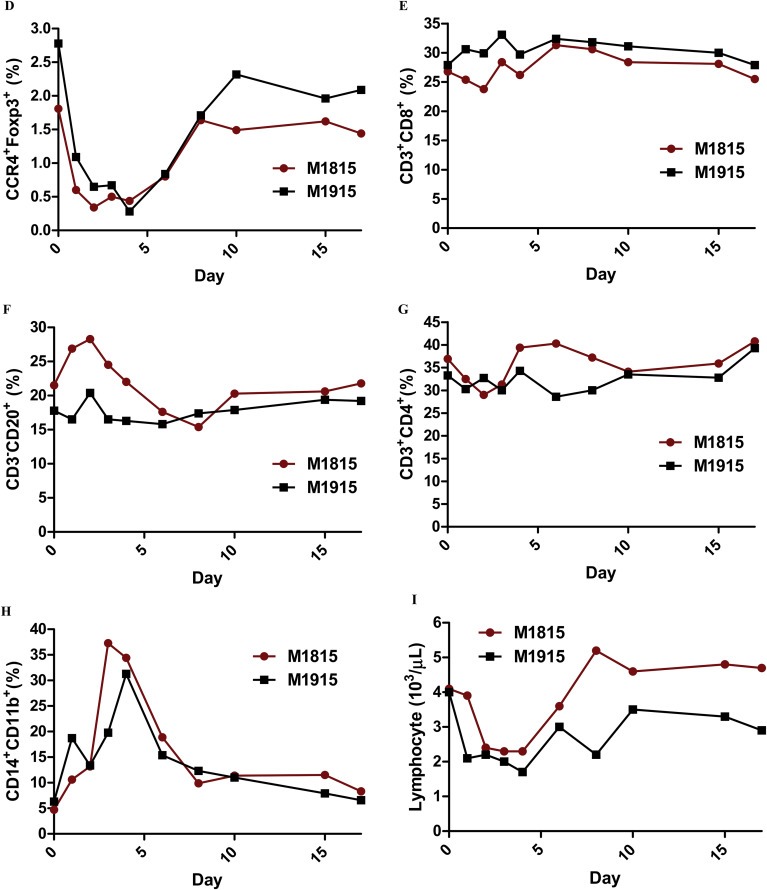
In vivo depletion studies were performed in two naive cynomolgous monkeys. Based on the results of previous experiments (Wang et al., 2015 and Figure 2), the foldback diabody anti‐human CCR4 immunotoxin was chosen for the in vivo monkey Treg depletion experiments. The anti‐human CCR4 immunotoxin was administered intravenously at a dose of 25 μg/kg, twice daily, 6 h apart for 4 consecutive days. This dosing strategy was chosen based on our previous experience with another recombinant diphtheria toxin based immunotoxin that targets CD3+ T cells in NHP [A‐dmDT390‐scfbDb(C207)] (Kim et al., 2007). The CD3 immunotoxin was constructed in a similar fashion as the CCR4 immunotoxin with the DT390 domain being identical for both. This dosing strategy using CD3 immunotoxin demonstrated efficacy while showing minimal toxicity (Kim et al., 2007; Nishimura et al., 2011). Depletion of Tregs was monitored by flow cytometry. With the described dosing strategy, up to 80% depletion of monkey CCR4+ cells in the peripheral blood was achieved and the depletion lasted for approximately 10 days (Figure 3A–B). In the peripheral blood, 78–89% of CCR4+Foxp3+ Tregs were depleted with similar duration (Figure 3C–D). Other cell populations including CD8+ T cells, other CD4+ T cells, B cells and NK cells were unaffected (Figure 3E–G and data not shown). The CD4+ cell population was minimally affected as CCR4+ Tregs and CCR4+ Th2 cells only occupy a small fraction of the entire CD4+ cell population. Remarkably, 82–88% of monkey CCR4+ cells and 89–96% of CCR4+Foxp3+ monkey Tregs were depleted from the lymph nodes after the four‐day course of immunotoxin (Figure 4A–B). Most of the depleted lymph node CCR4+Foxp3+ Tregs belongs to CD45RA−Foxp3+ effector Tregs (Figure 4B). Consistent with the peripheral blood analysis, other cell populations (CD8+ T cells, CD20+ B cells and other CD4+ T cells) in the lymph nodes were not affected (Figure 4B). Taken together, NHP CCR4+Foxp3+ Tregs were effectively depleted in vivo from both peripheral blood and lymph nodes using the foldback diabody anti‐human CCR4 immunotoxin. To the best our knowledge, this is the first effective agent for NHP Treg depletion in vivo. The results of this study strongly support our initial hypothesis that an anti‐human CCR4 immunotoxin will effectively deplete human CCR4+Foxp3+ Tregs. This suggests potential clinical applications such as using this agent to directly target CCR4+ tumors or as an indirect immunotherapy via depleting CCR4+ Tregs for other advanced malignancy.
Figure 3.
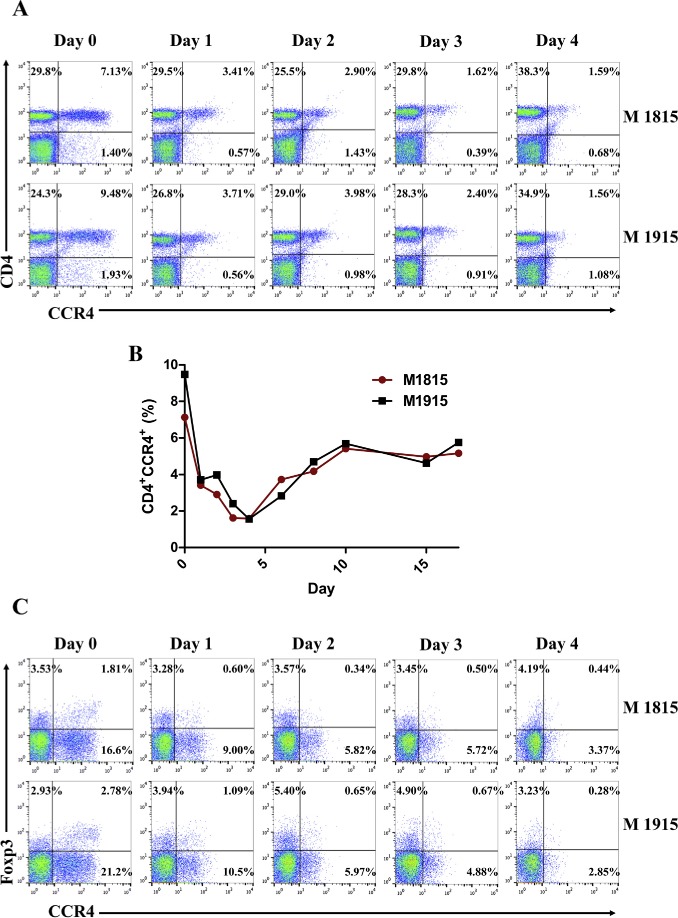
Monkey Treg depletion in the peripheral blood using the foldback diabody anti‐human CCR4 immunotoxin. The immunotoxin was injected at 25 μg/kg IV, BID for four consecutive days. M1815 and M1915: two cynomolgus monkeys. (A) Representative flow cytometry analysis (day 0 to day 4) of the CCR4+ cell depletion in the peripheral blood using the antibodies against CD4 and CCR4 (CD4+CCR4+). (B) The CCR4+ cell depletion in the peripheral blood was monitored by flow cytometry using the antibodies against human CD4 and CCR4 (CD4+CCR4+). (C) Representative flow cytometry analysis (day 0 to day 4) of the CCR4+Foxp3+ cell depletion in the peripheral blood using the antibodies against CCR4 and Foxp3 (CCR4+Foxp3+ among the gated CD4+ cells). (D) The CCR4+Foxp3+ Treg depletion in the peripheral blood was monitored by flow cytometry using the antibodies against human CCR4 and Foxp3 (CCR4+Foxp3+ among the gated CD4+ cells). (E) The CD8+ T cells in the peripheral blood were monitored by flow cytometry using the antibodies against human CD3 and CD8 (CD3+CD8+). (F) The CD20+ B cells in the peripheral blood were monitored by flow cytometry using antibodies against human CD3 and CD20 (CD3−CD20+). (G) The CD4+ cells in the peripheral blood were monitored by flow cytometry using the antibodies against human CD3 and CD4 (CD3+CD4+). (H) The CD14+CD11b+ monocytes in the peripheral blood were monitored by flow cytometry using antibodies against human CD14 and CD11b (CD14+CD11b+). (I) The entire lymphocyte was monitored by complete blood count analysis using HESKA Veterinary Hematology System.
Figure 4.
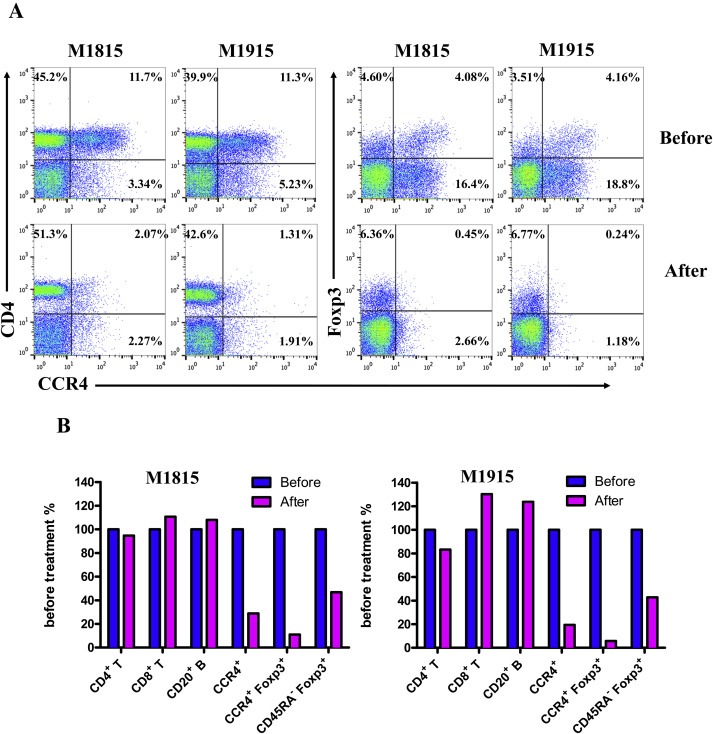
Monkey Treg depletion in the lymph nodes using the foldback diabody anti‐human CCR4 immunotoxin. The lymph node biopsies were performed before and after the immunotoxin treatment. (A) Representative flow cytometry analysis of the lymph node biopsy samples using antibodies against CD4, CCR4 and Foxp3 (CD4+CCR4+, CCR4+Foxp3+ among the gated CD4+ cells) before and after immunotoxin treatment. (B) The lymph node Treg depletion was monitored by flow cytometry using the antibodies against CD4, CCR4, CD45RA and Foxp3 (CCR4+ cells: CD4+CCR4+, CCR4+ Tregs: CCR4+Foxp3+ among the gated CD4+ cells, effector Tregs: CD45RA−Foxp3+ among the gated CD4+ cells). The other cell populations in lymph node were monitored by flow cytometry using antibodies against CD3, CD4, CD8 and CD20 (CD4+ T cells: CD3+CD4+, CD8+ T cells: CD3+CD8+, CD20+ B cells: CD3−CD20+).
Figure 3.
(Continued).
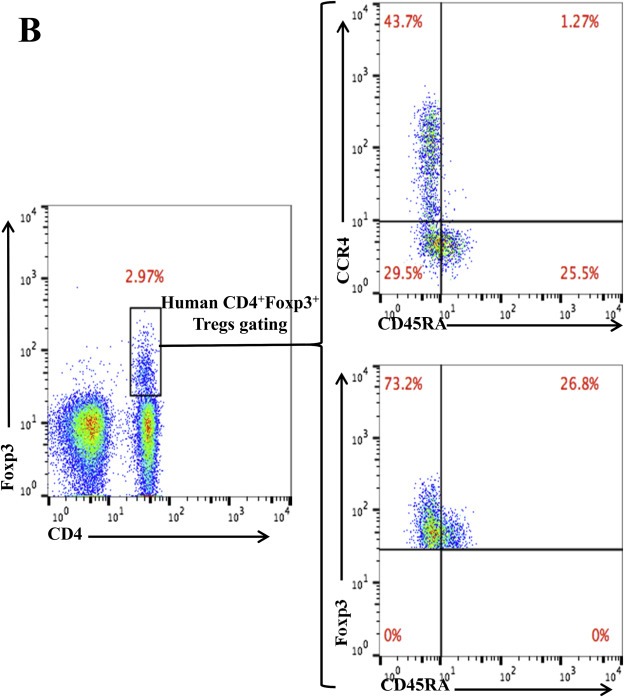
As shown in Figure 4B, CD45RA−Foxp3+ Tregs were depleted lower than that of CCR4+Foxp3+ Tregs. In order to explore the possible reasons, we have checked the expression pattern of Foxp3 versus CD45RA and CCR4 versus CD45RA among the gated monkey CD4+Foxp3+ Tregs (Figure 5A). The data demonstrated that ∼27% (16.8% of 61.2%) of CD45RA−Foxp3+ effector Tregs are CCR4 negative, which explained why CD45RA−Foxp3+ effector Tregs were depleted lower than that of Foxp3+CCR4+ Tregs in Figure 4B. In order to check whether this expression pattern is only in monkey PBMC, we have also checked the expression pattern of Foxp3 versus CD45RA and CCR4 versus CD45RA among the gated human CD4+Foxp3+ Tregs. As shown in Figure 5B, the data also demonstrated that ∼40% (29.5% of 73.2%) of CD45RA−Foxp3+ effector Tregs are CCR4 negative.
Figure 5.
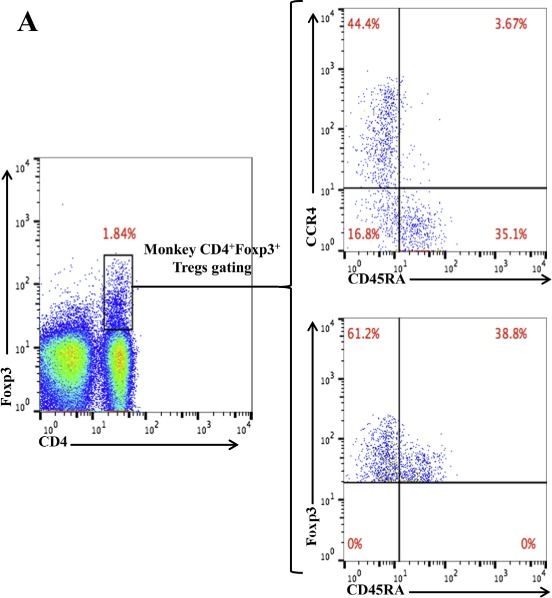
Flow cytometry analysis of the CCR4 versus CD45RA expression and Foxp3 versus CD45RA expression among the gated CD4+Foxp3+ monkey (A) or human (B) Tregs. The data are representative of two individual experiments.
Figure 5.
(Continued)
Clinically, the animals were healthy without any adverse effects from the immunotoxin for the entire duration of the study. Transient decreased appetite was observed and was most likely due to procedural sedations as the appetite loss was also observed during sedation for blood draw without medication administration. We speculate that there remains room for dose escalation and increased duration, which may improve the Treg depletion further. Additional experiments are necessary to optimize the dosing regimen.
The CCR4 immunotoxin had an inverse effect on the monocyte population, which rose as CCR4+Foxp3+ Tregs were depleted (Figure 3H). This monocyte expansion is a possible functional indication of the Treg depletion (Mayer et al., 2014). This observation suggests that monkey CCR4+ Tregs may directly suppress the maturation of monocyte similar to the previously reported effect of CCR4+ Tregs on suppression of dendritic cell (DC) maturation (Bayry et al., 2008). Further characterization of the effects on monocyte and DC using this unique CCR4 immunotoxin may shed light on the interaction between Tregs and maturation of important antigen‐presenting cell populations.
The entire lymphocyte population was significantly decreased during the course of the study (Figure 3I). Therefore we could not use the absolute count to present the peripheral blood flow cytometry data. However this transient lymphopenia is a possible functional indication of the Treg depletion as reported by Moltedo et al., 2014. It is also possible in part due to the twice daily administration of Ketamine for the procedural sedation (Kim et al., 2005). A possible mechanism that has been described is that Ketamine may cause a redistribution of lymphocytes from the circulation into the extravascular pool (Kim et al., 2005). In addition, the depletion of CCR4+ non‐Treg T cells might also partially contribute to the transient lymphopenia.
It is a general concern with DT‐based immunotoxins for the pre‐existing neutralizing antibodies due to the vaccination against diphtheria. It is known that majority of the antibodies against DT vaccine is directed to the binding domain (last 150 amino acids) which is eliminated in the DT390‐based immunotoxins (Thompson et al., 1995). Therefore, although the neutralizing antibodies due to the vaccination against diphtheria may be present, the CCR4 immunotoxin will still be effective.
Ontak is a FDA approved DT based human IL‐2 fusion toxin. It was approved by FDA with repeated dosing (9 or 18 ug/kg/day by intravenous infusion over 30–60 min for 5 consecutive days every 21 days for 8 cycles). We speculate that the immunogenicity of the CCR4 immunotoxin will be similar with that of Ontak. However it is still necessary to study the immunogenicity of the CCR4 immunotoxin in vivo using animal models.
One limitation of this study is that the impact of this observed depletion on Treg function was not assessed. Future studies will investigate the effects on Treg function by performing in vitro functional assays and studying its potential to break long‐term transplantation tolerance in an established NHP transplantation model.
We should keep in mind that CCR4 is also expressed on other cell populations such as CD4+CCR4+ Th2 cells (Yoshi and Matsushima, 2014) and CD8+CCR4+ cells (Yamauchi et al., 2015). We need to evaluate the in vivo net effect after the Treg depletion with the CCR4 immunotoxin.
Acknowledgments
We would like to thank Joanne Morris for veterinary support and lymph node biopsies, Isabel M. Hanekamp, James A. Winter, Elena Shubina, Sarah Lofgren and Matthew Defazio for their technical assistance. This study was supported by NIH/NIAID (non‐human primate opportunities pool 2014) R00000000004616 (to Z. W. and D. H. S).
Wang Zhaohui, Pratts Shannon G., Zhang Huiping, Spencer Philip J., Yu Ruichao, Tonsho Makoto, Shah Jigesh A., Tanabe Tatsu, Powell Harrison R., Huang Christene A., Madsen Joren C., Sachs David H., Wang Zhirui, (2016), Treg depletion in non‐human primates using a novel diphtheria toxin‐based anti‐human CCR4 immunotoxin, Molecular Oncology, 10, doi: 10.1016/j.molonc.2015.11.008.
References
- Bayry, J. , Tchilian, E.Z. , Davies, M.N. , Forbes, E.K. , Draper, S.J. , Kaveri, S.V. , Hill, A.V. , Kazatchkine, M.D. , Beverley, P.C. , Flower, D.R. , Tough, D.F. , 2008. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc. Natl. Acad. Sci. U. S. A. 105, 10221–10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos, P.D. , Plitas, G. , Rudra, D. , Lee, S.Y. , Rudensky, A.Y. , 2013. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 210, 2435–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C.Y. , Lee, H.S. , Han, S.C. , Heo, J.D. , Kwon, M.S. , Ha, C.S. , Han, S.S. , 2005. Hematological and serum biochemical values in cynomolgus monkeys anesthetized with ketamine hydrochloride. J. Med. Primatol. 34, 96–100. [DOI] [PubMed] [Google Scholar]
- Kim, G.B. , Wang, Z. , Liu, Y.Y. , Stavrou, S. , Mathias, A. , Goodwin, K.J. , Thomas, J.M. , Neville, D.M. , 2007. A foldback single-chain diabody format enhances the bioactivity of an anti-monkey CD3 recombinant diphtheria toxin-based immunotoxin. Protein Eng. Des. Sel. 20, 425–432. [DOI] [PubMed] [Google Scholar]
- Klages, K. , Mayer, C.T. , Lahl, K. , Loddenkemper, C. , Teng, M.W. , Ngiow, S.F. , Smyth, M.J. , Hamann, A. , Huehn, J. , Sparwasser, T. , 2010. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 70, 7788–7799. [DOI] [PubMed] [Google Scholar]
- Li, X. , Kostareli, E. , Suffner, J. , Garbi, N. , Hämmerling, G.J. , 2010. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur. J. Immunol. 40, 3325–3335. [DOI] [PubMed] [Google Scholar]
- Mayer, C.T. , Ghorbani, P. , Kühl, A.A. , Stüve, P. , Hegemann, M. , Berod, L. , Gershwin, M.E. , Sparwasser, T. , 2014. Few Foxp3+ regulatory T cells are sufficient to protect adult mice from lethal autoimmunity. Eur. J. Immunol. 44, 2990–3002. [DOI] [PubMed] [Google Scholar]
- Moltedo, B. , Hemmers, S. , Rudensky, A.Y. , 2014. Regulatory T cell ablation causes acute T cell lymphopenia. PLoS One. 9, e86762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, H. , Scalea, J. , Wang, Z. , Shimizu, A. , Moran, S. , Gillon, B. , Sachs, D.H. , Yamada, K. , 2011. First experience with the use of a recombinant CD3 immunotoxin as induction therapy in pig-to-primate xenotransplantation: the effect of T-cell depletion on outcome. Transplantation. 92, 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino, J.S. , Hermanrud, C.E. , Springett, L. , Zhang, H. , Li, G. , Srinivasan, S. , Gusha, A. , Sachs, D.H. , Huang, C.A. , Wang, Z. , 2012. Expression and characterization of recombinant soluble porcine CD3 ectodomain molecules: mapping the epitope of an anti-porcine CD3 monoclonal antibody 898H2-6-15. Cell Immunol. 276, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, D. , Nishikawa, H. , Maeda, Y. , Nishioka, M. , Tanemura, A. , Katayama, I. , Ezoe, S. , Kanakura, Y. , Sato, E. , Fukumori, Y. , Karbach, J. , Jäger, E. , Sakaguchi, S. , 2013. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. U. S. A. 110, 17945–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, M.W. , Ngiow, S.F. , von Scheidt, B. , McLaughlin, N. , Sparwasser, T. , Smyth, M.J. , 2010. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 70, 7800–7809. [DOI] [PubMed] [Google Scholar]
- Thompson, J. , Hu, H. , Scharff, J. , Neville, D.M. , 1995. An anti-CD3 single-chain immunotoxin with a truncated diphtheria toxin avoids inhibition by pre-existing antibodies in human blood. J. Biol. Chem. 270, 28037–28041. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Wei, M. , Zhang, H. , Chen, H. , Germana, S. , Huang, C.A. , Madsen, J.C. , Sachs, D.H. , Wang, Z. , 2015. Diphtheria-toxin based anti-human CCR4 immunotoxin for targeting human CCR4+ cells in vivo . Mol. Oncol. 9, 1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi, J. , Coler-Reilly, A. , Sato, T. , Araya, N. , Yagishita, N. , Ando, H. , Kunitomo, Y. , Takahashi, K. , Tanaka, Y. , Shibagaki, Y. , Nishioka, K. , Nakajima, T. , Hasegawa, Y. , Utsunomiya, A. , Kimura, K. , Yamano, Y. , 2015. Mogamulizumab, an anti-CCR4 antibody, targets human T-lymphotropic virus type 1-infected CD8+ and CD4+ T cells to treat associated myelopathy. J. Infect Dis. 211, 238–248. [DOI] [PubMed] [Google Scholar]
- Yoshi, O. , Matsushima, K. , 2014. CCR4 and its ligands: from bench to bedside. Int. Immunol. 27, 11–20. [DOI] [PubMed] [Google Scholar]


