To the Editor
Eosinophilic esophagitis (EoE) is a chronic, food antigen mediated disease associated with esophageal fibrosis and dysmotility (1). Esophageal studies using ultrasounds and endoscopic functional luminal probes show increased esophageal rigidity and thickening of the smooth muscle layers, indicating hypertrophy (2–4). During EoE progression, the esophagus undergoes substantial tissue remodeling with fibrosis leading to clinical complications including strictures and food impactions (1, 4). EoE is induced by inflammation but the consequences of tissue remodeling can remain even after the inflammation is treated, suggesting the role of a fibrotic extracellular matrix (ECM) in the chronic nature of the disease. Fibrosis promotes remodeling of ECM, a substrate for adherent cells composing the tissue, often resulting in increased ECM rigidity. Changes in substrate rigidity have been shown to affect multiple cellular functions important for development, tissue homeostasis and progression of some diseases including cancer (5–7). Given the increased esophageal rigidity in EoE, we investigated the effect of cell substrate rigidity on the morphology, size, and gene transcription of cultured normal longitudinal smooth muscle (LSM) cells.
To control the rigidity of the substrate for primary human donor esophagus derived LSM cells we used SoftSubstrates silicone gels (MuWells, San Diego, CA) of defined, physiologically relevant rigidities (Young’s Moduli, E, of 0.8, 3, 24, and 48 kPa) (see Methods in online supplement). We found that esophageal LSM cells cultured on stiffer substrate exhibited, decreased cell elongation, more pronounced actin filaments, and larger size compared to cells plated on softer substrates (Figure 1a, b). Cell spreading area was also significantly greater on increasingly rigid matrix (p=0.003) (Figure 1c). Taken together, our data indicate that smooth muscle (SM) cells on rigid matrix have a phenotype consistent with increased contraction and/or hypertrophy.
Figure 1.
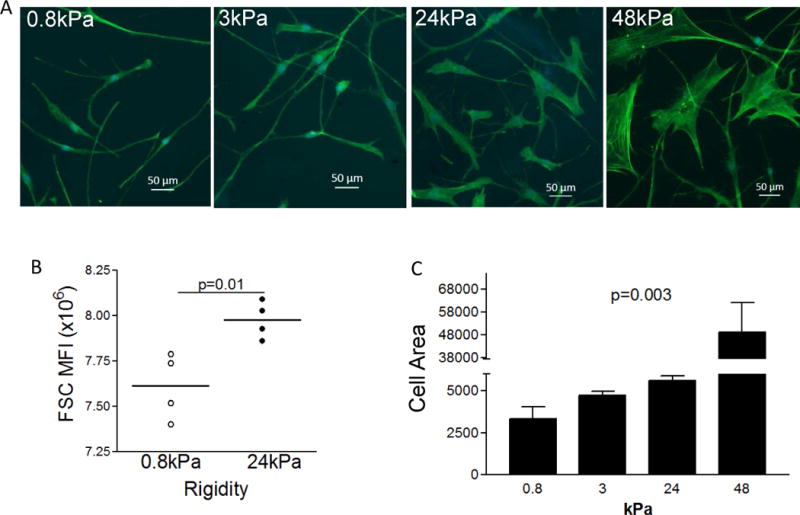
Morphology of LSM cells on substrates of different rigidity. Representative image of phalloidin stain (green) of cells cultured on silicone gels of indicated elastic moduli (0.8, 3, 24 and 48kPa) (A). FACS analysis for cell size (relative units) was done using forward scatter and mean fluorescence intensity on live cells using Flowjo software (Tree Star) using cells cultured on 0.8 and 24kPa substrates. Each dot represents a biological replicate. Lines represent mean. p value was calculated using an unpaired t test (B). Quantification of cell area by the ratio of the area of phalloidin staining and cell numbers (quantified by blue nuclei) on substrates of indicated rigidities (0.8 to 48kPa). p value was calculated using ANOVA test (C).
Phospholamban (PLN), an integral membrane protein regulating the sarcoendoplasmic reticulum calcium channel Serca2, is essential for cardiac contractility and is up-regulated in the esophageal muscularis mucosa in pediatric EoE subjects (8, 9). Our recent work has also identified PLN as an important player in TGFβ1-mediated esophageal smooth muscle cell and myofibroblast contraction (8). To investigate the effects of rigid matrix on PLN expression substrate rigidity, we measured the transcriptional level of PLN in LSM cells plated on substrates of E=0.8, 3, 24, and 48 kPa and found it to be monotonically increased by substrate rigidity (Figure 2a). To understand the role of PLN in regulating SM cell size and morphology, we employed a stable transgenic primary human esophageal SM cell line expressing PLN under the control of a doxycycline inducible promoter (see online Methods). Using these cells, we found that SM cells overexpressing PLN had decreased elongation and increased cell size on flow cytometry analysis (Figure 2b, c). Therefore, upregulation of PLN expression is sufficient to induce morphological changes in SM similar to those observed on rigid substrates.
Figure 2.
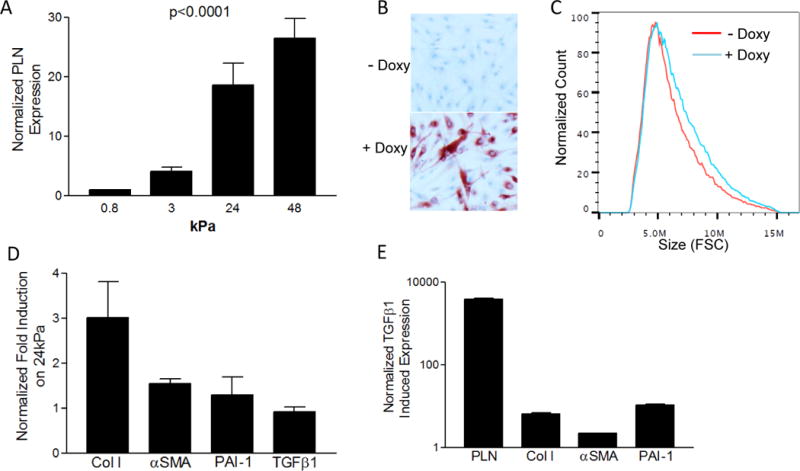
Gene Transcription and cell size in response to rigid substrate, PLN, or TGFβ1. PLN induction in LSM cells cultured on substrates of indicated rigidities (0.8 to 48kPa). p value was calculated using ANOVA test (A). Doxycycline (Doxy) treatment of PLN inducible transgenic esophageal SM cells shows up-regulation of PLN expression (red stain) (B) and increased cell size using forward scatter (C). Normal longitudinal smooth muscle cells have increased gene transcription of indicated genes expressed as a ratio gene induction on hard (E=24 kPa) versus soft (E=0.8 kPa) substrates (D). Fold increase in LSM gene expression induced by TGFβ1 treatment (E). mRNA levels for all conditions were normalized to the housekeeping gene RPL13A.
The pattern of gene expression in LSM cultured on rigid cell substrate was strikingly reminiscent of a subset of TGFβ1-induced genes including PLN, collagen I, and αSMA (Figure 2d, e). However the expression of TGFβ1 itself and other TGFβ1-induced genes such as plasminogen activator inhibitor-1 (PAI-1) were not affected by the rigidity of the cell substrate (Figure 2d). Therefore cellular responses to substrate rigidity are likely to be controlled by signaling pathways with upstream molecular player(s) other than TGFβ1.
In summary, we report here that a rigid cell substrate induces morphological and transcriptional changes in SM cells of the esophagus consistent with EoE. This finding challenges the paradigm of EoE pathogenesis as almost exclusively inflammation-dependent. In addition, the induction of collagen I, a structural component of the esophageal ECM, by a rigid matrix suggests that rigidity can potentially cause a positive feedback loop for fibrosis. We also demonstrate that PLN may be a key molecular player in rigid substrate-induced cellular hypertrophy since its expression increased monotonically with increasingly rigid substrate in parallel with cell size. Moreover, we demonstrated PLN overexpression is sufficient to promote SM cell hypertrophy. These results suggest that successful EoE treatment might require therapies that reverse the fibrotic changes in ECM to minimize the effects of rigid matrix on SM and that PLN may be a potential molecular target for these therapies. A focus on both inflammation-independent and inflammation-dependent esophageal rigidity may be needed to limit the onset, halt the progression, or reverse the path to a fibrostenotic esophagus in EoE.
Supplementary Material
Acknowledgments
Donor tissue was obtained from NDRI and ARORA. This research was funded by the following sources Funding Sources: NIH/NIAID AI092135 (S.A), ART/APFED HOPE Award (S.A.), DOD FA100044 (S.A.), Hearst Foundation (R.D.), AHA SDG (E.T.), NIH/NCRR/NCATS UL1TR000039, Arkansas Biosciences Institute (R.K.). We acknowledge Lisa Beppu for technical assistance. LV vectors were cloned and produced by the UCSD Vector Development Core Laboratory
Funding Sources: NIH/NIAID AI092135 (S.A), ART/APFED HOPE Award (S.A.), DOD FA100044 (S.A.), Hearst Foundation (R.D.), AHA SDG (E.T.), NIH/NCRR/NCATS UL1TR000039, Arkansas Biosciences Institute (R.K.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: ET has shares in MuWells (supplier of elastic cell substrates).
References
- 1.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2007 Jan;119(1):206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Fox VL, Nurko S, Teitelbaum JE, Badizadegan K, Furuta GT. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointestinal endoscopy. 2003 Jan;57(1):30–6. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010 Nov;139(5):1526–37. doi: 10.1053/j.gastro.2010.07.048. 37 e1. [DOI] [PubMed] [Google Scholar]
- 4.Nicodeme F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2013 Sep;11(9):1101–7 e1. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006 Aug 25;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, et al. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Current biology: CB. 2009 Sep 29;19(18):1511–8. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indra I, Undyala V, Kandow C, Thirumurthi U, Dembo M, Beningo KA. An in vitro correlation of mechanical forces and metastatic capacity. Physical biology. 2011 Feb;8(1):015015. doi: 10.1088/1478-3975/8/1/015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beppu LY, Anilkumar AA, Newbury RO, Dohil R, Broide DH, Aceves SS. TGF-beta1- induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2014 Nov;134(5):1100–7 e4. doi: 10.1016/j.jaci.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003 Feb 28;299(5611):1410–3. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


