SUMMARY
The cJun NH2-terminal kinase (JNK) signaling pathway is required for the development of hepatitis and hepatocellular carcinoma. A role for JNK in liver parenchymal cells has been proposed, but more recent studies have implicated non-parenchymal liver cells as the relevant site of JNK signaling. Here we tested the hypothesis that myeloid cells mediate this function of JNK. We show that mice with myeloid cell-specific JNK-deficiency exhibit reduced hepatic inflammation and suppression of both hepatitis and hepatocellular carcinoma. These data identify myeloid cells as a site of pro-inflammatory signaling by the JNK signaling pathway that can promote liver pathology. Targeting myeloid cells with a drug that inhibits JNK may therefore provide therapeutic benefit for the treatment of inflammation-related liver disease.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major cause of human cancer death (Fitzmorris et al., 2014; Mikhail et al., 2014). The world-wide incidence of HCC has increased in recent years, but the management of patients with HCC has not dramatically changed. Primary treatment options for early stage disease include surgical resection and liver transplantation. Unresectable disease is treated with loco-regional therapies and/or systemic chemotherapy and is associated with poor rates of survival. New treatment options for patients with HCC are therefore critically important.
The development of HCC appears to require hepatocyte death that triggers disease progression from hepatitis associated with a number of liver insults, including steatosis, hepatotoxins, viral infection, and autoimmune disease (Luedde et al., 2014). These changes are associated with the development of inflammation, fibrosis, and cirrhosis. Recent studies have demonstrated that inflammation is a hallmark of liver disease that may represent a cause of HCC development (Sun and Karin, 2013). This insight suggests that targeting hepatic inflammation may provide therapeutic benefit for the treatment of HCC.
Inflammatory responses are frequently associated with MAP kinases, including the cJun NH2-terminal kinase (JNK) signaling pathway that is activated by inflammatory cytokines, endotoxin, and physical-chemical stress (Davis, 2000). This signaling pathway is mediated by ubiquitously expressed JNK isoforms that are encoded by the Mapk8 and Mapk9 genes (also known as Jnk1 and Jnk2) (Gupta et al., 1996). JNK inhibition therefore represents a potential mechanism for decreasing hepatic inflammation and preventing hepatitis and HCC.
Studies using JNK-deficient mice have confirmed that JNK plays a key role in the development of hepatitis and HCC. For example, Mapk8−/− and Mapk9−/− mice were reported to be resistant to hepatitis (Maeda et al., 2003), although subsequent studies indicated that JNK1 may play a primary role in this response (Kamata et al., 2005; Chang et al., 2006). Moreover, Mapk8−/− mice are resistant to the development of HCC (Sakurai et al., 2006; Hui et al., 2008). Together, these studies demonstrate that JNK plays a key role in hepatitis and the development of HCC in mice.
To test whether JNK in liver parenchymal cells (hepatocytes) is required for hepatitis and HCC development, studies have been performed using tissue-specific JNK knockout mice. These studies demonstrated that JNK in parenchymal cells is not required for the development of hepatitis or HCC (Das et al., 2009; Das et al., 2011). Nevertheless, JNK-deficiency in both parenchymal and non-parenchymal cells did protect against hepatitis and HCC (Das et al., 2009; Das et al., 2011). These data indicate that non-parenchymal cells may represent the site of JNK function that is required for the development of hepatitis and HCC. However, the identity of the relevant non-parenchymal cell population is unclear. These hepatic cells include stellate cells, endothelial cells that form blood vessels and bile ducts, and other cells that mediate innate and adaptive immune responses.
We considered it likely that myeloid cells may represent a site of JNK function during the development of hepatitis and HCC because of the association of inflammation with liver disease (Shirabe et al., 2012; Sun and Karin, 2013) and the role of JNK in the promotion of inflammation (Han et al., 2013). The purpose of this study was to test this hypothesis. We report that mice with myeloid cell-specific JNK-deficiency are resistant to hepatitis and the development of HCC. These data identify myeloid cells as a key site of JNK function in inflammation-related liver disease. This information is important for the design of potential therapies based on the use of small molecules that target JNK because the relevant cell population is now established (myeloid cells). Moreover, relevant biomarkers for dose-ranging studies (myeloid inflammatory molecules) can be defined. Together with previous studies, this analysis confirms JNK inhibition as a possible therapeutic strategy for the treatment of inflammation-related liver disease.
RESULTS
Mice with JNK-deficiency in myeloid cells
We established mice with myeloid cell-specific ablation of the Mapk8 plus Mapk9 genes (LysM-cre+ Mapk8LoxP/LoxP Mapk9LoxP/LoxP mice) and control mice (LysM-cre+). To test for Mapk8 and Mapk9 gene ablation, we isolated Kupffer cells, macrophages, and neutrophils from control (ØWT) and JNK-deficient (ØKO) mice. Genotype analysis demonstrated ablation of the Mapk8 and Mapk9 genes in each cell population (Figure S1A). Immunoblot analysis confirmed similar JNK expression in non-myeloid cells of ØWT and ØKO mice, including B cells, T cells, and hepatocytes (Figure S1A). Immunophenotyping demonstrated that similar numbers of CD45+ leukocytes, dendritic cells (DC), B cells, T cells, NKT cells, NK cells, and monocytes in the blood of ØWT and ØKO mice (Figure S1B). Small changes in the number of B cells, T cells, and NK cells were detected in bone marrow and lymph nodes, but no significant differences in these cell populations were observed in the spleen or liver (Figure S1B). This analysis demonstrates that myeloid cell JNK-deficiency does not cause major changes in leukocyte cell numbers. We conclude that ØKO mice represent a model for studies of JNK-deficiency in myeloid cells (Han et al., 2013).
JNK promotes infiltration of the liver by inflammatory cells
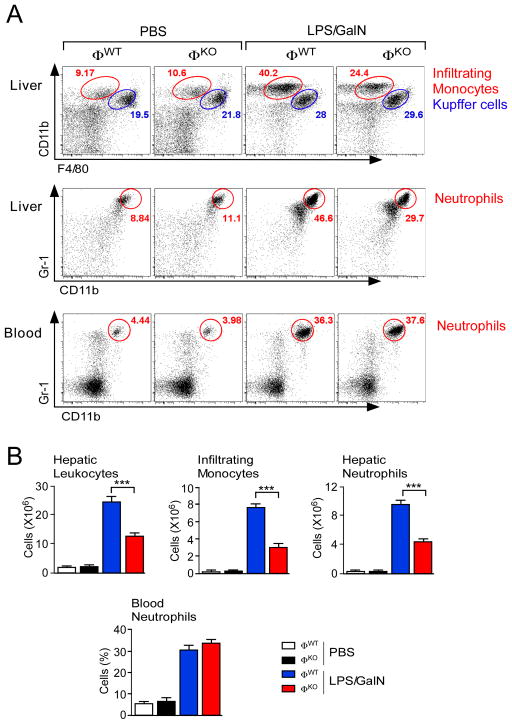
We examined the hepatotoxic response of ØWT and ØKO mice exposed to lipopolysaccharide (LPS) plus N-acetyl-galactosamine (GalN). Treatment with LPS/GalN caused a marked increase in the total number of hepatic leukocytes in ØWT mice (Figure 1). This increase in hepatic leukocytes was strongly suppressed (p<0.001) in LPS/GalN-treated ØKO mice (Figure 1). Flow cytometry using CD11b and F4/80 antibodies identified populations of Kupffer cells (CD11blow F4/80hi) and infiltrating monocytes (CD11bhi F4/80low) (Movita et al., 2012). No significant change in the Kupffer cell population was detected (Figure 1). In contrast, the infiltrating monocyte population was increased in LPS/GalN-treated ØWT mice and this increase was suppressed (p<0.001) in ØKO mice (Figure 1).
Figure 1. Myeloid JNK promotes hepatic infiltration by monocytes and neutrophils.
(A) Mice (ØWT and ØKO) were treated with PBS or LPS/GalN (5.5 h). Representative flow cytometry data of hepatic leukocytes stained with antibodies to CD11b and F4/80 (red, infiltrating monocytes; blue, Kupffer cells) are presented (upper panels). Representative flow cytometry data of neutrophils stained with antibodies to CD11b and Gr-1 (red) within total hepatic leukocytes (middle panels) and blood (lower panels) are presented.
(B) The total number of hepatic leukocytes is presented (mean ± SEM; PBS ØWT, n = 3; PBS ØKO, n = 4; LPS/GalN ØWT, n = 13; LPS/GalN ØKO, n = 12). The number of total hepatic leukocytes corresponding to infiltrating monocytes, infiltrating neutrophils, and Kupffer cells is presented (mean ± SEM; PBS ØWT, n = 5; PBS ØKO, n = 6; LPS/GalN ØWT, n = 11 (except neutrophils, n = 16); LPS/GalN ØKO, n = 11 (except neutrophils, n = 15)). The percentage of total blood leukocytes corresponding to neutrophils is also presented (mean ± SEM). PBS groups, n = 3; LPS/GalN groups, n = 9). Statistically significant differences between ØWT and ØKO mice are indicated (***, p<0.001).
See also Figure S1.
We also examined neutrophils in the ØWT and ØKO mice. Flow cytometry demonstrated that treatment with LPS/GalN caused a similar increase in the number of neutrophils (Gr-1hi Cd11b+) circulating in the blood of ØWT and ØKO mice (Figure 1). However, the increased hepatic neutrophil population in LPS/GalN-treated ØWT mice was suppressed in ØKO mice (p<0.001) (Figure 1). Together, these data indicate that while JNK-deficiency does not alter the LPS/GalN-stimulated mobilization of neutrophils from the bone marrow, JNK-deficiency does suppress the infiltration of neutrophils into the liver.
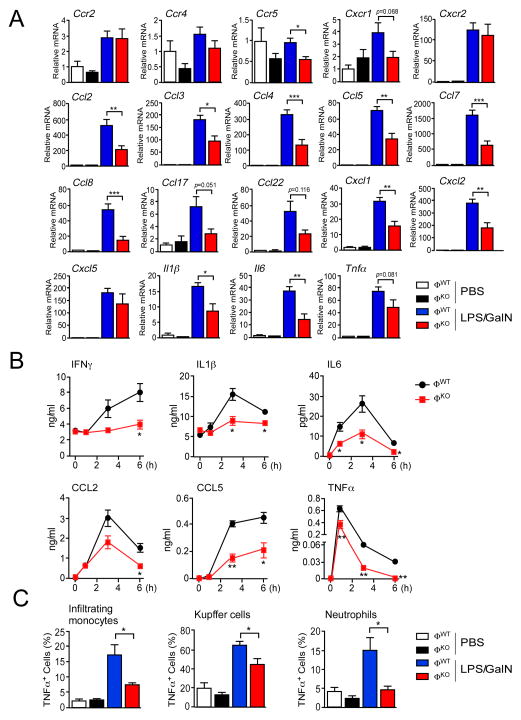
The JNK-mediated promotion of hepatic infiltration by monocytes and neutrophils (Figure 1) may be caused by chemokines (Marra and Tacke, 2014). We therefore examined whether myeloid cell JNK-deficiency disrupted chemokine signaling networks. The monocyte chemokine receptor CCR2 binds ligands (CCL2, CCL7, and CCL8) that are expressed at low levels in the liver of LPS/GalN-treated ØKO mice compared with LPS/GalN-treated ØWT mice (Figure 2A). A similar reduction in expression of ligands CCL3, CCL4, CCL5, and CCL8 that bind the chemokine receptor CCR5 (Figure 2A), the ligands CCL2, CCL4, CCL5, CCL17 and CCL22 that bind the chemokine receptor CCR4, and the ligands CXCL1, CXCL2 and CXCL5 that bind the chemokine receptors CXCR1 and CXCR2 (Figure 2A) were detected in the liver of LPS/GalN-treated ØKO mice compared with LPS/GalN-treated ØWT mice. These changes in chemokine signaling may contribute to reduced hepatic infiltration by inflammatory cells detected in ØKO mice compared with ØWT mice.
Figure 2. Myeloid JNK-deficiency suppresses expression of inflammatory cytokines.
(A) Hepatic expression of the chemokine receptors (Ccr2, Ccr4, Ccr5, Cxcr1 and Cxcr2), ligands (Ccl2, Ccl3, Ccl4, Ccl5, Ccl7, Ccl8, Ccl17, Ccl22, Cxcl1, Cxcl2 and Cxcl5) and inflammatory cytokines (Il1β , Il6, and Tnfα ) is presented. The data shows relative mRNA expression (mean ± SEM; n = 8) measured by quantitative RT-PCR assays using total RNA isolated from ØWT and ØKO mice treated (6 h) with PBS or LPS/GalN. Statistically significant differences between ØWT and ØKO mice are indicated (*, p<0.05; **, p<0.01; ***, p<0.001).
(B) The concentration of inflammatory cytokines and chemokines in the blood of ØWT and ØKO mice treated with LPS/GalN was measured by multiplexed ELISA (mean ± SEM; n = 10). Statistically significant differences between ØWT and ØKO mice are indicated (*, p<0.05; **, p<0.01).
(C) The expression of TNFα by hepatic myeloid cells (infiltrating monocytes, Kupffer cells, and neutrophils) was measured by intracellular staining. The percentage TNFα-positive cells was quantitated (mean ± SEM; PBS, n = 3; LPS/GalN, n = 6). Statistically significant differences between ØWT and ØKO mice are indicated (*, p<0.05).
See also Figure S2.
JNK promotes the expression of inflammatory cytokines
The requirement of JNK for normal myeloid cell infiltration of the liver following treatment of mice with LPS/GalN (Figure 1) suggests that JNK plays an important role in the promotion of hepatic inflammation. To explore the role of JNK in inflammation, we examined the hepatic expression of inflammatory cytokines in mice treated with LPS/GalN. We found reduced amounts of inflammatory cytokines (IFNγ , IL1β , IL6, and TNFα ) and chemokines (CCL2 and CCL5) circulating in the blood of LPS/GalN-treated ØKO mice compared with ØWT mice (Figure 2B). This reduction in cytokine and chemokine expression is consistent with the reduced expression of chemokine mRNA (Figure 2A) and inflammatory cytokine mRNA (Figure 2A) detected in the liver of ØKO mice compared with ØWT mice.
Myeloid cells represent a source of inflammatory cytokines (e.g. TNFα ) in mice treated with LPS/GalN. We therefore performed intracellular staining to detect the expression of TNFα by hepatic leukocytes. We found that JNK-deficiency caused significantly reduced TNFα expression (p<0.05) by Kupffer cells, infiltrating monocytes, and neutrophils (Figures 2C & S2). Together, these data demonstrate that multiple myeloid cell types may contribute to the suppression of TNFα expression caused by JNK-deficiency.
JNK promotes the development fulminant hepatitis
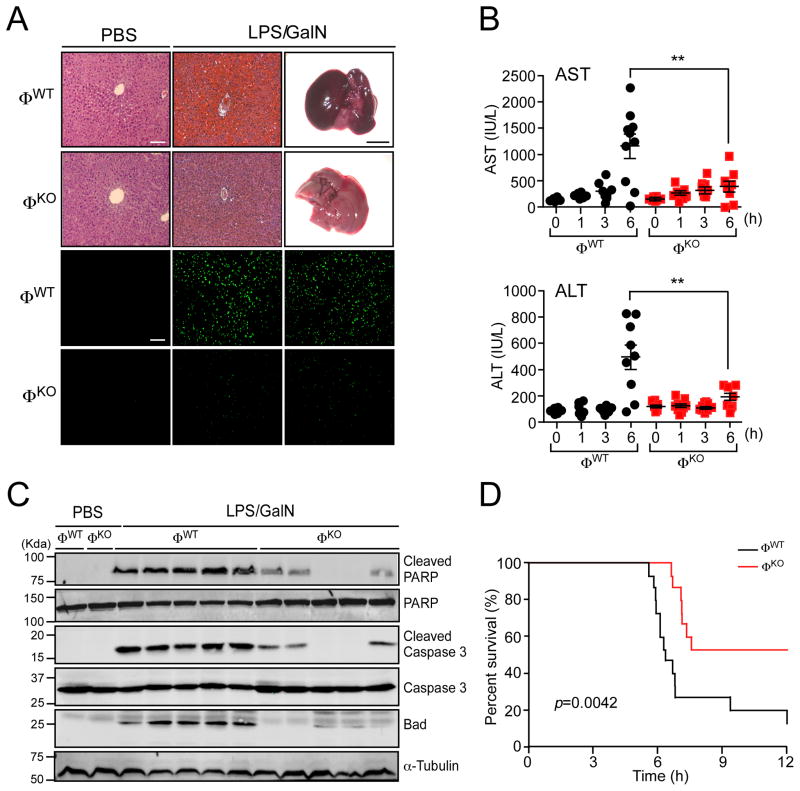
The reduction of hepatic infiltration by myeloid cells (Figure 1) and the decreased expression of inflammatory cytokines (Figure 2) suggest that LPS/GalN-induced hepatitis may be suppressed in ØKO mice compared with ØWT mice. Indeed, we found that myeloid cell JNK-deficiency caused reduced hepatic hemorrhage and reduced TUNEL-staining of apoptotic cells (Figure 3A). We also examined serum aminotransferases (a marker for hepatic damage) in LPS/GalN-treated ØWT and ØKO mice. This analysis demonstrated that JNK-deficiency significantly (p<0.01) reduced the amount of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in ØKO mice compared with ØWT mice (Figure 3B). Immunoblot analysis of liver extracts demonstrated reduced expression of Bad, a pro-apoptotic BH3-only protein implicated in LPS/GalN-induced hepatitis (Takamura et al., 2007), in liver extracts prepared from in LPS/GalN-treated ØWT and ØKO mice. Moreover, we found reduced caspase 3 activation and reduced cleavage of PARP (a caspase 3 substrate) in ØKO mice compared with ØWT mice (Figure 3C). Consistent with these observations, the survival of ØKO mice was significantly increased (p<0.005) compared with ØWT mice following treatment with LPS/GalN (Figure 3D). Together, these data demonstrate that JNK in myeloid cells can function to promote the development of fulminant hepatitis.
Figure 3. Myeloid JNK-deficiency suppresses the development of fulminant hepatitis.
(A) Mice (ØWT and ØKO) were treated with PBS or LPS/GalN (6 h). Representative images of the dissected liver (scale bar, 5 mm) and hematoxylin & eosin-stained liver sections are presented (upper panels). Apoptotic cells in the liver sections were examined by TUNEL assay (lower panels). Scale bars, 100 μm.
(B) The accumulation of hepatic aminotransferases (ALT and AST) in the blood of ØWT and ØKO mice was measured (mean ± SEM; n = 10, except ALT at 6 h, n = 9). Statistically significant differences between LPS/GalN-treated ØWT and ØKO mice are indicated (**, p<0.01).
(C) Liver extracts prepared from mice treated with PBS or LPS/GalN (6 h) were examined by immunoblot analysis using antibodies to α-Tubulin, Bad, Caspase 3, cleaved Caspase 3, PARP and cleaved PARP.
(D) Kaplan-Meier analysis of survival following treatment of ØWT and ØKO mice with LPS/GalN (n = 15). See also Figure S3.
It is established that the LPS/GalN model of hepatitis depends upon the interaction of soluble TNFα with TNF-R1 (Nowak et al., 2000). Our analysis suggests that reduced TNFα expression (Figure 2) may account for the resistance of ØKO mice to LPS/GalN-induced hepatitis compared with ØWT mice. However, it is also possible that ØKO mice exhibit a defect in TNFα signaling. To test this hypothesis, we compared the response of ØWT and ØKO mice to treatment with TNFα /GalN. We found that TNFα /GalN caused similar hepatic hemorrhage (Figure S3A) and similar concentrations of serum aminotransferases (ALT & AST) in ØWT and ØKO mice (Figure S3B). Moreover, biochemical analysis of liver extracts demonstrated similar caspase 3 activation and PARP cleavage in ØWT and ØKO mice (Figure S3C). No significant difference in the blood concentration of inflammatory cytokines (IL6 and TNFα ) or chemokines (CCL2 and CCL5) between ØWT and ØKO mice was detected (Figure S3D). Flow cytometry demonstrated that TNFα /GalN caused similar hepatic infiltration by inflammatory cells in ØWT and ØKO mice (Figure S3E). Together, these data demonstrate that JNK-deficiency in myeloid cells suppresses TNFα expression (Figure 2), but does not suppress TNFα signaling (Figure S3) in a mouse model of hepatitis, including increased hepatic infiltration by myeloid cells (Figure S3E).
JNK in myeloid cells promotes the development of HCC
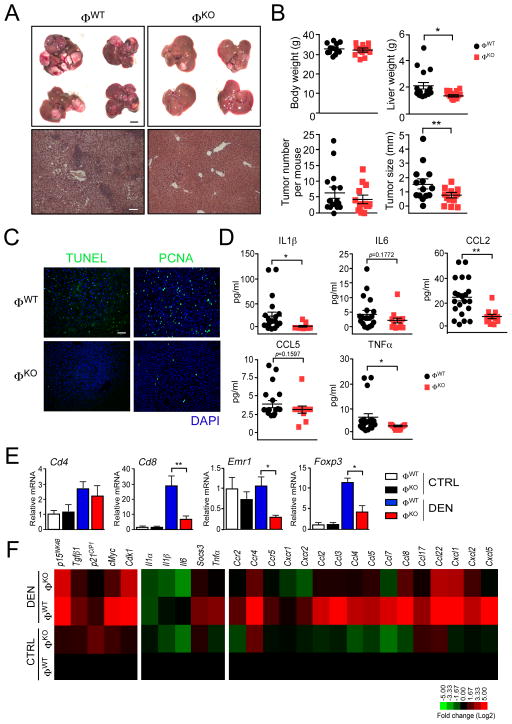
It is established that the development of HCC is increased by inflammation (Sun and Karin, 2013). Since hepatic inflammation is suppressed in ØKO mice compared with ØWT mice (Figures 1–3), we anticipated that JNK-deficiency in myeloid cells might protect mice against the development of HCC. To test this hypothesis, we examined the development of HCC in ØWT and ØKO mice using the diethlynitrosamine (DEN) model of liver cancer. The mice were exposed to the carcinogen DEN at age 2 wk and tumor development was examined at age 38 wk (Figure 4A). JNK-deficiency in myeloid cells caused significantly decreased liver mass (p<0.05) and decreased tumor size (p<0.01), but did not cause a significant change in the number of hepatic tumors detected by macroscopic examination (Figure 4B). Examination of liver sections by a pathologist demonstrated that myeloid cell JNK-deficiency decreased the development of adenoma and carcinoma (Figure S4A). Control studies indicated that HCC in ØWT and ØKO mice expressed similar amounts of JNK (Figure S4B). Together, these data demonstrate that JNK in myeloid cells is not required for tumor formation, but myeloid cell JNK acts to promote tumor development by increasing both tumor grade and tumor mass. To test this conclusion, we examined a different model of hepatic tumor development. We found that liver tumors caused by intrasplenic injection of B16 melanoma cells were markedly suppressed in ØKO mice compared with ØWT mice (Figure S4C,D). This observation confirms the conclusion that JNK in myeloid cells can promote tumor development in the liver.
Figure 4. Myeloid JNK promotes the development of HCC.
(A) Mice (ØWT and ØKO) were treated with diethynitrosamine (DEN) at age 2 wks. and euthanized at age 38 wks. Representative images of livers (scale bar, 500 μm) and hematoxylin & eosin-stained liver sections (scale bar, 100 μm) are presented.
(B) The total body mass, liver mass, surface tumor number, and tumor size are presented (mean ± SEM). Body mass: ØWT, n = 17; ØKO, n = 14. Liver mass: ØWT, n = 18; ØKO, n = 14. Tumor number and size: ØWT, n = 19; ØKO, n = 14. Statistically significant differences between ØWT and ØKO mice are indicated (*, p<0.05; **, p<0.01).
(C) Representative sections of liver stained with DAPI (blue) and by TUNEL assay (green) or with an antibody to PCNA (green) are presented. Scale bar, 100 μm.
(D) The concentration of blood cytokines (IL1β , IL6, and TNFα ) and chemokines (CCL2 and CCL5) was measured by multiplexed ELISA (mean ± SEM). ØWT, n = 21; ØKO, n = 13). Statistically significant differences between ØWT and ØKO mice are indicated (*, p<0.05; **, p<0.01).
(E) Hepatic mRNA associated with immune cell sub-sets (Cd4, Cd8, Foxp3, and Emr1 (F4/80)) was examined by quantitative RT-PCR assays (mean ± SEM; n = 3). Statistically significant differences between ØWT and ØKO mice are indicated (*, p<0.05; **, p<0.01).
(F) The expression of hepatic mRNA for inflammatory cytokines (Il1α , Il1β , Il6, and Tnfα ) and the cytokine target gene Socs3, the chemokine receptors (Ccr2, Ccr4, Ccr5, Cxcr1 and Cxcr2), the chemokine ligands (Ccl2, Ccl3, Ccl4, Ccl5, Ccl7, Ccl8, Ccl17, Ccl22, Cxcl1, Cxcl2 and Cxcl5) and proliferation-associated genes (Cdk1, p15INK4B, p21CIP1, cMyc and Tgfβ 1) are presented as a heat map of log2 transformed data normalized to control ØWT mice (mean; n = 3).
See also Figure S4.
Liver sections stained by TUNEL assay and an antibody to the proliferation marker PCNA demonstrated that myeloid cell JNK-deficiency caused both decreased hepatocyte cell death and decreased proliferation in the DEN model of HCC (Figure 4C). This observation is consistent with a role for JNK-mediated inflammation in damage-induced regeneration during development of HCC (Das et al., 2011). To test whether JNK in myeloid cells contributes to tumor-associated inflammation, we examined the expression of cytokines and chemokines. Blood analysis demonstrated decreased amounts of chemokines and inflammatory cytokines (Figure 4D). It is likely that the observed reduction in cytokine and chemokine expression reflects both expression by myeloid cells and also actions of myeloid cells on expression by different cell types, including other immune cells and tumor cells. Indeed, we found decreased hepatic expression of markers of CD8 T cells (but not CD4 T cells), Tregs (Foxp3), and macrophages (Emr1 (F4/80)) (Fig. 4E) together with inflammatory cytokine mRNA (Il1β , Il6, and Tnfα ) and chemokines /chemokine receptor mRNA, including Ccr2 (and ligands Ccl2, Ccl7, and Ccl8), Ccr4 (and ligands Ccl2, Ccl4, Ccl5, Ccl17 and Ccl22), Ccr5 (and ligands Ccl3, Ccl4, Ccl5 and Ccl8), and Cxcr1/2 (and ligands Cxcl1, Cxcl2 and Cxcl5) in ØKO tumors compared with ØWT tumors (Figure 4F). These data confirm that JNK in myeloid cells promotes tumor-associated inflammation.
Studies of cell cycle regulatory gene expression demonstrated that myeloid cell JNK-deficiency caused decreased tumor-associated expression of Cdk1, cMyc, p15INK4B, p21CIP1 and Tgfβ 1 mRNA (Figure 4F). The finding that ØKO tumors express decreased amounts of Cdk1 and cMyc compared with ØWT tumors is consistent with the larger tumor burden of control mice compared with myeloid cell JNK-deficient mice.
DISCUSSION
It is established that immunosurveillance plays a key role in tumor development (Hanahan and Weinberg, 2011) that can lead to either prevention or promotion of cancer (Schreiber et al., 2011). Pro-tumorigenic inflammation is generally associated with tumor infiltration by M2-like macrophages, myeloid-derived suppressor cells, Th2 cells, and Treg cells (Melief and Finn, 2011). In contrast, anti-tumorigenic inflammation is most often associated with tumor infiltration by M1-like macrophages, Th1 cells, and CD8+ cytotoxic T cells (Melief and Finn, 2011). The balance of these inflammatory mechanisms represents one factor that controls tumor formation.
Interestingly, both M1-like and M2-like macrophages (and also mixed-phenotype macrophages) are present in tumors, although there appears to be regional localization with M1-like macrophages primarily located in the peri-tumoral stroma and M2-like macrophages within the growing tumor (Kuang et al., 2007). Tumor development is associated with the progressive replacement of M1-like macrophages during early stages by M2-like macrophages within established tumors (Capece et al., 2013). The mechanism of macrophage polarization to the M2 phenotype in tumors may involve multiple mechanisms, including IL4 produced by T cells (DeNardo et al., 2009) and lactic acid produced by tumor cells (Colegio et al., 2014), that regulate signaling pathways that control macrophage polarization (Zhou et al., 2014).
The JNK signaling pathway is implicated in the control of macrophage polarization (Zhou et al., 2014). Consequently, reduced JNK signaling causes decreased expression of the M1-associated pro-inflammatory cytokines TNFα , IL1β , and IL6 (Han et al., 2013) and reduced expression of the M2-associated chemokines CCL17 and CCL22 (Hefetz-Sela et al., 2014). These observations are significant because TNFα , IL1β , and IL6 promote hepatic inflammation, hepatitis, and HCC development (Park et al., 2010; Negash and Gale, 2015) and CCL17/CCL22 can act as chemoattractants for tumor-associated Foxp3+ Tregs that regulate anti-tumor immunity (Nishikawa and Sakaguchi, 2010). Consequently, decreased expression of chemokines and inflammatory cytokines by JNK-deficient myeloid cells may contribute to reduced hepatic infiltration by inflammatory cells and the suppression of both hepatitis and HCC detected in ØKO mice compared with ØWT mice (Figure 3 & 4). Moreover, JNK in myeloid cells may influence the overall inflammatory microenvironment through immunoregulatory cells (Figure 4E), including CD8+ Foxp3+ Tregs that are associated with high grade HCC development (Yang et al., 2010).
In conclusion, we identify myeloid cells as a site of pro-inflammatory signaling by the JNK signaling pathway that can promote liver pathology. Targeting myeloid cells with a drug that inhibits JNK may therefore provide therapeutic benefit for the treatment of inflammation-related liver disease. A challenge for the design of such therapy is the potential for altered innate and adaptive immune responses caused by defects in macrophage function.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6J mice (stock number 000664) and B6.129P2-Lyz2tm1(cre)Ifo/J mice (stock number 004781) (Clausen et al., 1999) were obtained from The Jackson Laboratory. We have described Mapk8LoxP/LoxP mice (Das et al., 2007) and Mapk9 LoxP/LoxP mice (Han et al., 2013). The mice were backcrossed to the C57BL/6J strain (ten generations) and housed in a specific pathogen free facility (accredited by the American Association for Laboratory Animal Care) at 20°C using laminar flow cages. The Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School approved all studies using animals.
Hepatitis was induced by intraperitoneal (i.p.) injection (male mice at age 8 – 10 wks.) with 35 μg/kg E. coli 0111:B LPS (Sigma) plus 750 mg/kg N-acetyl-galactosamine (GalN) (Sigma) or 20 μg/kg TNFα (R&D Systems) plus 750 mg/kg GalN (Galanos et al., 1979; Wallach et al., 1988). Kaplan-Meier analysis was performed using mice treated with 25 μg/kg LPS plus 750 mg/kg GalN. Carcinogen-induced HCC was induced by i.p. injection (male mice at age 2 wks.) with single dose (25 mg/kg) of DEN (Sigma) diluted in glyceryl trioctanoate (Sigma); the mice were euthanized at age 38 wks. Control mice were injected with solvent alone. Liver metastasis was examined using mice (6–7 wk old) intrasplenically injected with 1 x 106 B16 melanoma cells in 100μl PBS (Kitajima et al., 2008); the mice were euthanized on day 12 post-injection.
Statistical analysis
Data are expressed as the mean ± SEM to assess whether the variance of each group was similar. The statistical significance of differences between groups was examined using Student’s test (two-sided) or analysis of variance (ANOVA) with the Fisher’s test. Kaplan-Meier analysis was performed using the Log-rank test.
Supplementary Material
Acknowledgments
We thank Dr. David Garlick for analysis of liver sections, Jamie Kady and Meghan Blackwood for expert technical assistance, and Kathy Gemme for administrative assistance. RJD is an investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://www.cell.com/cell-reports.
AUTHOR CONTRIBUTIONS
M.S.H. and R.J.D. designed the study; M.A.B., M.S.H. and T.B. performed experiments; and M.S.H. and R.J.D. analyzed data and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Jiang F, Sluss HK, Zhang C, Shokat KM, Flavell RA, Davis RJ. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci U S A. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Sabio G, Jiang F, Rincon M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNFα. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmorris P, Shoreibah M, Anand BS, Singal AK. Management of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014 doi: 10.1007/s00432-014-1806-0. [DOI] [PubMed] [Google Scholar]
- Galanos C, Freudenberg MA, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hefetz-Sela S, Stein I, Klieger Y, Porat R, Sade-Feldman M, Zreik F, Nagler A, Pappo O, Quagliata L, Dazert E, et al. Acquisition of an immunosuppressive protumorigenic macrophage phenotype depending on c-Jun phosphorylation. Proc Natl Acad Sci U S A. 2014;111:17582–17587. doi: 10.1073/pnas.1409700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Abe T, Miyano-Kurosaki N, Taniguchi M, Nakayama T, Takaku H. Induction of natural killer cell-dependent antitumor immunity by the Autographa californica multiple nuclear polyhedrosis virus. Mol Ther. 2008;16:261–268. doi: 10.1038/sj.mt.6300364. [DOI] [PubMed] [Google Scholar]
- Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–595. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783. e764. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. e571. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- Melief CJ, Finn OJ. Cancer immunology. Curr Opin Immunol. 2011;23:234–236. doi: 10.1016/j.coi.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Mikhail S, Cosgrove D, Zeidan A. Hepatocellular carcinoma: systemic therapies and future perspectives. Expert Rev Anticancer Ther. 2014;14:1205–1218. doi: 10.1586/14737140.2014.949246. [DOI] [PubMed] [Google Scholar]
- Movita D, Kreefft K, Biesta P, van Oudenaren A, Leenen PJ, Janssen HL, Boonstra A. Kupffer cells express a unique combination of phenotypic and functional characteristics compared with splenic and peritoneal macrophages. J Leukoc Biol. 2012;92:723–733. doi: 10.1189/jlb.1111566. [DOI] [PubMed] [Google Scholar]
- Negash AA, Gale M., Jr Hepatitis regulation by the inflammasome signaling pathway. Immunol Rev. 2015;265:143–155. doi: 10.1111/imr.12279. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK, 3rd, Moldawer LL. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1202–1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- Sun B, Karin M. Inflammation and liver tumorigenesis. Front Med. 2013;7:242–254. doi: 10.1007/s11684-013-0256-4. [DOI] [PubMed] [Google Scholar]
- Takamura M, Matsuda Y, Yamagiwa S, Tamura Y, Honda Y, Suzuki K, Ichida T, Aoyagi Y. An inhibitor of c-Jun NH2-terminal kinase, SP600125, protects mice from D-galactosamine/lipopolysaccharide-induced hepatic failure by modulating BH3-only proteins. Life Sci. 2007;80:1335–1344. doi: 10.1016/j.lfs.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Wallach D, Holtmann H, Engelmann H, Nophar Y. Sensitization and desensitization to lethal effects of tumor necrosis factor and IL-1. J Immunol. 1988;140:2994–2999. [PubMed] [Google Scholar]
- Yang ZQ, Yang ZY, Zhang LD, Ping B, Wang SG, Ma KS, Li XW, Dong JH. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol. 2010;71:1180–1186. doi: 10.1016/j.humimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.