Abstract
The process by which prostate cancer cells non-randomly disseminate to the bone to form lethal metastases remains unknown. Metastasis is the ultimate consequence of the long-range dispersal of a cancer cell from the primary tumor to a distant secondary site. In order to metastasize, the actively emigrating cell must move. Movement ecology describes an individual’s migration between habitats without the requirement of conscious decision-making. Specifically, this paradigm describes four interacting components that influence the dynamic process of metastasis: (1) the microenvironmental pressures exerted on the cancer cell, (2) how the individual cell reacts to these external pressures, (3) the phenotypic switch of a cell to gain the physical traits required for movement, and (4) the ability of the cancer cell to navigate to a specific site. A deeper understanding of each of these components will lead to the development of novel therapeutics targeted to interrupt previously unidentified steps of metastasis.
Keywords: metastasis, microenvironment, dispersal, transmogrification, epithelial-mesenchymal-transition, homing
1.Introduction
The necessary characteristics of lethal cancer cells and the discrete steps required for metastasis have been well described [10, 23, 37, 51], but it remains fundamentally unclear how and why prostate cancer cells metastasize to the skeleton with near 100% efficiency. Metastatic prostate cancer is responsible for approximately 28,000 deaths annually in the United States. Five-year survival in patients with localized disease is near 100%, but metastatic disease remains incurable [12]. Novel paradigms to better understand the metastatic process are necessary to instigate the development of new therapeutic strategies to treat metastatic disease.
Ultimately, to metastasize, a cancer cell must move. Metastasis represents a long-distance migration of a cancer cell from the primary tumor to a distant secondary site. While cells from the primary tumor may be passively sloughed into circulation, it is unlikely that these cancer cells have the necessary machinery to successfully invade a secondary site as a disseminated tumor cell and, ultimately, metastasis. It is more likely that cells that move and actively emigrate from the primary tumor are phenotypically suited to establish a metastatic colony.
Over the last decade, it has been suggested that cancer can be best understood by combining the concepts of Darwinian evolution of malignant cells and the selective pressures of the microenvironment through the science of ecology [1, 19, 25, 30-32]. This framework has opened up a new understanding of cancer as an invasive species, establishing itself in a primary organ ecosystem, and then spreading (metastasizing) to form metacommunities of interconnecting ecosystems throughout the patient host [2, 9, 20, 21, 36].
Physical movement is critical both to an individual’s survival as well as the survival of a species as a whole. Movement ecology describes an individual’s movement, encompassing (1) the external pressures of the habitat on the organism, (2) the necessary biomechanical processes of motion, (3) the organism’s intrinsic motivations to move or to stay, and (4) the abilities of the individual to sense navigational direction. The dynamic interactions of these four components result in a defined movement path (Figure 1, Table 1) [33]. Applying this framework of movement ecology to the actively emigrating metastatic cancer cell will allow a better understanding of the upstream pressures and resultant phenotype of the metastatic clone.
Figure 1. Movement paradigm for metastatic prostate cancer.
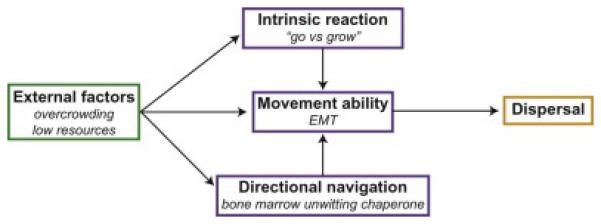
A general paradigm to describe the dynamic interactions of four critical factors that contribute to cancer cell metastasis. In green, the external factors encompass all of the influences within the ecosystem of the primary microenvironment, including other cells and abiotic factors, that influence an individual cell movement. In purple are the interrelated factors of the individual cancer cell. Intrinsic reaction describes the cell’s altered movement goals based on the pressures of external factors. Movement ability is the biomechanical requirements for locomotion. Directional navigation is the ability of the cell for site-directed movement. All of these factors interact to influence Dispersal, in orange, ultimately resulting in metastasis.
Table 1.
Paradigm of movement ecology in cancer
| Term | Definition | Ecological example | Cancer biology example |
|---|---|---|---|
|
External
factors |
The other organisms and factors in the ecosystem. |
Resource exhaustion | Hypoxia, acidity, and nutrient poverty of the “cancer swamp.” |
|
Intrinsic
motivation |
The inherent movement goals of the individual. |
The plague locust disperses in response to high population density. |
The cancer cell disperses in response to the overcrowding of the “cancer swamp.” |
|
Movement
ability |
The biomechanical requirements to move. |
Transmogrification from stationary grasshopper to dispersing locust. |
Epithelial-to-mesenchymal transition. |
|
Directional
navigation |
The ability to orient dispersal to a specific location. |
Insects unwittingly carry mites over large distances to other flowering plants. |
Bone marrow cells unwittingly chaperone tumor cells to the bone microenvironment. |
2. Metastasis and the risks associated with dispersal
In ecologic systems, movement from a native ecosystem into an unknown habitat is a high-risk endeavor. Dispersal, analogous to metastasis, is a proportionally long-distance movement from the organism’s natal habitat into a largely unknown environment [3]. This type of long-range movement requires substantial sacrifice of short-term individual fitness, including loss of replicative ability, alteration of metabolism, and using energy to transmogrify into a dispersal-morph phenotype. Transit itself increases risk to the individual due to the disperser’s unfamiliarity with available resources as well as the hazards of the novel environment. In addition, the dispersing organism lacks prior knowledge of a suitable secondary ecosystem and thus accepts the burden of future risk associated with the possibility of a hostile environment with low resource levels or increased predation [6]. Dispersers typically have lower expected survival and fecundity compared to their counterparts that remain in the native ecosystem. Given these risks, it is clear why individuals of a species do not leave their native ecosystem unless conditions deteriorate to the point where the organism’s survival is at risk, or as a collective bet-hedging strategy.
Metastatic tumor cells experience the same risks as dispersing organisms when they enter the metastatic cascade. Cells that gain a metastatic phenotype alter their metabolism, lose proliferative advantage, and undergo a phenotypic switch from an epithelial cell to a mesenchymal cell [5, 43]. Once the cell initiates invasion and successfully intravasates into the vasculature, it also experiences increased risk of cell death in circulatory transit, including anoikis, damage due to shear stress, and failure of immune evasion [22]. These high risks associated with dispersal highlight the necessity of an external cue to initiate metastasis: the acquisition of migratory or invasive ability does not imply that the cancer cell will leave the native ecosystem of the primary tumor. Rather, the early metastatic migrants are most likely responding to a stimulus to leave the primary site and/or to migrate to a secondary site. The paradigm of movement ecology applied to metastasizing prostate cancer cells provides hints regarding these stimuli that alter both the inherent motivation as well as biomechanical potential of actively migrating cells.
3. External pressures of the primary tumor microenvironment influence all aspects of an individual’s movement
A defining characteristic of a malignant cell is its unregulated rapid rate of proliferation, resulting in a tumor mass. Secondary to this inherent phenotype of cancer, the tumor initiates ecosystem engineering, alters and ultimately destroys the native microenvironment, resulting in a pro-tumorigenic microenvironment [2, 51]. The local rapid cellular proliferation is decoupled from the native homeostatic angiogenic process, and the tumor quickly overcomes the host vasculature [16]. The tumor overwhelms the both the incoming and outgoing vasculature, resulting in local oxygen and nutrient exhaustion as well an accumulation of cellular and metabolic waste, resulting in the ecological equivalent of an unproductive toxic swamp [2].
This proliferative cancer-cell mediated process, termed autoeutrophication, is directly analogous to the ecological phenomenon of cultural eutrophication in eutrophication in ponds and stream habitats. The accelerated eutrophic ecological process is the result of nitrogen and phosphorus loading of watersheds, typical of untreated sewage and fertilizer runoff. The rapid increase in the limiting factors of photosynthesis leads to accelerated population growth of short-lived cyanobacteria, dinoflagellates, or diatoms resulting characteristic algal blooms and red tide phenomena [26]. Organic waste from these organisms accumulates and decomposition levels rise, resulting in high oxygen consumption. Native species are unable to survive in the hypoxic environment, and the ecosystem is overtaken by anaerobic decomposers. If this eutrophication process goes unchecked, the ecosystem becomes increasingly unstable and may undergo ecosystem collapse or shift permanently to a different, less desirable state [8].
The primary tumor microenvironment experiences a similar process as the tumor grows beyond the host vasculature. An important distinction is that the autoeutrophic phenomenon of the tumor ecosystem is self-initiated and self-maintained. Rapid cancer cell proliferation leads to exhaustion of the local glucose and oxygen while simultaneously polluting the habitat with cellular waste, lowering local pH levels [4, 15, 24, 44, 53]. This tumor-initiated and tumor-maintained process displaces the native homeostatic microenvironment with a hypoxic, nutrient-poor, and acidic microenvironment: the cancer swamp [2].
The external factors of an individual’s habitat – either in the setting of ecology or cancer biology – directly influences all aspects of an individual’s movement: the individual’s intrinsic motivation to disperse, the biomechanical requirements of movement, and the capacity of an individual to spatially direct migration (Figure 1).
4. The dispersing cancer cell reacts to the pressures of the external environment
In movement ecology, an individual’s intrinsic motivation describes its movement goals that are directly influenced by the factors within its ecosystem (Figure 1, Table 1). In a resource-rich environment, an organism receives the positive feedback to remain in the habitat and therefore not engage in high-risk dispersal. In contrast, however, as population density increases and resource availability wanes, the organism responds to this negative feedback, thus altering its movement goal from remaining stationary and competing for local resources to dispersing from its native ecosystem in search of more favorable habitats.
The phenomenon of undirected dispersal involves being “pushed” from a habitat by the individual’s assessment that current circumstances are unsuitable for survival or will reduce fecundity, thus reducing the fitness of future progeny [50]. This information of the current quality of the habitat and the current density of competitors may compel individuals to engage in long-range dispersal, particularly if these negative conditions are likely to be large-scale, and thus cannot to be overcome by local dispersal or territory expansion [11].
The prototypical example of such a large-scale undirected dispersal is the periodic migration of plague locusts in response to population flushes [28]. During most years, these insects remain restricted to their natal semi-arid habitats that provide the necessary resources to complete their lifecycle from the egg through nymph stages to reproductive adult with limited flight capacity. Typically, population densities remain modest and therefore do not stress the native resource availability. Approximately once a decade, however, high levels of rainfall in the drought-prone habitat results in unusual flushes of vegetation that extends for hundreds of miles beyond the grasshopper’s natal ecosystem. Coincident with this rapid increase in resources is an increase in grasshopper population density. Because these unusual resource-rich conditions will only last for one to two years, the inevitable resource crash upon the return of typical weather conditions results in the decimation of most of the offspring from the reproductive boom.
Avoidance of the inevitable overcrowding and subsequent competition for resources thus alters the insect’s inherent motivation to move from or to remain stationary in its native ecosystem. Nymphs born into the high-density population use abundant resources to develop into a larger adult form with large wings enabling fast, strong, and long-distance migration (as described below, Figure 2 A-B) [52]. The net effect of this alteration in individual inherent motivation is that the original natal colony will remain populated at a low-density, while the migratory locust plague will potentially result in the colonization of new natal sites.
Figure 2. The epigenetic transmogrification between morphs.
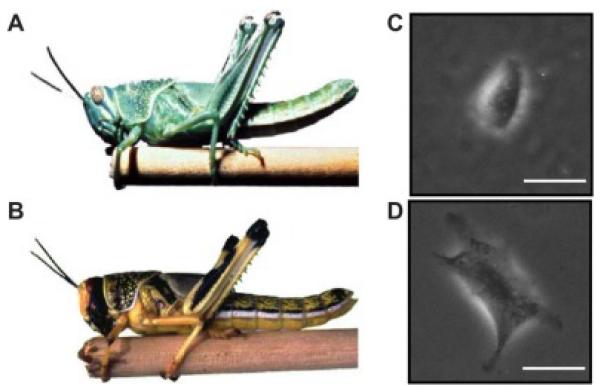
The (A) stay-at-home morph grasshopper and (B) dispersing locust morph share the same genetic background. (Adapted from a photograph courtesy of Compton Tucker, NASA.) The prostate cancer cell line PC3 was induced to undergo an epithelial-to-mesenchymal transition, resulting in two distinct stable cell lines, phenotypically epithelial PC3-epi (C) and mesenchymal PC3-emt (D) that share an identical genetic background. (Phase contrast images of cells plated on polyacrylamide gel. Scale bar = 50 μm. Image courtesy of Steven An, Johns Hopkins University.)
In a similar mechanism, cancer cells also go through a shift in inherent motivation from a proliferative stay-at-home morph to an actively migratory morph (Figure 2 C-D). As elegantly described in Hanahan and Weinburg’s landmark “Hallmarks of Cancer” paper, one of the critical characteristics of a malignant cell is its “limitless replicative potential” [23]. These cells are inherently programmed to proliferate, insensitive to anti-growth or pro-apoptotic signals. Similar to the grasshopper, the goal of the tumor cell is simple: to use the available resources to produce progeny. Eventually, however, the tumor cells experience overcrowding and, subsequently, resource limitation. Under such constrained circumstances, the risk of death in its native ecosystem of the primary tumor outweighs the risks associated with dispersal out of the tumor. This phase-shift in population density, resource allocation, and, therefore, risk assessment, alters the cancer cell’s intrinsic motivation and epithelial phenotype from one of stationary growth (“grow”) to one of a mesenchymal phenotype capable of adaptive movement (“go”). Ultimately, the tumor cells that are successful in the undirected dispersal event will invade a secondary site as a disseminated tumor cell. Once established in this favorable high-resource, low-population secondary ecosystem, the intrinsic motivation for movement of the cancer cell again shifts back to a goal of low-migration and local resource use.
These shifts in an individual’s intrinsic motivation in response to ecosystem influences are dependent on the capacity of the individual to biomechanically fulfill the movement goal (Figure 1).
5. Biomechanical movement abilities necessary for dispersal
In order to move, an individual must acquire the phenotypic traits necessary for locomotion. This movement ability encompasses all of the discrete traits that enable a fish to swim, a lion to run, or a slime mold to glide. The necessary movement machinery is highly variable, even within a single organism, and is influenced by the physical characteristics, the inherent motivation, and the movement goals of the individual. All of these can be directly and indirectly influenced by the other components of the ecosystem.
When a cell shifts from the goal of “growing” to the goal of “going,” it may undergo an epithelial-to-mesenchymal transition (EMT) in order to gain increased motility and invasive capabilities associated with a mesenchymal cell phenotype (Figure 2 C-D). Importantly, the ability to move or to remain stationary is entirely an epigenetic phenomenon – the running lion maintains the same genetic background as when it is stationary. This epigenetic plasticity holds true even in species that undergo a transmogrification with phenotypically distinct migratory- and stay-at-home-morphs, such as the locust morphs discussed above (Figure 2 A-B) [52].
The genetic clonal architecture of cancer cells suggests that most if not all cancer cells have the capacity to gain a metastatic phenotype [21, 34, 48], highlighting the necessity of phenotypic adaptation. This epigenetic adaptation arises not from genetic variation, but from variation in gene expression, thus altering function. There is a rich literature describing the influences of the external pressures of the “cancer swamp” – hypoxia, acidity, and nutrient poverty – on cancer cells. Lower-than-physiologic oxygen levels induce HIF1α expression that subsequently induces the expression of genetic drivers of EMT and angiogenesis [14, 27, 41]. Culturing cancer cell lines in acidic conditions increases mesenchymal cellular phenotype [29, 47]. Hypoxia, glucose starvation, and decreased pH all independently increase experimental metastasis in mouse models [39, 40].
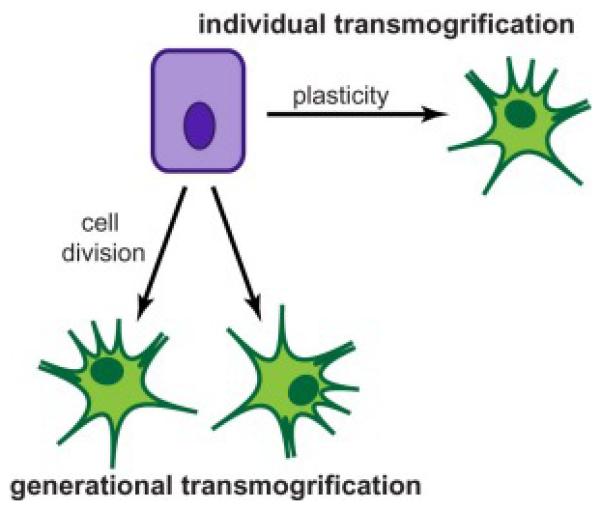
The complex transmogrification of the cancer cell between epithelial and mesenchymal phenotypes is not well understood. In ecology, such a phenotypic transformation between morphs may occur through a non-reversible developmental epigenetic trigger in the organism’s offspring (generational transmogrification) or through wholly individual phenotypic plasticity (individual transmogrification). It is unclear by which mechanism a cancer cell undergoes a transmogrification to gain movement ability (Figure 3).
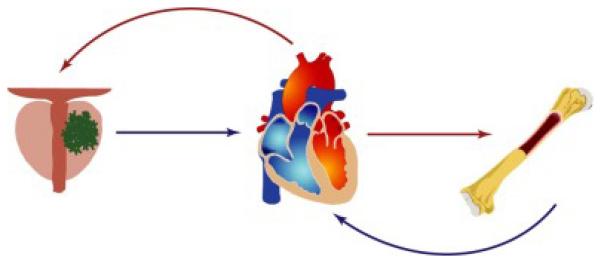
Figure 3. The circulatory path between the primary tumor and the bone metastatic site.
Oxygenated blood exits the heart through the arterial system (red arrows) to deliver oxygenated blood to the organs. Deoxygenated blood returns to the heart through the venous system (blue arrows) where it circulates through the lungs to become oxygenated and repeat the circuit.
In the case of the plague locust, the alteration from a stay-at-home grasshopper to a dispersal locust occurs only as a non-reversible developmental event. In essence, each individual is born into its phenotypic morph, so reverting back to a prior morph can only occur via offspring in a later generation. Nymphs that are born into a pressured habitat with high population density and the inevitability of future resource exhaustion mature into a locust-morph that has the biomechanical locomotion abilities for long-range dispersal. Once the locust dispersal-morph colonizes a secondary site, however, its progeny, when born into a low-density and resource-rich habitat, can either remain the same or develop into the stay-at-home grasshopper adult. Thus, the life history across multiple generations could, in theory, manifest as a stationary grasshopper giving birth to a dispersing locust that later gives birth to a stationary grasshopper. Notably, the alterations to biomechanical movement ability associated with each generational transition is not related to genetic variation, but is effected purely by epigenetic means, driven by the external factors of overcrowding and resource poverty and the intrinsic motivation for undirected dispersal (Figure 2 A-B) [52].
There are other species in which an individual may use external cues to transmogrify from one phenotypic form to another. For instance, the single-cell ciliate Tetrahymena vorax exists in two phenotypically distinct forms – the so-called Jekyll and Hyde morphs [35]. The smaller microstome form (“Jekyll”) feeds on bacteria, while the larger macrostome form (“Hyde”) eats other ciliates of self-similar size. The microstome is an obligate bacteria-eater while the macrostome can only depredate protists. As with the plague locust transition, preference of one morph or the other is dependent on availability of resources [7, 35]. A high density of ciliate prey provides preferential external pressure for the macrostome morph, and when ciliates are at low numbers the microstome morph predominates. In contrast to the generational morphological shift in grasshopper/locust insects, an individual microstome can physically alter its morphology and feeding structures to transmogrify into a macrostome, capable of ingesting large ciliates. Interestingly, however, the microstome morph can only be recovered through cell division.
In both the ecology of the animal kingdom and in the cancer ecosystem, an organism’s ability to move is influenced by factors in its habitat, most notably, population density and resource availability. There is high observable variation in individual movement ability, with some individuals migrating more efficiently or over larger distances than other morphs of the same genotype. In the cancer ecosystem, the epithelial-to-mesenchymal transition endows the cancer cell with the locomotion ability to disperse from the primary site. It is currently unclear if this transmogrification event occurs in an individual cell that undergoes a phenotype switch or whether it occurs with cell division by producing daughter cells of the opposite morph (Figure 3). Answering this question will open insights into how a cancer cell actively metastasizes and has important implications for prognosis as well as the development of therapeutic interventions.
6. Impossibility of directional dispersal through systemic gradients
A number of tumor types, most notably prostate cancer and breast cancer, preferentially home to bone to establish bone metastasis. This preferential homing implies that dispersal of cancer cells from the primary tumor cannot be entirely undirected. One of the most commonly cited explanations for this primary tumor-to-bone homing is the SDF1 chemokine gradient. Osteoblasts secrete SDF1, creating a chemo-attractant gradient for CXCR4-expressing cells, including prostate cancer cells [42, 45, 46]. Under physiologic conditions, this SDF1/CXCR4 gradient is used by hematopoietic stem cells (HSCs) to home to the bone. CXCR4 antagonism mobilizes HSCs from the endosteal niche into the blood stream. CXCR4 antagonism has likewise been used to evict prostate cancer cells (and other bone-homing cancer cells) from the HSC niche into the blood stream in preclinical models [42].
The CXCR4-binding of the SDF1 gradient is often cited as the stimulus for prostate cancer cells to migrate to the bone. This assumption of any systemic gradient, however, is incorrect. Oxygenated blood flows from the heart through arteries to distant organs. The blood then transfuses through a capillary system to deliver oxygen, and the deoxygenated blood finally enters the venous blood vessels for transit back to the heart (Figure 4). This one-way blood flow negates the possibility of a physical gradient of chemical signals through the circulation. The venous-arterial-venous circulation cannot carry gradient cues for a cancer cell to leave its primary tumor and head directly across the body to a distant suitable microenvironment.
Figure 4. Transmogrification of an epithelial cell to a mesenchymal cell.
Transmogrification resulting in phenotypically distinct morphs in ecology occurs both as result of a highly plastic individual transformation and as result of a developmental epigenetic trigger in an organism’s offspring. It is unclear whether the epithelial-to-mesenchymal transition occurs in a single cell with a highly plastic phenotype (individual transmogrification) or if a cell division is required for the mesenchymal morph to arise (generational transmogrification).
It is clear, however, that local SDF1 gradients play a critical role for CXCR4-expressing HSCs or tumor cells that are already in circulation to exit the blood vessel and establish within bone marrow niches. Indeed, such local chemogradients have been shown to provide essential cues for circulating cells to stop at a specific site to complete their physiologic function, such as circulating immune cells in response to inflammation or circulating endothelial progenitor cells in response to pro-angiogenic cues [18, 38]. Notably, however, these do not represent direct homing of the cells from their native organ to a site of injury. Rather, a chemical signal or local chemokine gradient provides a signal for a cell that has already vacated its host organ to stop and occupy a secondary site. The high efficiency with which prostate cancer cells seed the bone, however, implies that there still may be some signal for direct homing from the primary tumor to the bone microenvironment.
7. Unintentional chaperones orchestrate directional dispersal
The unidirectional flow of the circulatory system does not preclude the possibility of chemical signaling or direct cell-to-cell communication between the cells of the bone marrow niche and the cells of the primary tumor. As part of their physiologic function, numerous types of bone marrow cells emigrate from the bone and enter the circulation to surveil for injury and inflammation. Because these cells enter the circulation as part of their native function, they are inherently equipped to survive the severe forces they encounter in transit. As discussed previously, the mammalian arterial-venous circulatory system has unidirectional flow, but these bone marrow derived cells have the potential to encounter a primary tumor simply as a matter of chance. We hypothesize that these cells may unwittingly carry information or physically chaperone cancer cells to the bone marrow niche as they continue their path through the circulation and back to the bone marrow.
This phenomenon of unintentional chaperoning to mediate directed dispersal is common in ecology. For example, flower mites use pollinating animals such as hummingbirds, bats, and insects as carriers [13, 17, 49]. The inherent movement goal of the flower mite is for long-range dispersal to colonize distant plants or patches of flowers. As pollinators explore flowers to collect nectar, the mites have the opportunity to attach themselves to the animal to travel to a distant flower and then detach. Thus, the pollinator is an unwitting chaperone for the opportunistic flower mite. There are a number of critical features of this unwitting chaperone interaction: (1) the dispersal agent arrives at the mite’s habitat for reasons other than dispersing the mites, (2) the pollinator and the mite share an inherent motivation to find high quality habitats for nectar or for residence, and (3) the mite senses the pollinator and has the ability to physically attach as a means for directed dispersal.
It is likely that a similar phenomenon of directed dispersal by one or more unwitting chaperones plays a part in the non-random prostate cancer homing to bone. There is evidence for tumor cells to circulate in complex with host white blood cells (Figure 5). Many bone marrow cells, including myeloid derived suppressor cells, mesenchymal stem cells, and endothelial progenitor cells, circulate through the body to respond to localized inflammation and areas of wound healing. For example, myeloid derived suppressor cells accumulate in areas with high levels of interleukin-6 (IL-6) associated with inflammation in healthy individuals and with a tumor site in cancer patients [18]. In addition to their direct impacts on the localized tumor growth, it is also possible that these bone marrow cells unintentionally chaperone cancer cells from the primary tumor back to the bone marrow. As with the flower mite-pollinator carrier example, there are three key features of this unwitting chaperone interaction: (1) the dispersal agent, the bone marrow cell, arrives at the primary tumor site for reasons other than dispersing cancer cells, (2) the bone marrow cell and the cancer cell share an inherent motivation to find similar high quality habitats, and (3) the cancer cell senses the bone marrow cell and the cells have the capacity to physically attach as a means for directed dispersal. Engagement with the unintentional bone marrow chaperones, therefore, provides the emigrating cancer cells with the capacity for directed dispersal to the bone (Figure 1, Table 1). The identification of the host cells associated with cancer cells in circulating clusters should be an important goal for the field.
Figure 5. Tumor cells clustered with white blood cells in a patient with prostate cancer.
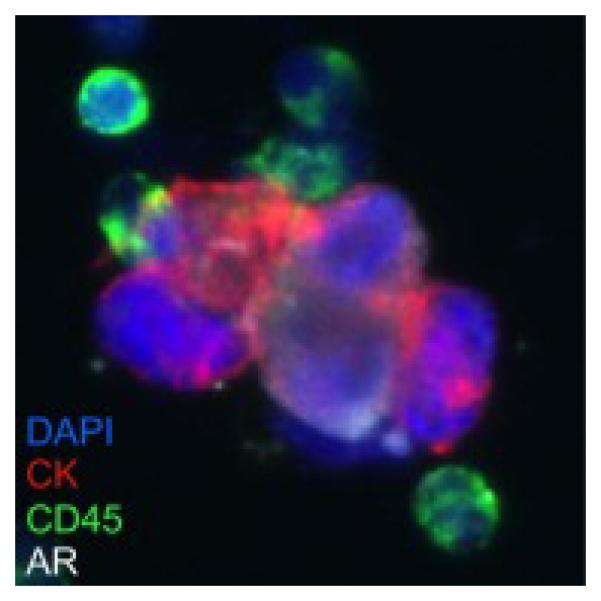
Prostate cancer cells (Cytokeratin+/androgen receptor+) are clustered with white blood cells (CD45+) in a bone marrow aspirate from a patient with metastatic castrate resistant prostate cancer. (Immunofluorescence microscopy image 40X; blue = 4’,6-diamidino-2-phenylindole [DAPI] nuclear stain; red = cytokeratin; green = CD45; white = androgen receptor. Image courtesy of Peter Kuhn and Anders Carlsson, University of Southern California.)
8. A novel paradigm for the movement ecology of bone metastatic cancer
Just because a cancer cell residing in a primary tumor acquires the physical machinery to move does not mean that it has to emigrate. Indeed, the science of ecology clearly demonstrates that species do not leave their native habitat if the ecosystem is healthy and is providing nutrients in a non-hostile environment. It is also clear that even in the hostile environment of a crowded, proliferating tumor that is outstripping its oxygen and nutrient supply that not all of the cancer cells undergo a phenotypic transmogrification and leave the tumor en mass. Determining the combination of environmental and cellular cues that endow a cell to become an active and successful emigrant remains a high priority for the cancer field. Similarly, it is now evident that many different cancer cell phenotypes can be found in the circulation. It has been speculated by many that cancer cells with a mesenchymal morph are the “active emigrants” and that cells with an epithelial morph are passive migrants that are destined to die during transit. The importance of circulating clusters remains unclear but their role as unwitting chaperones must be delineated.
Movement ecology provides a framework for a deeper understanding of metastasis. It describes, without requiring conscious thought or decision-making, an individual’s movement from one habitat to another. Applied to cancer, this framework encompasses (1) the external pressures of the habitat on the cell, (2) the biomechanics that a cell requires to move, (3) the cell’s intrinsic motivations to move or stay, and (4) the abilities of the cell to sense navigational direction (Figure 1) [33]. The dynamic interactions of these four components, taken together, define the factors necessary for the development of an active metastatic clone and the successful development of a clinical metastasis. Identifying the successful clones could provide prognostic information for the patient. Identifying the factors and processes involved (e.g., the transmogrification pressures of the primary ecosystem or the importance of circulating clusters) could lead to the development of new therapeutic interventions targeting previously unidentified steps in the metastatic process.
Highlights.
- The mechanisms of bone metastasis in prostate cancer remain unclear.
- Ecological models provide a rich resource to understand the metastatic cascade.
- A novel paradigm of cancer movement ecology frames the dynamic metastatic process.
- Is EMT due to individual cell plasticity or a generational transmogrification?
- What bone marrow cells orchestrate the directed dispersal of metastatic cancer cells?
Acknowledgements
This work was supported by NCI grant nos. U54CA143803, CA163124, CA093900 and CA143055. to K.J.P and the Provost’s Undergraduate Research Award at Johns Hopkins University to S.R. The authors thank the laboratories of Peter Kuhn and Steven An for use of images. The authors thank the members of the Brady Urological Institute, especially Dr. Donald S. Coffey and members of the Pienta laboratory, Dr. Robert Gatenby, and Mark Lloyd for thoughtful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None
References
- [1].Aktipis CA, Boddy AM, Gatenby RA, Brown JS, Maley CC. Life history trade-offs in cancer evolution, Nature reviews Cancer. 2013;13:883–892. doi: 10.1038/nrc3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amend SR, Pienta KJ. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget. 2015;6:9669–9678. doi: 10.18632/oncotarget.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baguette M, Van Dyck H. Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landscape ecology. 2007;22:1117–1129. [Google Scholar]
- [4].Barar J, Omidi Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. BioImpacts : BI. 2013;3:149–162. doi: 10.5681/bi.2013.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barriere G, Fici P, Gallerani G, Fabbri F, Rigaud M. Epithelial Mesenchymal Transition: a double-edged sword. Clinical and translational medicine. 2015;4:14. doi: 10.1186/s40169-015-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beier P. Dispersal of juvenile cougars in fragmented habitat. The Journal of Wildlife Management. 1995:228–237. [Google Scholar]
- [7].Buhse HE. An Analysis of Macrostome Production in Tetrahymena vorax Strain V2S- Type*. The Journal of protozoology. 1966;13:429–435. doi: 10.1111/j.1550-7408.1966.tb01934.x. [DOI] [PubMed] [Google Scholar]
- [8].Callisto M, Molozzi J, Barbosa JLE. Eutrophication: Causes, Consequences and Control. Springer; 2014. Eutrophication of Lakes; pp. 55–71. [Google Scholar]
- [9].Camacho DF, Pienta KJ. Disrupting the networks of cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2801–2808. doi: 10.1158/1078-0432.CCR-12-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen KW, Pienta KJ. Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theoretical biology & medical modelling. 2011;8:36. doi: 10.1186/1742-4682-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clobert J, Baguette M, Benton TG, Bullock JM, Ducatez S. Dispersal ecology and evolution. Oxford University Press; 2012. [Google Scholar]
- [12].DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- [13].Dobkin DS. Distribution patterns of hummingbird flower mites (Gamasida: Ascidae) in relation to floral availability on Heliconia inflorescences. Behavioral Ecology. 1990;1:131–139. [Google Scholar]
- [14].Esteban MA, Tran MG, Harten SK, Hill P, Castellanos MC, Chandra A, Raval R, O'Brien ST, Maxwell PH. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer research. 2006;66:3567–3575. doi: 10.1158/0008-5472.CAN-05-2670. [DOI] [PubMed] [Google Scholar]
- [15].Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- [16].Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspectives in biology and medicine. 1985;29:10–36. doi: 10.1353/pbm.1985.0049. [DOI] [PubMed] [Google Scholar]
- [17].Fronhofer EA, Sperr EB, Kreis A, Ayasse M, Poethke HJ, Tschapka M. Picky hitch- hikers: vector choice leads to directed dispersal and fat- tailed kernels in a passively dispersing mite. Oikos. 2013;122:1254–1264. [Google Scholar]
- [18].Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gallaher J, Anderson AR. Evolution of intratumoral phenotypic heterogeneity: the role of trait inheritance. Interface focus. 2013;3:20130016. doi: 10.1098/rsfs.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nature Reviews Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [23].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [24].Jain RK. Delivery of molecular and cellular medicine to solid tumors. Advanced drug delivery reviews. 2012;64:353–365. doi: 10.1016/j.addr.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kareva I. Biological stoichiometry in tumor micro-environments. PloS one. 2013;8:e51844. doi: 10.1371/journal.pone.0051844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khan MN, Mohammad F. Consequences and Control. Springer; 2014. Eutrophication: challenges and solutions, in: Eutrophication: Causes; pp. 1–15. [Google Scholar]
- [27].Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer research. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- [28].Lomer C, Bateman R, Johnson D, Langewald J, Thomas M. Biological control of locusts and grasshoppers. Annual review of entomology. 2001;46:667–702. doi: 10.1146/annurev.ento.46.1.667. [DOI] [PubMed] [Google Scholar]
- [29].Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clinical & experimental metastasis. 1996;14:176–-186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- [30].McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature cell biology. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process, Nature reviews. Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- [32].Nagy JD. The ecology and evolutionary biology of cancer: a review of mathematical models of necrosis and tumor cell diversity. Mathematical biosciences and engineering: MBE. 2005;2:381–418. doi: 10.3934/mbe.2005.2.381. [DOI] [PubMed] [Google Scholar]
- [33].Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–l28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- [35].Orlando PA, Brown JS, Buhse HE, Jr, Whelan CJ. Switching strategies, population dynamics, and mechanisms of co-existence in food webs with Jekyll-and-Hyde species. 2011.
- [36].Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Translational oncology. 2008;1:158–164. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pienta KJ, Robertson BA, Coffey DS, Taichman RS. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5849–5855. doi: 10.1158/1078-0432.CCR-13-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Real C, Caiado F, Dias S. Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovascular & Haematological Disorders-Drug Targets (Formerly Current Drug Targets-Cardiovascular & Hematological Disorders) 2008;8:185–192. doi: 10.2174/187152908785849071. [DOI] [PubMed] [Google Scholar]
- [39].Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer research. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- [40].Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. British journal of cancer. 1991;64:663–670. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends in pharmacological sciences. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. The Journal of clinical investigation. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shiraishi T, Verdone JE, Huang J, Kahlert UD, Hernandez JR, Torga G, Zarif JC, Epstein T, Gatenby R, McCartney A. Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget. 2015;6:130. doi: 10.18632/oncotarget.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer metastasis reviews. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. Journal of cellular biochemistry. 2003;89:462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- [47].Suzuki A, Maeda T, Baba Y, Shimamura K, Kato Y. Acidic extracellular pH promotes epithelial mesenchymal transition in Lewis lung carcinoma model. Cancer cell international. 2014;14:129. doi: 10.1186/s12935-014-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Talmadge JE. Clonal selection of metastasis within the life history of a tumor. Cancer research. 2007;67:11471–11475. doi: 10.1158/0008-5472.CAN-07-2496. [DOI] [PubMed] [Google Scholar]
- [49].Tschapka M, Cunningham SA. Flower Mites of Calyptrogyne ghiesbreghtiana (Arecaceae): Evidence for Dispersal Using Pollinating Bats1. Biotropica. 2004;36:377–381. [Google Scholar]
- [50].Venable D, Brown J. The population-dynamic functions of seed dispersal. Vegetatio. 1993;107:31–55. [Google Scholar]
- [51].Yang KR, Mooney SM, Zarif JC, Coffey DS, Taichman RS, Pienta KJ. Niche inheritance: a cooperative pathway to enhance cancer cell fitness though ecosystem engineering. Journal of cellular biochemistry. 2014;115:1478–1485. doi: 10.1002/jcb.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zera AJ, Denno RF. Physiology and ecology of dispersal polymorphism in insects. Annual review of entomology. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]
- [53].Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review) Oncology letters. 2012;4:1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]


