Abstract
Background
There is great debate on the possible adverse interaction between proton pump inhibitors (PPIs) and clopidogrel. In addition, whether the use of PPIs affects the clinical efficacy of ticagrelor remains less known. We aimed to determine the impact of concomitant administration of PPIs and clopidogrel or ticagrelor on clinical outcomes in patients with acute coronary syndrome (ACS) after percutaneous coronary intervention (PCI).
Methods
We retrospectively analyzed data from a “real world”, international, multi-center registry between 2003 and 2014 (n = 15,401) and assessed the impact of concomitant administration of PPIs and clopidogrel or ticagrelor on 1-year composite primary endpoint (all-cause death, re-infarction, or severe bleeding) in patients with ACS after PCI.
Results
Of 9429 patients in the final cohort, 54.8% (n = 5165) was prescribed a PPI at discharge. Patients receiving a PPI were older, more often female, and were more likely to have comorbidities. No association was observed between PPI use and the primary endpoint for patients receiving clopidogrel (adjusted HR: 1.036; 95% CI: 0.903–1.189) or ticagrelor (adjusted HR: 2.320; 95% CI: 0.875–6.151) (Pinteraction = 0.2004). Similarly, use of a PPI was not associated with increased risk of all-cause death, re-infarction, or a decreased risk of severe bleeding for patients treated with either clopidogrel or ticagrelor.
Conclusions
In patients with ACS following PCI, concomitant use of PPIs was not associated with increased risk of adverse outcomes in patients receiving either clopidogrel or ticagrelor. Our findings indicate it is reasonable to use a PPI in combination with clopidogrel or ticagrelor, especially in patients with a higher risk of gastrointestinal bleeding.
Keywords: Acute coronary syndrome, Clopidogrel, Outcome, Proton pump inhibitor, Ticagrelor
1. Introduction
The use of dual antiplatelet therapy (DAPT) with aspirin and P2Y12 receptor inhibitors is recommended by current clinical guidelines for at least 12 months in patients with acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI).[1],[2] Proton pump inhibitors (PPIs) are often prescribed together with DAPT to reduce the risk of gastrointestinal bleeding.[3],[4]
During the past few years, however, concerns have been raised about the potential for PPIs, especially omeprazole and esomeprazole, to attenuate the antiplatelet effects of clopidogrel.[5]–[7] This phenomenon may be interpreted by the ability of PPIs to competitively inhibit cytochrome P450 2C19 (CYP2C19) isoenzyme, which is involved in the conversion of clopidogrel to its active metabolite.[8]–[10] Observational studies showed conflicting data regarding the effects of concomitant use of clopidogrel and PPIs on cardiovascular events.[11]–[14] And the results of randomized controlled trial indicated no apparent cardiovascular interaction between clopidogrel and omeprazole.[15],[16] In contrast, ticagrelor is a direct P2Y12 receptor inhibitor without need of biotransformation.[17] Subgroup analysis of PLATO trial[18] revealed concurrent use of PPIs increased risk of adverse events in both clopidogrel and ticagrelor groups, but it may be just a marker for higher rates of cardiovascular events. Herein, whether the use of PPIs affects the clinical efficacy of ticagrelor remains less known.
Given the uncertainties as to a possible adverse interaction between PPIs and P2Y12 receptor inhibitors, we analyzed data from a large “real world” registry named BleeMACS (Bleeding complications in a Multicenter registry of patients discharged with diagnosis of Acute Coronary Syndrome) to determine the impact of concomitant administration of PPI and clopidogrel or ticagrelor on clinical outcomes in patients with ACS after PCI.
2. Methods
2.1. Study population
The details of the design and methods of the BleeMACS registry have been previously described.[19] In brief, The BleeMACS registry is an international, multi-center, investigator-initiated, retrospective observational registry, and aims to explore the real world burden of long-term bleeding in ACS patients. A total of 18,077 patients were enrolled from 16 centers in 11 countries: North America (Canada), South America (Brazil), Europe (Germany, Netherlands, Poland, Spain, Italy, Macedonia, Greece), and Asia (Japan and China). Data from one center (Macedonia, n = 2676) were excluded from BleeMACS registry because of high percentage of missing values. Therefore, the final BleeMACS database was constructed by merging the individual databases from the remaining 15 centers, making up a large cohort of 15,401 consecutive patients. Patients were eligible if they were at least 18 years old and were discharged alive with a diagnosis of ACS and treated with PCI from 2003 to 2014. Patients using P2Y12 receptor inhibitors (clopidogrel or ticagrelor) with or without PPIs at discharge were included in the final cohort (n = 9,429). More details may be consulted in clinicaltrials.gov (Identifier: NCT02466854).
The institutional review boards or ethics committees of each center approved participation in BleeMACS registry, and all patients gave written informed consent. The executive committee vouch for the integrity of the data. This study was approved by the institutional ethical committee of Beijing Anzhen Hospital, Capital Medical University (NO.2015009X). All authors have read and agreed to the final manuscript.
2.2. Data collection and follow-up
Patient demographics, history, clinical features, and diagnosis were collected at baseline. Laboratory tests, index PCI, and adjunctive therapy were also documented. At discharge, cardiovascular outcomes and bleeding events during index admission were recorded.
Patients were followed throughout one year after discharge. Data on cardiovascular and bleeding events were systematically obtained by telephone or face-to-face talk, and also reviewed by the medical records of the index events.
2.3. Primary endpoint
The primary endpoint was a composite of all-cause death, re-infarction, or severe bleeding events. Death included cardiac or non-cardiac death. Re-infarction was identified as ischemic symptoms (or new electrocardiographic changes) and new elevation of troponin and/or creatine kinase (CK) or CK-MB. Bleeding was defined as intracranial bleeding or any other bleeding leading to hospitalization and/or red blood transfusion. Bleeding and/or transfusion related with any type of surgery were excluded from the analysis.
2.4. Definitions
Prior bleeding included any episode of serious bleeding previous to the qualifying ACS hospitalization, and was defined as intracranial bleeding or any other bleeding leading to hospitalization and/or red blood transfusion. Malignancy indicated any active cancer or any non-active cancer who was treated during the last five years. The measurements of serum creatinine were standardized according to the recommendations of the National Kidney Disease Educational Program and the European Federation of Clinical Chemistry and Laboratory Medicine, to reduce inter-laboratory variation in creatinine assay calibration. Complete revascularization indicated a final angiographic result without coronary stenosis ≥ 70% in left anterior descending, left circumflex, or right coronary arteries, or stenosis ≥ 50% in left main coronary artery.
2.5. Statistical analysis
Baseline characteristics are presented as percentages (numbers) for categorical variables and as means ± SD for continuous variables. Categorical variables were compared by χ2 or Fisher's exact test and continuous variables by t test or Mann-Whitney test.
Kaplan-Meier analysis was used to illustrate composite primary endpoint. Patients were divided into four groups according to PPI use and use of clopidogrel or ticagrelor. Cox proportional hazard models were used to analyze the effects of PPIs versus no-PPIs within each P2Y12 receptor inhibitors (clopidogrel or ticagrelor) on clinical outcomes. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated.
To consolidate our findings, we carried out a propensity score adjusted cox model in which propensity score was included besides aforementioned factors. A logistic regression was used to estimate propensity score. In this logistic model, age, sex, diabetes mellitus, hypertension, peripheral arterial disease, history of cancer, serum creatinine at admission and hemoglobin at admission were included according to variables screening (backward method) or that were prespecified (sex, diabetes mellitus and hemoglobin at admission).
In order to further analyze the impact of concomitant use of PPI on composite primary end point, we performed a subgroup analysis in seven subsets. Cox model with or without propensity score adjustment was performed.
Since most subjects included in our study, whose records of PPI use (or not) are complete, have no missing data, we did not conduct an imputation. All P values were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.2 (SAS Institute Inc; Cary, NC) statistical software.
3. Results
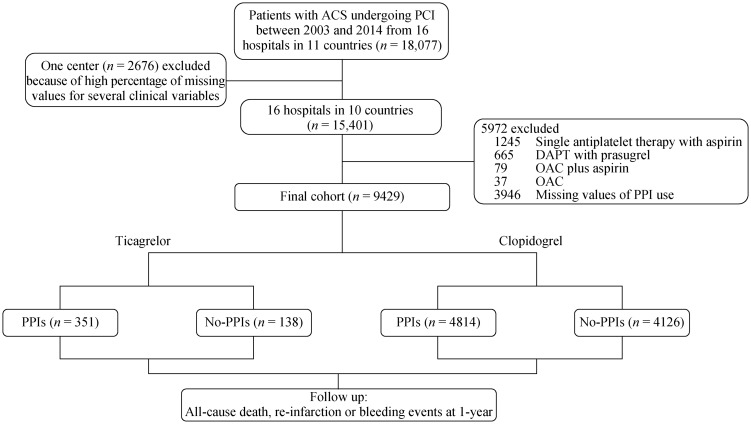
Figure 1 showed how the patients were selected for the final analytic cohort. Of the 15,401 patients enrolled in the BleeMACS registry, patients with single antiplatelet therapy with aspirin (n = 1245), DAPT with prasugrel (n = 665), oral anticoagulant (OAC) plus aspirin (n = 79), OAC (n = 37), and missing values of PPIs use (n = 3946) were excluded. Finally, 9429 patients using P2Y12 receptor inhibitors (clopidogrel or ticagrelor) with or without PPIs at discharge were included in the final analysis. Of these, 5165 patients (54.8%) claimed at least one prescription for PPIs at discharge. Patients characteristics at baseline are shown in Table 1. Patients who were treated with a PPI were older, were more often female, and were more likely to have a history of hypertension, dyslipidemia, diabetes mellitus, peripheral arterial disease, myocardial infarction, chronic kidney disease, peptic ulcer, coronary artery bypass grafting, prior bleeding or malignancy; were more likely to have an index diagnosis of unstable angina or non ST elevation myocardial infarction and had higher rate of Killip class ≥ 2; had lower baseline hemoglobin and higher baseline creatinine levels. The rate of complete revascularization was higher in patients receiving PPIs. Moreover, the use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers and statins were more common in patients with PPIs.
Figure 1. Patient flow chart for the study cohort.
ACS: acute coronary syndrome; DAPT: dual antiplatelet therapy; OAC: oral anticoagulants; PCI: percutaneous coronary intervention; PPIs: proton pump inhibitors.
Table 1. Baseline characteristics by PPIs use.
| Treated with PPIs (n = 5165) | Not treated with PPIs (n = 4264) | P | |
| *Age, yrs | 66.22 (56.39–73.80) | 61.25 (52.00–71.00) | < 0.001 |
| ≤75 yrs | 3855/5165 (74.64%) | 3606/4264 (84.57%) | 0.000 |
| Female | 1285/5165 (24.88%) | 894/4264 (20.97%) | 0.000 |
| Medical history | |||
| Hypertension | 3073/5165 (59.50%) | 2115/4264 (49.60%) | 0.000 |
| Dyslipidemia | 2498/5165 (48.36%) | 1792/4264 (42.03%) | 0.000 |
| Diabetes mellitus | 1349/5165 (26.12%) | 955/4264 (22.40%) | 0.000 |
| Peripheral arterial disease | 394/5165 (7.63%) | 231/4264 (5.42%) | 0.000 |
| Myocardial infarction | 619/5165 (11.98%) | 444/4264 (10.41%) | 0.016 |
| Congestive heart failure | 113/5165 (2.38%) | 68/4264 (2.25%) | 0.717 |
| Chronic kidney disease | 98/5165 (4.83%) | 30/4264 (2.83%) | 0.008 |
| Peptic ulcer | 115/5165 (5.58%) | 46/4264 (3.50%) | 0.006 |
| PCI | 542/5165 (10.49%) | 427/4264 (10.01%) | 0.445 |
| CABG | 178/5165 (3.45%) | 92/4264 (2.16%) | 0.000 |
| Prior bleeding | 253/5165 (4.90%) | 130/4264 (3.05%) | 0.000 |
| Malignancy | 398/5165 (7.71%) | 195/4264 (4.57%) | 0.000 |
| Index event type | |||
| STEMI | 2965/5165 (57.41%) | 3142/4264 (73.69%) | 0.000 |
| Unstable angina | 711/5165 (13.77%) | 448/4264 (10.51%) | |
| NSTEMI | 1489/5165 (28.82%) | 674/4264 (15.80%) | |
| Clinical characteristic | |||
| *Baseline hemoglobin, g/dL | 14.00 (12.80–15.10) | 14.20 (13.10–15.20) | < 0.001 |
| *Baseline creatinine, mg/dL | 0.86 (0.76–1.05) | 0.85 (0.74–1.02) | < 0.001 |
| Killip class ≥ 2 | 710/5165 (14.19%) | 491/4264 (11.58%) | 0.000 |
| *Left ventricular ejection fraction | 56.5 (45–60) | 57 (47–60) | 0.079 |
| Index PCI intervention | |||
| Drug-eluting stent | 2126/5165 (41.16%) | 2013/4264 (47.21%) | 0.000 |
| PTCA | 132/5165 (2.56%) | 180/4264 (4.22%) | 0.000 |
| Thrombolysis | 108/5165 (2.09%) | 81/4264 (1.90%) | 0.509 |
| Complete revascularization | 2923/5165 (62.01%) | 2403/4264 (58.28%) | 0.000 |
| Prescribed drugs | |||
| β-blocker | 4209/5165 (81.49%) | 3458/4264 (81.10%) | 0.626 |
| ACEI/ARB | 3946/5165 (76.40%) | 2953/4264 (69.25%) | < 0.001 |
| Statins | 4858/5165 (94.06%) | 3910/4264 (91.70%) | < 0.001 |
Values in parentheses are percentages unless indicated otherwise. *Values are median (25th, 75th percentiles). ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; CABG: coronary artery bypass grafting; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; PPIs: proton pump inhibitors; PTCA: percutaneous transluminal coronary angioplasty.
Patients were followed up throughout one year. Nine hundred and fourteen (9.7%) adjudicated all-cause deaths, re-infarctions or bleedings events were registered. The rate of composite primary endpoint was significantly higher in patients on a PPI than those not on a PPI (10.9% vs. 8.3%; unadjusted HR: 1.329; 95% CI: 1.163–1.518) (Table 2). However, using the propensity score generated from logistic regression models and adjusting for baseline covariates, there were no significant difference regarding the primary endpoints between patients with PPIs and those without PPIs (adjusted HR: 1.044; 95% CI: 0.912–1.196) (Table 2). In addition, the use of PPIs was associated with higher rate of re-infarction event (4.9% vs. 4.4%; unadjusted HR: 0.960; 95% CI: 0.789–1.168; adjusted HR: 0.808; 95% CI: 0.662–0.987), but was not associated with increased risk of all-cause death/re-infarction and all-cause death, or a decreased risk of bleeding (Table 2).
Table 2. Kaplan-Meier 1-year event rates according to PPIs use.
| Treated with PPIs (n = 5165) (%) | Not treated with PPIs (n = 4264) (%) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| All-cause death/re-infarction bleeding | 561/5165 (10.9%) | 353/4264 (8.3%) | 1.329 (1.163–1.518) | 1.044 (0.912–1.196) |
| All-cause death/re-infarction | 406/5165 (7.9%) | 260/4264 (6.1%) | 1.301 (1.113–1.520) | 1.002 (0.856–1.175) |
| All-cause death | 257/5165 (5.0%) | 134/4264 (3.1%) | 1.598 (1.297–1.969) | 1.111 (0.899–1.374) |
| Re-infarction | 251/5165 (4.9%) | 186/4264 (4.4%) | 0.960 (0.789–1.168) | 0.808 (0.662–0.987) |
| Bleeding | 198/5165 (3.8%) | 116/4264 (2.7%) | 1.427 (1.135–1.794) | 1.113 (0.881–1.406) |
HR (unadjusted and adjusted) showing the relationship between PPIs and clinical outcomes. HR: hazard ratios; PPIs: proton pump inhibitors.
For patients treated with clopidogrel, the rate of the composite primary endpoint was 11.1% for individuals on a PPI and 8.4% for those not on a PPI (unadjusted HR: 1.331; 95% CI: 1.161–1.524). For patients treated with ticagrelor, the rate of the composite primary endpoint was 8.0% for individuals on a PPI and 3.6% for those not on a PPI (unadjusted HR: 2.191; 95% CI: 0.846–5.674) (Figure 2, Table 3). After adjusting for potential confounders and the propensity to be on a PPI at discharge, no significant association remained between use of a PPI and the primary endpoint both for patients receiving clopidogrel (adjusted HR: 1.036; 95% CI: 0.903–1.189) and for those receiving ticagrelor (adjusted HR: 2.320; 95% CI: 0.875–6.151) (interaction between P2Y12 inhibitor treatment and PPI use, P = 0.2004) (Table 3). Similarly, use of a PPI was not associated with increased risk of all-cause death, re-infarction, or a decreased risk of bleeding for patients treated with either clopidogrel or ticagrelor (Table 3).
Figure 2. Kaplan-Meier analysis of 1-year primary endpoint (all-cause death/re-infarction/severe bleeding) in PPIs versus no-PPIs groups within each P2Y12 receptor inhibitors (clopidogrel or ticagrelor).
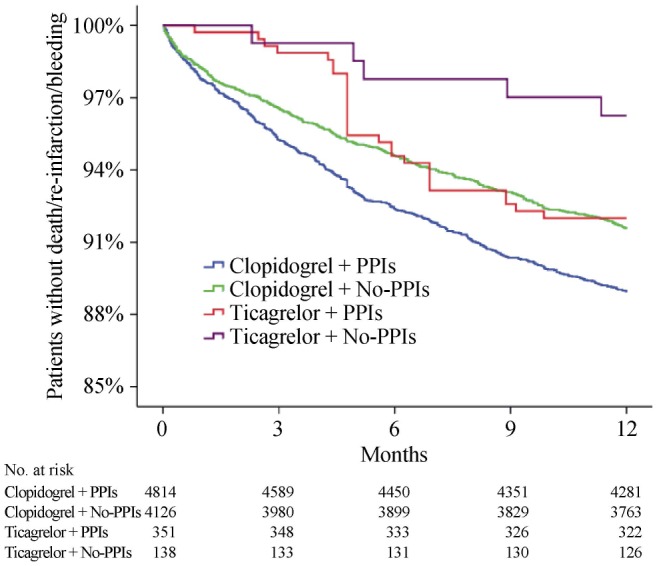
PPIs: proton pump inhibitiors.
Table 3. Unadjusted and propensity-score adjusted hazard ratios for 1-year endpoint in patients with PPIs versus no-PPIs within each P2Y12 receptor inhibitors (clopidogrel or ticagrelor).
| Clopidogrel |
Ticagrelor |
P, Interaction of treatment with PPIs use, unadjusted/adjusted | |||||||
| Treated with PPIs (n = 4814) | Not treated with PPIs (n = 4126) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Treated with PPIs (n = 351) | Not treated with PPIs (n = 138) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
| All-cause death/re-infarction bleeding | 533/4814 (11.1%) | 348/4126 (8.4%) | 1.331 (1.161–1.524) | 1.036 (0.903–1.189) | 28/351 (8.0%) | 5/138 (3.6%) | 2.191 (0.846–5.674) | 2.320 (0.875–6.151) | 0.3100/0.2004 |
| All-cause death/re-infarction | 395/4814 (8.2%) | 259/4126 (6.3%) | 1.320 (1.129–1.544) | 1.006 (0.858–1.181) | 11/351 (3.1%) | 1/138 (0.7%) | 4.345 (0.561–33.658) | 4.003 (0.502–31.932) | 0.2534/0.2001 |
| All-cause death | 248/4814 (5.2%) | 133/4126 (3.2%) | 1.614 (1.307–1.992) | 1.101 (0.889–1.364) | 9/3519 (2.6%) | 1/138 (0.7%) | 3.536 (0.448–27.888) | 3.199 (0.388–26.393) | 0.4546/0.3608 |
| Re-infarction | 213/4814 (4.3%) | 186/4126 (4.5%) | 1.604 (1.299–1.980) | 0.828 (0.677–1.012) | 2/351 (0.6%) | 0/138 (0.0%) | - | - | 0.9679/0.9566 |
| Bleeding | 181/4814 (3.8%) | 111/4126 (2.7%) | 1.416 (1.118–1.794) | 1.101 (0.865–1.401) | 17/351 (4.8%) | 5/138 (3.6%) | 1.331 (0.491–3.607) | 1.482 (0.533–4.117) | 0.9028/0.9748 |
HR (unadjusted and adjusted) showing the relationship between PPIs and clinical outcomes. HR: hazard ratios; PPIs: proton pump inhibitors.
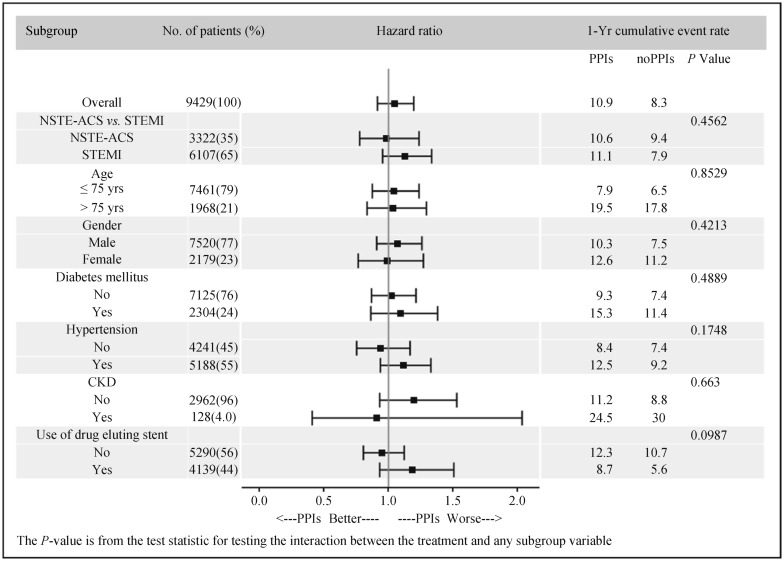
We also performed analyses in other seven subgroups and the results showed no significant association between PPIs use and the composite primary endpoint, which were consistent with the main results. In these analyses, there were no significant interactions with baseline or procedural variables, including the use of drug-eluting stent (Figure 3).
Figure 3. Subgroup analyses of 1-year primary endpoint (all-cause death/re-infarction/severe bleeding).
CKD: chronic kidney disease; NSTE-ACS: non-ST-segment elevation acute coronary syndrome; PPIs: proton pump inhibitiors; STEMI: ST-segment elevation myocardial infarction.
4. Discussion
In a large “real world” registry of patients with ACS undergoing PCI, concomitant use of PPI was not associated with increased risk of composite adverse clinical outcomes (all-cause death, re-infarction, or severe bleeding) in patients receiving either clopidogrel or ticagrelor. Our results are consistent with findings from propensity-matched analysis or randomized trial that reported no associated risk of cardiovascular events in patients treated with PPIs in combination with P2Y12 receptor inhibitors.[16],[20]
Current guidelines recommend use of PPIs in combination with DAPT in patients at higher than average risk of gastrointestinal bleeding.[1],[3],[4] However, several pharmacokinetic and pharmacodynamics studies have indicated that PPIs, especially omeprazole and esomeprazole, could blunt the antiplatelet effect of clopidogrel.[21]–[24] Conflicting data exist regarding use of PPIs on cardiovascular events in patients treated with clopidogrel. Most of observational studies showed PPIs increased risk of adverse events when co-administrated with clopidogrel.[13],[25]–[27] In the subgroup analysis of PLATO trial,[18] the use of PPIs was also associated with increased rate of cardiovascular events or even major bleeding in the clopidogrel group. It can be speculated that PPIs use is likely a marker for higher risk of cardiovascular events. High risk individuals are more prone to be prescribed a PPI.[28],[29] In our study, patients who were treated with PPIs were older and had more comorbidities, thus indicating a cluster of patients with high risk of ischemic and bleeding events.
After adjusting for potential confounders and the propensity to be treated with a PPI, our study demonstrated that concomitant use of PPIs and clopidogrel was not associated with a greater risk of net adverse clinical events. These results confirm and extend the findings of TRITON-TIMI 38,[20],[30] which showed no associated risk with clopidogrel and PPIs use. The only clinical trial COGENT[16] also failed to demonstrate a clinically significant cardiovascular interaction between clopidogrel and omeprazole. Therefore, despite the observed attenuation of in vitro anti-platelet effects of P2Y12 receptor inhibitors in patients treated with a PPI, it may not have significant effect on clinical outcomes.
In contrast to clopidogrel, ticagrelor is a direct-acting P2Y12 receptor inhibitor that does not require biotransformation and has no potential interaction with PPIs.[17] Yet, limited studies evaluated the impact of PPIs use on clinical benefit of ticagrelor. In the prespecified analysis of PLATO trial,[18] ticagrelor in combination with a PPI was also associated with increased risk of cardiovascular events. This finding was similar to that observed with clopidogrel and PPI use. In the present study, despite the observed tendency of higher risk of adverse events in the ticagrelor group when co-administrated with a PPI, the impact on clinical outcomes was non-significant after adjusting for potential confounders. To date, there is no pharmacologic or clinical evidence to support the interaction between ticagrelor and PPIs. Therefore, in the context of current guidelines, which recommend use of ticagrelor as first choice in ACS patients, concomitant use of a PPI is reasonable when the patients were at high risk of gastrointestinal bleeding.
4.1. Limitations
Our study has several limitations. First, as with other retrospective study, confoundings by unknown/unmeasured factors could not be entirely excluded. However, the components of the primary endpoint, including all-cause death, re-infarction, and bleeding, are hard endpoints and less subject to observation bias. Second, the use of a PPI was not randomized and depended on the decision of the treating physicians. Despite multivariable adjustment and propensity score matching for the PPI use, unobserved differences may still exist, which may lead to residual selection bias. However, this study enrolled consecutive patients from a retrospective, multi-center, observational registry, which may have less inclusion bias. Third, this study did not show an increased risk of primary endpoint in patients treated with concomitant ticagrelor and PPI, although there was a tendency toward a higher risk compared with no-PPI group. The small sample size and low incidence of all-cause death, re-infarction, and bleeding events in the ticagrelor subgroup may affect the power to detect such difference. Forth, although standard definitions were provided for the primary endpoints, events (including type of death) were not systematically validated or centrally adjudicated. Thus, the proportion of non-cardiovascular deaths may be overestimated. Finally, in most cases the follow-up information was based upon patient self-report and this could likely lead to under- and over-estimates of medical therapy, procedures, and events.
4.2. Conclusions
In conclusion, in a “real world” registry of patients with ACS following PCI, the use of PPIs in combination with clopidogrel was not associated with increased rate of adverse outcomes. Moreover, no association was observed between PPI use and adverse events in patients receiving ticagrelor. Therefore, in the context of current ACS guidelines with P2Y12 receptor inhibitors as first-line therapy, concomitant use of PPIs is reasonable, especially in patients with a higher risk for gastrointestinal bleeding.
Acknowledgments
The authors thank Dr. Xiao-Yan YAN and Dr. Yong-Pei YU (Medical Statistics, Peking University First Hospital, Beijing, China) for their technical assistance about data cleaning and statistical analysis.
Other BleeMACS investigators
Beijing Anzhen Hospital, Capital Medical University, Beijing, China: Zhang D and Chen YL; University Hospital Álvaro Cunqueiro, Vigo, Spain: García-Acuña JM; Dipartimento di Scienze Mediche, Divisione di Cardiologia, Città della Salute e della Scienza, Turin, Italy: Giordana F, Scarano S, and Gaita F; Libin Cardiovascular Institute of Alberta, Calgary, Canada: Southern DA; San Carlos Hospital, Madrid, Spain: Alfonso E and Terol B; Bellvitge Hospital, Barcelona, Spain: Garay A; University Patras Hospital, Athens, Greece: Xanthopoulou I; Kerckhoff Heart and Thorax Center, Frankfurt, Germany: Osman N and Möllmann H; University Clinical Hospital, Kyoto, Japan: Shiomi H; University Clinical Hospital, Warsaw, Poland: Kowara M and Filipiak KJ; Tokai University School of Medicine, Tokyo, Japan: Ikari Y; Kanazawa University Graduate School of Medicine, Kanazawa, Japan: Nakahayshi T, Sakata K, Yamagishi M; University Clinic of Cardiology, Skopje, Macedonia: Kalpak O.
References
- 1.Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 2.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82:E266–E355. doi: 10.1002/ccd.23390. [DOI] [PubMed] [Google Scholar]
- 4.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: The task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Gibson CM, Cheng S, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: Randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther. 2011;89:65–74. doi: 10.1038/clpt.2010.219. [DOI] [PubMed] [Google Scholar]
- 6.Frelinger AL, 3rd, Lee RD, Mulford DJ, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59:1304–1311. doi: 10.1016/j.jacc.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Shah BS, Parmar SA, Mahajan S, et al. An insight into the interaction between clopidogrel and proton pump inhibitors. Curr Drug Metab. 2012;13:225–235. doi: 10.2174/138920012798918390. [DOI] [PubMed] [Google Scholar]
- 8.Sofi F, Giusti B, Marcucci R, et al. Cytochrome p450 2c19*2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: A meta-analysis. Pharmacogenomics J. 2011;11:199–206. doi: 10.1038/tpj.2010.21. [DOI] [PubMed] [Google Scholar]
- 9.Holmes MV, Perel P, Shah T, et al. Cyp2c19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: A systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- 10.Simon T, Steg PG, Gilard M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: Results from the French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) registry. Circulation. 2011;123:474–482. doi: 10.1161/CIRCULATIONAHA.110.965640. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt M, Johansen MB, Robertson DJ, et al. Concomitant use of clopidogrel and proton pump inhibitors is not associated with major adverse cardiovascular events following coronary stent implantation. Aliment Pharmacol Ther. 2012;35:165–174. doi: 10.1111/j.1365-2036.2011.04890.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Ljung R, Lagergren J, et al. Prognosis of concomitant users of clopidogrel and proton-pump inhibitors in a high-risk population for upper gastrointestinal bleeding. BMC Pharmacol Toxicol. 2014;15:22. doi: 10.1186/2050-6511-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn SP, Steinhubl SR, Bauer D, et al. Impact of proton pump inhibitor therapy on the efficacy of clopidogrel in the CAPRIE and CREDO trials. J Am Heart Assoc. 2013;2:e004564. doi: 10.1161/JAHA.112.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLos One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng FH, Tunggal P, Chu WM, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol. 2012;107:389–396. doi: 10.1038/ajg.2011.385. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 17.Htun WW, Steinhubl SR. Ticagrelor: The first novel reversible p2y(12) inhibitor. Expert Opin Pharmacother. 2013;14:237–245. doi: 10.1517/14656566.2013.757303. [DOI] [PubMed] [Google Scholar]
- 18.Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978–986. doi: 10.1161/CIRCULATIONAHA.111.032912. [DOI] [PubMed] [Google Scholar]
- 19.D'Ascenzo F, Abu-Assi E, Raposeiras-Roubin S, et al. BleeMACS: Rationale and design of the study. J Cardiovasc Med (Hagerstown) 2016 doi: 10.2459/JCM.0000000000000362. Published Online First: Jan 27, 2016. [DOI] [PubMed] [Google Scholar]
- 20.O'Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: An analysis of two randomised trials. Lancet. 2009;374:989–997. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 21.Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: The randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–260. doi: 10.1016/j.jacc.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 22.Gilard M, Arnaud B, Le Gal G, et al. Influence of omeprazol on the antiplatelet action of clopidogrel associated to aspirin. J Thromb Haemost. 2006;4:2508–2509. doi: 10.1111/j.1538-7836.2006.02162.x. [DOI] [PubMed] [Google Scholar]
- 23.Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol. 2008;48:475–484. doi: 10.1177/0091270008315310. [DOI] [PubMed] [Google Scholar]
- 24.Siller-Matula JM, Spiel AO, Lang IM, et al. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J. 2009;157:148 e1–e5. doi: 10.1016/j.ahj.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Charlot M, Ahlehoff O, Norgaard ML, et al. Proton-pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: A nationwide cohort study. Ann Intern Med. 2010;153:378–386. doi: 10.7326/0003-4819-153-6-201009210-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 27.Harjai KJ, Shenoy C, Orshaw P, et al. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: An analysis from the Guthrie Health Off-Label Stent (GHOST) investigators. Circ Cardiovasc Interv. 2011;4:162–170. doi: 10.1161/CIRCINTERVENTIONS.110.958884. [DOI] [PubMed] [Google Scholar]
- 28.Shih CJ, Chen YT, Ou SM, et al. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177:292–297. doi: 10.1016/j.ijcard.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Hulot JS, Collet JP, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome p450 2c19*2 loss-of-function allele or proton pump inhibitor coadministration: A systematic meta-analysis. J Am Coll Cardiol. 2010;56:134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 30.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]