Abstract
Background
The coronary artery calcium (CAC) and aortic arch calcification (AoAC) are individually associated with cardiovascular disease and outcome. This study investigated the predictive value of AoAC combined with CAC for cardiovascular diagnosis and outcome in patients with angina.
Methods
A total of 2018 stable angina patients who underwent chest X-ray and cardiac multi-detector computed tomography were followed up for four years to assess adverse events, which were categorized as cardiac death, stroke, myocardial infarction, or repeated revascularization. The extent of AoAC on chest X-ray was graded on a scale from 0 to 3.
Results
During the four years of follow-up, 620 patients were treated by coronary stenting and 153 (7%) adverse events occurred. A higher grade of AoAC was associated with a higher CAC score. Cox regression showed that the CAC score, but not AoAC, were associated with adverse events. In patients with CAC score < 400, AoAC showed an additive predictive value in detecting significant coronary artery disease (CAD). A gradual increases in the risk of adverse events were noted if AoAC was present in patients with similar CAC score.
Conclusions
As AoAC is strongly correlated with the CAC score regardless of age or gender, careful evaluation of CAD would be required in patients with AoAC on conventional chest X-rays.
Keywords: Aortic arch, Atherosclerosis, Calcification, Coronary artery disease
1. Introduction
Atherosclerosis is a diffuse progressive disorder and the major cause of cardiovascular disease. Vascular calcification occurs as atherosclerosis advances and can be quantified readily using non-invasive radiographic imaging techniques. Abundant evidence has reproducibly shown that high levels of coronary artery calcium (CAC) are correlated with clinically significant coronary artery disease (CAD) and can identify patients at risk for adverse cardiac events.[1]–[4] However, routine CAC screening has not been recommended because of radiation hazards, cost and insufficient evidence.[5],[6] As vascular calcification would reflect overall systemic atherosclerotic burden, the association between coronary and extra-coronary calcification such as thoracic or abdominal aorta has been evaluated.[7]–[11] The results from these studies were obtained from lateral lumbar X-ray or CT procedures, which are not suitable for repeated assessments in clinical practice. Chest X-ray is a rapid screening tool that identifies the causes of chest pain or associated complications. Previous epidemiologic studies identified that aortic arch calcification (AoAC) detected on chest X-ray was associated with increased cardiovascular morbidity and mortality,[12]–[15] and AoAC was a strong independent predictor of cardiovascular events beyond traditional risk factors, including endothelial dysfunction.[12]–[15] These studies have some limitations that were small number in size and short-term follow-up in period. And then, it is not known whether AoAC correlates closely with the CAC score or whether AoAC could be additional benefit to predict adverse cardiac events compared with CAC score only. This study investigated the predictive value of AoAC combined with CAC for cardiovascular diagnosis and outcome in patients with angina.
2. Methods
2.1. Study population
This was a single center cohort study of stable angina patients who underwent cardiac multi-detector computed tomography (MDCT) and chest X-ray within one month of each other from April 2008 to July 2009. The total number of cardiac MDCT examinations during this time span was 3454. Patients were excluded from the study if they had a prior diagnosis of acute myocardial infarction (AMI), catheterization-defined CAD, or prior revascularization therapy. The final study population consisted of 2018 patients, and they were retrospectively evaluated for the rates of significant CAD requiring coronary revascularization and occurrence of death from all causes, MI, repeated coronary revascularizations, or stroke over a mean follow-up period of 3.8 ± 0.7 years (range 0.7–5.1 years).
The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the Kyung Hee University College of Medicine (KMC IRB 1119-03). The committee waived the need for written informed consent from the participants.
2.2. Assessment of aortic arch calcification
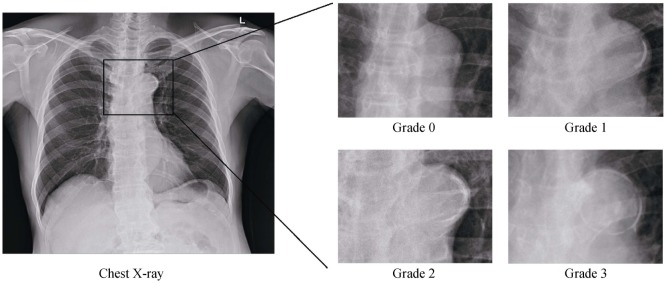
Two independent observers (observer A, 5 years of experience; observer B, 11 years of experience) blindly reviewed the postero-anterior chest X-rays of all subjects. Conflicts were resolved by discussion with the senior author. The extent of AoAC in each chest X-ray assessed is shown in Figure 1. The AoAC was graded semi-quantitatively on a 4-point scale using a modified method based on previous reports: grade 0, no visible calcification; grade 1, < 50% calcification in the arch; grade 2, > 50% calcification; grade 3, circumferential calcification.[14],[15] The concordance rate of this technique for grading was 94% in grade 0, 78% in grade 1, 74% in grade 2, and 96% in grade 3. Because grade 1 and 2 showed relatively low reproducibility, we categorized AoAC into three groups: grade 0, grade 1/2, and grade 3.
Figure 1. Assessment of aortic arch calcification from chest X-rays.
2.3. Assessment of coronary artery calcium score
CAC scoring was performed following the analysis of 64-slice cardiac MDCT scans (Brilliance 64, Philips Medical Systems, Best, the Netherlands) equipped with a standard cardiac reconstruction and post-processing package. Following scout chest radiography, a CAC score scan was performed using a 2.5-mm slice thickness, tube voltage of 120 kV, and tube current of 150 mA. Quantification of coronary calcification was performed using a dedicated 3D workstation (Extended Brilliance Workspace, Philips Medical Systems, Best, the Netherlands) by an experienced radiologist who was blinded to the clinical data of the participants. All pixels with a density > 130 Hounsfield units were automatically color marked, and the lesion was selected manually, followed by software recognition of the lesions on subsequent images. From the selected areas, the software calculated the lesion volume in cubic millimeters and the CAC score for each patient according to the Agatston method.[16] For further analysis, a CAC score was categorized into the either three groups (0–99, 100–399, ≥ 400) or two groups (< 100 and > 100).[17],[18]
2.4. Clinical outcomes and study end points
The standard clinic examination included a physician-performed interview and physical examination. Age was assessed at the time of the cardiac MDCT scan in 2008–2009 and cardiovascular risk factor data assessed during a clinical visit within the same time frame. Coronary angiography (CAG) and percutaneous coronary intervention (PCI), if indicated, were performed using standard techniques. All procedural and technical details and the choice of devices were left to the physician's judgment. Clinical follow-up was performed via an office visit or telephone contact by researchers blinded to cardiac MDCT and clinical data. Hospital records were screened for clinical events to confirm the obtained information. The primary end points were the predictive values of long-term adverse outcomes, including death from all causes, MI, stroke, unplanned coronary revascularizations (> 90 days after MDCT scan) and repeated PCI after index PCI. The secondary end point included the correlation between AoAC and CAC scores.
2.5. Statistical analysis
The analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL, USA). Differences were considered significant if the two-sided P value < 0.05. Continuous variables, presented as means ± SD, were evaluated for normal distribution and compared using analysis of variance. The continuous parameters with a skewed distribution were logarithmically transformed. Categorical variables, presented as frequencies and percentages, were compared using the Chi-square test or Fisher's exact test when appropriate. Correlations between two continuous variables were performed using the Pearson correlation coefficient or, if not normally distributed, the Spearman's rank correlation. Kaplan-Meier methods were used to describe survival curves according to AoAC and the CAC score. A multivariable logistic regression and cox proportional hazards model were used to estimate significant CAD and long-term clinical outcomes by model 1 (AoAC and CAC scores) and 2 (age, gender, diabetes, hypertension, smoking, dyslipidemia, chronic kidney disease, AoAC and CAC scores).
3. Results
The AoAC grades of the participants were distributed as follows: grade 0 (n = 1496, 74%), grade 1 (n = 256, 13%), grade 2 (n = 178, 9%) and grade 3 (n = 88, 4%). The mean CAC score was 143 (range: 0–7895). Baseline characteristics, cardiovascular risk factors, concomitant medications and laboratory findings of the study population according to the AoAC grades are summarized in Table 1. There were positive associations between AoAC grade and the following variables: age, systolic blood pressure, body mass index, high-sensitivity C-reactive protein, N-terminal pro-brain natriuretic peptide, hemoglobin A1c, current smoking, prevalence of diabetes, hypertension, and chronic kidney disease, as well as previous history of stroke and heart failure. The AoAC grade was negatively correlated with high-density lipoprotein cholesterol but not with other lipid parameters. A previous history of angina was not significantly associated with the AoAC grade.
Table 1. Demographics according to aortic arch calcification.
| Grade 0 (n = 1496) | Grade 1/2 (n = 434) | Grade 3 (n = 88) | P value | |
| Age, yrs | 58.9 ± 10.9 | 68.6 ± 8.7 | 72.2 ± 7.9 | < 0.001 |
| Male gender | 798 (53%) | 184 (42%) | 22 (25%) | < 0.001 |
| Framingham risk score | 12.6 ± 4.8 | 16.0 ± 3.6 | 17.6 ± 3.2 | < 0.001 |
| Hypertension | 843 (57%) | 333 (78%) | 71 (81%) | < 0.001 |
| Diabetes mellitus | 377 (26%) | 149 (35%) | 30 (34%) | < 0.001 |
| Dyslipidemia | 538 (51%) | 182 (60%) | 36 (54%) | 0.02 |
| Current smoker | 343 (32%) | 110 (31%) | 11 (14%) | 0.004 |
| Previous stroke | 153 (10%) | 72 (17%) | 13 (15%) | 0.001 |
| Chronic kidney disease | 77 (5%) | 60 (14%) | 27 (31%) | < 0.001 |
| History of heart failure | 47 (3%) | 31 (7%) | 8 (9%) | < 0.001 |
| Any antiplatelet agents | 648 (43%) | 250 (58%) | 50 (57%) | < 0.001 |
| β-blocker | 340 (23%) | 136 (32%) | 35 (40%) | < 0.001 |
| ACE inhibitor or ARB | 451 (30%) | 192 (44%) | 43 (49%) | < 0.001 |
| Calcium channel blocker | 417 (28%) | 170 (39%) | 32 (36%) | < 0.001 |
| Statins | 451 (30%) | 152 (35%) | 32 (36%) | 0.09 |
| Creatinine, mg/dL | 0.9 ± 0.9 | 1.1 ± 1.6 | 1.3 ± 1.7 | < 0.001 |
| Total cholesterol, mg/dL | 180.6 ± 42.6 | 177.2 ± 41.7 | 179.6 ± 48.4 | 0.37 |
| Triglyceride, mg/dL | 141.5 ± 76.9 | 138.0 ± 72.1 | 140.4 ± 71.9 | 0.71 |
| HDL-cholesterol, mg/dL | 50.1 ± 13.6 | 47.8 ± 13.0 | 45.2 ± 11.5 | < 0.001 |
| LDL-cholesterol, mg/dL | 110.9 ± 36.0 | 108.1 ± 37.1 | 110.1 ± 36.4 | 0.44 |
| Calcium, mg/dL | 8.9 ± 0.6 | 8.8 ± 0.8 | 8.8 ± 0.5 | 0.60 |
| Phosphate, mg/dL | 3.5 ± 0.6 | 3.5 ± 0.8 | 3.7 ± 0.6 | 0.04 |
| ALP, IU/L | 68.6 ± 25.4 | 70.7 ± 30.3 | 75.2 ± 26.7 | 0.04 |
| hsCRP, mg/L | 0.8 (0.4–2.1) | 1.0(0.5 – 3.2) | 0.9(0.5 – 2.5) | 0.003 |
| NT-proBNP, pg/mL | 54.6 (24.4 – 133.0) | 113.5 (47.1 – 463.0) | 295.0 (76.9 – 900.7) | < 0.001 |
| HbA1c, % | 6.3 ± 1.1 | 6.5 ± 1.3 | 6.4 ± 1.3 | 0.002 |
Data are presented as n (%), means ± SD or median (range) unless other indicated. ACE: angiotensin converting enzyme; ALP: alkaline phospatase; ARB: angiotensin receptor blocker; HbA1c: hemoglobin A1c; HDL: high density lipoprotein; hsCRP: high-sensitivity C-reactive protein; LDL: low density lipoprotein; NT-proBNP: N-terminal pro-brain natriuretic peptide.
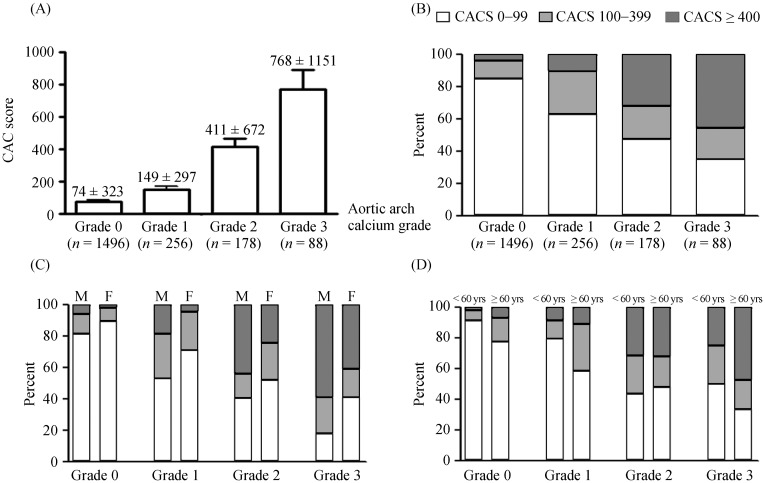
As shown in Figure 2A and 2B, subjects with lower grades of AoAC had lower CAC scores, and a greater number had a CAC score of 0–99. Subjects with higher grades of AoAC had higher CAC scores, and a greater number had CAC scores > 400. Regardless of gender (Figure 2C) and age (Figure 2D) differences, AoAC grades were positively associated with the CAC score.
Figure 2. Correlation between AoAC and the CAC score.
(A): Differences in the CAC score according to AoAC grades in all subjects; (B): distribution of the CAC score according to AoAC grades in all subjects; (C): distribution of the CAC score and AoAC grades according to gender; and (D) distribution of the CAC score and AoAC grades according to age (< 60 and ≥ 60 years). AoAC: aortic arch calcification; CAC: coronary artery calcium.
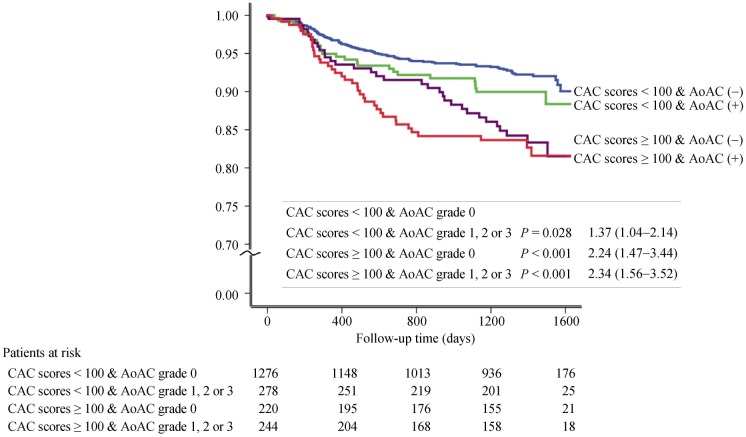
The results of clinical outcome are shown in Table 2. With increasing grades or scores of AoAC and CAC, there were significantly higher rates of CAD and total adverse events. In a regression model 1, AoAC and CAC score were independent predictors of significant CAD (Table 3), but the CAC score was an independent predictor of significant CAD adjusted by model 2 (age, gender, diabetes, hypertension, smoking, dyslipidemia, and chronic kidney disease). As shown in Table 3, the CAC score, but not AoAC, was an independent predictor of long-term adverse outcomes by model 1 and 2. Figure 3 shows the hazard ratio for total adverse events according to the CAC score with the presence or absence of AoAC using Kaplan-Meier analysis. If the CAC score cutoff value was set at 100, the presence of AoAC had a stepwise incremental predictive value for adverse events in patients with similar CAC scores.
Table 2. The incidence of significant coronary artery disease and clinical outcomes.
| Aortic arch calcification |
P value | Coronary artery calcium score |
P value | |||||
| Grade 0 (n = 1496) | Grade 1/2 (n = 434) | Grade 3 (n = 88) | 0−99 (n = 1554) | 100−399 (n = 278) | ≥= 400 (n = 186) | |||
| Significant CAD | 388 (25.9%) | 184 (42.4%) | 48 (54.5%) | < 0.001 | 355 (22.3%) | 144 (51.8%) | 121 (65.1%) | < 0.001 |
| Total adverse outcomes | 126 (8.4%) | 48 (11.1%) | 17 (19.3%) | < 0.001 | 120 (7.7%) | 35 (12.6%) | 36 (19.4%) | < 0.001 |
| Death | 1 (0.1%) | 1 (0.2%) | 0 | 0.61 | 1 (0.1%) | 0 | 1 (0.5%) | 0.13 |
| MI | 6 (0.4%) | 0 | 1 (1.1%) | 0.94 | 4 (0.3%) | 1 (0.4%) | 2 (1.1%) | 0.10 |
| Unplanned PCI | 61 (4.7%) | 17 (3.9%) | 5 (5.7%) | 0.74 | 51 (3.3%) | 15 (5.4%) | 17 (9.1%) | < 0.001 |
| Repeated PCI | 28 (1.9%) | 13 (3.0%) | 4 (4.5%) | 0.12 | 30 (1.9%) | 6 (2.2%) | 9 (4.8%) | 0.04 |
| CABG | 1 (0.1%) | 0 | 1 (1.1%) | 0.07 | 0 | 0 | 2 (1.1%) | < 0.001 |
| Stroke | 29 (1.9%) | 17 (3.9%) | 6 (6.8%) | < 0.001 | 34 (2.2%) | 13 (4.7%) | 5 (2.6%) | 0.05 |
Data are presented as n (%). CABG: coronary artery bypass graft surgery; CAD: coronary artery disease; CVD: cerebrovascular disease; MI: myocardial infarction; PCI: percutaneous coronary intervention.
Table 3. Univariate and multivariate Cox proportional hazards analysis.
| Prediction of significant CAD |
Prediction of total adverse events |
||||||||
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
||||||
| P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | ||
| Aortic arch calcification grade | < 0.001 | 0.006 | 0.001 | 0.26 | |||||
| Grade 0 | N/A | 1 | N/A | 1 | N/A | 1 | N/A | 1 | |
| Grade 1/2 | < 0.001 | 2.10 (1.68–2.63) | 0.005 | 1.42 (1.11–1.81) | 0.07 | 1.36 (0.98–1.89) | 0.65 | 1.08 (0.76–1.54) | |
| Grade 3 | < 0.001 | 3.42 (2.22–5.29) | 0.04 | 1.64 (1.01–2.67) | < 0.001 | 2.51 (1.51–4.17) | 0.10 | 1.59 (0.91–2.77) | |
| Coronary artery calcium score | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||
| 0–99 | N/A | 1 | N/A | 1 | N/A | 1 | N/A | 1 | |
| 100–399 | < 0.001 | 3.63 (2.79–4.72) | < 0.001 | 3.32 (2.54–4.35) | 0.009 | 1.65 (1.13–2.41) | 0.02 | 1.59 (1.08–2.34) | |
| ≥ 400 | < 0.001 | 6.29 (4.55–8.69) | < 0.001 | 5.21 (3.69–7.35) | < 0.001 | 2.84 (1.96–4.12) | < 0.001 | 2.50 (1.65–3.79) | |
CAD: coronary artery disease; N/A: not assessable.
Figure 3. Kaplan-meier analysis.
Total adverse events according to the CAC score with the presence or absence of AoAC. AoAC: aortic arch calcification; CAC: coronary artery calcium.
4. Discussion
The major findings of this cohort study were as follows: (1) AoAC evaluated on conventional chest X-rays strongly correlated with the CAC score on cardiac MDCT, regardless of age or gender; and (2) Although AoAC itself was not an independent predictor compared with CAC scores, AoAC evaluation could be valuable because the presence of AoAC had an additional benefit in subjects with similar CAC scores.
The correlation of calcifications in the coronary arteries and the aorta have been evaluated using several non-invasive imaging techniques, such as plain chest, abdomen, and lumbar X-rays, CT, electron beam CT (EBCT) and MDCT.[7]–[11] In addition, the prognostic implications of calcifications in the aortic arch, thoracic or abdominal aorta, alone or in combination, have been assessed for adverse cardiovascular events.[12],[13],[15],[19]–[25] This leads to questions concerning the level of calcification in the aorta that would predict future cardiovascular events greater than would the CAC score. While calcification of the thoracic aorta is associated with the CAC score, it was not shown to have a greater predictive value over CAC.[9],[10] In the abdominal aorta, a significant correlation with future cardiovascular events was found in 2467 Framingham Heart Study participants using plain abdominal X-ray during a 22-year period.[21],[22] There are no reports comparing the predictive value of AoAC or abdominal aorta calcification for cardiovascular events with that of the CAC score, although many studies have shown that AoAC or abdominal aorta calcification was positively associated with CAC scores and future cardiovascular events.[12]–[14],[15],[21],[22] It is generally accepted that a plain chest X-ray is a diagnostic baseline procedure in patients with chest discomfort. This study, as well as others, has demonstrated that assessment of AoAC on a chest X-ray is a simple and reliable method for risk assessment.
In contrast to the atherosclerotic features of coronary calcium, aortic calcification can be divided into two separate pathophysiological processes: intimal, which is primarily atherosclerotic, and medial, which is not atherosclerotic.[12],[23] Intimal calcification was associated with plaque vulnerability,[24] observed as a spotty and patchy radio-opaque finding. Medial calcification is usually associated with aging, end-stage renal disease and diabetes. It is seen as continuous linear deposits along the internal elastic lamina. However, it is difficult to distinguish these calcific changes in the arterial wall solely by radiographic techniques without using a pathologic approach. AoAC was correlated with carotid intima media thickness, pulse wave velocity, and poor flow mediated dilation,[15] suggesting that AoAC and CAC may have similar pathogeneses.
MDCT provides highly accurate information on coronary artery stenosis with excellent sensitivity and negative predictive value.[1]–[4] This commercially available scan also provides an accurate assessment of the amount of calcification for total or individual coronary arteries, and the result can be achieved quickly during a single breath-hold of a few seconds. Although CAC evaluation can provide additional information identifying patients at risk for adverse cardiac events,[1]–[4] it requires special equipment, is expensive to perform and is not suitable for repeated assessment in clinical practice. Additionally, routine CAC scanning of the asymptomatic adult population is not currently recommended, and there is little evidence determining the CAC score in an individual patient resulted in improved outcomes and reduced coronary events. Importantly, there are concerns regarding the associated radiation exposure.[25]–[27] Although the usual radiation dose for detecting CAC is relatively low (generally, 0.6–1.0 mSv for EBCT and 0.9–2.0 mSv for MDCT),[28] some MDCT imaging protocols are associated with estimated radiation doses > 10 mSv.[25] Einstein, et al.,[29] calculated that the risk for future cancer using MDCT at 14 mSv in a 20-year-old woman was estimated to be 1 in 219, compared with 1 in 715 in a 60-year-old woman and 1 in 1911 in a 60-year-old man (9 mSv). In comparison, chest X-rays are less expensive, easy to follow-up routinely and yield a radiation dose of 0.01–0.02 mSv.[26],[27] As AoAC is strongly correlated with the CAC score regardless of age or gender, careful evaluation of CAD would be required in patients with AoAC on conventional chest X-rays. In the present study, we examined AoAC using a simple standard chest X-ray method to investigate the prognostic features of cardiovascular events. As shown in Figure 3, the presence of AoAC showed additive predictive role in patients with CAC score < 100. It suggests AoAC evaluation would be more valuable in low to intermediate-risk probability groups. These could be valuable findings, since the current American Heart Association and European Society of Cardiology guidelines mention that CAC testing is not suitable for low-risk patients.
The recent PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial was performed with the enthusiasm evaluating the critical coronary stenosis to improve the prognosis of CAD patients. But anatomical approach did not achieve the superiority in clinical outcomes than functional testing strategy although we need to concern its inconclusive result due to limited statistical power.[30] In clinical practice, we could experience some patients with signs and symptoms of myocardial ischemia have normal or insignificant degree of coronary stenosis, and vise versa, others with severe CAD have neither any chest pain nor evidence of myocardial ischemia.[31] These suggest identification of anatomically obstructive CAD is not solely diagnostic work-up, rather understanding of atherosclerosis and functional status would improve patients' prognosis. But this study did not include the associations of AoAC and the results of functional tests. Other limitations were as follows: this study evaluated non-randomized, observational registry data. As in any observational cohort study, residual confounding is of concern. Additionally, only a small number of high-risk patients (23 patients with CACS ≥ 400) were included, which necessitates further studies using a larger group of patients.
Despite these drawbacks, AoAC combined with the CAC score is a valuable tool. In patients with similar CAC scores, AoAC was associated with an increasing risk of adverse events, suggesting that careful attention should be given to the presence of AoAC on plain chest X-ray.
Acknowledgments
This research was supported by the Bio Research & Development program through the National Research Foundation of Korea funded by grant 2012M3A9C6050507 from the Ministry of Education, Science and Technology (Seoul, Korea).
References
- 1.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 4.Silverman MG, Blaha MJ, Krumholz HM, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 7.Oei HH, Vliegenthart R, Hak AE, et al. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39:1745–1751. doi: 10.1016/s0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 8.Adler Y, Fisman EZ, Shemesh J, et al. Spiral computed tomography evidence of close correlation between coronary and thoracic aorta calcifications. Atherosclerosis. 2004;176:133–138. doi: 10.1016/j.atherosclerosis.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Wong ND, Gransar H, Shaw L, et al. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2:319–326. doi: 10.1016/j.jcmg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs PC, Prokop M, van der Graaf Y, et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209:455–462. doi: 10.1016/j.atherosclerosis.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Wong ND, Lopez VA, Allison M, et al. Abdominal aortic calcium and multi-site atherosclerosis: the Multiethnic Study of Atherosclerosis. Atherosclerosis. 2011;214:436–441. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 13.Odink AE, van der Lugt A, Hofman A, et al. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam study. Atherosclerosis. 2007;193:408–413. doi: 10.1016/j.atherosclerosis.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto H, Iijima K, Hashimoto M, et al. Validity and usefulness of aortic arch calcification in chest X-ray. J Atheroscler Thromb. 2009;16:256–264. doi: 10.5551/jat.e570. [DOI] [PubMed] [Google Scholar]
- 15.Iijima K, Hashimoto H, Hashimoto M, et al. Aortic arch calcification detectable on chest X-ray is a strong independent predictor of cardiovascular events beyond traditional risk factors. Atherosclerosis. 2010;210:137–144. doi: 10.1016/j.atherosclerosis.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 17.Rumberger JA, Sheedy PF, 2nd, Breen JF, et al. Electron beam computed tomography and coronary artery disease: scanning for coronary artery calcification. Mayo Clin Proc. 1996;71:369–377. doi: 10.4065/71.4.369. [DOI] [PubMed] [Google Scholar]
- 18.He ZX, Hedrick TD, Pratt CM, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–251. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 19.Walsh CR, Cupples LA, Levy D, et al. Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: the Framingham Heart Study. Am Heart J. 2002;144:733–739. doi: 10.1067/mhj.2002.124404. [DOI] [PubMed] [Google Scholar]
- 20.Chuang ML, Massaro JM, Levitzky YS, et al. Prevalence and distribution of abdominal aortic calcium by gender and age group in a community-based cohort (from the Framingham Heart Study) Am J Cardiol. 2012;110:891–896. doi: 10.1016/j.amjcard.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson PW, Kauppila LI, O'Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 22.Bastos Goncalves F, Voute MT, Hoeks SE, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–994. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 23.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 24.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 25.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188–1194. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 27.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 29.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 30.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzilli M, Merz CN, Boden WE, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol. 2012;60:951–956. doi: 10.1016/j.jacc.2012.02.082. [DOI] [PubMed] [Google Scholar]