Abstract
Doppler echocardiography is the gold standard for assessment of diastolic dysfunction, which is increasingly recognised as a cause of heart failure, especially in the elderly. Using a combination of Doppler echocardiography techniques, it is possible to identify grades of diastolic dysfunction, estimate left ventricular filling pressures and establish the chronicity of diastolic dysfunction. These physiologically-derived measures have been widely validated against invasive measurements of left heart pressures and have been shown to be prognostically valuable in a wide range of clinical settings. This review explores the mechanisms, and approaches to the assessment of diastolic dysfunction in the elderly. The challenge for clinicians is to identify pathophysiological changes from those associated with normal ageing. When used in combination, and taking age into account, Doppler echocardiographic parameters are helpful in the assessment of dyspnoea in older patients and provide prognostic insights.
Keywords: Diastolic dysfunction, Doppler, Echocardiography
1. Introduction
The ageing of population has become one of the most challenging global public health issues as advances in medicine and socioeconomic development have been leading to improved life expectancy worldwide.[1] Due to the progressive growing of the elderly population and to the increasing survival from all forms of cardiovascular disease, we can expect that the development of diastolic dysfunction (DD) will also rise. This review discusses the physiological contributors to diastolic function, the role of cardiac imaging for the diagnosis of DD and the prognostic implications of DD in the elderly.
The left ventricle (LV) has two sequential functions: systolic contraction and diastolic filling: both must be adequate in order to provide perfusion to the organs through forward cardiac output. Failure to maintain forward output and the consequent elevation of ventricular filling pressures are associated with symptoms of heart failure (HF). While systolic function is commonly assessed by measuring parameters of pump dysfunction, typically LV ejection fraction (LVEF), the evaluation of diastolic function remains challenging. Gold standards for diagnosing DD are measurements of LV diastolic pressure-volume relations and the rate of LV pressure fall during isovolumetric relaxation, usually recorded during cardiac catheterization. However, invasive methods are not feasible for daily clinical practice and Doppler echocardiography is routinely used for this purpose since Doppler measurements compare favourably with invasive measurements.[2] Doppler echocardiography is now commonly applied and accepted in a wide variety of settings as a reliable non-invasive tool to evaluate LV filling pressures and diastolic proprieties.[3]
Doppler assessment of diastolic function should be an integral part of a standard echocardiographic examination, especially in patients referred with symptoms or signs of HF (mainly dyspnoea and peripheral oedema): in this set of patients, up to half of all them will be found to have an LVEF within the normal range (so-called heart failure with preserved ejection fraction, HF-PEF).[4]
2. Diastolic function assessment
The current echocardiographic approach to LV diastolic function assessment aims to estimate the degree of LV filling pressure elevation. Alteration of LV relaxation is detected in hypertensive patients in association with LV remodelling, and it may be responsible for symptoms or signs of HF even when LVEF is preserved.[5] In the most recent European Society of Cardiology guidelines, HF diagnosis and therapeutic guidance is based on imaging, and the use of echocardiography is a Class I Level C recommendation due to its accuracy, availability and safety.[6] LV DD is thought to be the underlying pathophysiological alteration in patients with HF-PEF, and its identification is therefore crucial.[7] Noticeably, no single parameter is good enough to diagnose DD, and, in order to grade the severity of the diastolic filling pattern, a comprehensive evaluation of several echocardiographic measurements is required.
2.1. Mitral valve Doppler
The first step in assessment of DD by echocardiography is the mitral valve diastolic blood flow. The inflow velocity profile of blood moving down a pressure gradient from the left atrium (LA) to the LV can be used to characterize LV filling dynamics. Primary measurements include: the peak of early passive filling velocity (E wave), the late active diastolic filling velocity (A wave) which occurs in response to contraction of the atria, the ratio of early to late filling (E/A), and the deceleration time (DT) of the early filling E wave velocity. An additional index of diastolic function derived by mitral flow analysis is the isovolumetric relaxation time (IVRT), which is the interval from the end of the aortic ejection, signified as the closure of the aortic valve, to the onset of the mitral inflow, representing opening of the mitral valve. IVRT provides a good estimation of LA pressure and it becomes shortened when LV pressure is elevated, since the mitral valve opens in response to a high pressure gradient between LA and LV.[8] In addition to diastolic function, other conditions influence mitral inflow such as cardiac output, rhythm and heart rate. Moreover, a slower myocardial relaxation which occurs in the ageing heart is correlated with age-related changes in diastolic function,[9] as evidenced by reduced passive filling and more dependence on active filling (E: A < 1) and prolonged isovolumic relaxation and deceleration times.
2.2. Mitral annular tissue Doppler
More recently, the addition of tissue Doppler imaging to diastolic assessment through measurement of early diastolic velocity (e') using pulsed wave tissue Doppler imaging (TDI) of the mitral annulus has allowed clinicians to draw inferences about LV relaxation and to predict LV filling pressure. The myocardial fiber arrangement is complex and increasing evidence supports the importance of longitudinal systolic function and diastolic rebound in assessing myocardial relaxation. When used in isolation, both mitral E velocity and annular e' are impacted by stroke volume, but when used in combination with the mitral E wave velocity (E/e') good correlation with invasive LV filling pressures has been demonstrated.[2] At rest, septal e' and lateral e' are > 10 cm/s and > 15 cm/s in healthy individuals respectively, and they increase with exercise reflecting the ability to achieve a lower minimal LV diastolic pressure to increase early diastolic filling, while the impaired relaxation of the ageing heart is characterised by lower e' velocity, which remains depressed during exercise.
2.3. Left atrial size
The LA exerts a fundamental and somewhat independent physiological role in cardiovascular performance as a mechanical contributor to forward output behaving as a reservoir chamber and acting as a contractile pump for LV filling. The size of the LA provides essential information regarding the chronicity of the LV filling abnormalities and exposure to elevated filling pressures. In a young heart, the ventricle is compliant enough to respond to large pressure changes that occur during the cardiac cycle. In fact, even with normal physiology, the LV is exposed to the largest pressure change of all the cardiac chambers. The peak systolic pressure is equivalent to systemic blood pressure (range 100–140 mmHg) and after emptying, the LV pressure in diastole steeply decreases (normal range 3–12 mmHg). This pressure change is easily accommodated in a compliant heart, leading physicians to describe the LV in a young person as a “sucker” since the blood is almost sucked into the LV through rapid relaxation of the LV myocardium. But in an older and stiffer ventricle, the LV pressure rises rapidly, the LA is exposed to the increasing pressure of the LV during diastole when the mitral valve is open and, therefore, LA pressure must augment to maintain adequate LV filling. Left atrial pressure rise is facilitated through atrial contraction, and thus the LA plays a significant role in diastolic filling of the LV in older people. In an older heart, the LA transforms into a “pusher”, literally pushing the blood forward into a non-compliant LV cavity that has lost its “sucker” attributes.[2] This leads to histological changes that result in progressive LA enlargement, in order to prevent direct transmission of pressure to the pulmonary circulation. LA dilatation is detected in different scenarios, from mitral valve disease to atrial fibrillation and, in terms of diastolic function evaluation, it has been demonstrated to be a marker of long standing pressure.[10]
2.4. Pulmonary venous Doppler
In order to empty properly, the LA chamber must first fill efficiently. Filling is achieved through four pulmonary veins that are largely passive conduits for blood returning from the pulmonary circulation. Pulmonary venous (PV) blood flow, evaluated by pulsed wave Doppler, occurs in two phases that correlate with the systolic and diastolic phases of the cardiac cycle (peak systolic and diastolic PV flow velocity).[11] Blood flow to the LA is always along a pressure gradient from the PVs to the LA and in normal subject both phases are significant determinants of left heart filling, although most of the anterograde flow to the LA occurs when the LA pressures are lower during atrial relaxation. In ventricular diastole, the atrium acts as an open conduit through which blood flows directly from the pulmonary veins through the mitral valve into the left ventricle. This diastolic phase becomes the main determinant of atrial filling when the LA pressures increase.[12] There is a brief episode of retrograde blood flow from the LA to the pulmonary veins that corresponds to atrial systole, and the peak atrial reversal velocity (AR) and duration (ARdur) of this flow are directly related to LA pressure. The pulmonary veins offer a path of lower resistance and therefore the degree to which this atrial reversal of blood flow occurs is a measure of elevated LV filling pressure. Moreover, an ARdur longer than 30 ms compared to A wave duration of the mitral inflow is predictive of an increased LA pressure.[13]
2.5. Grading diastolic function
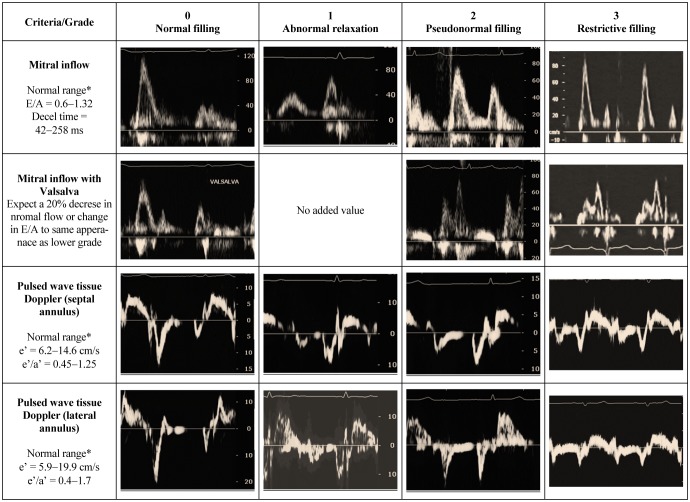
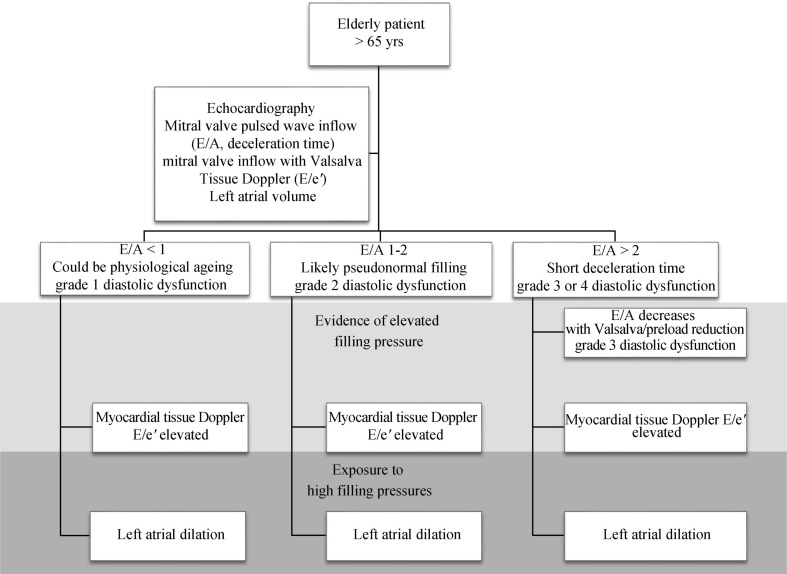
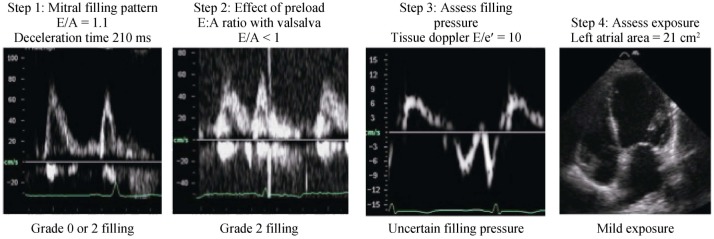
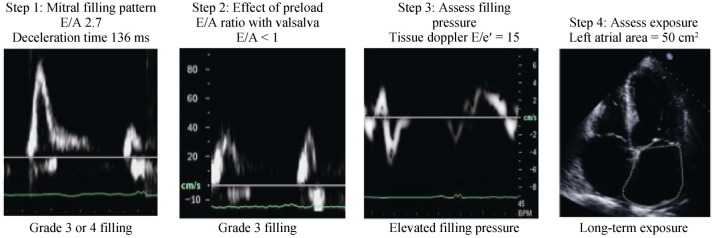
The combined analysis of the multiple parameters of diastolic filling can be used collectively to stratify degrees of DD in individual patients. One suggested approach describes the filling phase as grades: normal (grade 0), abnormal relaxation (grade 1), pseudonormal (grade 2) and restrictive filling pattern (grade 3) (Figure 1), which can be further classified as reversible or non-reversible with a preload reduction provocation. Assessment should commence with an instantaneous assessment of filling pattern (based on E/A ratio and deceleration time). Once mitral filling grade is established, this is this is followed by an estimate of filling pressure (E/e') and a measure of exposure (LA size). This approach (Figure 2) provides a quick and comprehensive assessment of the current and overall diastolic filling of the left ventricle. An impaired relaxation of the LV resulting in impaired early-diastolic filling is the main pathophysiologic feature of grade 1 diastolic dysfunction. Doppler LV inflow curve displays a reduced E velocity and early diastolic deceleration slope with an increased A velocity and a prolonged isovolumic relaxation time. The mitral annular TDI shows a reduced e' while the pulmonary vein flow is characterised by a decreased ratio of systolic to diastolic pulmonary venous flow. As the extent of diastolic dysfunction progresses, a reduced diastolic compliance of the LV is detected as well as an increasing in the LA pressure resulting in an increased pressure gradient from the LA to the LV at mitral valve opening. Thus, grade 2 diastolic dysfunction is present with an E/A ratio > 1 and a rapid deceleration slope. PV blood flood shows an increased diastolic phase and a reduced systolic phase. In addition, the increased resistance to LV filling results in an increase in the velocity and duration of atrial flow reversal. This is in contrast to the normal or grade 0 pattern, which is characterized by nearly equal systolic and diastolic PV flow velocities and reduced and short atrial reversal flow. Late in the disease course, a restrictive pattern of LV filling occurs with an increased E velocity and reduced A velocity, and shortened isovolumic relaxation time and DT. The increased ratio of peak flow velocity in early to late diastole is associated with elevated LV filling pressure. This approach can be seen in two cases, both men > 65 years presenting with shortness of breath on exertion. The first patient appears to have a normal filling pattern, which we may presume to be pseudonormal based on his age (Figure 3). This is confirmed by the reversal of the E/A with preload reduction (Valsalva) and the tissue Doppler (e'< a'), with no definite evidence of elevated filling pressure and a normal-mild left atrial area. This pattern suggests very early signs of DD. In contrast, the second patient's E/A suggests restrictive filling (Figure 4), which is confirmed to be reversible restriction with preload reduction (Valsalva). The high E/e' suggests a high filling pressure and the atrial area suggests that the left atrium has been exposed to high filling pressure for some time. This pattern suggests significant DD.
Figure 1. Diastolic filling grades based on mitral inflow and tissue Doppler indices.
*Normal values for > 60 years from Nagueh, et al. [3].
Figure 2. Algorithm to assess diastolic function in an elderly patient.
Figure 3. Case example of a stepped approach to the assessment of mild diastolic function.
Figure 4. Case example of a stepped approach to the assessment of severe diastolic function.
3. Invasive validation of Doppler
Doppler echocardiography does not measure diastolic function, but it does provide an estimate of filling pressure since many of the Doppler indices have been correlated with invasive direct measurements of left heart pressures and as such these Doppler indices are regarded as surrogates for filling pressure. The E/A ratio has been correlated with LVDP and mitral E wave deceleration time is negatively correlated with LV stiffness and filling pressures and associated with higher neurohormonal activity.[14] In a cohort of patients with coronary artery disease and EF < 50%, Yamamoto, et al.[15] found that E wave DT predicts LV filling pressures whereas it is inaccurate in patients with preserved systolic function. Despite the absence of atrial contraction, the interactions of the transmitral velocity with LA pressure in atrial fibrillation are similar to those observed in sinus rhythm and DT can then be used to predict LV filling pressure in both patients with systolic impairment or without systolic dysfunction.[16] The difference between ARdur and AR is correlated with LV filling pressure in patients and this relationship is observed in higher grades of diastolic filling abnormalities, regardless of EF.[15] These relationships are valid in AF where pulmonary capillary wedge pressure is negatively correlated with mitral deceleration time, IVRT and pulmonary deceleration time,[17],[18] and have been validated in patients with normal EF and mild systolic impairment[19] and evaluated under manipulated loading conditions.[20]
4. Why diastolic function matters?
Many of the elderly patients who present with HF are found with normal systolic function on presentation. This is widely referred to as heart failure with preserved ejection fraction (HF-PEF) and in the absence of coronary artery disease and other pathology the symptoms of patients presenting with HF-PEF are often attributed to DD.[21] Much interest in DD over recent years has possibly over-implicated DD as the pathophysiologic cause of heart failure symptoms and this is in part because many of the individual diastolic parameters have been shown to be predictive of outcome, independently of systolic function, in a wide variety of clinical settings.[22] Recording a normal filling pattern in patients with HF symptoms delineates a very good prognosis, whereas an abnormal diastolic function indicates patients with a higher risk of mortality, and this risk is seen to increase progressively as the diastolic function becomes worst.[23],[24] Some studies have reported an increasing mortality in patients with abnormal relaxation pattern after acute myocardial infarction.[25],[26] In a population of heart failure patients admitted to the hospital for exacerbation of symptoms, pseudonormal filling pattern was associated with hospital admission rates and mortality similar to those seen with restrictive filling.[27] Severe diastolic impairment, or restrictive filling pattern, has been linked to a 2-fold increase in mortality in patients post acute myocardial infarction (AMI) independently of Killip class and LVEF.[24] Similarly, restrictive filling has been linked to prognosis in patients with HF regardless of age and LVEF.[28] Thus, diastolic function is not innocuous; when severe diastolic function is present in hospitalized elderly patients, the rate of cardiovascular and all-cause mortality is comparable to that observed in subjects with systolic dysfunction, and furthermore it provides independent and incremental prognostic information of all-cause mortality.[29]
Tissue Doppler derived indices have also been linked to poor prognosis in other patient populations, for example, the mitral annulus velocity in e' provides incremental predictive power for cardiac mortality compared to clinical data and standard echocardiographic measurements in patients with impaired LV systolic function,[30] and contributes to a comprehensive risk stratification in hypertensive patients under treatment.[31] Furthermore, left ventricular diastolic pressure (LVDP) has been correlated with the ratio of early transmitral flow/early annulus velocity, and this last parameter was found to predict poorer survival after AMI independently of LVEF.[32] The prognostic power of E/e' has also been established in a cohort of patients without either systolic or diastolic dysfunction assessed with conventional echocardiographic tools.[33]
Lastly, LA size is a powerful predictor of outcome in the general population,[34] and in a wide variety of cardiac diseases.[35],[36] Among HF patients, LA area demonstrates a strong association with mortality, even after adjustment for LVEF, DT, clinical symptoms and age,[37] and LA volume index predicts survival after AMI, providing prognostic information which is incremental to clinical data and standard evaluation of LV systolic function.[38] Patients with LV systolic dysfunction due to either ischemic or idiopathic dilated cardiomyopathy with a diastolic predominant pulmonary venous flow (defined by a systolic to diastolic peak velocity ratio < 1) are at higher risk of heart failure hospitalisations and death from end-stage heart failure, and moreover this blunted pulmonary venous flow had more prognostic power compared to reduced LVEF, older age and increased heart rate.[39]
5. Physiology of the ageing heart
Just like many other tissues, myocardial histological changes do occur in the ageing heart. Myocyte loss due to apoptosis or necrosis has been detected, and this decrease in muscle mass is accompanied by a cellular hypertrophic response in the remaining myocytes.[40] The enlargement of cardiomyocites has been detected at autopsy in elderly patients who died without apparent cardiovascular disease and poor reaction of stem cells prevents adequate compensation for the myocyte damage, either caused by ageing or due to myocardial injury and ischemia.[41] Moreover, increased collagen and extracellular matrix deposition has been reported, along with a change in the collagen proprieties, mostly due to non-enzymatic cross-link.[42] This increased collagen turnover has been correlated with a worse diastolic filling pattern and subsequently with a poor prognosis in a cohort of patients with dilated cardiomyopathy, independently of LA size and LVEF.[43] This process is even associated with development of fibrosis, which is promoted by several mechanisms, many of which occur in patients with HF: higher hormonal and immune activation, mostly due to angiotensin II, aldosterone, and inflammatory cytokines,[44],[45] are considered to be the key factors in the progression of LV dysfunction and HF. Overall, these changes lead to an increased prevalence of LV hypertrophy and impaired relaxation, with lower elasticity and reduced ability to respond to pressure variation (compliance).[46] This hypertension-related LV hypertrophy and concentric remodelling of the LV finally leads to diastolic HF. Furthermore, age-related changes affect the whole cardiovascular system, with an increasing atherosclerotic process that involves arterial walls, which are thicker and stiffer.
Arterial changes are easily assessed using carotid ultrasound scanning. With high-resolution systems, it is now possible to measure the intima-media complex of the carotid arterial wall. The extent of carotid intima-media thickness is an independent predictor of cardiac and cerebrovascular events, adding value in stratification of patients beyond assessment of traditional risk factors.[47] Increasing stiffness is responsible for the high pulse pressure (the difference between systolic and diastolic blood pressure values) that is detected in elderly hypertensive patients, which has been reported to have a possible additional prognostic role.[48] Moreover, the calcification process that could be considered one of the last effect of the pathway leading to fibrosis, affects the heart valves too, especially the aortic valve, which is often sclerotic or stenotic. A sclerotic aortic valve was found to be a significant predictor of carotid atherosclerosis, independently of other clinical and echocardiographic risk factors,[49] and it is associated with all-cause and cardiovascular mortality in a high cardiovascular risk population (type 2 diabetic patients).[50]
6. Assessment of diastolic function in the elderly
Age is a fundamental parameter when defining normal diastolic values and different cut-offs have to be considered.[2] With the increasing stiffness of the ageing heart, diastolic impairment can be detected. Age is strongly associated with diastolic velocity parameters and the mitral inflow velocity profile changes with a progressively decreased E wave, while the atrial contribution becomes more vigorous,[51] leading to an equalization of E and A velocities at approximately age 50 years and to a reversed E/A after that age and occurs independent of cardiovascular disease and other confounding physiologic variables. Deceleration time increases since a longer time is needed to accommodate blood volume during the diastole in a LV cavity with reduced compliance. Age was found to be the strongest independent determinant of LV filling in a large cohort of healthy volunteers.[52] LA filling is altered by many of the same variables that affect LV filling and a reduction in the diastolic filling phase and a compensatory increase in the systolic one can be detected with ageing. In addition, a more prominent atrial reversal is common in elderly patients.[53] TDI measurements have been reported to be age-dependent too: recently it has been documented a decrease of e' of approximately 1 cm/s per decade and an increase of E/e' of about 1 unit per decade in a cohort of asymptomatic patients referred for a routine echocardiography. This trend has been observed both in patients with and without LV hypertrophy due to hypertension and it suggests that different cut-off values should be used to assess diastolic dysfunction through tissue Doppler in elderly patients.[54]
Some of the clinical conditions that are associated with DD are also frequently detected in elderly patients.[55] LV hypertrophy is common in the older population, since the high prevalence of hypertension, and hypertensive heart disease is the most common abnormality leading to diastolic heart failure.[56] DD occurs before the structural changes associated with hypertensive heart disease,[57] and regarding the molecular changes, there is evidence that the increasing collagen deposition that occurs with LV hypertrophy leads to passive structural changes which are detected in the stiff aged heart.[58] Atrial fibrillation is an age-related condition,[59] and it is correlated to many physiologic and pathologic factors which can be detected in DD, such as hypertension,[60] obesity,[61] and diabetes.[62] Up to 30% of patients with DD and new onset of HF symptoms are found to have atrial fibrillation,[63] and recently DD has been described to be a predictor of first documented non valvular atrial fibrillation in a cohort of elderly patients referred for echocardiography with the gradient of risk which is correlated with the severity of the DD itself.[64]
The challenge comes for clinicians to differentiate what changes are anticipated from expected ageing of the heart from those associated with pathophysiological changes. In people over 65 years of age, some degree of myocardial stiffness may be anticipated and, therefore, an abnormal relaxation pattern (grade 1 diastolic dysfunction) could be expected. This has been described as “physiologic” by some and “normal for age” by others. However, this pattern (E/A < 1) should not be regarded as “normal” but perhaps more appropriately as “expected”. An E/A > 1 would be unusual in this group and pseudonormal filling should always be considered. Recently, our group showed that in the setting of acute myocardial infarction in patients over 65, E/A > 1 was associated with worse prognosis.[65]
7. Conclusions
Diastolic dysfunction is a common finding in elderly patients referred for echocardiography and is linked to many concomitant conditions that are also age-related. And whilst, an abnormal relaxation pattern should be anticipated in responses to normal ageing of the heart, more advanced diastolic filling rarely occurs in response to age alone and in patients over 65 years, E/A> 1 should always be considered abnormal. In large population studies, diastolic dysfunction has been consistently shown to be independently related to outcome and should be assessed irrespective of the patient's age. In large population studies, diastolic dysfunction has been consistently shown to be independently related to outcome and should be assessed irrespective of the patient's age.
References
- 1.Suzman R, Beard JR, Boerma T, et al. Health in an ageing world-what do we know? Lancet. 2014;9967:484–486. doi: 10.1016/S0140-6736(14)61597-X. [DOI] [PubMed] [Google Scholar]
- 2.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 3.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 4.Rigolli M, Whalley GA. Heart failure with preserved ejection fraction. J Geriatr Cardiol. 2013;10:360–376. doi: 10.3969/j.issn.1671-5411.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee P, Banerjee T, Khand A, et al. Diastolic heart failure: neglected or misdiagnosed? J Am Coll Cardiol. 2002;39:138–141. doi: 10.1016/s0735-1097(01)01704-1. [DOI] [PubMed] [Google Scholar]
- 8.Oh JK, Appleton CP, Hatle LK, et al. The noninvasive assessment of left ventricular diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 1997;10:246–270. doi: 10.1016/s0894-7317(97)70062-2. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med. 2006;16:273–227. doi: 10.1016/j.tcm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lancellotti P, Henri C. The left atrium: an old ‘barometer’ which can reveal great secrets. Eur J Heart Fail. 2014;16:1047–1048. doi: 10.1002/ejhf.155. [DOI] [PubMed] [Google Scholar]
- 11.Keren G, Sherez J, Megidish R, et al. Pulmonary venous flow pattern--its relationship to cardiac dynamics. A pulsed Doppler echocardiographic study. Circulation. 1985;71:1105–1112. doi: 10.1161/01.cir.71.6.1105. [DOI] [PubMed] [Google Scholar]
- 12.Rossvoll O, Hatle LK. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: Relation to left ventricular diastolic pressures. J Am Coll Cardiol. 1993;21:1687–1696. doi: 10.1016/0735-1097(93)90388-h. [DOI] [PubMed] [Google Scholar]
- 13.Keren G, Milner M, Lindsay J, et al. Load dependence of left atrial and left ventricular filling dynamics by transthoracic and transesophageal Doppler echocardiography. Am J Card Imaging. 1996;10:108–116. [PubMed] [Google Scholar]
- 14.Margulies KB, Jaffer S, Pollack PS, et al. Physiological significance of early deceleration time prolongation in asymptomatic elderly subjects. J Card Fail. 1999;5:92–99. doi: 10.1016/s1071-9164(99)90031-3. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto K, Nishimura RA, Chaliki HP, et al. Determination of left ventricular filling pressure by Doppler echocardiography in patients with coronary artery disease: Critical role of left ventricular systolic function. J Am Coll Cardiol. 1997;30:1819–1826. doi: 10.1016/s0735-1097(97)00390-2. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Kopelen HA, Quinones MA. Assessment of left ventricular filling pressures by doppler in the presence of atrial fibrillation. Circulation. 1996;94:2138–2145. doi: 10.1161/01.cir.94.9.2138. [DOI] [PubMed] [Google Scholar]
- 17.Traversi E, Cobelli F, Pozzoli M. Doppler echocardiography reliably predicts pulmonary artery wedge pressure in patients with chronic heart failure even when atrial fibrillation is present. Eur J Heart Fail. 2001;3:173–181. doi: 10.1016/s1388-9842(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 18.Chirillo F, Brunazzi MC, Barbiero M, et al. Estimating mean pulmonary wedge pressure in patients with chronic atrial fibrillation from transthoracic Doppler indexes of mitral and pulmonary venous flow velocity. J Am Coll Cardiol. 1997;30:19–26. doi: 10.1016/s0735-1097(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 19.Poerner TC, Goebel B, Unglaub P, et al. Non-invasive evaluation of left ventricular filling pressures in patients with abnormal relaxation. Clin Sci. 2004;106:485–494. doi: 10.1042/CS20030169. [DOI] [PubMed] [Google Scholar]
- 20.Choong CY, Herrmann HC, Weyman AE, et al. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800–808. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 21.Hart CY, Redfield MM. Diastolic heart failure in the community. Curr Cardiol Rep. 2000;2:461–469. doi: 10.1007/s11886-000-0061-y. [DOI] [PubMed] [Google Scholar]
- 22.Badano LP, Albanese MC, De Biaggio P, et al. Prevalence, clinical characteristics, quality of life, and prognosis of patients with congestive heart failure and isolated left ventricular diastolic dysfunction. J Am Soc Echocardiogr. 2004;17:253–261. doi: 10.1016/j.echo.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 24.Møller JE, Whalley GA, Dini FL, et al. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation. 2008;117:2591–2598. doi: 10.1161/CIRCULATIONAHA.107.738625. [DOI] [PubMed] [Google Scholar]
- 25.Møller JE, Egstrup K, Køber L, et al. Prognostic importance of systolic and diastolic function after acute myocardial infarction. Am Heart J. 2003;145:147–153. doi: 10.1067/mhj.2003.46. [DOI] [PubMed] [Google Scholar]
- 26.Quintana M, Edner M, Kahan T, et al. Is left ventricular diastolic function an independent marker of prognosis after acute myocardial infarction? Int J Cardiol. 2004;96:183–189. doi: 10.1016/j.ijcard.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Whalley GA, Doughty RN, Gamble GD, et al. Pseudonormal mitral filling pattern predicts hospital re-admission in patients with congestive heart failure. J Am Coll Cardiol. 2002;39:1787–1795. doi: 10.1016/s0735-1097(02)01868-5. [DOI] [PubMed] [Google Scholar]
- 28.Meta-analysis Research Group in Echocardiography (MeRGE) Heart Failure Collaborators. Doughty RN, Klein AL, et al. Independence of restrictive filling pattern and LV ejection fraction with mortality in heart failure: An individual patient meta-analysis. Eur J Heart Fail. 2008;10:786–792. doi: 10.1016/j.ejheart.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Safar ME, Iaria P, et al. Prevalence and prognosis of left ventricular diastolic dysfunction in the elderly: The PROTEGER Study. Am J Cardiol. 2010;160:471–478. doi: 10.1016/j.ahj.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Yp G, Yu CM, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005;45:272–277. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Yip GW, Wang AY, et al. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens. 2005;23:183–191. doi: 10.1097/00004872-200501000-00029. [DOI] [PubMed] [Google Scholar]
- 32.Hillis GS, Møller JE, Pellikka PA, et al. Noninvasive estimation of left ventricular filling pressure by E/e' is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004;43:360–367. doi: 10.1016/j.jacc.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 33.Mogelvang R, Sogaard P, Pedersen SA, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 34.Benjamin EJ, D'Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 35.Rossi A, Tomaino M, Golia G, et al. Usefulness of left atrial size in predicting postoperative symptomatic improvement in patients with aortic stenosis. Am J Cardiol. 2000;86:567–570. doi: 10.1016/s0002-9149(00)01019-5. [DOI] [PubMed] [Google Scholar]
- 36.Ammash NM, Seward JB, Bailey KR, et al. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation. 2000;101:2490–2496. doi: 10.1161/01.cir.101.21.2490. [DOI] [PubMed] [Google Scholar]
- 37.Rossi A, Temporelli PL, Quintana M, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure) Eur J Heart Fail. 2009;11:929–936. doi: 10.1093/eurjhf/hfp112. [DOI] [PubMed] [Google Scholar]
- 38.Moller JE, Hillis GS, Oh JK, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 39.Dini FL, Dell'Anna R, Micheli A, et al. Impact of blunted pulmonary venous flow on the outcome of patients with left ventricular systolic dysfunction secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2000;85:1455–1460. doi: 10.1016/s0002-9149(00)00794-3. [DOI] [PubMed] [Google Scholar]
- 40.Olivetti G, Melissari M, Capasso JM, et al. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 41.Wong LSM, van der Harst P, de Boer RA, et al. Aging, telomeres and heart failure. Heart Fail Rev. 2010;15:479–486. doi: 10.1007/s10741-010-9173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 43.Rossi A, Cicoira M, Golia G, et al. Amino-terminal propeptide of type III procollagen is associated with restrictive mitral filling pattern in patients with dilated cardiomyopathy: a possible link between diastolic dysfunction and prognosis. Heart. 2004;90:650–654. doi: 10.1136/hrt.2002.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brilla CG, Zhou G, Matsubara L, et al. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994;26:809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- 45.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2002;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 46.Stern S, Behar S, Gottlieb S. Cardiology patient pages. Aging and diseases of the heart. Circulation. 2003;108:e99–e101. doi: 10.1161/01.CIR.0000086898.96021.B9. [DOI] [PubMed] [Google Scholar]
- 47.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 48.Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- 49.Rossi A, Faggiano P, Amado AE, et al. Mitral and aortic valve sclerosis/calcification and carotid atherosclerosis: results from 1065 patients. Heart Vessels. 2014;29:776–783. doi: 10.1007/s00380-013-0433-z. [DOI] [PubMed] [Google Scholar]
- 50.Rossi A, Targher G, Zoppini G, et al. Aortic and mitral annular calcifications are predictive of all-cause and cardiovascular mortality in patients with type 2 diabetes. Diabetes Care. 2012;35:1781–1786. doi: 10.2337/dc12-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitzman DW, Sheikh KH, Beere PA, et al. Age-related alterations of Doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol. 1991;18:1243–1250. doi: 10.1016/0735-1097(91)90542-h. [DOI] [PubMed] [Google Scholar]
- 52.Benjamin EJ, Levy D, Anderson KM, et al. Determinants of Doppler indexes of left ventricular diastolic function in normal subjects (the Framingham Heart Study) Am J Cardiol. 1991;70:508–515. doi: 10.1016/0002-9149(92)91199-e. [DOI] [PubMed] [Google Scholar]
- 53.Klein AL, Burstow DJ, Tajik AJ, et al. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc. 1994;69:212–224. doi: 10.1016/s0025-6196(12)61059-3. [DOI] [PubMed] [Google Scholar]
- 54.De Sutter J, De Backer J, Van de Veire N, et al. Effects of age, gender, and left ventricular mass on septal mitral annulus velocity (E') and the ratio of transmitral early peak velocity to E' (E/E') Am J Cardiol. 2005;95:1020–1023. doi: 10.1016/j.amjcard.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Zabalgoitia M, Rahman SN, Haley WE, et al. Comparison in systemic hypertension of left ventricular mass and geometry with systolic and diastolic function in patients < 65 to > or = 65 years of age. Am J Cardiol. 1998;82:604–608. doi: 10.1016/s0002-9149(98)00404-4. [DOI] [PubMed] [Google Scholar]
- 56.Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665–671. doi: 10.1002/ejhf.304. [DOI] [PubMed] [Google Scholar]
- 57.Aeschbacher BC, Hutter D, Fuhrer J, et al. Diastolic dysfunction precedes myocardial hypertrophy in the development of hypertension. Am J Hypertens. 2001;14:106–113. doi: 10.1016/s0895-7061(00)01245-0. [DOI] [PubMed] [Google Scholar]
- 58.Villari B, Campbell SE, Hess OM, et al. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–1484. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- 59.Nagarakanti R, Ezekowitz M. Diastolic dysfunction and atrial fibrillation. J Interv Card Electrophysiol. 2008;22:111–118. doi: 10.1007/s10840-008-9203-8. [DOI] [PubMed] [Google Scholar]
- 60.Palmiero P, Zito A, Maiello M, et al. Left ventricular diastolic function in hypertension: methodological considerations and clinical implications. J Clin Med Res. 2015;7:137–144. doi: 10.14740/jocmr2050w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study) J Am Coll Cardiol. 2010;55:2319–2327. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falcão-Pires I, Palladini G, Gonçalves N, et al. Distinct mechanisms for diastolic dysfunction in diabetes mellitus and chronic pressure-overload. Basic Res Cardiol. 2011;106:801–814. doi: 10.1007/s00395-011-0184-x. [DOI] [PubMed] [Google Scholar]
- 63.Chen HH, Lainchbury JG, Senni M, et al. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fai. 2002;8:279–287. doi: 10.1054/jcaf.2002.128871. [DOI] [PubMed] [Google Scholar]
- 64.Tsang TSM, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 65.Rigolli M, Rossi A, Quintana M, et al. The prognostic impact of diastolic dysfunction in patients with chronic heart failure and post-acute myocardial infarction: Can age-stratified E/A ratio alone predict survival? Int J Cardiol. 2015;181:362–368. doi: 10.1016/j.ijcard.2014.12.051. [DOI] [PubMed] [Google Scholar]