Abstract
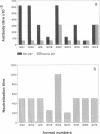
At present immunization against Theileria parva is by infection with live sporozoites and simultaneous treatment with a long-acting oxytetracycline. This method has major limitations in that live organisms are used and the immunity engendered is parasite stock specific. In an attempt to develop an alternative immunization procedure, the gene encoding p67, a major surface antigen of sporozoites, has been expressed by using the plasmid expression vector pMG1. The gene, which has been characterized previously, encodes 709 amino acid residues, contains a single intron of 29 base pairs, and is only transcribed during sporogony. The recombinant p67 sequences were fused to the first 85 amino acid residues derived from a nonstructural gene (NS1) of influenza virus A. Immunization with a partially purified recombinant antigen emulsified in 3% saponin induced sporozoite neutralizing antibodies in cattle and provided protection in six of nine animals on homologous challenge with T. parva sporozoites. This recombinant antigen is therefore a candidate for development of a vaccine against T. parva.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbet A. F., McGuire T. C. Crossreacting determinants in variant-specific surface antigens of African trypanosomes. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1989–1993. doi: 10.1073/pnas.75.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. G., Radley D. E., Cunningham M. P., Kirimi I. M., Morzaria S. P., Musoke A. J. Immunization against East Coast fever (Theileria parva infection of cattle) by infection and treatment: chemoprophylaxis with N-pyrrolidinomethyl tetracycline. Tropenmed Parasitol. 1977 Sep;28(3):342–348. [PubMed] [Google Scholar]
- Cunningham M. P., Brown C. G., Burridge M. J., Purnell R. E. Cryopreservation of infective particles of Theileria parva. Int J Parasitol. 1973 Sep;3(5):583–587. doi: 10.1016/0020-7519(73)90082-9. [DOI] [PubMed] [Google Scholar]
- Dobbelaere D. A., Spooner P. R., Barry W. C., Irvin A. D. Monoclonal antibody neutralizes the sporozoite stage of different Theileria parva stocks. Parasite Immunol. 1984 Jul;6(4):361–370. doi: 10.1111/j.1365-3024.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Dolan T. T., Young A. S., Losos G. J., McMilian I., Minder C. E., Soulsby K. Dose dependent responses of cattle to Theileria parva stabilate. Int J Parasitol. 1984 Feb;14(1):89–95. doi: 10.1016/0020-7519(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Egan J. E., Weber J. L., Ballou W. R., Hollingdale M. R., Majarian W. R., Gordon D. M., Maloy W. L., Hoffman S. L., Wirtz R. A., Schneider I. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science. 1987 Apr 24;236(4800):453–456. doi: 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- Emery D. L. Adoptive transfer of immunity to infection with Theileria parva (East Coast fever) between cattle twins. Res Vet Sci. 1981 May;30(3):364–367. [PubMed] [Google Scholar]
- Eugui E. M., Emery D. L. Genetically restricted cell-mediated cytotoxicity in cattle immune to Theileria parva. Nature. 1981 Mar 19;290(5803):251–254. doi: 10.1038/290251a0. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Doxsey S., Stagg D. A., Young A. S. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Electron microscopic observations. Eur J Cell Biol. 1982 Apr;27(1):10–21. [PubMed] [Google Scholar]
- Goddeeris B. M., Katende J. M., Irvin A. D., Chumo R. S. Indirect fluorescent antibody test for experimental and epizootiological studies on East coast fever (Theileria parva infection in cattle). Evaluation of a cell culture schizont antigen fixed and stored in suspension. Res Vet Sci. 1982 Nov;33(3):360–365. [PubMed] [Google Scholar]
- Goddeeris B. M., Morrison W. I., Teale A. J., Bensaid A., Baldwin C. L. Bovine cytotoxic T-cell clones specific for cells infected with the protozoan parasite Theileria parva: parasite strain specificity and class I major histocompatibility complex restriction. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5238–5242. doi: 10.1073/pnas.83.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin A. D., Mwamachi D. M. Clinical and diagnostic features of East Coast fever (Theileria parva) infection of cattle. Vet Rec. 1983 Aug 27;113(9):192–198. doi: 10.1136/vr.113.9.192. [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., Crighton G. W., Pirie H. M. Theileria parva: kinetics of replication. Exp Parasitol. 1969 Feb;24(1):9–25. doi: 10.1016/0014-4894(69)90215-x. [DOI] [PubMed] [Google Scholar]
- Katende J. M., Goddeeris B. M., Morzaria S. P., Nkonge C. G., Musoke A. J. Identification of a Theileria mutans-specific antigen for use in an antibody and antigen detection ELISA. Parasite Immunol. 1990 Jul;12(4):419–433. doi: 10.1111/j.1365-3024.1990.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mack S. R., Vanderberg J. P., Nawrot R. Column separation of Plasmodium berghei sporozoites. J Parasitol. 1978 Feb;64(1):166–168. [PubMed] [Google Scholar]
- Maimone M. M., Morrison L. A., Braciale V. L., Braciale T. J. Features of target cell lysis by class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1986 Dec 1;137(11):3639–3643. [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J., Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979 Oct;38(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Rurangirwa F. R., Buscher G. Evidence for a common protective antigenic determinant on sporozoites of several Theileria parva strains. Immunology. 1984 Jun;52(2):231–238. [PMC free article] [PubMed] [Google Scholar]
- Radley D. E., Brown C. G., Burridge M. J., Cunningham M. P., Peirce M. A., Purnell R. E. East Coast fever: quantitative studies of Theileria parva in cattle. Exp Parasitol. 1974 Oct;36(2):278–287. doi: 10.1016/0014-4894(74)90067-8. [DOI] [PubMed] [Google Scholar]
- Shatzman A. R., Rosenberg M. The pAS vector system and its application to heterologous gene expression in Escherichia coli. Hepatology. 1987 Jan-Feb;7(1 Suppl):30S–35S. doi: 10.1002/hep.1840070706. [DOI] [PubMed] [Google Scholar]
- Shaw M. K., Tilney L. G., Musoke A. J. The entry of Theileria parva sporozoites into bovine lymphocytes: evidence for MHC class I involvement. J Cell Biol. 1991 Apr;113(1):87–101. doi: 10.1083/jcb.113.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J., Duffus W. P. Trypanosoma theileri: antibody-dependent killing by purified populations of bovine leucocytes. Clin Exp Immunol. 1982 May;48(2):289–299. [PMC free article] [PubMed] [Google Scholar]
- Tsuji M., Romero P., Nussenzweig R. S., Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990 Nov 1;172(5):1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. G., Duffus W. P., Burridge M. J. The specific immunoglobulin response in cattle immunized with isolated Theileria parva antigens. Parasitology. 1974 Aug;69(1):43–53. doi: 10.1017/s0031182000046163. [DOI] [PubMed] [Google Scholar]
- Webster P., Dobbelaere D. A., Fawcett D. W. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Immunoelectron microscopic observations. Eur J Cell Biol. 1985 Mar;36(2):157–162. [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P., Ferguson B., Shatzman A. R., Rosenberg M. Efficient expression of influenza virus NS1 nonstructural proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6105–6109. doi: 10.1073/pnas.80.19.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]