Abstract
SIRT6 has been demonstrated to exert protective effects on endothelial cells and is closely associated with lipid metabolism, glucose metabolism, and obesity, indicating an important role in the pathogenesis and progression of coronary artery disease (CAD). Nonetheless, the biological significance of SIRT6 variants on CAD is far to be elucidated. Here we aimed to investigate the influence of SIRT6 polymorphisms on individual susceptibility and severity of CAD. Multivariate logistic regression analysis exhibited no significant association between these five polymorphisms and CAD risk in the genotype and allele frequencies. However, we found that the rs352493 polymorphism in SIRT6 exhibited a significant effect on the severity of CAD; C allele (χ 2 = 7.793, adjusted P = 0.013) and the combined CC/CT genotypes (χ 2 = 5.609, adjusted P = 0.031) presented the greater CAD severity. In addition, A allele (χ 2 = 5.208, adjusted P = 0.046) and AA (χ 2 = 4.842, adjusted P = 0.054) of rs3760908 were also associated with greater CAD severity in Chinese subjects. Our data provided the first evidence that SIRT6 tagSNPs rs352493 and rs3760908 play significant roles in the severity of CAD in Chinese Han subjects, which might be useful predictors of the severity of CAD.
1. Introduction
Coronary artery disease (CAD) poses the predominant health threat to the public and is the leading cause of death and morbidity worldwide. Previous studies and clinical trials have demonstrated that various environmental factors contribute to the development of CAD, including obesity, diabetes, alcohol intake, tobacco use, hypercholesterolemia, dyslipidemia, and hypertension [1, 2]. Moreover, apart from these modifiable risk factors, accumulating evidences have established that genetic variants or polymorphisms in candidate genes are closely associated with CAD pathogenesis, which have been estimated to account for 40–60% of the risk for CAD in epidemiology, family, and twin studies [3, 4].
SIRT6, one of the seven members of the NAD(+)-dependent histone deacetylase family of sirtuins, has been considered as one of the most important longevity genes [5–7]. Transgenic mice overexpressing SIRT6 exhibit a significantly longer life span, while the SIRT6-deficient mice have severe metabolic defects, developed ageing-like phenotypes by 2-3 weeks of age, and eventually died at about 4 weeks [5, 8]. In addition to the life span regulation function, a growing body of evidences have unraveled that SIRT6 also exerts crucial effects on cell metabolism and DNA damage repair by directly influencing the expression of target genes [9–13]. Among these, the association between SIRT6 and lipid metabolism has been well established. SIRT6 has been reported to serve as a negative regulator of TG synthesis and low-density lipoprotein (LDL) cholesterol homeostasis. SIRT6 deficiency resulted in accumulation of TG and LDL cholesterol levels [14–16]. Vice versa, SIRT6-overexpressing mice exhibit a decrease in visceral fat accumulation and improved blood lipid profile [17]. Moreover, the sterol-regulatory element binding protein, a key regulator of cholesterol biosynthesis, has been validated as a substrate of SIRT6 [18]. SIRT6 was found to repress the expression of sterol-regulatory element binding protein by suppressing its promoter activity and protein cleavage and activating the AMPK signal pathway [19]. Knockout of SIRT6 caused elevated serum cholesterol levels, while ectopic expression of SIRT6 diminished the serum cholesterol levels, thus improving hypercholesterolemia and atherosclerosis [20, 21]. Therefore, it was reasonable to speculate that SIRT6 might exert an important role in CAD pathogenesis and progression.
Single nucleotide polymorphisms (SNPs) are the most frequent genetic variants in the human genome, which have been demonstrated to affect individual susceptibility for a variety of human diseases. Mounting evidences have suggested that SNPs within the candidate genes may potentially contribute to the development of CAD [22–24]. Nonetheless, the genetic causes and underlying molecular mechanisms of these candidate genes for CAD pathogenesis are still far to be elucidated. As SIRT6 plays crucial roles in cellular senescence, genome integrity, and lipid metabolism and improves hypercholesterolemia and atherosclerosis [25], we speculated that the SNPs within the SIRT6 gene might influence the susceptibility and severity of CAD as well. Thus, we herein conducted a case-control study to investigate the associations of five tagSNPs (rs11878868, rs107251, rs352493, rs4807546, and rs3760908) in SIRT6 gene with the risk and severity of CAD. Our results uncovered that rs352493 and rs3760908 polymorphisms within SIRT6 gene were associated with the higher CAD severity in Chinese Han subjects.
2. Materials and Methods
2.1. Study Subjects
The study population consisted of 1129 subjects (655 CAD-free controls and 474 CAD patients). All enrolled subjects were recruited from the Affiliated Hospital of Guangdong Medical University (Zhanjiang, China) and the First People's Hospital of Foshan (Foshan, China) from March 2011 to October 2014. All CAD subjects were previously untreated and newly diagnosed. The diagnosis of CAD was confirmed by coronary angiography performed with the Judkins technique. CAD was defined as angiographic evidence of at least one segment of a major epicardial coronary artery with more than 50% organic stenosis. Patients were divided into three groups (1-vessel, 2-vessel, and 3-vessel stenosis) according to the number of significantly stenotic vessels. A total of 655 control individuals were consecutively recruited from the two hospitals for regular physical examinations when CAD subjects were recruited. The unaffected controls were defined to be free of CAD by clinical examination, medical history, electrocardiography, and questionnaires. Subjects with peripheral vascular disease, rheumatic heart disease, congestive heart failure, pulmonary heart disease, hepatic disease, chronic kidney, or any malignancy were excluded from the study. All enrolled subjects were genetically unrelated Han Chinese. The diagnosis of hypertension was established if the individual's systolic blood pressure (SBP) was above 140 mm Hg or diastolic blood pressure (DBP) was above 90 mm Hg or if the patient was on antihypertensive medication, respectively. Diabetes mellitus was defined as fasting blood glucose (FBG) being above 7.0 mmol/L or patient being on antidiabetic drug treatment. Subject was defined as smoker if he/she smoked once a day for over 1 year. Dyslipidemia was defined as triglyceride (TG) concentration being >1.70 mmol/L or total cholesterol (TC) concentration being > 5.72 mmol/L or patient being on lipid-lowering therapy. This study was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University and the First People's Hospital of Foshan, and informed consent was obtained from all recruited subjects. A structured questionnaire was administered to collect information on demographic data and the risk factors related to CAD.
2.2. Biochemical Analysis
An approximately 2 mL blood sample was collected from each participant into tubes containing EDTA. The blood sample was centrifuged at 2000 ×g for 15 min after collection and then stored at −80°C until analysis. Serum concentrations of TC, TG, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured enzymatically on a chemistry analyzer (Olympus, Japan). Glucose was measured via the glucose oxidase method (Abbott Laboratories, USA).
2.3. DNA Extraction
Genomic DNA was isolated from peripheral whole blood using TIANamp blood DNA extraction kit (TianGen Biotech, Beijing, China). All DNA samples were dissolved in water and then stored at −20°C until use.
2.4. TagSNP Selection and Genotyping
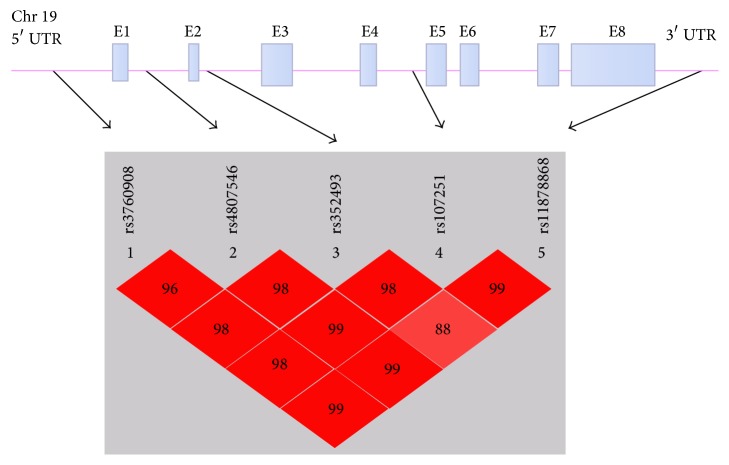
The Chinese Han population's SNP data of SIRT6 gene (8,427 bp, 8 exons) plus 3 kb upstream and downstream was obtained in the HapMap data release 27 (http://www.hapmap.org/) [26]. Further analysis of these data was performed utilizing Haploview software version 4.2. Using linkage disequilibrium patterns with r 2 > 0.8 as a cutoff, five tagSNPs (rs11878868, rs107251, rs352493, rs4807546, and rs3760908) in SIRT6 gene were selected for genotyping. All of the tagSNPs have a MAF (minor allele frequency) > 0.05 in the HapMap Chinese Han population. The positions of SNPs were shown in Figure 1. These five tagSNPs would capture the information of 7 known SIRT6 SNPs with a MAF > 0.05. The r 2 information for the five tagSNPs and alleles captured accordingly was exhibited in Table S1 (see Supplementary Material available online at http://dx.doi.org/10.1155/2016/1628041). The potential functions of these SNPs were predicted by a platform freely available online (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.htm). The haplotypic blocks of the five tagSNPs were estimated by the Haploview software version 4.2 and then the haplotype analysis was done by using the SHEsis software (http://analysis.bio-x.cn/myAnalysis.php)[27]. Six common haplotypes (frequency > 3%) derived from the five tagSNPs accounted for almost 100% of the haplotype variations. The allelic sequence in the haplotypes is in the following order: rs11878868, rs107251, rs352493, rs4807546, rs3760908. For example, haplotype GCCTA represents rs11878868G-rs107251C-rs352493C-rs4807546T-rs3760908A.
Figure 1.
Schematic of SIRT6 gene structure and pairwise LD between the five tagSNPs. SIRT6 gene consists of 8 exons (boxes, E1~E8) separated by 7 introns and spans a region of 8,427 bp. Filled boxes indicate the coding regions. The arrows indicate the locations of single nucleotide polymorphism. D′ values are plotted as a graph to show linkage disequilibrium between the five tagSNPs.
Genomic DNA was genotyped by PCR-LDR method (Shanghai Biowing Applied Biotechnology Company). The sequences of probes and primers were summarized in Table S2. The PCR was performed on the GeneAmp PCR system 9600 (Perkin Elmer, Norwalk, CT, USA) in 20 μL total volume including 50 ng genomic DNA, 1x PCR buffer, 1 U Taq polymerase, 2 mM dNTPs, 3 mM MgCl2, and 0.5 μM primer mix. The cycling parameters were as follows: 95°C for 2 min; 40 cycles at 94°C for 90 s, 56°C for 90 s, and 65°C for 30 s; and a final extension step at 65°C for 10 min. The ligation reaction for each PCR product was performed with a final volume of 10 μL containing 4 μL of PCR product, 1 μL 1x buffer, 2 U Taq DNA ligase, and 2 pmol probe mixture. The LDR parameters were as follows: 95°C for 2 min and 40 cycles at 94°C for 15 s and 50°C for 25 s. Following the LDR reaction, 1 μL LDR reaction product was mixed with 1 μL loading buffer and 1 μL ROX. The mixture was then analyzed by the ABI Prism 3730 DNA Sequencer (Applied Biosystems, USA). About 10% of these samples were randomly selected for repeated genotyping for confirmation, and the results were 100% concordant.
2.5. Statistical Analysis
The statistical analyses were performed using the SPSS software package (version 21). The haplotype analysis on the polymorphisms was undertaken by using the SHEsis online webserver. All the five SIRT6 tagSNPs were tested for confirmation within Hardy-Weinberg expectations through a goodness-of-fit χ 2 test in the control subjects. Qualitative variables were presented as percentages, and quantitative variables were expressed as means ± standard deviation. Student's t-test was used for continuous variables and χ 2 test was used for categorical variables in the differences of the demographic characteristics between the CAD and control groups. The association of SIRT6 tagSNPs with CAD severity was evaluated by χ 2 test. The association of SIRT6 polymorphisms with the severity of CAD has been determined by logistic regression model with adjustment by age, sex, smoking, drinking, hypertension, diabetes, and hyperlipidemia. Association between the risk for CAD and the tagSNP was evaluated via logistic regression analysis, adjusted by conventional risk factors (age, sex, drinking, smoking, diabetes, hypertension, and hyperlipidemia). P values of less than 0.05 were considered statistically significant for all statistical tests.
3. Results
3.1. Characteristics of Study Subjects
The baseline characteristics of the case and control subjects were presented in Table 1. Compared with the controls, a higher rate of patients with CAD were male, smoking and alcohol consumers, with prevalence of hypertension, dyslipidemia, and diabetes, and with higher levels of SBP, FBG, TG, and LDL-C, but lower HDL-C. These data demonstrated that male gender, alcohol intake, smoking, hyperlipidemia, hypertension, and diabetes mellitus were the important risk factors for developing CAD in this study.
Table 1.
The characteristics of CAD cases and controls.
| Variable | Controls (n = 655) | Cases (n = 474) | P a |
|---|---|---|---|
| Age (years) | 61.50 ± 12.50 | 63.19 ± 11.68 | 0.021 |
| Sex (male) | 379 (57.9%) | 362 (76.4%) | <0.001 b |
| Smoking | 154 (23.5%) | 286 (60.3%) | <0.001 |
| Alcohol consumption | 99 (15.1%) | 123 (25.9%) | <0.001 |
| Hypertension | 221 (33.7%) | 293 (61.8%) | <0.001 |
| Diabetes | 104 (15.9%) | 229 (48.3%) | <0.001 |
| Hyperlipidemia | 239 (36.5%) | 314 (66.2%) | <0.001 |
| Systolic BP (mm Hg) | 131.83 ± 19.38 | 139.70 ± 19.80 | <0.001 |
| Diastolic BP (mm Hg) | 72.78 ± 10.63 | 73.42 ± 11.24 | 0.332 |
| FPG (mM) | 5.79 ± 1.95 | 6.27 ± 1.64 | <0.001 |
| Triglycerides (mM) | 1.48 ± 0.81 | 1.90 ± 1.09 | <0.001 |
| Total cholesterol (mM) | 4.59 ± 1.15 | 4.67 ± 1.21 | 0.266 |
| HDL cholesterol (mM) | 1.39 ± 0.67 | 1.24 ± 0.44 | <0.001 |
| LDL cholesterol (mM) | 2.61 ± 0.92 | 2.88 ± 0.92 | <0.001 |
aTwo-sided chi-square test or independent-samples t-test.
b P values under 0.05 were indicated in bold font.
3.2. Multivariate Associations of SIRT6 tagSNPs with the Risk of CAD
Five SIRT6 tagSNPs (rs11878868, rs107251, rs352493, rs4807546, and rs3760908) were genotyped in 474 CAD patients and 655 control subjects. The primary information for these tagSNPs was shown in Table 2. MAFs for all five tagSNPs in our controls were similar to those for Chinese subjects in HapMap database (Table 2). Genotype frequencies did not deviate from the Hardy-Weinberg equilibrium in the controls (all P values ≥ 0.05, Table 2), providing no evidence of population stratification within the dataset. After adjustment for the risk factors including age, gender, drinking, smoking, hypertension, hyperlipidemia, and diabetes, there was no significant difference between CAD cases and controls in the genotype and allele frequencies in these five tagSNPs (all P values ≥ 0.05, Table 3).
Table 2.
Primary information for tagSNPs in SIRT6 gene.
| Genotyped SNPs | rs11878868 | rs107251 | rs352493 | rs4807546 | rs3760908 |
|---|---|---|---|---|---|
| Chr Pos (genome build 106) | 4173640 | 4176088 | 4180839 | 4182063 | 4184515 |
| Pos in SIRT6 gene | 3′ UTR | Intron 4 | Intron 2 | Intron 1 | 5′ UTR |
| MAFa for Chinese (CHB) population in HapMap | 0.110 | 0.310 | 0.240 | 0.390 | 0.430 |
| MAF in controls (n = 655) | 0.097 | 0.296 | 0.254 | 0.365 | 0.434 |
| P value for HWEb test in controls | 0.411 | 0.773 | 0.785 | 0.839 | 0.093 |
aMAF: minor allele frequency.
bHWE: Hardy-Weinberg equilibrium.
Table 3.
Multivariate associations of tagSNPs in SIRT6 gene with the risk of CAD.
| Type | Controls (n = 655) | Cases (n = 474) | OR (95% CI)a | P a |
|---|---|---|---|---|
| Number (%) | Number (%) | |||
| rs11878868 | ||||
| Additive | ||||
| G | 1183 (90.3) | 856 (90.3) | 1 | |
| T | 127 (9.7) | 92 (9.7) | 0.97 (0.69–1.36) | 0.870 |
| Dominant | ||||
| GT + GG | 647 (98.8) | 471 (99.4) | 1 | |
| TT | 8 (1.2) | 3 (0.6) | 0.60 (0.14–2.57) | 0.487 |
| Recessive | ||||
| GG | 536 (81.8) | 385 (81.2) | 1 | |
| GT + TT | 119 (18.2) | 89 (18.8) | 1.00 (0.69–1.45) | 0.993 |
|
| ||||
| rs107251 | ||||
| Additive | ||||
| T | 388 (29.6) | 279 (29.4) | 1 | |
| C | 922 (70.4) | 669 (70.6) | 1.13 (0.90–1.41) | 0.293 |
| Dominant | ||||
| TT | 59 (9.0) | 37 (7.8) | 1 | |
| CC + CT | 596 (91.0) | 437 (92.2) | 1.65 (0.98–2.76) | 0.060 |
| Recessive | ||||
| CT + TT | 329 (50.2) | 242 (51.1) | 1 | |
| CC | 326 (49.8) | 232 (48.9) | 1.04 (0.78–1.39) | 0.774 |
|
| ||||
| rs352493 | ||||
| Additive | ||||
| T | 977 (74.6) | 721 (76.1) | 1 | |
| C | 333 (25.4) | 227 (23.9) | 0.99 (0.78–1.25) | 0.921 |
| Dominant | ||||
| CC | 41 (6.3) | 30 (6.3) | 1 | |
| CT + TT | 614 (93.7) | 444 (93.7) | 0.98 (0.54–1.78) | 0.944 |
| Recessive | ||||
| CT + CC | 292 (44.6) | 197 (41.6) | 1 | |
| TT | 363 (55.4) | 277 (58.4) | 0.99 (0.74–1.32) | 0.929 |
|
| ||||
| rs4807546 | ||||
| Additive | ||||
| C | 478 (36.5) | 352 (37.1) | 1 | |
| T | 832 (63.5) | 596 (62.9) | 0.94 (0.76–1.16) | 0.565 |
| Dominant | ||||
| TT | 263 (40.2) | 189 (39.9) | 1 | |
| CT + CC | 392 (59.8) | 285 (60.1) | 0.96 (0.72–1.28) | 0.763 |
| Recessive | ||||
| CT + TT | 569 (86.9) | 407 (85.9) | 1 | |
| CC | 86 (13.1) | 67 (14.1) | 0.86 (0.57–1.30) | 0.478 |
|
| ||||
| rs3760908 | ||||
| Additive | ||||
| G | 741 (56.6) | 562 (59.3) | 1 | |
| A | 569 (43.4) | 386 (40.7) | 0.95 (0.77–1.17) | 0.616 |
| Dominant | ||||
| AA | 113 (17.3) | 86 (18.1) | 1 | |
| AG + GG | 542 (82.7) | 388 (81.9) | 0.88 (0.60–1.29) | 0.506 |
| Recessive | ||||
| GG | 199 (30.4) | 174 (36.7) | 1 | |
| AG + AA | 456 (69.6) | 300 (63.3) | 0.83 (0.61–1.12) | 0.212 |
aAdjusted for age, sex, smoking, drinking, hypertension, diabetes, and hyperlipidemia.
3.3. Association between the Haplotypes of SIRT6 tagSNPs with the Risk of CAD
Haplotype analyses were performed to further investigate the combinational effects of these tagSNPs on CAD risk. As shown in Figure 1, the five tagSNPs were located in one haplotypic block. We thus further assessed the haplotype frequencies of the five tagSNPs between CAD group and controls. Haplotype with frequency over 0.03 will be considered in statistical analysis [28]. Six common haplotypes derived from the five tagSNPs accounted for almost 100% of the haplotype variations. Among the six common haplotypes, no haplotype was found to be associated with the risk for CAD (Table 4).
Table 4.
Association between haplotypes of tagSNPs in SIRT6 gene with the risk of CAD.
| Haplotypea | Controls | Cases | OR (95% CI) | P |
|---|---|---|---|---|
| Number (%) | Number (%) | |||
| Total | n = 655 | n = 474 | ||
| GCCTA | 328.69 (25.1) | 223.42 (23.6) | 0.93 (0.76–1.12) | 0.432 |
| GCTCG | 86.43 (6.6) | 71.83 (7.6) | 1.17 (0.84–1.61) | 0.356 |
| GCTTA | 108.25 (8.3) | 62.54 (6.6) | 0.79 (0.57–1.09) | 0.145 |
| GCTTG | 266.76 (20.4) | 214.82 (22.7) | 1.15 (0.94–1.41) | 0.174 |
| GTTCG | 385.25 (29.4) | 274.28 (28.9) | 0.98 (0.82–1.18) | 0.846 |
| TCTTA | 125.02 (9.5) | 90.91 (9.6) | 1.01 (0.76–1.34) | 0.950 |
aThe allelic sequence in the haplotypes is in the following order: rs11878868, rs107251, rs352493, rs4807546, rs3760908.
3.4. The Association of SIRT6 tagSNPs with the Severity of CAD
The severity of CAD was evaluated according to the number of vessels with significant stenosis. To analyze a possible association between SIRT6 tagSNPs and the severity of CAD, patients were grouped into those with 1- and 2-vessel disease and 3-vessel disease (50% luminal obstruction) coronary arteries. The association of SIRT6 tagSNPs with the severity of disease was presented in Table 5. By χ 2 test and logistic regression analysis, we found that rs352493 polymorphism in SIRT6 exhibited a significant effect on the severity of CAD in the Chinese Han population; C allele (χ 2 = 7.793, P = 0.005, and adjusted P = 0.013) and the combined CC/CT genotypes (χ 2 = 5.609, P = 0.018, and adjusted P = 0.031) tended to have greater CAD severity. In addition, A allele (χ 2 = 5.208, P = 0.022, and adjusted P = 0.046) and AA (χ 2 = 4.842, P = 0.028, and adjusted P = 0.054) of rs3760908 also presented the greater CAD severity in the Chinese Han population.
Table 5.
SIRT6 tagSNPs in association with diseased vessel (n = 474).
| Type | 1VD and 2VDa (n = 292) | 3VD (n = 182) | χ 2 | P | OR (95% CI)c | P c |
|---|---|---|---|---|---|---|
| Number (%) | Number (%) | |||||
| rs11878868 | ||||||
| G | 530 (90.8) | 326 (89.6) | 0.364 | 0.546 | 1 | 0.563 |
| T | 54 (9.2) | 38 (10.4) | 1.14 (0.73–1.80) | |||
| GG | 240 (82.2) | 145 (79.7) | 0.467 | 0.494 | 1 | 0.501 |
| GT + TT | 52 (17.8) | 37 (20.3) | 1.18 (0.73–1.90) | |||
|
| ||||||
| rs107251 | ||||||
| C | 405 (69.3) | 264 (72.5) | 1.091 | 0.296 | 1 | 0.463 |
| T | 179 (30.7) | 100 (27.5) | 0.89 (0.66–1.21) | |||
| CC | 139 (47.6) | 93 (51.1) | 0.548 | 0.459 | 1 | 0.647 |
| CT + TT | 153 (52.4) | 89 (48.9) | 0.92 (0.63–1.34) | |||
|
| ||||||
| rs352493 | ||||||
| T | 462 (79.1) | 259 (71.2) | 7.793 | 0.005 b | 1 | 0.013 |
| C | 122 (20.9) | 105 (28.8) | 1.47 (1.09–2.00) | |||
| TT | 183 (62.7) | 94 (51.6) | 5.609 | 0.018 | 1 | 0.031 |
| CC + CT | 109 (37.3) | 88 (48.4) | 1.52 (1.04–2.24) | |||
|
| ||||||
| rs4807546 | ||||||
| T | 360 (61.6) | 236 (64.8) | 0.978 | 0.232 | 1 | 0.443 |
| C | 224 (38.4) | 128 (35.2) | 0.90 (0.68–1.18) | |||
| TT | 109 (37.3) | 80 (44.0) | 2.054 | 0.152 | 1 | 0.177 |
| CT + CC | 183 (62.7) | 102 (56.0) | 0.77 (0.52–1.13) | |||
|
| ||||||
| rs3760908 | ||||||
| A | 221 (37.8) | 165 (45.3) | 5.208 | 0.022 | 1 | 0.046 |
| G | 363 (62.2) | 199 (54.7) | 0.76 (0.59–1.00) | |||
| AA | 44 (15.1) | 42 (23.1) | 4.842 | 0.028 | 1 | 0.054 |
| GG + AG | 248 (84.9) | 140 (76.9) | 0.62 (0.38–1.01) | |||
a1VD: 1-vessel disease; 2VD: 2-vessel disease; 3VD: 3-vessel disease. b P values under 0.05 were indicated in bold font. The P c values and ORc were adjusted for age, sex, smoking, drinking, hypertension, diabetes, and hyperlipidemia.
4. Discussion
CAD is a complex multifactorial, polygenic disorder that results from both the various environmental factors and the individual's genetic makeup. Recent studies have demonstrated the critical roles of SIRT6 in DNA damage repair, lipid metabolism, and atherosclerosis, providing evidences that SIRT6 may play an important role in the CAD pathogenesis [20, 21]. Nonetheless, the association of SNPs in SIRT6 gene with CAD risk and severity is largely unknown. In the present study, we proceeded with a genetic association analysis on the five SIRT6 tagSNPs (rs11878868, rs107251, rs352493, rs4807546, and rs3760908) in 474 CAD cases and 655 controls. Our results revealed that C allele of rs352493 and A allele of rs3760908 conferred the increased severity of CAD patients in Chinese subjects, though all the five SNPs and haplotypes showed no significant effect on the risk of CAD. Our study suggested that SIRT6 rs352493 and rs3760908 SNPs might play roles in the progression of CAD, which could be useful as predictors of the severity of CAD.
The function of SIRT6 on various physiological and pathological processes has been widely studied, and SIRT6 deficiency or dysregulation is closely associated with diverse human diseases including cardiac hypertrophy, adipocyte disorders, and atherosclerosis [18, 29–31]. Nonetheless, the relationship between SIRT6 variants and the risk of these diseases remains largely unknown. Previous study has revealed that T-carriers of rs107251 polymorphism in SIRT6 exhibited an enhanced risk of carotid plaque [32], while individuals with either CC or CT genotype at rs107251 within SIRT6 displayed >5-year mean survival advantages compared to TT genotype [33], which is consistent with the protective role of SIRT6. Alternatively, SIRT6 tagSNP rs4807546 showed no significant association with the risk of Parkinson's disease in a Spanish population [34], and the SIRT6 polymorphisms (rs350852, rs7246235, rs107251, and rs350844) were not associated with diabetic nephropathy in a combined meta-analysis as well [35]. Thus, it was not unexpected that the five tagSNPs (rs11878868, rs107251, rs352493, rs4807546, and rs3760908) in this study exhibited no significant association with the risk of CAD.
Multiple variants or polymorphisms within candidate genes have been reported to be associated with the severity of CAD, which is defined by the number of vessels with significant stenosis [36]. CT or TT genotype of rs7903146 polymorphism in TCF7L2 exhibited a higher prevalence and severity of CAD [37]; and the T allele of rs5065 polymorphism in ANP showed a decreased severity of CAD in an Iranian population [38]. We herein presented the evidence that C allele of rs352493 and A allele of rs3760908 polymorphisms conferred the increased severity of CAD patients in the Chinese Han population, further expanding the knowledge of the associations between polymorphisms and the progression of CAD. Nonetheless, the underlying molecular mechanisms of SIRT6 polymorphisms on the severity of CAD still warrant further investigations.
Several limitations in this study still need to be addressed. First, the CAD patients and control subjects enrolled from hospitals may not represent the general population. Nonetheless, the genotype distribution of the control subjects was in Hardy-Weinberg equilibrium. Second, the relatively moderate sample size limited the statistical power of our study. Finally, further studies in different population could help to further verify the significance of the association between the rs352493 and rs3760908 polymorphisms and the severity of CAD. However, our data provided valuable insights into this area.
5. Conclusion
In aggregate, our study unraveled that tagSNPs rs352493 and rs3760908 in SIRT6 gene exhibited a significant effect on the severity of CAD in the Chinese Han population, while all the five tagSNPs and haplotypes showed no significant effect on CAD risk. Further studies with diverse ethnic populations and larger sample size are needed to confirm the general validity of our findings.
Supplementary Material
The information for alleles captured by rs11878868, rs107251, rs352493, rs4807546 and rs3760908 was shown in TABLE S1. The sequences of the primers and probes used to genotype the rs11878868, rs107251, rs352493, rs4807546 and rs3760908 polymorphisms were shown in TABLE S2.
Acknowledgments
The authors thank the First People's Hospital of Foshan and the Affiliated Hospital of Guangdong Medical University, Guangdong Province, China, for their kind assistance in collecting the samples and data. This work was supported by Grants from the National Natural Science Foundation of China (81370456), the Natural Science Foundation of Guangdong Province (2014A030311015 and 2014A030310027), the Medical Scientific Research Foundation of Guangdong Province (A2015288), the Science and Technology Planning Project of Dongguan City (2013108101057), and Scientific Research Foundation of Guangdong Medical University (B2013002).
Disclosure
Sai-sai Tang and Shun Xu are co-first authors of the paper.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Sai-sai Tang and Shun Xu contributed equally to this work.
References
- 1.Zhang X.-H., Lu Z. L., Liu L. Coronary heart disease in China. Heart. 2008;94(9):1126–1131. doi: 10.1136/hrt.2007.132423. [DOI] [PubMed] [Google Scholar]
- 2.Prins B. P., Lagou V., Asselbergs F. W., Snieder H., Fu J. Genetics of coronary artery disease: genome-wide association studies and beyond. Atherosclerosis. 2012;225(1):1–10. doi: 10.1016/j.atherosclerosis.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Kangas-Kontio T., Huotari A., Ruotsalainen H., et al. Genetic and environmental determinants of total and high-molecular weight adiponectin in families with low HDL-cholesterol and early onset coronary heart disease. Atherosclerosis. 2010;210(2):479–485. doi: 10.1016/j.atherosclerosis.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Zdravkovic S., Wienke A., Pedersen N. L., Marenberg M. E., Yashin A. I., De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. Journal of Internal Medicine. 2002;252(3):247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 5.Mostoslavsky R., Chua K. F., Lombard D. B., et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Lombard D. B., Schwer B., Alt F. W., Mostoslavsky R. SIRT6 in DNA repair, metabolism and ageing. Journal of Internal Medicine. 2008;263(2):128–141. doi: 10.1111/j.1365-2796.2007.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawahara T. L. A., Michishita E., Adler A. S., et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanfi Y., Naiman S., Amir G., et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 9.McCord R. A., Michishita E., Hong T., et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging. 2009;1(1):109–121. doi: 10.18632/aging.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong L., Mostoslavsky R. SIRT6: a master epigenetic gatekeeper of glucose metabolism. Transcription. 2010;1(1):17–21. doi: 10.4161/trns.1.1.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao Z., Hine C., Tian X., et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332(6036):1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G., Su L., Singhal S., Liu X. Emerging roles of SIRT6 on telomere maintenance, DNA repair, metabolism and mammalian aging. Molecular and Cellular Biochemistry. 2012;364(1-2):345–350. doi: 10.1007/s11010-012-1236-8. [DOI] [PubMed] [Google Scholar]
- 13.Mao Z., Tian X., Van Meter M., Ke Z., Gorbunova V., Seluanov A. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(29):11800–11805. doi: 10.1073/pnas.1200583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao R., Xiong X., DePinho R. A., Deng C.-X., Dong X. C. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. Journal of Biological Chemistry. 2013;288(41):29252–29259. doi: 10.1074/jbc.m113.481473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H.-S., Xiao C., Wang R.-H., et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metabolism. 2010;12(3):224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S. J., Choi J. M., Chang E., Park S. W., Park C.-Y. Sirt1 and Sirt6 mediate beneficial effects of rosiglitazone on hepatic lipid accumulation. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0105456.e105456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanfi Y., Peshti V., Gil R., et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9(2):162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 18.Masri S., Rigor P., Cervantes M., et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell. 2014;158(3):659–672. doi: 10.1016/j.cell.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elhanati S., Kanfi Y., Varvak A., et al. Multiple regulatory layers of SREBP1/2 by SIRT6. Cell Reports. 2013;4(5):905–912. doi: 10.1016/j.celrep.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Stöhr R., Mavilio M., Marino A., et al. ITCH modulates SIRT6 and SREBP2 to influence lipid metabolism and atherosclerosis in ApoE null mice. Scientific Reports. 2015;5, article 9023 doi: 10.1038/srep09023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao R., Xiong X., DePinho R. A., Deng C.-X., Dong X. C. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. Journal of Lipid Research. 2013;54(10):2745–2753. doi: 10.1194/jlr.m039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong X.-D., Cho M., Cai X.-P., et al. A common variant in pre-miR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2014;761:15–20. doi: 10.1016/j.mrfmmm.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Cheng J., Cho M., Cen J.-M., et al. A TagSNP in SIRT1 gene confers susceptibility to myocardial infarction in a Chinese Han population. PLoS ONE. 2015;10(2) doi: 10.1371/journal.pone.0115339.e0115339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S., Cheng J., Chen Y.-N., et al. The LRP6 rs2302685 polymorphism is associated with increased risk of myocardial infarction. Lipids in Health and Disease. 2014;13(1, article 94) doi: 10.1186/1476-511x-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kugel S., Mostoslavsky R. Chromatin and beyond: the multitasking roles for SIRT6. Trends in Biochemical Sciences. 2014;39(2):72–81. doi: 10.1016/j.tibs.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett J. C., Fry B., Maller J., Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y. Y., He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Research. 2005;15(2):97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 28.Li L., Pan Y., Dai L., Liu B., Zhang D. Association of genetic polymorphisms on vascular endothelial growth factor and its receptor genes with susceptibility to coronary heart disease. Medical Science Monitor. 2016;22:31–40. doi: 10.12659/MSM.895163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte J. H. Osteoarthritis: SIRT6 prevents chondrocyte senescence and DNA damage. Nature Reviews Rheumatology. 2015;11(5):p. 260. doi: 10.1038/nrrheum.2015.52. [DOI] [PubMed] [Google Scholar]
- 30.Ailixiding M., Aibibula Z., Iwata M., et al. Pivotal role of Sirt6 in the crosstalk among ageing, metabolic syndrome and osteoarthritis. Biochemical and Biophysical Research Communications. 2015;466(3):319–326. doi: 10.1016/j.bbrc.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Cardus A., Uryga A. K., Walters G., Erusalimsky J. D. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovascular Research. 2013;97(3):571–579. doi: 10.1093/cvr/cvs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong C., Della-Morte D., Wang L., et al. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027157.e27157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.TenNapel M. J., Lynch C. F., Burns T. L., et al. SIRT6 minor allele genotype is associated with >5-year decrease in lifespan in an aged cohort. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0115616.e115616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jesús S., Gómez-Garre P., Carrillo F., et al. Genetic association of sirtuin genes and Parkinson's disease. Journal of Neurology. 2013;260(9):2237–2241. doi: 10.1007/s00415-013-6970-7. [DOI] [PubMed] [Google Scholar]
- 35.Maeda S., Koya D., Araki S.-I., et al. Association between single nucleotide polymorphisms within genes encoding sirtuin families and diabetic nephropathy in Japanese subjects with type 2 diabetes. Clinical and Experimental Nephrology. 2011;15(3):381–390. doi: 10.1007/s10157-011-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guney A. I., Ergec D., Kirac D., et al. Effects of ACE polymorphisms and other risk factors on the severity of coronary artery disease. Genetics and Molecular Research. 2013;12(4):6895–6906. doi: 10.4238/2013.December.19.8. [DOI] [PubMed] [Google Scholar]
- 37.Sousa A. G. P., Marquezine G. F., Lemos P. A., et al. TCF7L2 polymorphism rs7903146 is associated with coronary artery disease severity and mortality. PLoS ONE. 2009;4(11) doi: 10.1371/journal.pone.0007697.e7697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziaee S., Kalayinia S., Boroumand M. A., et al. Association between the atrial natriuretic peptide rs5065 gene polymorphism and the presence and severity of coronary artery disease in an Iranian population. Coronary Artery Disease. 2014;25(3):242–246. doi: 10.1097/mca.0000000000000075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information for alleles captured by rs11878868, rs107251, rs352493, rs4807546 and rs3760908 was shown in TABLE S1. The sequences of the primers and probes used to genotype the rs11878868, rs107251, rs352493, rs4807546 and rs3760908 polymorphisms were shown in TABLE S2.