Abstract
Background:
Anterior cruciate ligament (ACL) injury increases the risk of meniscus and articular cartilage damage, but the causes are not well understood. Previous in vitro studies were static, required extensive knee dissection, and likely altered meniscal and cartilage contact due to the insertion of pressure sensing devices.
Hypothesis:
ACL deficiency will lead to increased translation of the lateral meniscus and increased deformation of the medial meniscus as well as alter cartilage contact location, strain, and area.
Study Design:
Descriptive laboratory study.
Methods:
With minimally invasive techniques, six 1.0-mm tantalum beads were implanted into the medial and lateral menisci of 6 fresh-frozen cadaveric knees. Dynamic stereo x-rays (DSXs) were obtained during dynamic knee flexion (from 15° to 60°, simulating a standing squat) with a 46-kg load in intact and ACL-deficient states. Knee kinematics, meniscal movement and deformation, and cartilage contact were compared by novel imaging coregistration.
Results:
During dynamic knee flexion from 15° to 60°, the tibia translated 2.6 mm (P = .05) more anteriorly, with 2.3° more internal rotation (P = .04) with ACL deficiency. The medial and lateral menisci, respectively, translated posteriorly an additional 0.7 mm (P = .05) and 1.0 mm (P = .03). Medial and lateral compartment cartilage contact location moved posteriorly (2.0 mm [P = .05] and 2.0 mm [P = .04], respectively).
Conclusion:
The lateral meniscus showed greater translation with ACL deficiency compared with the medial meniscus, which may explain the greater incidences of acute lateral meniscus tears and chronic medial meniscus tears. Furthermore, cartilage contact location moved further posteriorly than that of the meniscus in both compartments, possibly imparting more meniscal stresses that may lead to early degeneration. This new, minimally invasive, dynamic in vitro model allows the study of meniscus function and cartilage contact and can be applied to evaluate different pathologies and surgical techniques.
Clinical Relevance:
This novel model illustrates that ACL injury may lead to significant meniscus and cartilage abnormalities acutely, and these parameters are dynamically measurable while maintaining native anatomy.
Keywords: knee, meniscus, meniscal deformation, meniscal movement, cartilage contact
The anterior cruciate ligament (ACL) is an important restraint against anterior tibial translation, medial translation, and internal rotation, working in concert with other soft tissues and bony geometry to guide tibiofemoral kinematics during knee motion.2,13 The medial and lateral menisci deform along the tibiofemoral joint line to guide tibiofemoral contact and improve load distribution and play a pivotal role in maintaining joint health after ACL injury, with or without reconstruction. Injury to the ACL is associated with high risk for subsequent meniscal injury and degenerative changes of the articular surfaces.6,8,15,19,20 Because ACL injury alters knee kinematics, meniscal loading and movement may also be altered, possibly compromising knee joint load distribution and contact congruity.2,6,26 There are, however, few data to support this conjecture.
Numerous biomechanical studies have attempted to evaluate meniscal function and injury by measuring tibiofemoral contact area or stress; however, few have directly examined meniscal motion and deformation as well as cartilage parameters in healthy and ACL-deficient knees. Previous studies assessing tibiofemoral contact and/or meniscal motion have employed methods requiring extensive dissections that disrupt the native knee anatomy and function.3,18,23 Many of these have used sensing methods such as strain gauges to characterize meniscal strain or, more commonly, pressure sensors to measure contact. It is likely that the insertion of these types of devices alters the contact mechanics and the devices themselves result in unrepresentative measurements and an inaccurate model of the joint’s true dynamics.16 Studies evaluating the meniscus have used static in vivo magnetic resonance imaging (MRI) at various flexion angles but with low loads and in varying positions, limiting their clinical application.10,27,31 Furthermore, although previous studies have evaluated these parameters individually, none have evaluated the complex dynamic interaction between knee kinematics, cartilage contact, meniscal motion, and deformation in the intact knee as well as changes in these relationships after ACL injury of the same knee.
Based on these shortcomings, the purpose of this study was to develop a new cadaveric model that was physiologically loaded, dynamic, and simulated a functional activity while maintaining native knee anatomy. We hypothesized that with ACL deficiency there will be greater lateral meniscal translation and greater medial meniscal deformation as well as changes in cartilage contact location, strain, and area.
Methods
Specimen Preparation
Six fresh-frozen human cadaveric knees (mean age, 44 years; range, 39-49 years) were screened with fluoroscopy (Philips BV Pulsera) to ensure they were free of osteoarthritis and abnormal ligamentous laxity. Six 1.0 mm–diameter tantalum beads were implanted into the medial and lateral menisci using minimally invasive surgical techniques. For each bead, a vertical incision was made and dissection was carried down to the meniscus, being careful to maintain native ligamentous structures and minimize capsular trauma; then the meniscus was marked with a marking pen. The bead was placed in a hip arthroscopy needle with a bone wax plug in the tip and the needle pushed into the meniscus. The plunger was then used to evacuate the bead (Figure 1A). Two beads were placed in each of 3 regions: anterior horn, midportion, and posterior horn. Bead locations were confirmed with fluoroscopy, and the capsule and other soft tissues were sutured (Figure 1, B and C). Three additional beads were implanted into the femur and tibia for bone tracking by dissecting down to bone, where a pilot hole was drilled. The beads were placed in different planes, and locations were confirmed with fluoroscopy. Bone wax was again placed during implantation as well as over the pilot hole to secure the bead, and the overlying soft tissue was sutured. The quadriceps tendon was isolated, and the femur and tibia were potted in custom molds using fiberglass resin (Bondo Body Filler).
Figure 1.
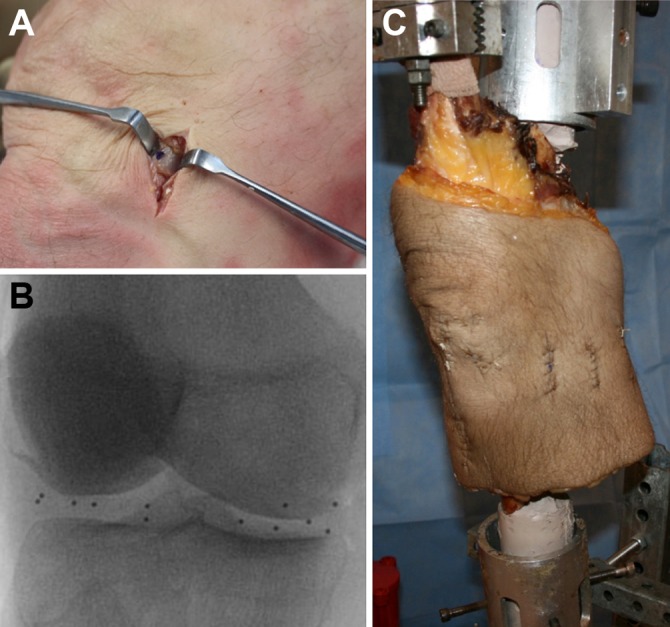
(A) Minimally invasive bead implantation technique. (B) Meniscus bead locations confirmed (n = 12) with fluoroscopy. (C) Knee in testing apparatus with quadriceps clamp after 12 meniscus beads and 6 bone beads were implanted.
Computed Tomography and Magnetic Resonance Imaging
3-T MRIs (0.6-mm isotropic voxels; 3D double-echo steady-state [DESS]; Siemens Trio) were acquired to generate 3-dimensional (3D) bone and meniscal models and to confirm bead placement and identify meniscal root insertions. Cartilage was manually segmented on MRI 3D DESS sequences and models were created using Mimics Software (Materialise).28 Computed tomography (CT) images (0.6-mm slices; GE LightSpeed 16) were obtained to identify bone bead placement and to create 3D femur and tibia models.
Bead migration was evaluated by comparing bead-to-bead distance on MRI and all dynamic and static trials. DSX data were collected before knees were loaded, between trials without load, and without load after all trials were completed. If any change in bead-to-bead distance occured between these unloaded trials, these data were not used. Two knees were excluded due to bead migration and quadriceps failure at this load. Only knees with no bead migration were included.
Dynamic Stereo X-ray Testing
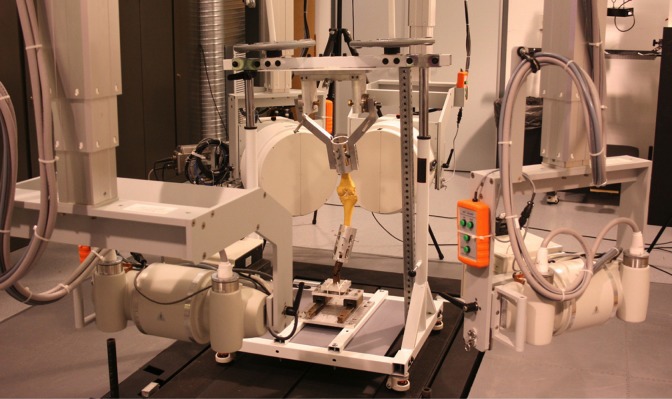
The knees were secured in a custom-designed Oxford-style knee apparatus (5 degrees of freedom) with actuator-controlled quadriceps tendon force (Figures 1C and 2). The apparatus was unlocked and the actuator was tensioned so the knee was held in extension while a 46-kg axial load was applied on the top of the knee apparatus. This load represents approximately 1.3× body weight during a 2-legged squat of a person weighing 80 kg and is within the limits of the quadriceps clamp used in the study. The quadriceps was then allowed to lengthen, providing an eccentric load, using a remote control via the actuator. This allowed the knee to undergo controlled dynamic flexion by the 46-kg axial load on top of the apparatus from 15° to 60°, simulating a squat, all while high-speed dynamic stereo x-ray (DSX) images (90 kVp, 160 mA) were collected at 30 frames per second. The 60° of flexion angle was chosen as it is a common knee flexion angle during daily activities and was within the range of our testing apparatus.21 The ACL was then arthroscopically transected while the knee remained in the apparatus, and testing was repeated using a goniometer to insure the same starting position.
Figure 2.
Custom Oxford-style knee apparatus in the dynamic stereo x-ray system.
Data Analysis
DSX data were transferred to custom computerized software, and radiographic images were corrected for distortion and intensity gradient. As previously described, the positions of the implanted bone and meniscal beads were tracked and reconstructed into the 3D laboratory coordinate system in custom software with 0.1-mm precision (Figure 3).4,5,26 The CT scans were segmented and reconstructed into 3D specimen-specific femur and tibia models, and anatomic coordinate systems were manually established.25 Knee kinematics (femoral and tibial bone positions) was determined by 3D locations of bone beads. Meniscal kinematics and deformation were determined by evaluating movement of meniscal beads relative to the tibia using custom MATLAB software (MathWorks Inc).
Figure 3.
Images obtained from dynamic stereo x-ray testing.
An ellipse, using the least-squares method, was mathematically fit to the 6 meniscal beads and the meniscal root insertions for each meniscus, which were identified on MRI as described (Figure 4). The overall meniscal translation was characterized by the movement of the center of the ellipse and was compared between the ACL-intact and ACL-deficient states. The change in arc length between beads was used to measure circumferential meniscal deformation in the anterior, middle, and posterior regions in the 2 states. Radial deformation was measured between the center of the ellipse and the bead locations in these same regions. Finally, we measured vertical deformation, or meniscal compression, by the vertical movement of the beads relative to the underlying tibia, which was converted to a percentage relative to the unloaded knee. Each knee was compared with its unloaded static state.
Figure 4.
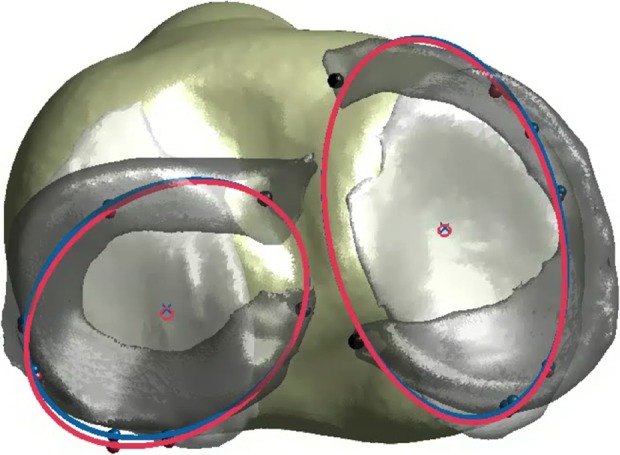
Model created from combining computed tomography, magnetic resonance imaging, and dynamic stereo x-ray testing data that shows the meniscus beads with the mathematically fitted ellipse (used for evaluation of meniscal deformation) and its center (used for evaluation of meniscal translation). Blue, anterior cruciate ligament (ACL)–intact; pink, ACL-deficient.
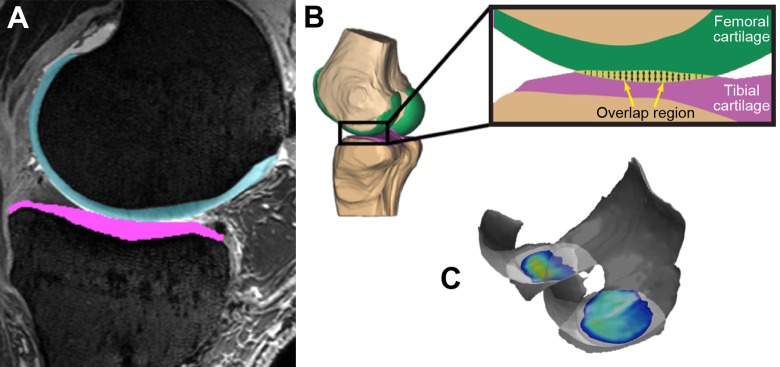
MRI bone and cartilage models as well as CT bone models were coregistered with DSX data, and the overlap of the cartilage models was evaluated using a previously validated method to determine centroid weighted cartilage contact location, mean strain, and mean area of both the ACL-intact and ACL-deficient states (Figure 5).9,14,28 Two-way repeated-measures analysis of variance (ANOVA) using SPSS (IBM Corp) was utilized to compare the 2 states at angle increments of 5° of flexion (P < .05).
Figure 5.
(A) Manually segmented cartilage from magnetic resonance imaging. (B) A nondeformable cartilage model is created and coregistered with computed tomography and dynamic stereo x-ray testing data. The model’s overlap region is calculated, which allows determination of contact location, area, and strain. (C) Example of evaluation of the overlapped region for 1 specimen.
Results
Kinematics
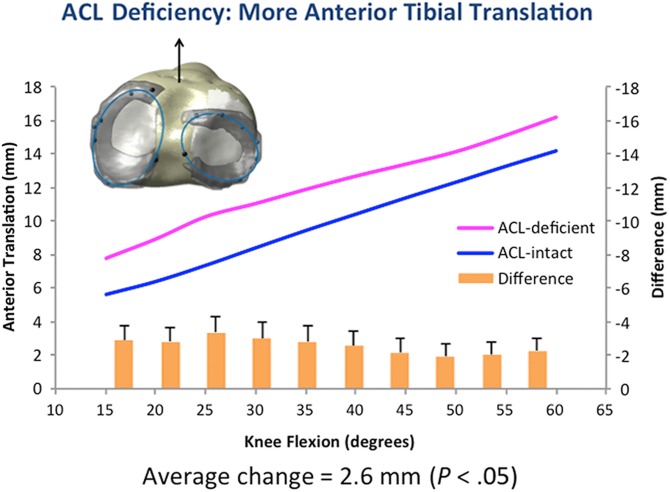
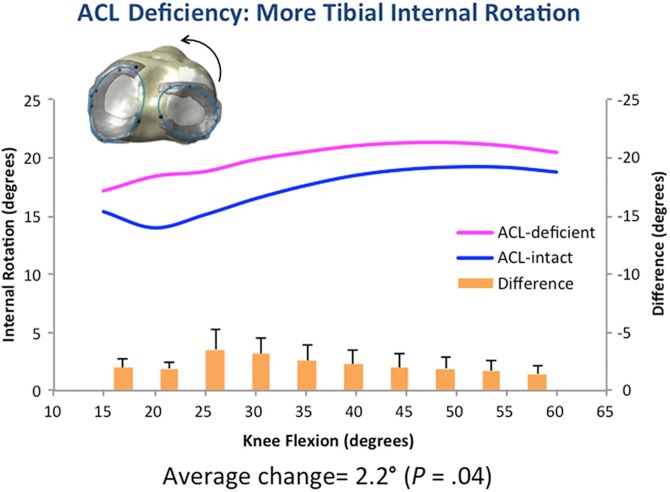
During a simulated squat, with ACL deficiency, the tibia translated 2.6 ± 0.26 mm more anteriorly (P = .05) with 2.3° ± 0.34° more internal rotation (P = .04), with the highest values seen from 25° to 35° with both parameters (Figures 6 and 7).
Figure 6.
Anterior tibial translation, used to evaluate knee kinematics, was increased with anterior cruciate ligament (ACL) deficiency, which is consistent with in vivo literature, indicating this model is viable.
Figure 7.
Tibial internal rotation, used to evaluate knee kinematics, was increased with anterior cruciate ligament (ACL) deficiency.
Meniscal Movement and Deformation
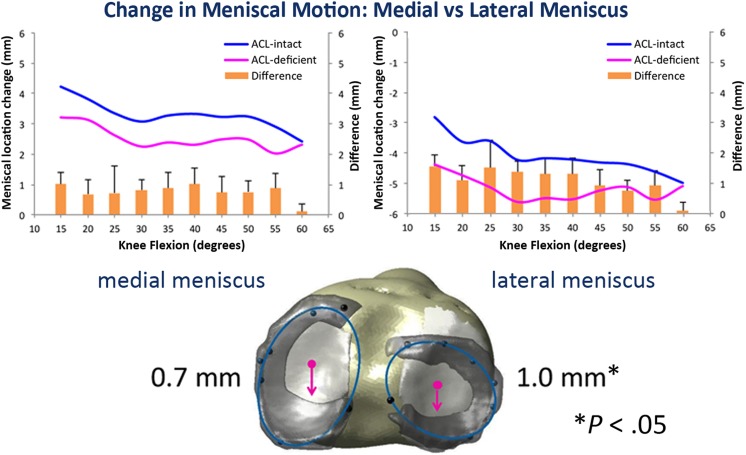
The medial meniscus translated, on average, 2.3 mm posteriorly during dynamic knee flexion while the lateral meniscus translated 3.2 mm posteriorly in the intact knee. In the ACL-deficient state, the medial and lateral meniscus translated an additional 0.7 ± 0.12 mm (P = .08) and 1.0 ± 0.16 mm (P = .05) in the posterior direction; in the lateral meniscus, this change was statistically significant. Vertical deformation, or meniscal compression, increased with ACL deficiency—anterior (14% ± 1.1%, P = .08), middle (12% ± 0.90%, P = .09), and overall—but was not significantly different. The change in circumferential and radial deformation was minimal. Dynamic data can be seen in Figure 8.
Figure 8.
Change in meniscal motion between anterior cruciate ligament (ACL)–intact and ACL-deficient knees: The lateral meniscus translated significantly more posterior with ACL deficiency compared with the intact state, indicating the lateral meniscus is at risk with ACL deficiency.
Cartilage Contact
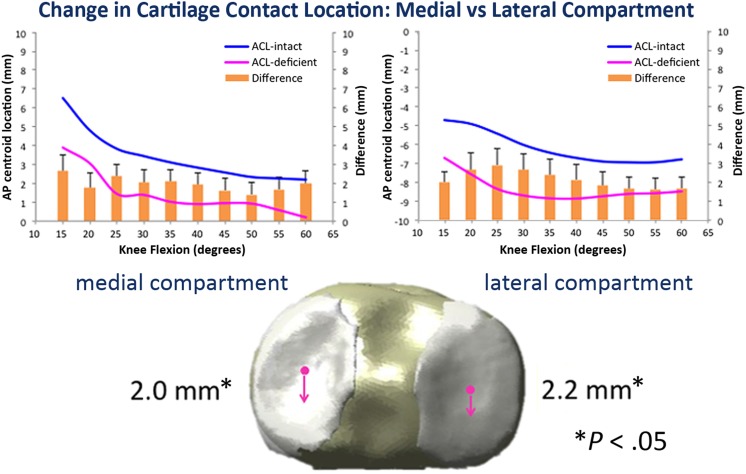
With ACL deficiency, medial and lateral compartment cartilage contact location moved significantly posteriorly (2.0 ± 0.20 mm [P = .05] and 2.0 ± 0.23 mm [P = .04]). Cartilage-to-cartilage contact percent area decreased by 2.2% ± 0.32% in the lateral compartment but was not significant (P = .06), while the medial compartment showed no change in contact area. Medial-to-lateral cartilage contact location movement was no different between the 2 states. These differences from the intact state were greater at lower knee flexion angles. Cartilage strain was not significantly different in either compartment. Dynamic data can be seen in Figure 9.
Figure 9.
Change in cartilage contact location between anterior cruciate ligament (ACL)–intact and ACL-deficient knees: Both the medial and lateral compartment contact locations moved significantly more posterior when comparing intact with ACL-deficient states. This was greater than meniscal translation, possibly imparting more stresses on the meniscus.
Discussion
The majority of our results are consistent with previous static in vitro and in vivo studies and our hypothesis; however, the model and means are novel. ACL-deficient knees had greater anterior tibial translation and internal rotation compared with knees in the intact state.12,24 Meniscal translation was greater in the lateral compartment than in the medial compartment in the intact state and increased with ACL deficiency, and cartilage contact location was more posterior in both compartments after ACL transection. These dynamic results confirm our hypotheses and qualitatively are consistent with static in vitro studies.12,17
We expect that modifications to the testing protocol would elicit more pronounced changes in these parameters with ACL deficiency. The flexion angles of this initial study were limited to 60° maximum, and the applied weight was limited to 46 kg due to limitations of the quadriceps clamp and the testing apparatus used. Deeper flexion, we believe, will lead to greater meniscal displacements and deformations,33 as will increased loads. Although our load was considerably greater than that of other studies, for future work we plan to develop an improved quadriceps clamp that can sustain increased loads. However, the peak quadriceps force measured during testing was similar to that of squatting.30 Furthermore, our knee apparatus limits us to only test the activity of a squat. A greater number of specimens may provide better statistical data: a post hoc power analysis suggested a much greater sample size (>100) for detecting a significant difference in the deformation results with a power of >0.8. Standardized bead placement was difficult due to the minimally invasive technique as was securing the beads. This may impart some form of experimental error; however, the algorithm used mathematically minimized that error. For future testing, we plan to use better bead fixation techniques and also increase knee flexion angle and axial loading.
The changes in meniscus translation observed with ACL deficiency appear to reflect the greater mobility of the lateral meniscus, possibly having implications for meniscal tears, both acutely and chronically. Although not able to be concluded from this study, the authors believe 1 or more millimeters of shift in the meniscus and/or cartilage may lead to degeneration overtime due to loss of normal contact between the articular cartilage of the tibia and femur. During dynamic knee flexion in the intact knee, the lateral meniscus translated more than the medial meniscus, and as knee flexion angle increased so did lateral meniscus posterior translation. With ACL deficiency, both menisci showed increased posterior translation, with a greater increase in the lateral compared with the medial meniscus. A greater difference was seen at low knee flexion angles. These results are consistent with our hypotheses; as the tibia translates anteriorly with knee flexion, the menisci are pulled posteriorly on the tibia, with greater effect on the more mobile lateral meniscus.24,29,33
Using different methodologies and low loads, previous studies found highly variable normal meniscal translations, making their interpretation difficult.22,27 Furthermore, studies comparing knee pathologies are sparse. Our finding of increased meniscal translation with ACL deficiency is contrary to limited other studies that found meniscal translation was influenced by loading and not by ACL deficiency.22,24,32 Those studies, however, were static and were further limited by MRI slice thickness or invasive techniques.27 Dynamic meniscal evaluation, as performed in this study, likely is a better representation of true motion due to the complex nature of the meniscus and knee. The authors do not know if the observed increase in meniscal movement may lead to greater rates of meniscal tears. However, the greater change in translation of the lateral versus medial meniscus may explain the increased incidences of acute lateral meniscus tears and chronic medial meniscus tears. The medial meniscus likely sees greater stresses, and with time, likely experiences greater tear rates.24 It is known, however, that meniscal translation is essential to prevent stress concentrations between the cartilage surfaces during knee range of motion.1
When evaluating deformation of the menisci during dynamic knee flexion, although not statistically significant, vertical deformation or meniscal compression increased while the change in radial and circumferential deformation was minimal. These changes may lead to early meniscal degeneration. These results may be secondary to inconsistent medial-lateral and rotatory tibiofemoral kinematics with ACL deficiency, as described in the literature; however, studies evaluating meniscal deformation are limited, and the authors are not aware of any doing so dynamically.7,17 With flexion angles limited to 60° and a 46-kg load, forces may not have been sufficient to produce marked changes in meniscal deformation. Again, we expect that with deeper flexion angles and larger loading, the circumferential fibers would be further stressed and therefore show more significant meniscal deformation.
Cartilage contact location is altered with ACL deficiency, possibly leading to abnormal wear and osteoarthritis. Consistent with our hypothesis, we found cartilage contact location to be significantly more posterior with ACL deficiency in both the medial and lateral compartments and exceeded the change in meniscal translation. This difference is likely due to meniscal roots and capsular attachments, which constrain the movement of the meniscus.27 These results illustrate that the lateral compartment is more at risk for injury with ACL deficiency acutely, as seen with the pivot-shift phenomenon and the common bone bruise pattern, and chronically may lead to early osteoarthritis.11 We found minimal differences in cartilage contact area and strain between ACL intact and deficient states. The lack of change in acute contact area and strain is consistent with the thought that the menisci are secondary restraints, so the menisci likely are picking up the forces that the ACL usually experiences,27 thereby reducing the effects on the cartilage. These results are also consistent with the theory that chronically overloading the meniscus leads to degeneration and subsequent changes to the cartilage.32,33 Our dynamic study evaluated only the acute phase without meniscal injury or degeneration, and therefore, cartilage changes have not yet occurred. For this reason, quantitative values found in our study are slightly different than others in the literature.
Some differences can be noted between the results of this study and those of a previous in vivo investigation of changes in tibiofemoral cartilage contact after ACL reconstruction.17 During a static lunge with cartilage contact positions estimated at knee flexion angles of 0°, 30°, 60°, and 90° in 9 patients, Li et al17 reported a similar posterior shift of the contact point in ACL-deficient knees, but only in the medial compartment and only at flexion angles less than 30°. These discrepancies are most likely due to significant differences in the loading scenarios for the 2 studies. While the true axial load for the in vivo studies is unknown, the lunge position most likely requires active stabilization, with cocontraction between the quadriceps and hamstrings to maintain a static knee flexion angle. Hamstrings activation would tend to reduce anterior tibial translation (and the associated posterior shift of tibial cartilage contact) relative to the present cadaver study, which utilized only quadriceps force to control knee flexion. While it could be argued that in vivo studies are more relevant to actual function, Li et al17 were unable to assess meniscal function in their model. Interactions between tibiofemoral kinematics, meniscal motion, and cartilage contact were a key goal for this study.
This study overcomes many limitations of previous cadaver models by enabling dynamic evaluation of meniscal function in a loaded knee with minimal disruption to native anatomy. The core technical innovation is the unique combination of implanted radiopaque markers, DSX, and CT/MRI-based 3D geometric modeling to assess meniscal movement and deformation during dynamic knee flexion, while maintaining native anatomy without implantation of devices within the joint that likely alter meniscal and cartilage contact mechanics. The authors are not aware of any previous studies that investigate the complex dynamic interplay between knee kinematics, meniscal motion and deformation, and cartilage contact in the intact knee as well as changes in these relationships after ACL injury. Combining simultaneous assessment of multiple parameters is essential to elucidate the role of the meniscus in the pathway from ACL injury to osteoarthritis. This knowledge is fundamental for developing and evaluating optimal treatments that preserve long-term knee joint health.
This study indicates that ACL deficiency leads to altered meniscal and cartilage mechanics. Can these changes be reversed with surgical intervention? We plan to utilize this methodology to better characterize the effects of different knee injuries and different surgical treatments such as ACL reconstruction, extra-articular tenodesis (iliotibial band), posterior cruciate ligament deficiency and reconstruction, as well as meniscal injury, surgery, and transplantation.
In summary, the lateral meniscus showed greater translation with ACL deficiency compared with the medial meniscus, which may explain the greater incidences of acute lateral meniscus tears and chronic medial meniscus tears. Furthermore, cartilage contact location moved further posterior than that of the meniscus in both medial and lateral compartments. This study introduces a novel minimally invasive dynamic in vitro model to study knee kinematics, meniscus function, and cartilage contact, which found significant changes with ACL deficiency and will be applied to study the effects of different pathologies and surgical techniques in restoring joint function.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was sponsored by the 2013 American Orthopaedic Society for Sports Medicine (AOSSM) Young Investigator Award.
References
- 1. Aagaard H, Verdonk R. Function of the normal meniscus and consequences of meniscal resection. Scand J Med Sci Sports. 1999;9:134–140. [DOI] [PubMed] [Google Scholar]
- 2. Abebe ES, Utturkar GM, Taylor DC, et al. The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. J Biomech. 2011;44:924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. 2008;90:1922–1931. [DOI] [PubMed] [Google Scholar]
- 4. Anderst W, Zauel R, Bishop J, Demps E, Tashman S. Validation of three-dimensional model-based tibio-femoral tracking during running. Med Eng Phys. 2009;31:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderst WJ, Tashman S. A method to estimate in vivo dynamic articular surface interaction. J Biomech. 2003;36:1291–1299. [DOI] [PubMed] [Google Scholar]
- 6. Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. [DOI] [PubMed] [Google Scholar]
- 7. Barrance PJ, Williams GN, Snyder-Mackler L, Buchanan TS. Altered knee kinematics in ACL-deficient non-copers: a comparison using dynamic MRI. J Orthop Res. 2006;24:132–140. [DOI] [PubMed] [Google Scholar]
- 8. Borchers JR, Kaeding CC, Pedroza AD, Huston LJ, Spindler KP, Wright RW. Intra-articular findings in primary and revision anterior cruciate ligament reconstruction surgery: a comparison of the MOON and MARS study groups. Am J Sports Med. 2011;39:1889–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carey RE, Zheng L, Aiyangar AK, Harner CD, Zhang X. Subject-specific finite element modeling of the tibiofemoral joint based on CT, magnetic resonance imaging and dynamic stereo-radiography data in vivo. J Biomech Eng. 2014;136(4). doi:10.1115/1.4026228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen HN, Yang K, Dong QR, Wang Y. Assessment of tibial rotation and meniscal movement using kinematic magnetic resonance imaging. J Orthop Surg Res. 2014;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deangelis JP, Spindler KP. Traumatic bone bruises in the athlete’s knee. Sports Health. 2010;2:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Defrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34:1240–1246. [DOI] [PubMed] [Google Scholar]
- 13. Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79. [DOI] [PubMed] [Google Scholar]
- 14. Hosseini A, Van de Velde S, Gill TJ, Li G. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. J Orthop Res. 2012;30:1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ichiba A, Kishimoto I. Effects of articular cartilage and meniscus injuries at the time of surgery on osteoarthritic changes after anterior cruciate ligament reconstruction in patients under 40 years old. Arch Orthop Trauma Surg. 2009;129:409–415. [DOI] [PubMed] [Google Scholar]
- 16. Jones RS, Keene GC, Learmonth DJ, et al. Direct measurement of hoop strains in the intact and torn human medial meniscus. Clin Biomech (Bristol, Avon). 1996;11:295–300. [DOI] [PubMed] [Google Scholar]
- 17. Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–1834. [DOI] [PubMed] [Google Scholar]
- 18. Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2009;37:124–129. [DOI] [PubMed] [Google Scholar]
- 19. Neuman P, Englund M, Kostogiannis I, Friden T, Roos H, Dahlberg LE. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36:1717–1725. [DOI] [PubMed] [Google Scholar]
- 20. Noyes FR, Barber-Westin SD. Treatment of meniscus tears during anterior cruciate ligament reconstruction. Arthroscopy. 2012;28:123–130. [DOI] [PubMed] [Google Scholar]
- 21. Rowe PJ, Myles CM, Walker C, Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12:143–155. [DOI] [PubMed] [Google Scholar]
- 22. Scholes C, Houghton ER, Lee M, Lustig S. Meniscal translation during knee flexion: what do we really know? Knee Surg Sports Traumatol Arthrosc. 2015;23:32–40. [DOI] [PubMed] [Google Scholar]
- 23. Seitz AM, Lubomierski A, Friemert B, Ignatius A, Durselen L. Effect of partial meniscectomy at the medial posterior horn on tibiofemoral contact mechanics and meniscal hoop strains in human knees. J Orthop Res. 2012;30:934–942. [DOI] [PubMed] [Google Scholar]
- 24. Shefelbine SJ, Ma CB, Lee KY, et al. MRI analysis of in vivo meniscal and tibiofemoral kinematics in ACL-deficient and normal knees. J Orthop Res. 2006;24:1208–1217. [DOI] [PubMed] [Google Scholar]
- 25. Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. [DOI] [PubMed] [Google Scholar]
- 26. Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. [DOI] [PubMed] [Google Scholar]
- 27. Thompson WO, Thaete FL, Fu FH, Dye SF. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am J Sports Med. 1991;19:210–215. [DOI] [PubMed] [Google Scholar]
- 28. Thorhauer E, Tashman S. Validation of a method for combining biplanar radiography and magnetic resonance imaging to estimate knee cartilage contact. Med Eng Phys. 2015;37:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tienen TG, Buma P, Scholten JG, van Kampen A, Veth RP, Verdonschot N. Displacement of the medial meniscus within the passive motion characteristics of the human knee joint: an RSA study in human cadaver knees. Knee Surg Sports Traumatol Arthrosc. 2005;13:287–292. [DOI] [PubMed] [Google Scholar]
- 30. Van Haver A, De Roo K, Claessens T, De Beule M, Verdonk P, De Baets P. Pilot validation study on a quasi-static weight-bearing knee rig. Proc Inst Mech Eng H. 2013;227:229–233. [DOI] [PubMed] [Google Scholar]
- 31. Vedi V, Williams A, Tennant SJ, Spouse E, Hunt DM, Gedroyc WM. Meniscal movement. An in-vivo study using dynamic MRI. J Bone Joint Surg Br. 1999;81:37–41. [DOI] [PubMed] [Google Scholar]
- 32. von Eisenhart-Rothe R, Bringmann C, Siebert M, et al. Femoro-tibial and menisco-tibial translation patterns in patients with unilateral anterior cruciate ligament deficiency—a potential cause of secondary meniscal tears. J Orthop Res. 2004;22:275–282. [DOI] [PubMed] [Google Scholar]
- 33. Yao J, Lancianese SL, Hovinga KR, Lee J, Lerner AL. Magnetic resonance image analysis of meniscal translation and tibio-menisco-femoral contact in deep knee flexion. J Orthop Res. 2008;26:673–684. [DOI] [PubMed] [Google Scholar]