Abstract
This review was based on a literature search of PubMed and Scielo databases using the keywords “quercetin, rutin, isoquercitrin, isoquercitin (IQ), quercetin-3-glucoside, bioavailability, flavonols and favonoids, and cancer” and combinations of all the words. We collected relevant scientific publications from 1990 to 2015 about the absorption, bioavailability, chemoprevention activity, and treatment effects as well as the underlying anticancer mechanisms of isoquercitin. Flavonoids are a group of polyphenolic compounds widely distributed throughout the plant kingdom. The subclass of flavonols receives special attention owing to their health benefits. The main components of this class are quercetin, rutin, and IQ, which is a flavonoid and although mostly found as a glycoside, is an aglycone (lacks a glycoside side chain). This compound presents similar therapeutic profiles to quercetin but with superior bioavailability, resulting in increased efficacy compared to the aglycone form. IQ has therapeutic applications owing to its wide range of pharmacological effects including antioxidant, antiproliferative, anti-inflammatory, anti-hypertensive, and anti-diabetic. The protective effects of IQ in cancer may be due to actions on lipid peroxidation. In addition, the antitumor effect of IQ and its underlying mechanism are related to interactions with Wnt signaling pathway, mixed-lineage protein kinase 3, mitogen-activated protein kinase, apoptotic pathways, as well proinflammatory protein signaling. This review contributed to clarifying the mechanisms of absorption, metabolism, and actions of IQ and isoquercitrin in cancer.
Keywords: Drug screening assays, Antitumor, Cancer, Flavonoids, Flavonols, Antioxidants, Neoplasms
Core tip: Flavonoids have gained a great deal of attention over the years, and their health benefits are the focus of the new research studies. This review contributed to clarifying the mechanisms of absorption and metabolism of isoquercitin, as well as emphasizing its chemopreventive and therapeutics effects in cancer. Overall, we presented a hypothesis of the underlying mechanisms of this biocompound in cancer.
INTRODUCTION
Flavonoids, which are widely distributed throughout the plant kingdom, are commonly found in fruits and vegetables. In addition, they are an accessible alternative preventive therapy or supplement for the treatment of numerous diseases. The importance of flavonoids is even more evident in developing countries where the technological challenges often hinder access to modern medicines and limit their therapeutic effectiveness[1].
Flavonoids are a group of polyphenolic compounds and as a part of the human diet, they have very low toxicity in addition to various beneficial health effects[2]. Among the numerous groups of flavonoids, the flavonols have recently been the focus of special attention for their potential actions on different organic reactions and biological pathways. Structurally, flavonols have one hydroxyl (OH) group on the third carbon (C3) and one carbonyl group (C=O) on the fourth carbon (C4) of the C ring (Figure 1)[2,3].
Figure 1.
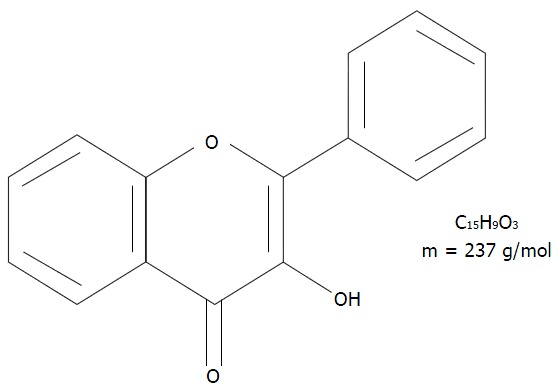
Basic structure of Flavonols. Flavonols have one hydroxyl (OH) group on the third carbon (C3) and one carbonyl group (C=O) on the fourth carbon (C4) of the C ring [2]2.
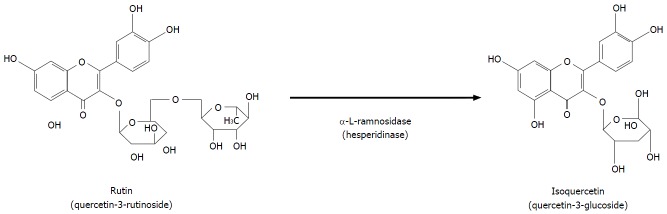
The best-known flavonols are (1) quercetin, an aglycone molecule widely found in nature; (2) rutin, a hydrophilic molecule; and (3) isoquercitin (IQ), a naturally occurring glycoside of quercetin also known as hirsutrin, isoquercetrin, quercetin-3-glucoside (Q3G), quercetin-3-O-β-D-glucoside (Figure 2). The molecular formula and molar mass of IQ are C21H20O12 and 464.38 g/mol, respectively. IQ is also sometimes called isoquercetin, which is a nearly identical quercetin-3-monoglucoside. Although they are technically different because isoquercetin has a pyranose ring whereas IQ has a furanose ring, functionally, the two molecules are indistinguishable. Published literature often considers them to be the same compound and uses the names interchangeably, as is the case in this present review. In addition, quercetin and IQ differ in their structure, bioavailability, absorption, and biological actions[2,4].
Figure 2.
Enzymatic hydrolysis of rutin and formation of isoquercitin. The rutin (quercitin-3-rytinoside) is a hydrophilic molecule and isoquercitin (quercitin-3-glucoside), a naturally occurring glycoside of quercetin also known as isoquercetrin. Isoquercetrin can be obtained by enzymatic hydrolysis of rutin with hesperedinase, which has α-l-ramnosidase activity and removes the rhamnose radical of the rutin molecule.
The absorption of flavonoids in the small intestine is limited owing to their molecular weight and hydrophilicity of their glycosides. Naturally occurring quercetin compounds are mainly glycosides such as IQ and are commonly found in plants and the human diet. Glycosides are not easily absorbed in the digestive tract, and most of their absorption occurs after transformation to the aglycone form[2].
IQ can be obtained by enzymatic hydrolysis of rutin with hesper kinase (produced by Penicillium sp), which has α-l-ramnosidase activity when applied at 58 °C for 30 min, and removes the rhamnose radical of the rutin molecule (Figure 2). Enzymatically modified IQ is the product of this procedure and consists of a mixture that includes Q3G, Q4G, rutin, and other small metabolites[5-7].
Recent in vitro and in vivo studies with flavonols, particularly IQ, have demonstrated their potential activities including antioxidants, anti-inflammatory, anti-allergic, and antiproliferative among other effects[2,7]. This review contributed to clarifying the absorption, bioavailability, and chemopreventive effects of IQ as well as its use in the treatment of cancer. In addition, we present a hypothesis of the underlying anticancer mechanism of this biocompound.
RESEARCH
This study was based on literature published between the years 1990 and 2015, found in online databases such as Scielo and Pubmed. We used search terms including “quercetin, rutin, isoquercitrin, isoquercitin, Q3G, bioavailability, flavonols, flavonoids, and cancer” as well as a combination of all the terms.
ABSORPTION OF IQ
Several studies have attempted to elucidate the pathway of IQ intestinal absorption. Thus far, the most widely accepted hypothesis involves lactase phlorizin hydrolase (LPH) as the major step[8,9,10], and sodium-dependent glucose transporter 1 (SGLT1) as a second step. LPH is an extracellular enzyme localized on the outer surface of the small intestinal brush-border membrane (BBM)[10,11]. When IQ is ingested, it is first hydrolyzed in the small intestine by the lactase domain of LPH, releasing the quercetin aglycone[12,13], which then passively diffuses to the enterocity throughout the BBM (apical surface). The deglycosylation of IQ leads to a higher concentration of the aglycone at the apical enterocyte membrane, thereby increasing the rate of absorption[9]. A small amount of IQ is transported by the SGLT1[8] present in the BBM of the small intestine, thereby transporting the intact glycoside into the cell[14]. In enterocytes, cytosolic β-glycosidase hydrolyzes the intact IQ, which is then transported via the SGLT1 route, into the quercetin aglycone. Quercetin and other flavonoids are substrates for uridine diphosphate-glucuronosyltransferases (UDP-GT) in the human intestine[14]. UDP-GT glucuronidates the quercetin aglycone into quercetin glucuronides (conjugated quercetin metabolites) and then it finally reaches the bloodstream[4,9] (Figure 3).
Figure 3.
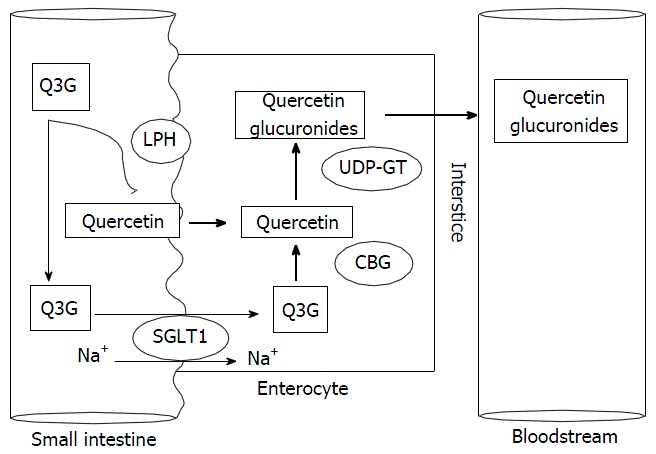
Accepted hypothesis pathway of isoquercitin intestinal absorption. The most widely accepted hypothesis of isoquercitin intestinal absorption involves lactase phlorizin hydrolase (LPH) as the major step, and sodium-dependent glucose transporter 1 (SGLT1) as a second step. LPH is an extracellular enzyme localized on the outer surface of the small intestinal brush-border membrane. When quercetin-3-glucoside (Q3G) is ingested, it is first hydrolyzed in the small intestine by the lactase domain of LPH, releasing the quercetin aglycone, which then passively diffuses to the enterocity throughout the apical surface. The deglycosylation of Q3G leads to a higher concentration of the aglycone at the apical enterocyte membrane, thereby increasing the rate of absorption. A small amount of Q3G is transported by the SGLT1 present in the apical surface of the small intestine, thereby transporting the intact glycoside into the cell. In enterocytes, cytosolic β-glycosidase (CBG) hydrolyzes the intact Q3G, which is then transported via the SGLT1 route, into the quercetin aglycone. UDP-GT glucuronidates the quercetin aglycone into quercetin glucuronides (conjugated quercetin metabolites and then it finally reaches the bloodstream. UDP-GT: Uridine diphosphate-glucuronosyltransferases.
Intact quercetin glucoside is not detected in the plasma and portal blood[10] even shortly after consumption because the quercetin glucuronides are the main metabolites[14]. Lactase supplementation increases the hydrolysis and thereby the bioavailability of IQ and hence, may be a useful strategy to improve the metabolism and absorption of IQ[9]. The strategy might be especially useful in lactose-intolerant individuals with a diminished capacity to achieve this hydrolytic action[9]. A fat-enriched diet enhances quercetin-3-O-glucoside bioavailability, which might be owing to improved solubility and enhanced absorption of the lipophilic quercetin aglycone via lipid micelles, as well as delayed elimination of quercetin from the plasma owing to a prolonged enterohepatic circulation[12]. It is important to note that following the enzymatic hydrolysis by hesperidinase, rutin is mainly bioconverted into Q3G[4,7,8,10-12], thereby increasing the absorption[15] and bioavailability[16].
BIOAVAILABILITY OF IQ
Bioavailability is a measure of the extent to which a therapeutically active drug reaches the systemic circulation and is available at the site of action. Some studies have attempted to elucidate the bioavailability of IQ (Table 1); however, we were, unfortunately, unable to access these studies while other aspects were not found in the literature such as drug specificity (the availability at different sites of action or excretion data). Nevertheless, peak plasma time (first and maximum) was found in some studies, which are described in Table 1.
Table 1.
Studies performed to elucidate bioavailability of isoquercitin and time to attain peak plasma time
| Ref. | Animal | Ingestion form | Compound |
Peak plasma time (min) |
Half-life of excretion (min) | |
| First | Maximum | |||||
| Olthof et al[15], 2000 | Human | Pure compound | Isoquercitin | - | 37 ± 12 | 1110 ± 48 |
| Chang et al[17], 2005 | Rat | Pure compound | Isoquercitin | - | 10 | - |
| Lesser et al[18], 2006 | Pig | Standard diet | Quercitin (standard) | ± 30 | 95 | - |
| Quercitin (high fat content) | 65 | |||||
| Lan et al[19], 2007 | Rat | Pure compound | Quercitin | - | 114-294 | - |
| Krogholm et al[20], 2010 | Human | "Juice mix" | Quercitin | - | 216 ± 96 | - |
| Berger et al[21], 2012 | Cow | Standard diet | Quercitin | - | 30.0 ± 0.0 | - |
| Gohlke et al[22], 2013 | Cow | Standard diet | Quercitin | 60-90 | About 115 | - |
| Cermak et al[23], 2003 | Pig | Standard diet (supplemented) | Isoquercitin (standard) | < 60 | 210 | - |
| Isoquercitin (high fat content) | > 90 | 30 | ||||
| Quercitin | - | 120 | - | |||
| Lesser et al[12], 2004 | Pig | Diet (different % of fat) | Isoquercitin (3%) | 30 | 70 ± 7.9 | - |
| -32% | 45.0 ± 7.9 | |||||
| Quercitin (3%) | - | 102.9 ± 8.0 | - | |||
| -32% | 51.4 ± 8.0 | |||||
| Wiczkowski et al[24], 2008 | Human | Shallot flesh | Isoquercitin | 15 | 13980 ± 30 | - |
| Dry shallot skin | Quercitin | 15 | 16680 ± 9 | - | ||
| Reinboth et al[16], 2010 | Dog | Standard diet (supplemented) | Isoquercitin | 48 | 246 | - |
| Quercitin | 72 | 234 | - | |||
| Lee et al[25], 2012 | Human | Mixture of apple peel and onion powder enriched applesauce | Isoquercitin | - | 144 ± 90 | - |
| + | ||||||
| Quercitin | - | 144 ± 90 | - | |||
ANTIOXIDANT AND CHEMOPREVENTIVE EFFECTS OF IQ IN CANCER
The antioxidant effect is the best-described property of phenolic compounds[1]. Antioxidant effect is based on the ability of certain molecules to retard or inhibit oxidative damage, which is responsible for cell dysfunction as well as the onset of various health problems such as cardiovascular diseases, neurodegenerative diseases, autoimmune disorders, diabetes, and cancer. It is well established that one of the mechanisms for cancer development is oxidative stress and therefore, the chemopreventive effects of IQ could be attributed to its antioxidant and oxidative damage preventive activities.
The role of antioxidants is to block oxidative reactions induced by highly reactive oxidant molecules that damage other molecules by capturing electrons and modifying the chemical structure. These damaging molecules are the so-called free radicals or reactive oxygen species (ROS). The antioxidant properties of substances such as vitamins, minerals, enzymes, plant pigments, and other natural compounds protect the membrane and other components of the cellular structure.
IQ exerts antioxidative effects by inhibiting lipid peroxidation via interference with enzyme activity (xanthine oxidase), chelation of redox-active metals, increased absorption of vitamin C, and direct scavenging of ROS such as singlet oxygen, hydroxyl, peroxyl, peroxynitrite, and superoxide radicals[1,2,7,26,27].
In vitro studies comparing the antioxidant effect of flavonols (glycosides and aglycones) confirmed that their protective efficacy against lipid peroxidation reaction were mediated by free radical-scavenging activities[3,7]. However, it has been reported that these substances may also be cytotoxic and damage the cell membrane (mainly erythrocytes) in the presence of ROS, and thereby become extremely harmful to living organisms themselves[28]. Boligon et al[29] disagreed with this study and, therefore, analyzed the protective effects of flavonoids against chromosome damage induced by hydrogen peroxide (H2O2) n human lymphocytes. They concluded that flavonoids decreased the chromosomal damage by reducing the oxidative stress. Furthermore, Razavi et al[30] showed that Q3G has cytotoxic and antibacterial effects that are lower than its antioxidant potential, which justifies its application as a pharmacological agent to protect against ROS responsible for exacerbating tissue damage.
In vitro studies comparing the antioxidative effects of quercetin, IQ and other quercetin glycosides in liposomal phospholipid suspensions also demonstrated that the quercetin aglycone is a more efficient antioxidant than its glycoside[7,31,32]. This result should not reduce the credibility of IQ as an antioxidant compound since quercetin (aglycone) is not the dominant form in nature and has an inferior bioavailability to Q3G[2,33].
In 2004, Murota et al[4] measured the antioxidant activity of IQ, Q3G, and Q4G against ion-induced lipid peroxidation of rat intestinal mucosa. Q4G showed higher inhibition of lipid peroxidation than the other compounds did, although Q3G possessed a greater ion chelation capacity.
The protective effects of IQ against lipid peroxidation have been demonstrated several times. This action is mediated by numerous mechanisms including the following: Protecting the cell membrane by inhibiting LDH efflux[34]; maintaining erythrocyte deformability, which plays an essential role in circulatory system by preventing vascular diseases[35]; accumulating in the arterial wall and preventing hyperlipidemia[36]; inhibiting fatty acid formation resulting from lipid peroxidation[37]; protecting neural cells against oxidative stress induced by H2O2 by decreasing lipid peroxidation and increasing cellular cholesterol concentrations[38]; reducing glutathione depletion and decreasing catalase and glutathione peroxidase 1 activities[39]; ROS and nitrite-scavenging activities[40]; and by regulating the oxidative complex NADPH and NO production[41], lipid-lowering activities in hepatocytes[42], induction of apolipoprotein A-I gene expression[43], and free radical scavenging[44-64].
IQ AND CANCER TREATMENT
An alternative strategy for reducing the risk of cancer is a dietary modification. Although phytochemicals naturally occur as complex mixtures, little information is available regarding possible additive, synergistic, or antagonistic interactions among these compounds. The cytotoxicity of quercetin, hyperoside (quercetin 3-O-galactoside), IQ (quercetin 3-O-glucoside), quercitrin (quercetin 3-O-rhamnoside), and spiraeoside (quercetin 4’-O-glucoside) were compared in vitro using cancer cell viability assays. The results indicate that IQ is a promising drug candidate for cancer therapy because its glycosylation conferred more advantageous pharmacological changes than quercetin had[65].
Amado et al[66] described the action of flavonoids on the Wnt signaling pathway, which plays an important role in the control of cellular differentiation, proliferation, and death as well as organogenesis and homeostasis in adults. The cancers most commonly associated with this pathway are colorectal[67], melanoma, hepatocellular carcinoma, gastric carcinoma, glioblastoma, leukemia, and breast cancers[66]. The main component of this pathway is β-catenin protein, which mainly functions to induce nuclear transcription. In the absence of Wnt ligands, β-catenin is phosphorylated by various enzyme complexes, and is able to bond with the ubiquitin-proteasome complex, which degrades it. However, in the presence of Wnt ligands, a cascade of reactions stabilizes cytoplasmic β-catenin, which can then translocate to the nucleus and mediate the stimulation of transcription factors. Numerous have studies demonstrate that inappropriate regulation and activation of this pathway is related to various diseases including cancer. The antiproliferative activity of flavonoids is related to their ability to modulate the activity of Wnt signaling at different levels. Thus, each flavonoid acts at different levels of the pathway making its actions specific against tumor cell lines. IQ for example, acts directly by inhibiting the nuclear translocation of β-catenin protein and exhibits significant anti-proliferative effects on glioblastoma cells[67].
DNA topoisomerases are important targets for cancer chemotherapy. Extracts of Helichrysum pamphylicum considerably inhibited the mammalian type I DNA topoisomerase activity in a dose-dependent manner and this effect might be correlated with the antioxidant capacity of the plant[68]. There are few studies on the carcinogenicity induced by IQ. Studies in F344/DuCrj rats to evaluate the potential carcinogenicity of enzymatically modified IQ showed no apparent effects on the development of kidney neoplasms, hyperplasias, chronic nephropathy, or mammary fibroadenomas[69].
The first report we found in PubMed on IQ and cancer was published in the year 2000. It was about the protective effects of flavonoid glycosides (quercitrin-3-glucoside and isrhamnetin-4-glicoside) against an anion meal. The study showed that increases in flavonoid plasma levels were associated with high resistance of lymphocyte DNA to strand breakage[70]. After this paper, some other in vivo and in vitro reports were published with conflicting results. The antiproliferative activities of quercetin derivatives and rutin were compared using different cancer cell lines and the results demonstrated that IQ had more potent antiproliferative effects than the other flavonoids did, especially in colon, breast, and hepatocellular cancers[71]. Similar studies demonstrated that hydrolyzed rutin, which is obtained following the enzymatic hydrolysis of rutin by an hesperidinase and mainly composed of IQ (70%), exerted markedly potent antiproliferative effect in vitro compared to the effects of quercetin and rutin in various cancer cell lines including ovarian adenocarcinoma (OVCAR-3), breast adenocarcinoma (MCF-7), and glioma (U-251). It is suggested that the most potent antiproliferative effect induced by IQ mixtures might be related to its specific glucose transport carrier SGLT-1[7].
There are some studies about effect of flavonoids on specific cancer, see as following content.
Fibrosarcoma cancer
The inhibitory effects of Salicornia herbacea on matrix metalloproteinase-9 and 2 were evaluated in a human fibrosarcoma cell line (HT1080). The study suggested that the flavonoid glycosides in this plant have potential value as natural chemopreventive agents[72].
Prostatic cancer
Polyphenols purified from the Brazilian aroeira plant (Schinus terebinthifolius, Raddi), which contain IQ, induced apoptotic and autophagic cell death of an androgen-insensitive DU145 human prostatic carcinoma cell line[73].
Pancreatic cancer
Recently in 2015, in vivo and in vitro studies investigated the effect of IQ on the progression of pancreatic cancer. In vitro, IQ inhibited proliferation, promoted apoptosis, and induced cell cycle arrest in the G1 phase in pancreatic cancer cells. IQ activated caspase-3, 8, and 9 and reduced the mitochondrial membrane potential. In addition, IQ inhibited the expression level of the δ opioid receptor; however, it had no effect on the κ and μ opioid receptors. Furthermore, IQ inhibited the phosphorylation of extracellular signal-regulated kinase (ERK) and promoted the phosphorylation of c-Jun N-terminal kinase (JuNK). In vivo, IQ inhibited xenograft growth in nude mice[74].
Breast cancer
In 2005, Matysik et al[75] showed that Calendulae officinalis flos (Asteraceae), which contains IQ, had two biological effects in human skin fibroblast and human breast cancer cells (T47D). Extracts at concentrations above 25 μg/mL, stimulated cell proliferation and cellular metabolism by increasing mitochondrial activity. However, concentrations higher than 75 μg/mL were cytotoxic[75]. Yang et al[76] assessed the synergistic effects of Q3G and apple extract in breast cancer cells. They proved that this combination significantly increased the antiproliferative activity against the growth of MCF-7 cells in vitro, compared to the effects of each compound alone. The antiproliferative activity of the apple extracts and Q3G combination was assessed by measuring the inhibition of MCF-7 human breast cancer cell proliferation, and the results demonstrated a synergistic effect against cell proliferation[76].
Cerebral cancer
Q3G pretreatment elevated both the expression and activation of sterol regulatory element-binding protein-2 (SREBP-2) and protected SH-SY5Y cells against H2O2-induced oxidative stress in neuroblastoma/SH-SY5Y cells. This data suggested a novel mechanism involving SREBP-2-mediated sterol synthesis that decreases lipid peroxidation by maintaining membrane integrity in the presence of oxidative stress[39].
IQ isolated from the aerial parts of Hyptis fasciculata was evaluated based on its ability to interfere with glioblastoma cell growth and the results suggested that beta-catenin-mediated signaling may be involved in the antiproliferative activity of IQ[77].
Lymphoproliferative cancer
Abnormal Savda Munziq is an herbal preparation used in Traditional Uighur Medicine for the treatment and prevention of diabetes, cardiovascular diseases, chronic asthma, and cancer. IQ and other flavonoids were identified as constituents of the preparation. The compound demonstrated antiproliferative activity in leukemia HL-60 cells[78].
Flavonoids (quercetin, quercitrin, IQ, and rutin) isolated from the leaves of Scutia buxifolia were evaluated against chromosomal damage induced by H2O2 in human lymphocytes and the result demonstrated that quercetin, IQ, and rutin recovered the mitotic index and chromosomal instability more than quercitrin did after treatment with H2O2[30].
Studies intended to evaluate the plant-associated quercetin glycosides and human phase II quercetin metabolites, actually found by analyzing human biological fluids that following the consumption of quercetin containing foods, these compounds can interact with the estrogen receptors (ER). It showed that IQ displays ERα- and ERβ-dependent estrogenic activity, and the results correlated with the protective activity of diets rich in quercetin glycosides[78].
Colon cancer
In 2002, Salucci et al[79] suggested that the antioxidant activity of flavonoids was not involved in their inhibition of the growth of colon adenocarcinoma cells (Caco2). They concluded that IQ is a promising antioxidant agent but that it did not inhibit Caco2 cellular growth[79].
In 2005, Kern et al[80] reported a perspective on the antiproliferative effect and demonstrated the potent EGFR-inhibitory properties of polyphenol-rich apple juice extract in adenocarcinoma colon in vitro model (HT29)[80].
Another study suggested that the beneficial effects of red wine on human health were attributable to the flavonoid content, which channeled the body metabolism towards O-methylation to yield compounds with potential protective effects against cancer[81].
In vivo assays in Xenopus embryos, a functional model of canonical Wnt signaling studies, and in vitro models were used to demonstrate the inhibitory effect of IQ on Wnt/β-catenin. The flavonoid was shown to act downstream of the translocation of β-catenin to the nuclei. IQ affects Xenopus axis establishment, reverses the double axes and LiCl hyperdorsalization phenotype, and reduces Xnr3 expression. IQ showed antitumoral effects on colon cancer cells (SW480, DLD-1, and HCT116) whereas it exerted no significant effect on non-tumor colon cells (IEC-18), suggesting a specific effect on tumor cells in vitro[82].
Liver cancer
In 2008, a study in F344 rats demonstrated the chemopreventive potential of food additives containing IQ against liver carcinogenesis[83]. Similarly, H. fasciculata and IQ, which was identified in this species, showed activity as 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavengers, suggesting their antioxidant potential in human cancer[84].
Flavonoids are also foreign compounds (xenobiotics), which can potentially modulate the activity of cytochrome P450s (CYPs), the xenobiotic-metabolizing enzymes involved in the activation and detoxification of food and environmental carcinogens. In 2009, a study investigated the effects of model glycosylated and deglycosylated flavonoids IQ and rutin, respectively, on CYPs in the rat liver. The study demonstrated their differential effects on CYP expression, suggesting a possible increase in the risk of cancer development in humans and the danger associated with the consumption of these compounds in large quantities[85].
To clarify whether enzymatically modified IQ or melatonin supplementation reduces the oxidative stress-mediated hepatocellular tumor-promoting effect of oxfendazole, a benzimidazole, anthelmintic study was conducted in male rats. They were administered a single intraperitoneal injection of N-diethylnitrosamine and fed a diet containing oxfendazole with or without EMIQ or MLT in drinking water. It was concluded that co-administration of EMIQ or MLT suppressed the hepatocellular tumor-promoting activity of oxfendazole by decreasing ROS production via activation of CYPs[5].
In the same year, another study suggested that co-administration of enzymatically modified IQ suppresses the hepatocellular tumor-promoting activity of the synthetic flavonoid derivative β-naphthoflavone (BNF) in rats by the anti-inflammatory effects of enzymatically modified IQ. In addition, the combination was shown to restore the cellular redox balance altered by BNF. The study compared the antiproliferative effects of EMIQ against the pro-oxidative and pro-tumor potential of the synthetic flavonoid derivative (BNF) in rat liver tissues. BNF is responsible for the maintenance of oxidative stress via production of ROS and activation of CYPs through the aryl hydrocarbon receptor. The results showed that the oxidative activity of BNF contributed to the formation of liver pre-neoplastic lesions while EMIQ demonstrated a protective effect by reducing the by reducing glutathione S-transferase P (GST-P)-positive cellular foci and cyclooxygenase (COX)-2 production. In addition, it suppressed encoding genes for proteins such as glutathione S-transferase mu 1 (GSTM1, drugs metabolizing enzyme), serpine1, COX-2, and nuclear factor kappa-light-chain-enhancer of activated B cells (Nf-κB), which are enzymes involved in inflammatory processes[86].
BNF is a strong inducer of CYP1A enzymes and exerts liver tumor-promoting activity through the enhancement of oxidative stress responses in rats. In 2011, a study investigated the role of the tissue environment surrounding hepatocellular preneoplastic lesions in the early tumor promotion stage induced by BNF in rats, using enzymatically modified IQ as an antioxidative chemopreventive agent. The results showed that most of the changes induced by BNF had disappeared after or were suppressed by IQ treatment, as well as the inhibition of tumor promotion. IQ also suppressed the transcription of tumor necrosis factor (TNF) induced by BNF. This observation suggested that BNF-induced oxidative stress causes single liver cell toxicity. This action further facilitated subsequent concomitant apoptosis and regeneration involving inflammatory responses including TNFα-signaling, which contributed to tumor promotion. In rat tissue, EMIQ reduces proinflammatory proteins (TNF-α) expression and cell cycle regulation molecules (Cdc20 and Cdkn2b)[87].
Enzymatically modified IQ suppressed the liver tumor-promoting activity of phenobarbital by inhibiting nuclear translocation of constitutive active/androstane receptor (CAR), and not by suppressing oxidative stress in rats. Morita et al[88] investigated the action of EMIQ in an experimental model of liver tumor induced by phenobarbital, a sedative and antiepileptic drug. The phenobarbital increased oxidative stress with the formation of ROS and activation of CAR. The group treated with EMIQ showed a significant decrease in oxidative stress markers in addition to inhibition of MAPK and CAR pathways, confirming the antioxidant and antiproliferative actions of this molecule[88,89].
A study investigated the effects of enzymatically modified IQ on preneoplastic liver cell lesions induced by thioacetamide promotion in a hepatocarcinogenesis rat model. The results suggested that thioacetamide (TAA)-induced tumor promotion involves activation of hepatic macrophages that produce proinflammatory factors. IQ may suppress the TAA-induced tumor-promoting activity by an anti-inflammatory mechanism mediated by suppressing the activation of these macrophages[90,91]. In addition, it may suppress tumor-promoting activity differentially between the inside and outside of GST-P(+) foci[90,91].
TAA induces oxidative stress and hepatocarcinogenicity in rats and downregulates p16 (Ink4a), which is associated with intraexonic hypermethylation in hepatocellular proliferative lesions. A study investigated the contribution of cell cycle aberrations associated with early hepatocarcinogenic processes induced by TAA using antioxidants, enzymatically modified IQ, and α-lipoic acid in a rat hepatocarcinogenesis model. The results suggested that downregulation of p16 (Ink4a) may allow the selective proliferation of preneoplastic cells promoted by TAA. However, antioxidants did not counteract this gene control. Moreover, effective suppression of TAA-induced cellular population changes within preneoplastic lesions by antioxidants, may reflect the facilitation of cell cycling and accumulation of DNA damage[91]. This damage activates cell cycle checkpoints, leading to G2 and M phase arrest at the early stages of the hepatocarcinogenesis promoted by TAA[91].
In 2014, Huang et al[92] investigated the impact of IQ from Bidens bipinnata L. extract on the progression of liver cancer in vitro and in vivo. IQ was shown to strongly inhibit proliferation, promote apoptosis, and induce G1 phase cell cycle arrest in human liver cancer cells. Additionally, IQ activated caspase-3, -8 and -9 while it inhibited expression levels of ERK and p38MAPK protein phosphorylation, promoted the phosphorylation of JuNK, and reduced the expression level of protein kinase C (PKC) in human liver cancer cells. Furthermore, in vivo experiments showed that IQs also significantly inhibited the growth of transplanted tumors in nude mice. The authors concluded that the molecular mechanism of IQ might be closely associated with the MAPK and PKC signaling pathways[92].
A study investigated the protective effect of bilberry extracts and enzymatically modified IQ on the hepatocarcinogenic process involving oxidative stress responses. The study used a two-stage hepatocarcinogenesis model in N-diethylnitrosamine-induced and piperonyl butoxide (PBO)-induced rats. They examined the modifying effect of co-administration of bilberry extracts and IQ on the liver tissue environment. Specifically, they determined their effects on oxidative stress responses, cell proliferation, and apoptosis. In addition, they also examined phosphatase and tensin homolog (PTEN)/Akt and transforming growth factor (TGF)-β/Smad signaling and evaluated the effects of all these phenomenon on the induction mechanism of preneoplastic lesions during early stages of hepatocellular tumor promotion. Their results suggested that PBO-induced tumor promotion potentiates PTEN/Akt and disrupts TGF-β/Smad signaling pathways, and is not related to oxidative stress responses. However, this promotion was suppressed by co-treatment with bilberry extracts and IQ, which suppressed the proliferation activity of preneoplastic liver cells[93].
ANTITUMOR MECHANISMS OF IQ
The antitumor effect of IQ and its underlying mechanism of action remain unclear. However, based on literature reports it is possible to speculate on some likely mechanisms and create a plausible hypothesis. MAPK is a protein kinase family involved in various cell processes, and the most notable act attributed to these kinases involves cell proliferation and apoptosis signaling. Currently, three sub-families of MAPK have been discovered including ERK, JuNK/MAPK, and p38 MAPK and they each have their specific cellular targets and action sites. The activation of cell adhesion recognition sites is extremely important in the molecular adhesion of both extracellular membranes and nuclear structures. Cell adhesion recognition is mostly associated with catenin-cadherin complexes and play an important role in adhesion and pathway signaling. Numerous extracellular stimuli activate MAPK pathways, generating a chain of reactions leading to intracellular remodeling and biochemical cascades. These pathways have been associated with several diseases including cancer[88,89,92].
Mutations in the MLK3 gene were first described in gastric and colorectal cancer[94] and the oncogenic effect was related to invasive induction of tumor cells. The study evaluated signaling pathways underlying the effect of MLK3 gene. It described new Wnt pathway regulation mechanisms, which may be responsible for the induction of colorectal cancer invasion related to MLK3 mutations. A reasonable hypothesis for the IQ antitumor mechanism would be the involvement of signaling pathways mediating its modulation of Wnt signaling[74], which would reaffirm data presented by other studies[67,77]. The Wnt pathway in turn, is related to the p53 pathway (via apoptosis), and published literature has demonstrated its mediation of the apoptosis-inducing action of IQ[73]. The induction of apoptosis may be related to caspases, inhibition of ERK phosphorylation, and promotion of JuNK phosphorylation[74]. Another possibility with the apoptotic pathway would be an association with the GST-P(+) foci. IQ facilitates the apoptosis of preneoplastic cells by upregulating DR5. Outside the GST-P(+) foci, IQ suppresses apoptosis of non-transformed liver cells[90].
In addition, other possible signaling interactions of IQ could involve changes in the reduction of proinflammatory proteins such as TNF-α and other cytokines such as TGF-β[87]. It is evident that numerous mechanisms are possible mediators of the actions of IQ. Therefore, further studies are crucial to clarify the specific mechanism underlying the anticancer effects of IQ, which currently remain unclear.
CONCLUSION
Flavonoids have gained a great deal of attention over the years, and their health benefits are the focus of the new research studies. Flavonoids, which are widely distributed throughout the plant kingdom, commonly occur in fruits and vegetables. Therefore, these compounds are an accessible alternative to conventional therapies in the prevention or supplementation of treatments for numerous diseases. Flavonoids are particularly important in developing countries where the technological challenges often hinder access to modern medicines, limiting their therapeutic effectiveness[1,2,27].
This review contributed to clarifying the mechanisms of absorption and metabolism of IQ, as well as highlighting the possible mechanisms of its various physiological effect. In addition, we corroborated its wide therapeutic applicability and stressed the importance of further studies to elucidate its specific anticancer mechanisms.
Based on our findings, the flavonoid IQ possesses adequate bioavailability and inhibits the pathogenesis of various diseases and, therefore, warrants further study to enhance the understanding of its beneficial actions. In addition, further studies would facilitate the discovery of new therapeutic applications and highlight the essential contribution of IQ to ensuring a more balanced diet, which would contribute to a healthier lifestyle. Further studies on the metabolism of flavonoids, their actions, and effects of their metabolites in various tissues are necessary to facilitate their development and better application in clinical management.
Footnotes
Supported by São Paulo Research Foundation - FAPESP, No. 2012-04634-1.
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 14, 2015
First decision: September 17, 2015
Article in press: February 16, 2016
P- Reviewer: Su CC, Vetvicka V S- Editor: Kong JX L- Editor: A E- Editor: Li D
References
- 1.Dhiman A, Nanda A, Ahmad S. A quest for staunch effects of flavonoids: Utopian protection against hepatic ailments. Arab J Chem. 2012:In press. [Google Scholar]
- 2.Appleton J. Evaluating the Bioavailability of Isoquercetin. Nat Med J. 2010;2:1–6. [Google Scholar]
- 3.Masuda T, Iritani K, Yonemori S, Oyama Y, Takeda Y. Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Biosci Biotechnol Biochem. 2001;65:1302–1309. doi: 10.1271/bbb.65.1302. [DOI] [PubMed] [Google Scholar]
- 4.Murota K, Shimizu S, Chujo H, Moon JH, Terao J. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line Caco-2. Arch Biochem Biophys. 2000;384:391–397. doi: 10.1006/abbi.2000.2123. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura J, Saegusa Y, Dewa Y, Jin M, Kawai M, Kemmochi S, Harada T, Hayashi SM, Shibutani M, Mitsumori K. Antioxidant enzymatically modified isoquercitrin or melatonin supplementation reduces oxidative stress-mediated hepatocellular tumor promotion of oxfendazole in rats. Arch Toxicol. 2010;84:143–153. doi: 10.1007/s00204-009-0497-9. [DOI] [PubMed] [Google Scholar]
- 6.Emura K, Yokomizo A, Toyoshi T, Moriwaki M. Effect of enzymatically modified isoquercitrin in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:68–74. doi: 10.3177/jnsv.53.68. [DOI] [PubMed] [Google Scholar]
- 7.de Araújo ME, Moreira Franco YE, Alberto TG, Sobreiro MA, Conrado MA, Priolli DG, Frankland Sawaya AC, Ruiz AL, de Carvalho JE, de Oliveira Carvalho P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013;141:266–273. doi: 10.1016/j.foodchem.2013.02.127. [DOI] [PubMed] [Google Scholar]
- 8.Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G. Absorption of quercetin-3-glucoside and quercetin-4’-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem Pharmacol. 2003;65:1199–1206. doi: 10.1016/s0006-2952(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 9.Boyer J, Brown D, Liu RH. In vitro digestion and lactase treatment influence uptake of quercetin and quercetin glucoside by the Caco-2 cell monolayer. Nutr J. 2005;4:1. doi: 10.1186/1475-2891-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sesink AL, Arts IC, Faassen-Peters M, Hollman PC. Intestinal uptake of quercetin-3-glucoside in rats involves hydrolysis by lactase phlorizin hydrolase. J Nutr. 2003;133:773–776. doi: 10.1093/jn/133.3.773. [DOI] [PubMed] [Google Scholar]
- 11.Gee JM, DuPont MS, Day AJ, Plumb GW, Williamson G, Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- 12.Lesser S, Cermak R, Wolffram S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J Nutr. 2004;134:1508–1511. doi: 10.1093/jn/134.6.1508. [DOI] [PubMed] [Google Scholar]
- 13.Cermak R, Landgraf S, Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br J Nutr. 2004;91:849–855. doi: 10.1079/BJN20041128. [DOI] [PubMed] [Google Scholar]
- 14.Sesink AL, O’Leary KA, Hollman PC. Quercetin glucuronides but not glucosides are present in human plasma after consumption of quercetin-3-glucoside or quercetin-4’-glucoside. J Nutr. 2001;131:1938–1941. doi: 10.1093/jn/131.7.1938. [DOI] [PubMed] [Google Scholar]
- 15.Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4’-glucoside do not differ in humans. J Nutr. 2000;130:1200–1203. doi: 10.1093/jn/130.5.1200. [DOI] [PubMed] [Google Scholar]
- 16.Reinboth M, Wolffram S, Abraham G, Ungemach FR, Cermak R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br J Nutr. 2010;104:198–203. doi: 10.1017/S000711451000053X. [DOI] [PubMed] [Google Scholar]
- 17.Chang Q, Zuo Z, Chow MS, Ho WK. Difference in absorption of the two structurally similar flavonoid glycosides, hyperoside and isoquercitrin, in rats. Eur J Pharm Biopharm. 2005;59:549–555. doi: 10.1016/j.ejpb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Lesser S, Cermak R, Wolffram S. The fatty acid pattern of dietary fat influences the oral bioavailability of the flavonol quercetin in pigs. Br J Nutr. 2006;96:1047–1052. doi: 10.1017/bjn20061953. [DOI] [PubMed] [Google Scholar]
- 19.Lan K, Jiang X, He J. Quantitative determination of isorhamnetin, quercetin and kaempferol in rat plasma by liquid chromatography with electrospray ionization tandem mass spectrometry and its application to the pharmacokinetic study of isorhamnetin. Rapid Commun Mass Spectrom. 2007;21:112–120. doi: 10.1002/rcm.2814. [DOI] [PubMed] [Google Scholar]
- 20.Krogholm KS, Bredsdorff L, Knuthsen P, Haraldsdóttir J, Rasmussen SE. Relative bioavailability of the flavonoids quercetin, hesperetin and naringenin given simultaneously through diet. Eur J Clin Nutr. 2010;64:432–435. doi: 10.1038/ejcn.2010.6. [DOI] [PubMed] [Google Scholar]
- 21.Berger LM, Wein S, Blank R, Metges CC, Wolffram S. Bioavailability of the flavonol quercetin in cows after intraruminal application of quercetin aglycone and rutin. J Dairy Sci. 2012;95:5047–5055. doi: 10.3168/jds.2012-5439. [DOI] [PubMed] [Google Scholar]
- 22.Gohlke A, Ingelmann CJ, Nürnberg G, Starke A, Wolffram S, Metges CC. Bioavailability of quercetin from its aglycone and its glucorhamnoside rutin in lactating dairy cows after intraduodenal administration. J Dairy Sci. 2013;96:2303–2313. doi: 10.3168/jds.2012-6234. [DOI] [PubMed] [Google Scholar]
- 23.Cermak R, Landgraf S, Wolffram S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J Nutr. 2003;133:2802–2807. doi: 10.1093/jn/133.9.2802. [DOI] [PubMed] [Google Scholar]
- 24.Wiczkowski W, Romaszko J, Bucinski A, Szawara-Nowak D, Honke J, Zielinski H, Piskula MK. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr. 2008;138:885–888. doi: 10.1093/jn/138.5.885. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Mitchell AE. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J Agric Food Chem. 2012;60:3874–3881. doi: 10.1021/jf3001857. [DOI] [PubMed] [Google Scholar]
- 26.Liang T, Yue W, Li Q. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int J Mol Sci. 2010;11:4452–4464. doi: 10.3390/ijms11114452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 28.Vellosa JCR, Regasini LO, Khalil NM, Bolzani VS, Khalil OAK, Manente FA, Netto HP, Oliveira OMMF. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclet Quim. 2001;36:7–20. [Google Scholar]
- 29.Boligon AA, Sagrillo MR, Machado LF, de Souza Filho O, Machado MM, da Cruz IB, Athayde ML. Protective effects of extracts and flavonoids isolated from Scutia buxifolia Reissek against chromosome damage in human lymphocytes exposed to hydrogen peroxide. Molecules. 2012;17:5757–5769. doi: 10.3390/molecules17055757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razavi SM, Zahri S, Zarrini G, Nazemiyeh H, Mohammadi S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Bioorg Khim. 2009;35:414–416. doi: 10.1134/s1068162009030133. [DOI] [PubMed] [Google Scholar]
- 31.Ioku K, Tsushida T, Takei Y, Nakatani N, Terao J. Antioxidative activity of quercetin and quercetin monoglucosides in solution and phospholipid bilayers. Biochim Biophys Acta. 1995;1234:99–104. doi: 10.1016/0005-2736(94)00262-n. [DOI] [PubMed] [Google Scholar]
- 32.Noroozi M, Angerson WJ, Lean ME. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am J Clin Nutr. 1998;67:1210–1218. doi: 10.1093/ajcn/67.6.1210. [DOI] [PubMed] [Google Scholar]
- 33.Williamson G, Plumb GW, Uda Y, Price KR, Rhodes MJ. Dietary quercetin glycosides: antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis. 1996;17:2385–2387. doi: 10.1093/carcin/17.11.2385. [DOI] [PubMed] [Google Scholar]
- 34.Yokozawa T, Dong E, Kawai Y, Gemba M, Shimizu M. Protective effects of some flavonoids on the renal cellular membrane. Exp Toxicol Pathol. 1999;51:9–14. doi: 10.1016/S0940-2993(99)80050-5. [DOI] [PubMed] [Google Scholar]
- 35.Begum AN, Terao J. Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. J Nutr Biochem. 2002;13:265–272. doi: 10.1016/s0955-2863(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 36.Kamada C, da Silva EL, Ohnishi-Kameyama M, Moon JH, Terao J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic Res. 2005;39:185–194. doi: 10.1080/10715760400019638. [DOI] [PubMed] [Google Scholar]
- 37.Dufour C, Loonis M. Flavonoids and their oxidation products protect efficiently albumin-bound linoleic acid in a model of plasma oxidation. Biochim Biophys Acta. 2007;1770:958–965. doi: 10.1016/j.bbagen.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Soundararajan R, Wishart AD, Rupasinghe HP, Arcellana-Panlilio M, Nelson CM, Mayne M, Robertson GS. Quercetin 3-glucoside protects neuroblastoma (SH-SY5Y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis. J Biol Chem. 2008;283:2231–2245. doi: 10.1074/jbc.M703583200. [DOI] [PubMed] [Google Scholar]
- 39.Jung SH, Kim BJ, Lee EH, Osborne NN. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells) Neurochem Int. 2010;57:713–721. doi: 10.1016/j.neuint.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Li R, Yuan C, Dong C, Shuang S, Choi MM. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:437–445. doi: 10.1007/s00210-011-0613-2. [DOI] [PubMed] [Google Scholar]
- 41.Kim BH, Choi JS, Yi EH, Lee JK, Won C, Ye SK, Kim MH. Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-κB/CRE/AP-1 signaling in murine macrophages. Mol Cells. 2013;35:410–420. doi: 10.1007/s10059-013-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan W, Rongyin G, Daoud A, Ding L, Wang L, Liu J, Shang J. Reduced oxidative stress contributes to the lipid lowering effects of isoquercitrin in free fatty acids induced hepatocytes. Oxid Med Cell Longev. 2014;2014:313602. doi: 10.1155/2014/313602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas MJ, Onstead-Haas LM, Szafran-Swietlik A, Kojanian H, Davis T, Armstrong P, Wong NC, Mooradian AD. Induction of hepatic apolipoprotein A-I gene expression by the isoflavones quercetin and isoquercetrin. Life Sci. 2014;110:8–14. doi: 10.1016/j.lfs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Chen P, Wu D, Pan Y. Separation and purification of antioxidants from Ampelopsis heterophylla by counter-current chromatography. J Sep Sci. 2013;36:3660–3666. doi: 10.1002/jssc.201300917. [DOI] [PubMed] [Google Scholar]
- 45.Maleš Z, Sarić D, Bojić M. Quantitative Determination of Flavonoids and Chlorogenic Acid in the Leaves of Arbutus unedo L. Using Thin Layer Chromatography. J Anal Methods Chem. 2013;2013:385473. doi: 10.1155/2013/385473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraujalis P, Venskutonis PR, Kraujalienė V, Pukalskas A. Antioxidant properties and preliminary evaluation of phytochemical composition of different anatomical parts of amaranth. Plant Foods Hum Nutr. 2013;68:322–328. doi: 10.1007/s11130-013-0375-8. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Li F, Zhang X, Wang L, Zhou Z, Wang M. Phenolic antioxidants from Rosa soulieana flowers. Nat Prod Res. 2013;27:2055–2058. doi: 10.1080/14786419.2013.811660. [DOI] [PubMed] [Google Scholar]
- 48.Sukito A, Tachibana S. Isolation of hyperoside and isoquercitrin from Camellia sasanqua as antioxidant agents. Pak J Biol Sci. 2014;17:999–1006. doi: 10.3923/pjbs.2014.999.1006. [DOI] [PubMed] [Google Scholar]
- 49.Kim DS, Kang YM, Jin WY, Sung YY, Choi G, Kim HK. Antioxidant activities and polyphenol content of Morus alba leaf extracts collected from varying regions. Biomed Rep. 2014;2:675–680. doi: 10.3892/br.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang TT, Lu CL, Jiang JG. Bioactivity evaluation of ingredients identified from the fruits of Amomum tsaoko Crevost et Lemaire, a Chinese spice. Food Funct. 2014;5:1747–1754. doi: 10.1039/c4fo00169a. [DOI] [PubMed] [Google Scholar]
- 51.Vlase L, Benedec D, Hanganu D, Damian G, Csillag I, Sevastre B, Mot AC, Silaghi-Dumitrescu R, Tilea I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules. 2014;19:5490–5507. doi: 10.3390/molecules19055490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Cao X, Jiang H, Qi Y, Chin KL, Yue Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules. 2014;19:21226–21238. doi: 10.3390/molecules191221226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liaudanskas M, Viškelis P, Raudonis R, Kviklys D, Uselis N, Janulis V. Phenolic composition and antioxidant activity of Malus domestica leaves. ScientificWorldJournal. 2014;2014:306217. doi: 10.1155/2014/306217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orzel J, Daszykowski M, Kazura M, de Beer D, Joubert E, Schulze AE, Beelders T, de Villiers A, Malherbe CJ, Walczak B. Modeling of the total antioxidant capacity of rooibos (Aspalathus linearis) tea infusions from chromatographic fingerprints and identification of potential antioxidant markers. J Chromatogr A. 2014;1366:101–109. doi: 10.1016/j.chroma.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Mocan A, Crișan G, Vlase L, Crișan O, Vodnar DC, Raita O, Gheldiu AM, Toiu A, Oprean R, Tilea I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules. 2014;19:15162–15179. doi: 10.3390/molecules190915162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cárdenas-Rodríguez N, González-Trujano ME, Aguirre-Hernández E, Ruíz-García M, Sampieri A, Coballase-Urrutia E, Carmona-Aparicio L. Anticonvulsant and antioxidant effects of Tilia americana var. mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid Med Cell Longev. 2014;2014:329172. doi: 10.1155/2014/329172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taiwo BJ, Igbeneghu OA. Antioxidant and antibacterial activities of flavonoid glycosides from Ficus exasperata Vahl-Holl (moraceae) leaves. Afr J Tradit Complement Altern Med. 2014;11:97–101. doi: 10.4314/ajtcam.v11i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brito A, Areche C, Sepúlveda B, Kennelly EJ, Simirgiotis MJ. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules. 2014;19:10936–10955. doi: 10.3390/molecules190810936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Ma SS, Ibrahim SA, Li EH, Yang H, Huang W. Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chem. 2015;185:159–164. doi: 10.1016/j.foodchem.2015.03.117. [DOI] [PubMed] [Google Scholar]
- 60.Marksa M, Radušienė J, Jakštas V, Ivanauskas L, Marksienė R. Development of an HPLC post-column antioxidant assay for Solidago canadensis radical scavengers. Nat Prod Res. 2016;30:536–543. doi: 10.1080/14786419.2015.1027703. [DOI] [PubMed] [Google Scholar]
- 61.Andriamadio JH, Rasoanaivo LH, Benedec D, Vlase L, Gheldiu AM, Duma M, Toiu A, Raharisololalao A, Oniga I. HPLC/MS analysis of polyphenols, antioxidant and antimicrobial activities of Artabotrys hildebrandtii O. Hffm. extracts. Nat Prod Res. 2015;29:2188–2196. doi: 10.1080/14786419.2015.1007458. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez JE, Zambrano R, Sepúlveda B, Kennelly EJ, Simirgiotis MJ. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015;176:106–114. doi: 10.1016/j.foodchem.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 63.Zorzetto C, Sánchez-Mateo CC, Rabanal RM, Lupidi G, Petrelli D, Vitali LA, Bramucci M, Quassinti L, Caprioli G, Papa F, et al. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium) Fitoterapia. 2015;100:95–109. doi: 10.1016/j.fitote.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 64.Bhullar KS, Rupasinghe HP. Antioxidant and cytoprotective properties of partridgeberry polyphenols. Food Chem. 2015;168:595–605. doi: 10.1016/j.foodchem.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 65.Park SH, Kim HJ, Yim SH, Kim AR, Tyagi N, Shen H, Kim KK, Shin BA, Jung DW, Williams DR. Delineation of the role of glycosylation in the cytotoxic properties of quercetin using novel assays in living vertebrates. J Nat Prod. 2014;77:2389–2396. doi: 10.1021/np500231g. [DOI] [PubMed] [Google Scholar]
- 66.Amado NG, Fonseca BF, Cerqueira DM, Neto VM, Abreu JG. Flavonoids: potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011;89:545–554. doi: 10.1016/j.lfs.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Martinez NP, Kanno DT, Pereira JA, Cardinalli IA, Priolli DG. Beta-catenin and E-cadherin tissue “content” as prognostic markers in left-side colorectal cancer. Cancer Biomark. 2011;8:129–135. doi: 10.3233/DMA-2011-0843. [DOI] [PubMed] [Google Scholar]
- 68.Topcu Z, Ozturk B, Kucukoglu O, Kilinc E. Flavonoids in Helichrysum pamphylicum inhibit mammalian type I DNA topoisomerase. Z Naturforsch C. 2008;63:69–74. doi: 10.1515/znc-2008-1-213. [DOI] [PubMed] [Google Scholar]
- 69.Salim EI, Kaneko M, Wanibuchi H, Morimura K, Fukushima S. Lack of carcinogenicity of enzymatically modified isoquercitrin in F344/DuCrj rats. Food Chem Toxicol. 2004;42:1949–1969. doi: 10.1016/j.fct.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Boyle SP, Dobson VL, Duthie SJ, Kyle JA, Collins AR. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur J Nutr. 2000;39:213–223. doi: 10.1007/s003940070014. [DOI] [PubMed] [Google Scholar]
- 71.You HJ, Ahn HJ, Ji GE. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J Agric Food Chem. 2010;58:10886–10892. doi: 10.1021/jf102871g. [DOI] [PubMed] [Google Scholar]
- 72.Kong CS, Kim YA, Kim MM, Park JS, Kim JA, Kim SK, Lee BJ, Nam TJ, Seo Y. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol In Vitro. 2008;22:1742–1748. doi: 10.1016/j.tiv.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Queires LC, Fauvel-Lafètve F, Terry S, De la Taille A, Kouyoumdjian JC, Chopin DK, Vacherot F, Rodrigues LE, Crépin M. Polyphenols purified from the Brazilian aroeira plant (Schinus terebinthifolius, Raddi) induce apoptotic and autophagic cell death of DU145 cells. Anticancer Res. 2006;26:379–387. [PubMed] [Google Scholar]
- 74.Chen Q, Li P, Li P, Xu Y, Li Y, Tang B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol Rep. 2015;33:840–848. doi: 10.3892/or.2014.3626. [DOI] [PubMed] [Google Scholar]
- 75.Matysik G, Wójciak-Kosior M, Paduch R. The influence of Calendulae officinalis flos extracts on cell cultures, and the chromatographic analysis of extracts. J Pharm Biomed Anal. 2005;38:285–292. doi: 10.1016/j.jpba.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Liu RH. Synergistic effect of apple extracts and quercetin 3-beta-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem. 2009;57:8581–8586. doi: 10.1021/jf8039796. [DOI] [PubMed] [Google Scholar]
- 77.Amado NG, Cerqueira DM, Menezes FS, da Silva JF, Neto VM, Abreu JG. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs. 2009;20:543–552. doi: 10.1097/CAD.0b013e32832d1149. [DOI] [PubMed] [Google Scholar]
- 78.Kizaibek M, Popescu R, Prinz S, Upur H, Singhuber J, Zehl M, Kopp B. Towards modernization of the formulation of the traditional uighur medicine herbal preparation abnormal savda munziq. Evid Based Complement Alternat Med. 2012;2012:863101. doi: 10.1155/2012/863101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salucci M, Stivala LA, Maiani G, Bugianesi R, Vannini V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2) Br J Cancer. 2002;86:1645–1651. doi: 10.1038/sj.bjc.6600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kern M, Tjaden Z, Ngiewih Y, Puppel N, Will F, Dietrich H, Pahlke G, Marko D. Inhibitors of the epidermal growth factor receptor in apple juice extract. Mol Nutr Food Res. 2005;49:317–328. doi: 10.1002/mnfr.200400086. [DOI] [PubMed] [Google Scholar]
- 81.Dragoni S, Gee J, Bennett R, Valoti M, Sgaragli G. Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro. Br J Pharmacol. 2006;147:765–771. doi: 10.1038/sj.bjp.0706662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amado NG, Predes D, Fonseca BF, Cerqueira DM, Reis AH, Dudenhoeffer AC, Borges HL, Mendes FA, Abreu JG. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J Biol Chem. 2014;289:35456–35467. doi: 10.1074/jbc.M114.621599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yokohira M, Yamakawa K, Saoo K, Matsuda Y, Hosokawa K, Hashimoto N, Kuno T, Imaida K. Antioxidant effects of flavonoids used as food additives (purple corn color, enzymatically modified isoquercitrin, and isoquercitrin) on liver carcinogenesis in a rat medium-term bioassay. J Food Sci. 2008;73:C561–C568. doi: 10.1111/j.1750-3841.2008.00862.x. [DOI] [PubMed] [Google Scholar]
- 84.Silva CG, Raulino RJ, Cerqueira DM, Mannarino SC, Pereira MD, Panek AD, Silva JF, Menezes FS, Eleutherio EC. In vitro and in vivo determination of antioxidant activity and mode of action of isoquercitrin and Hyptis fasciculata. Phytomedicine. 2009;16:761–767. doi: 10.1016/j.phymed.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 85.Křížková J, Burdová K, Stiborová M, Křen V, Hodek P. The effects of selected flavonoids on cytochromes P450 in rat liver and small intestine. Interdiscip Toxicol. 2009;2:201–204. doi: 10.2478/v10102-009-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimada Y, Dewa Y, Ichimura R, Suzuki T, Mizukami S, Hayashi SM, Shibutani M, Mitsumori K. Antioxidant enzymatically modified isoquercitrin suppresses the development of liver preneoplastic lesions in rats induced by beta-naphthoflavone. Toxicology. 2010;268:213–218. doi: 10.1016/j.tox.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Kuwata K, Shibutani M, Hayashi H, Shimamoto K, Hayashi SM, Suzuki K, Mitsumori K. Concomitant apoptosis and regeneration of liver cells as a mechanism of liver-tumor promotion by β-naphthoflavone involving TNFα-signaling due to oxidative cellular stress in rats. Toxicology. 2011;283:8–17. doi: 10.1016/j.tox.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 88.Morita R, Shimamoto K, Ishii Y, Kuwata K, Ogawa B, Imaoka M, Hayashi SM, Suzuki K, Shibutani M, Mitsumori K. Suppressive effect of enzymatically modified isoquercitrin on phenobarbital-induced liver tumor promotion in rats. Arch Toxicol. 2011;85:1475–1484. doi: 10.1007/s00204-011-0696-z. [DOI] [PubMed] [Google Scholar]
- 89.Rowlands TM, Symonds JM, Farookhi R, Blaschuk OW. Cadherins: crucial regulators of structure and function in reproductive tissues. Rev Reprod. 2000;5:53–61. doi: 10.1530/ror.0.0050053. [DOI] [PubMed] [Google Scholar]
- 90.Fujii Y, Kimura M, Ishii Y, Yamamoto R, Morita R, Hayashi SM, Suzuki K, Shibutani M. Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats. Toxicology. 2013;305:30–40. doi: 10.1016/j.tox.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Kimura M, Fujii Y, Yamamoto R, Yafune A, Hayashi SM, Suzuki K, Shibutani M. Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model. Exp Toxicol Pathol. 2013;65:979–988. doi: 10.1016/j.etp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Huang G, Tang B, Tang K, Dong X, Deng J, Liao L, Liao Z, Yang H, He S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol Rep. 2014;31:2377–2384. doi: 10.3892/or.2014.3099. [DOI] [PubMed] [Google Scholar]
- 93.Hara S, Morita R, Ogawa T, Segawa R, Takimoto N, Suzuki K, Hamadate N, Hayashi SM, Odachi A, Ogiwara I, et al. Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model. Exp Toxicol Pathol. 2014;66:225–234. doi: 10.1016/j.etp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Velho S, Pinto A, Licastro D, Oliveira MJ, Sousa F, Stupka E, Seruca R. Dissecting the signaling pathways associated with the oncogenic activity of MLK3 P252H mutation. BMC Cancer. 2014;14:182. doi: 10.1186/1471-2407-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]