Abstract
AIM: To study the indications for sentinel lymph node biopsy (SLNB) in clinically-detected ductal carcinoma in situ (CD-DCIS).
METHODS: A retrospective analysis of 20 patients with an initial diagnosis of pure DCIS by an image-guided core needle biopsy (CNB) between June 2006 and June 2012 was conducted at King Faisal Specialist Hospital. The accuracy of performing SLNB in CD-DCIS, the rate of sentinel and non-sentinel nodal metastasis, and the histologic underestimation rate of invasive cancer at initial diagnosis were analyzed. The inclusion criteria were a preoperative diagnosis of pure DCIS with no evidence of invasion. We excluded any patient with evidence of microinvasion or invasion. There were two cases of mammographically detected DCIS and 18 cases of CD-DCIS. All our patients were diagnosed by an image-guided CNB except two patients who were diagnosed by fine needle aspiration (FNA). All patients underwent breast surgery, SLNB, and axillary lymph node dissection (ALND) if the SLN was positive.
RESULTS: Twenty patients with an initial diagnosis of pure DCIS underwent SLNB, 2 of whom had an ALND. The mean age of the patients was 49.7 years (range, 35-70). Twelve patients (60%) were premenopausal and 8 (40%) were postmenopausal. CNB was the diagnostic procedure for 18 patients, and 2 who were diagnosed by FNA were excluded from the calculation of the underestimation rate. Two out of 20 had a positive SLNB and underwent an ALND and neither had additional non sentinel lymph node metastasis. Both the sentinel visualization rate and the intraoperative sentinel identification rate were 100%. The false negative rate was 0%. Only 2 patients had a positive SLNB (10%) and neither had additional metastasis following an ALND. After definitive surgery, 3 patients were upstaged to invasive ductal carcinoma (3/18 = 16.6%) and 3 other patients were upstaged to DCIS with microinvasion (3/18 = 16.6%). Therefore the histologic underestimation rate of invasive disease was 33%.
CONCLUSION: SLNB in CD-DCIS is technically feasible and highly accurate. We recommend limiting SLNB to patients undergoing a mastectomy.
Keywords: Non-invasive tumor, Sentinel lymph node biopsy, Ductal carcinoma in situ, Diagnosis, Breast cancer
Core tip: While most ductal carcinoma in situ (DCIS) cases present with a radiologically detected abnormality, our sample represented a rare group of ductal carcinoma which was detected clinically. This study had a specific objective to determine the indications for sentinel lymph node in clinically detected DCIS. There are very few studies worldwide tracking this specific group, and there is no screening program in our community for breast cancer. This study will help communities who have no screening program to put protocols in place for such a specific group of patients.
INTRODUCTION
Ductal carcinoma in situ (DCIS) is a non-invasive disease that does not have the metastatic potential to spread to the axillary lymph nodes. However, DCIS commonly coexists with microinvasion and invasive disease[1,2].
The preoperative diagnosis of pure DCIS can be achieved either by an image-guided core needle biopsy (CNB) or by a surgical biopsy. Image-guided CNB carries a risk of sampling error and underestimation of the presence of occult invasive disease. However, patients diagnosed with pure DCIS by a surgical biopsy show fewer sampling errors and underestimation because the whole lesion is examined to exclude the presence of invasive disease. Therefore, the diagnosis is more definitive. About 8.8%-51.5% of pure DCIS diagnosed by CNB are upstaged to DCIS with microinvasion (DCIS-MI) or invasive disease on final pathology[3]. Thus, it is of value to stage the axilla in patients diagnosed with pure DCIS by a CNB.
The simplicity of performing sentinel lymph node biopsy (SLNB), the positive SLN metastasis rate (0.39%-20%), and the high underestimation rate (8.8%-51.5%) associated with CNB has encouraged surgeons to perform SLNB in patients diagnosed preoperatively with pure DCIS[4,5]. Another argument in favor of this approach is that a positive SLN is an indirect means of diagnosing occult microinvasion or invasive disease in the breast specimen, since DCIS can coexist with microinvasion or invasive disease. Although SLNB is a less invasive procedure compared with axillary lymph node dissection (ALND), it is associated with a certain degree of morbidity and may lead to overdiagnosis and overtreatment[6]. Paresthesias, lymphedemas, and seromas have also been reported[7,8]. Therefore, the role of SLNB in DCIS is controversial and the indications for its use are not clear. In this study, we aimed to evaluate the indications for the use of SLNB in clinically-detected DCIS (CD-DCIS) with a preoperative image-guided CNB diagnosis of pure DCIS by measuring the following parameters: (1) the accuracy of performing SLNB in CD-DCIS; (2) sentinel and non-sentinel nodal positivity rate; and (3) the underestimation rate.
MATERIALS AND METHODS
We describe a single surgeon’s experience with SLNB in 20 cases of CD-DCIS treated between June 2006 and June 2012 at King Faisal Specialist Hospital (KFSH) and Research Centre (RC). All data were collected prospectively and analyzed retrospectively. The inclusion criteria were a preoperative diagnosis of pure DCIS and no evidence of invasion. We excluded any patient with evidence of microinvasion or invasion. There were two cases of mammographically detected DCIS and 18 cases of CD-DCIS. All patients were diagnosed by an image-guided CNB except for 2 patients who were diagnosed by fine needle aspiration (FNA). All patients underwent breast surgery, SLNB, and ALND if the SLN was positive.
The following clinical and pathological data were collected from the medical records: Age at diagnosis; menopausal status; initial diagnostic method; type of surgery; whether they presented mammographically or clinically; type of clinical presentation; the presence of multicentricity and multifocality; tumor size; nuclear grade; hormone receptor status; type of histology; and initial and final pathological status. Patient, clinical, treatment, and pathological characteristics are outlined in Table 1.
Table 1.
Patient characteristics
| Character | NO. | % |
| Age | ||
| Mean | 49.7 | |
| Range | 35-70 | |
| Menopausal status | ||
| Pre | 12 | 60 |
| Post | 8 | 40 |
| Clinical presentation | ||
| Mammographically detected | 2 | 10 |
| Clinically detected | 18 | 90 |
| Palpable mass | 15 | 83.30 |
| Nipple discharge | 1 | 5.50 |
| Paget’s | 2 | 11.10 |
| Multicentricity/mulifocality | 5 | 25 |
| Initial diagnostic tool | ||
| FNA | 2 | 10 |
| CNB | 18 | 90 |
| Type of surgery | ||
| Lumpectomy | 5 | 25 |
| Simple mastectomy | 10 | 50 |
| Skin sparing mastectomy | 5 | 25 |
| Tumor size | ||
| < 3 cm | 3 | 15 |
| > 3 cm < 6 cm | 12 | 60 |
| > 6 cm | 5 | 25 |
| Nuclear grade | ||
| Low | 0 | |
| Intermediate | 9 | 45 |
| High | 11 | 55 |
| Histology | ||
| With central necrosis | 11 | 55 |
| Without central necrosis | 9 | 45 |
| Final histology | ||
| Pure DCIS | 14 | 70 |
| DCIS + MIC | 3 | 15 |
| DCIS + IDC | 3 | 15 |
| Hormonal receptors | ||
| ER+ PR+ | 6 | 30 |
| ER- PR- | 10 | 50 |
| Unknown | 4 | 1 |
| Her2/neu | ||
| Her2/neu+ | 9 | 45 |
| Her2/neu- | 4 | 1 |
| Unknown | 7 | 35 |
| Adjuvant radiotherapy | ||
| Yes | 5 | 25 |
| No | 15 | 75 |
FNA: Fine needle aspiration; CNB: Core needle biopsy; DCIS: Ductal carcinoma in situ; IDC: Invasive ductal carcinoma; MIC: Microinvasion; ER: Estrogen receptor; PR: Progesterone receptor.
Preoperative lymphatic mapping
Lymphatic mapping was performed by a peri-areolar intradermal injection of 4 deposits of 0.1 mL each with 10 MBq technetium-labeled (Tc-99m) nano-colloid in each quadrant of the areola. This was performed in the nuclear medicine suite 1-4 h before surgery. Static lymphoscintigraphy was performed to visualize and localize the sentinel node.
Surgery SLNB
A hand-held gamma detector probe was used during surgery to identify the sentinel node/s. The highest radioactive node count relative to the background was regarded as the sentinel node. The SLN identification procedure at KFSH involves the sole use of Tc-99m injection and no blue dye is used during surgery.
Pathological assessment
The entire lymph node/s was submitted for intraoperative pathological examination. The node/s was sliced into 2 mm thick sections along its longitudinal axis while nodes less than 5 mm were processed uncut. Three levels were obtained from the frozen section and stained by hematoxylin and eosin (H and E). Three additional H and E-stained slices at 200 μm intervals were made on formalin-fixed, paraffin-embedded leftover frozen section tissue. Immunohistochemical stain for cytokeratin (AE1/AE3) was performed on equivocal cases. Pathological assessment for the presence of metastasis was categorized as macrometastasis (size > 2.0 mm), micrometastasis (> 0.2 mm but no larger than 2.0 mm), or isolated tumor cells (< 0.2 mm).
RESULTS
The mean age of the 20 female patients with an initial diagnosis of pure DCIS was 49.7 years (range: 35-70), 12 (60%) were premenopausal and 8 (40%) were postmenopausal. Of these patients, 18 (90%) were clinically detected and 2 (10%) were mammographically detected. Among the CD-DCIS, 15 (83.3%) presented with a palpable mass, and one with nipple discharge and 2 with Paget’s disease. Eighteen (90%) patients had their initial diagnosis by CNB and 2 by FNA (10%).
The final histopathology was pure DCIS in 14 (70%) cases, DCIS with microinvasion in 3 (15%) cases, and DCIS with invasive disease in 3 (15%) cases. High nuclear grade and central necrosis was present in 11 (55%) cases and intermediate nuclear grade without central necrosis was present in 9 (45%) cases. Eleven (55%) patients had high nuclear grade and 9 (45%) patients had intermediate grade. Five (25%) patients had multicentricity or multifocality, and 17 (85%) patients had tumors larger than 3 cm.
Of the 20 patients studied, 15 (75%) had either a simple mastectomy or skin sparing mastectomy, and 5 (25%) had a lumpectomy. Postoperative radiotherapy was offered to patients who underwent a lumpectomy. Hormonal therapy was given only to patients with DCIS with microinvasion or invasive carcinoma if they were hormone receptor positive. The mastectomy rate was high because patients either had Paget’s disease, multifocality, multicentricity, or extensive disease. All had a SLNB and only 2 had an ALND.
Accuracy
The accuracy of performing SLNB in DCIS was as follows (Table 2): The mean number of SLN’s removed was 2 (range: 1-3); the SLN visualization rate was 100%; the intraoperative SLN identification rate was 100%; and the false negative rate was zero. All 18 cases of negative sentinels were also negative on final pathology.
Table 2.
Accuracy of sentinel lymph node
| Accuracy | NO. | % |
| Total number | 20 | |
| SLN removed | ||
| Range | 1-3 | |
| Mean | 2 | |
| SLN positive | 2-20 | 10 |
| Non-SLN positive | 0/2 | 0 |
| SLN visualization rate | 20 | 100 |
| SLN identification rate | 20 | 100 |
| False negative rate | 0 out of 18 | 0 |
SLN: Sentinel lymph node.
Sentinel and non-sentinel nodal positivity rate
There were 2 positive SLNs and 18 negative SLNs (Table 3). One positive SLN was detected in a pure DCIS case (1/14) and the other in a patient with invasive disease (1/3). Two ALNDs were performed for the 2 positive SLNs, and in both cases the SLN was the only positive node. Therefore, the SLN positivity rate was 10%, and the non-SLN positivity rate was zero.
Table 3.
Pathology of sentinel lymph node and non-sentinel lymph node
| Final diagnosis | n | % |
SLN positive |
Non-SLN positive |
||
| n | % | n | % | |||
| Pure DCIS | 14 | 70 | 1/14 | 7.1 | None | 0 |
| DCIS/MIC | 3 | 15 | 0/3 | 0 | ||
| DCIS/IDC | 3 | 15 | 1/3 | 33.3 | None | 0 |
| Total | 20 | 2/20 | 10 | 0/2 | 0 | |
DCIS: Ductal carcinoma in situ; MIC: Microinvasion; IDC: Invasive ductal carcinoma; SLN: Sentinel lymph node.
Underestimation rate
The underestimation rate of microinvasion and occult invasive foci is outlined in Table 4. Eighteen patients had their initial diagnosis achieved by CNB and 2 by FNA. After definitive surgery, 3 patients were upstaged to invasive ductal carcinoma (3/18 = 16.6%) and 3 to DCIS with microinvasion (3/18 = 16.6%). The 2 cases diagnosed by FNA were excluded from the calculation of the underestimation rate. Therefore image-guided CNB was associated with a 33% (6/18) underestimation rate in 18 cases with an initial diagnosis of pure DCIS.
Table 4.
Comparison between initial and final pathology “underestimation rate” n (%)
| Initial diagnosis | Final diagnosis |
Underestimation rate |
|
| NO. | % | ||
| Pure DCIS | Pure DCIS 14 (70) | ||
| DCIS/MIC 3 (15) | 6/18 | 33% | |
| DCIS/IDC 3 (15) | |||
DCIS: Ductal carcinoma in situ; MIC: Microinvasion; IDC: Invasive ductal carcinoma.
DISCUSSION
DCIS in Saudi Arabia differs from that in Western societies in several aspects. Our patient population of DCIS present clinically, whereas DCIS in the West present with mammographically detected disease as a result of the widespread implementation of screening programs. Paget’s disease, bloody nipple discharge, and extensive and palpable disease formed a large proportion of our study population.
Saudi Arabia does not have a population-based screening program, therefore, the incidence is low (2.6%)[9] compared with the incidence of DCIS in Western societies (20%-30%)[10-12]. In Western societies during the pre-screening era, the incidence of DCIS did not exceed 5%, and patients would present with CD-DCIS similar to the current situation in Saudi Arabia[11].
The incidence and clinical significance of nodal metastasis in DCIS has been evaluated in both the pre- and the post-screening era. In the pre-screening era, ALND was not performed in DCIS because of the low incidence of axillary metastasis (1%-2%), and the high morbidity associated with ALND[12,13]. This low rate of axillary lymph node involvement in pure DCIS was attributed to missed diagnosis of invasion in the final pathology of the breast specimen[2,12]. Currently, the reported high incidence (0.39%-20%) of SLN metastasis in pure DCIS is different from that reported historically (1%-2%)[4,5,12]. The extent of the disease and the methods used for the diagnosis of DCIS are different between the 2 periods.
In the pre-screening era, a surgical biopsy was used to achieve a diagnosis, and it is a more definitive method of diagnosing pure DCIS because the whole specimen is examined to exclude the presence of invasive disease. However a CNB is currently used for the diagnosis of DCIS and is associated with sampling error and histologic underestimation of the presence of invasive disease that varies between 8.8% and 51%[3]. There are two problems associated with the diagnosis of pure DCIS. First, a definitive preoperative diagnosis of pure DCIS cannot be achieved with an image-guided CNB. Second, even a postoperative diagnosis of pure DCIS is difficult, especially when the lesion is large and extensive[4]. The presence of occult microinvasion and invasive disease cannot be ruled out without complete tissue processing. Hence, the method of tissue diagnosis plays a central role in deciding whether or not to perform a SLNB in DCIS.
The reported high underestimation rate of (8.8%-51.5%) associated with image-guided CNB diagnosis of pure DCIS is the main factor that explains the large difference between the historically reported incidence of lymph node metastasis during the pre-screening era (1%-2%) and the currently reported incidence of SLN metastasis (0.39%-20%). To our knowledge, there is no published data on the use of SLNB in CD-DCIS. Our patient population is unique, characterized by having a large palpable DCIS with extensive disease. Despite the presence of extensive DCIS, our study has shown that the use of SLNB in CD-DCIS is highly accurate and technically feasible. The false negative rate was zero and both the SLN intraoperative identification rate and SLN visualization rate were 100%.
The SLN identification procedure at KFSH and RC involves the sole use of Tc-99m injection. We have achieved high identification rates with the use of the gamma probe technique and we believe like others (European Institute of Oncology in Milan) that the combination approach (blue dye and Tc-99m) is not worthwhile[14]. The success of this approach is obvious with the rates outlined in (Table 2).
In our study, the incidence of SLN metastasis was 10% and the incidence of non-SLN metastasis was zero. Neither of our 2 patients with a positive SLN had additional disease in the axillary nodes following complete axillary dissection. Therefore, the risk of additional metastasis after a positive SLNB in pure DCIS was nil in our experience. Also, none of our patients with DCIS developed an axillary recurrence. We had one case of pure DCIS with a positive SLNB. The only explanation would be a missed undetected occult invasive focus in the breast specimen. This reflects that even the most meticulous complete tissue processing with serial sectioning can miss the an invasive component responsible for the sentinel node metastasis[15,16]. This is a very rare event that does not justify performing routinely SLNB routinely in pure DCIS. We had one additional case of DCIS with a positive SLN who had an occult invasive focus.
The low positive rate of SLN metastasis in this study (10%) is in line with that reported in the literature and is consistent with other published reports[17], suggesting the safety of delaying a SLNB until a definitive diagnosis of invasive disease is achieved. The single largest institutional study of 854 patients with DCIS treated with SLNB at the European Institute of Oncology had an incidence of SLN metastasis of only 1.4% and when patients with micrometastasis were excluded the incidence was only 0.6%[14]. A meta-analysis conducted by Ansari et al[14] has shown that the incidence of SLN metastasis was 7.4% in patients with a preoperative CNB diagnosis of DCIS, compared to 3.7% in patients with a definitive postoperative diagnosis of DCIS.
The reported rate of underestimation of invasive disease following a CNB diagnosis of pure DCIS varies among different institutions (8.8%-51.5%)[3]. This wide range reflects the different methods used for core biopsy, pathological assessment, and different patient populations. Image-guided CNB in our institution was associated with a high rate of underestimation (33%). After definitive surgery, 3 patients were upstaged to invasive ductal carcinoma (16.6%) and 3 to DCIS with microinvasion (16.6%). Therefore the total histologic underestimation rate of invasive disease was 33%. This is similar to other published studies (8.8%-51.5%)[3]. However, this high rate should not be used as an argument favoring the routine use of SLNB because it will expose about 67% of our patients to an unnecessary SLNB. CD-DCIS and widespread DCIS are expected to be associated with a higher risk of occult microinvasion and invasive disease than localized screening detected DCIS[5,12].
The risk of microinvasion and occult invasive ductal carcinoma correlate with the extent and size of DCIS, nuclear grade, and histologic type, and our results support this as the majority of our patients had large volume tumors and a 33% probability of containing either microinvasion or occult invasive disease. Therefore the high underestimation rate encountered in this study may be explained by the larger tumor size and greater extent of disease.
Advocates of the routine use of SLNB in pure DCIS diagnosed by image-guided CNB base their rationale on 4 main facts. First, the reported high incidence of SLN metastasis (0.39%-20%) in DCIS[4,5]. Second, the reported high rate of underestimation (8.8%-51.5%) associated with CNB[3]. Third, SLN metastasis is an indirect method of diagnosing occult invasive disease that may be missed on routine postoperative examination of the final specimen[17]. Fourth, an immediate SLNB will spare patients a second surgery if they are found to have invasive disease on final pathology[17,18]. Opponents of the routine use SLNB in DCIS summarize their reasoning according to the following 5 reasons. First, SLNB should not be performed for patients with pure DCIS because of its non-invasive biological behavior[1]. Second, a SLNB will be unnecessary in the majority of patients with a preoperative image-guided CNB diagnosis of DCIS because the final postoperative histology will only contain pure DCIS and will not contain any invasive disease[2,19]. Third, although SLNB is a less morbid surgical procedure compared with ALND, it is also associated with complications[7,8]. Fourth, SLNB can also lead to excessive and unnecessary treatment by providing misleading information about the axillary status. The disease detected in the SLN may not be clinically significant and may lead to unnecessary axillary dissection and adjuvant chemotherapy[6]. Finally a redo SLNB may be inaccurate and less successful in patients who later develop invasive disease[17].
The following is our suggested algorithm for SLNB in DCIS (Figure 1). The aim is to minimize the morbidity as much as possible and to limit SLNB to only those who will benefit from the procedure. We believe that the method of diagnosis (CNB and surgical biopsy), the risk of invasion, and the type of surgery (lumpectomy and mastectomy) are the 3 major determinants for the need for SLNB in DCIS. The high underestimation rate (33%), the low positive SLN rate (10%), and the morbidity associated with SLNB has encouraged us to limit the procedure to those who will undergo a mastectomy or immediate reconstruction or a wide local excision involving the upper outer quadrant. This is because of the difficulty of performing a SLN procedure after these surgical procedures as they may disrupt the lymphatic pathways toward the axilla. All patients presenting with DCIS and microinvasion or occult invasive disease should have a SLNB because of the risk of SLN metastasis. Patients with a diagnosis of pure DCIS following an excisional biopsy do not need a SLNB because the diagnosis of pure DCIS is definitive.
Figure 1.
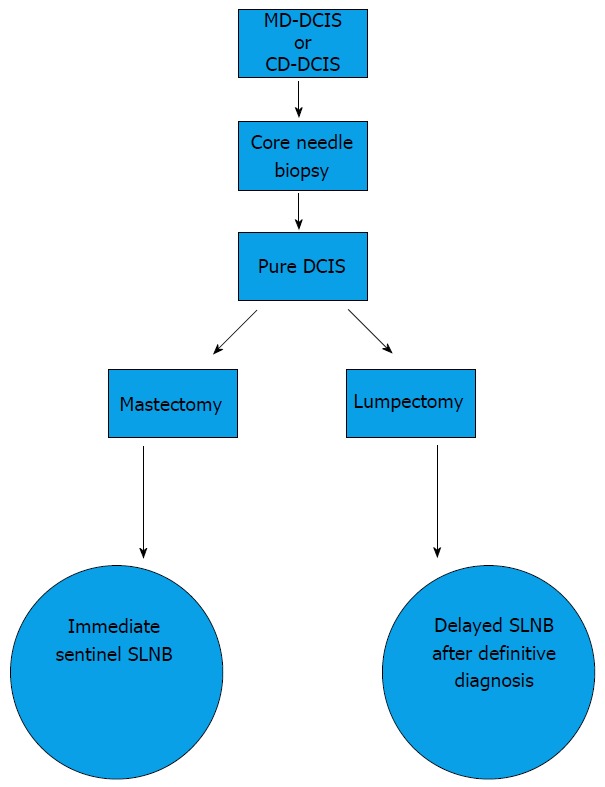
Ductal carcinoma in situ algorithm at King Faisal Specialist Hospital and Research Centre. MD-DCIS: Mammographically detected ductal carcinoma in situ; CD-DCIS: Clinically-detected ductal carcinoma in situ; SLNB: Sentinel lymph node biopsy.
This approach of limiting SLNB to these patients is consistent with our understanding of the natural history of pure DCIS, i.e., its inability to metastasize to the lymph nodes. Patients with a diagnosis of pure DCIS following a CNB have a high rate of underestimation (8.8%-51.5%), as in our patient population (33%), and may require a SLNB because the diagnosis of pure DCIS is not definitive. They can either have an immediate SLNB at the same setting of their conservative surgery, or they can have a delayed SLNB after a definitive diagnosis of invasive disease is made following a diagnostic and therapeutic lumpectomy. Both options are valid, but we recommend the delayed SLNB approach as this will limit axillary staging to only those who have an invasive component and will spare patients with pure DCIS an unnecessary SLNB. The main disadvantage of an immediate SLNB is unnecessarily subjecting the majority of our patients with pure DCIS to SLNB. This corresponds to 67% of our DCIS population. The main advantage of an immediate SLNB is in patients with DCIS with microinvasion and DCIS with occult invasive foci, hence avoiding a revisit to the operating theatre. This corresponds to (33%) in our patient population of DCIS.
Delayed SLNB has the advantage of avoiding an unnecessary SLNB in pure DCIS (67% in our population), thus limiting SLNB to DCIS with microinvasion or occult invasive disease. Also, it will not interfere with a future SLNB if needed in case of invasive recurrence. The only disadvantage is a second visit to the operating room in cases of upstaging to occult invasive disease.
Theoretically, there is concern that a wide local excision in the upper outer quadrant will disrupt the lymphatic drainage into the sentinel lymph node and therefore will negatively affect the accuracy of performing a SLNB after such a surgical procedure[19,20]. Unfortunately, the literature in this area is limited and controversial[20]. Therefore, we would recommend performing an immediate SLNB for patients having a wide local excision in the upper outer quadrant, in order to minimize the risk of an unsuccessful delayed SLNB. For patients undergoing a limited small lumpectomy in the upper outer quadrant that will not interfere with the lymphatic drainage of the SLN, a delayed SLNB is appropriate.
In conclusion, SLNB in CD-DCIS is technically feasible and highly accurate. Although the majority of our patients presented with extensive DCIS and had a high rate of underestimation of invasive cancer (33%), the overall rate of SLN positivity was only 10%. Therefore, we recommend limiting SLNB to patients with pure DCIS undergoing a mastectomy or a wide local excision in the upper outer quadrant.
COMMENTS
Background
Ductal carcinoma in situ (DCIS) is a non-invasive disease that does not have the metastatic potential to spread to the axillary lymph nodes. DCIS commonly coexists with microinvasion and invasive disease. The main diagnostic tool for DCIS is core needle biopsy (CNB) which is associated with a high underestimation rate, which indicates the coexistence of invasive disease. The simplicity of the sentinel lymph node biopsy (SLNB) encourages surgeons to perform SLNB. SLNB is a surgical procedure not devoid of complications. Hence, the use of SLNB in DCIS is controversial. The authors aimed to evaluate the indications of SLNB in clinically detected (CD) DCIS by measuring accuracy (100%), nodal positivity (10%), and underestimation rate (33%).
Research frontiers
The use of SLNB in CD-DCIS which is diagnosed by CNB prior to excision in this study is indicated in patients undergoing mastectomy or wide local excision in the upper outer quadrant.
Innovations and breakthroughs
The results of this study regarding accuracy, nodal positivity, and underestimation rate are similar to other reported studies. The authors had a high identification rate for SLN with use of Tc-99m without a dye like some other centers in the world, and the authors believe the combined technique (radioisotope and dye) is not worthwhile.
Applications
The use of SLNB in CD-DCIS is feasible but should not be indicated for all patients with such a diagnosis. The criteria are patients who are undergoing mastectomy or a wide local excision in the upper outer quadrant.
Terminology
DCIS is a noninvasive form of ductal carcinoma, limited to the confines of the basement membrane of the duct (also referred to as intraductal carcinoma). CD-DCIS is DCIS which is palpable or has specific signs which can be detected clinically. SLN is the first node on the lymphatic drainage pathway from a primary. SLNB is a technique using a detector material (dye, radioisotope material) to identify the SLN.
Peer-review
This is an interesting small clinical study on the use of SLNB for carcinoma in situ of the breast. The study is of particular interest for settings where screening programs are not in place and DCIS is diagnosed clinically.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of King Faisal Specialist Hospital and Research Centre.
Informed consent statement: Patients were not required to give informed consent for the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. For full disclosure, the details of the study are published on the home page of Fukushima Medical University.
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 5, 2015
First decision: July 26, 2015
Article in press: January 29, 2016
P- Reviewer: Voutsadakis IA S- Editor: Gong XM L- Editor: Cant MR E- Editor: Li D
References
- 1.Boler DE, Cabioglu N, Ince U, Esen G, Uras C. Sentinel Lymph Node Biopsy in Pure DCIS: Is It Necessary? ISRN Surg. 2012;2012:394095. doi: 10.5402/2012/394095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Intra M, Rotmensz N, Veronesi P, Colleoni M, Iodice S, Paganelli G, Viale G, Veronesi U. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast: the experience of the European institute of oncology on 854 patients in 10 years. Ann Surg. 2008;247:315–319. doi: 10.1097/SLA.0b013e31815b446b. [DOI] [PubMed] [Google Scholar]
- 3.Miyake T, Shimazu K, Ohashi H, Taguchi T, Ueda S, Nakayama T, Kim SJ, Aozasa K, Tamaki Y, Noguchi S. Indication for sentinel lymph node biopsy for breast cancer when core biopsy shows ductal carcinoma in situ. Am J Surg. 2011;202:59–65. doi: 10.1016/j.amjsurg.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Tada K, Ogiya A, Kimura K, Morizono H, Iijima K, Miyagi Y, Nishimura S, Makita M, Horii R, Akiyama F, et al. Ductal carcinoma in situ and sentinel lymph node metastasis in breast cancer. World J Surg Oncol. 2010;8:6. doi: 10.1186/1477-7819-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guth AA, Mercado C, Roses DF, Darvishian F, Singh B, Cangiarella JF. Microinvasive breast cancer and the role of sentinel node biopsy: an institutional experience and review of the literature. Breast J. 2008;14:335–339. doi: 10.1111/j.1524-4741.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- 6.McMasters KM, Chao C, Wong SL, Martin RC, Edwards MJ. Sentinel lymph node biopsy in patients with ductal carcinoma in situ: a proposal. Cancer. 2002;95:15–20. doi: 10.1002/cncr.10641. [DOI] [PubMed] [Google Scholar]
- 7.Tunon-de-Lara C, Giard S, Buttarelli M, Blanchot J, Classe JM, Baron M, Monnier B, Houvenaeghel G. Sentinel node procedure is warranted in ductal carcinoma in situ with high risk of occult invasive carcinoma and microinvasive carcinoma treated by mastectomy. Breast J. 2008;14:135–140. doi: 10.1111/j.1524-4741.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 8.Rönkä R, von Smitten K, Tasmuth T, Leidenius M. One-year morbidity after sentinel node biopsy and breast surgery. Breast. 2005;14:28–36. doi: 10.1016/j.breast.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 9.King faisal specialist hospital and research center. Tumor registry annual report 2011. Available from: http://www.kfshrc.edu.sa/oncology/Tumor%202011%20New%206%20Final.pdf.
- 10.Klauber-DeMore N, Tan LK, Liberman L, Kaptain S, Fey J, Borgen P, Heerdt A, Montgomery L, Paglia M, Petrek JA, et al. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann Surg Oncol. 2000;7:636–642. doi: 10.1007/s10434-000-0636-2. [DOI] [PubMed] [Google Scholar]
- 11.Sundara Rajan S, Verma R, Shaaban AM, Sharma N, Dall B, Lansdown M. Palpable ductal carcinoma in situ: analysis of radiological and histological features of a large series with 5-year follow-up. Clin Breast Cancer. 2013;13:486–491. doi: 10.1016/j.clbc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Sakr R, Barranger E, Antoine M, Prugnolle H, Daraï E, Uzan S. Ductal carcinoma in situ: value of sentinel lymph node biopsy. J Surg Oncol. 2006;94:426–430. doi: 10.1002/jso.20578. [DOI] [PubMed] [Google Scholar]
- 13.Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer. 2003;98:2105–2113. doi: 10.1002/cncr.11761. [DOI] [PubMed] [Google Scholar]
- 14.Ansari B, Ogston SA, Purdie CA, Adamson DJ, Brown DC, Thompson AM. Meta-analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg. 2008;95:547–554. doi: 10.1002/bjs.6162. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Xu F, Tao K, Qian N, Toi M. Clinical applications of sentinel lymph node biopsy in ductal carcinoma in situ of the breast: a dilemma. Tohoku J Exp Med. 2011;224:1–5. doi: 10.1620/tjem.224.1. [DOI] [PubMed] [Google Scholar]
- 16.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 17.Cox CE, Nguyen K, Gray RJ, Salud C, Ku NN, Dupont E, Hutson L, Peltz E, Whitehead G, Reintgen D, et al. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): why map DCIS? Am Surg. 2001;67:513–519; discussion 519-521. [PubMed] [Google Scholar]
- 18.Dominguez FJ, Golshan M, Black DM, Hughes KS, Gadd MA, Christian R, Lesnikoski BA, Specht M, Michaelson J, Smith BL. Sentinel node biopsy is important in mastectomy for ductal carcinoma in situ. Ann Surg Oncol. 2008;15:268–273. doi: 10.1245/s10434-007-9610-6. [DOI] [PubMed] [Google Scholar]
- 19.Luini A, Galimberti V, Gatti G, Arnone P, Vento AR, Trifirò G, Viale G, Rotmensz N, Fernandez JR, Gilardi D, et al. The sentinel node biopsy after previous breast surgery: preliminary results on 543 patients treated at the European Institute of Oncology. Breast Cancer Res Treat. 2005;89:159–163. doi: 10.1007/s10549-004-1719-8. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez Fernandez J, Martella S, Trifirò G, Caliskan M, Chifu C, Brenelli F, Botteri E, Rossetto F, Rotmensz N, Rietjens M, et al. Sentinel node biopsy in patients with previous breast aesthetic surgery. Ann Surg Oncol. 2009;16:989–992. doi: 10.1245/s10434-009-0349-0. [DOI] [PubMed] [Google Scholar]


