Abstract
Purpose
Ultrasound-guided fine needle aspiration (US-FNA) in thyroid nodules is presently most commonly used to identify whether these nodules are benign or malignant. However, atypical or follicular lesions of undetermined significance (AUS/FLUS), as categorized in the Bethesda System for reporting the results of FNA, cannot be classified as benign or malignant. Therefore, several clinical factors should be considered to assess the risk of malignancy in patients with AUS/FLUS. The purpose of the present study was to determine which clinical factor increased the risk of malignancy in patients with AUS/FLUS.
Methods
A retrospective study was done on 129 patients with fine needle aspiration categorized as AUS/FLUS from January 2011 through April 2015. Univariate and multivariate analyses were performed to assess the independent effect of risk factors such as age, sex, size of nodule, atypical descriptors, and ultrasonography criteria for malignancy.
Results
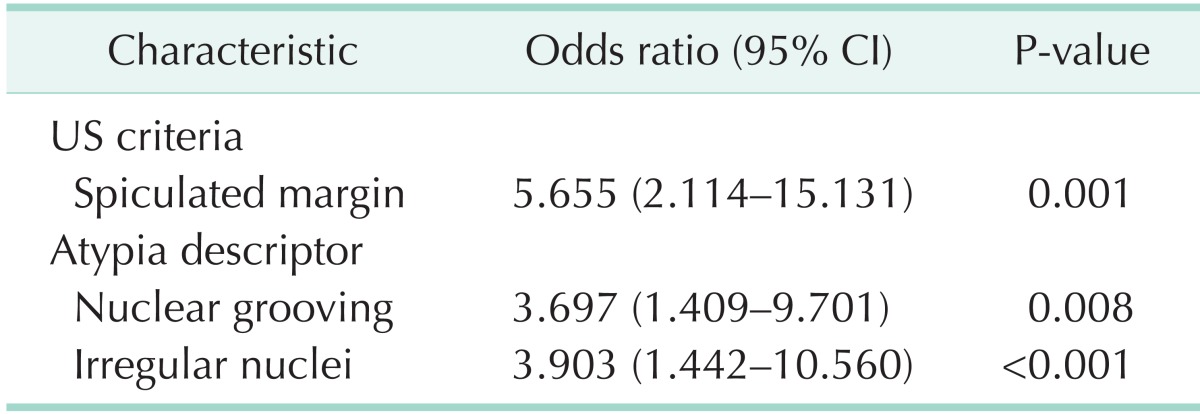
We identified that the presence of spiculated margin (odds ratio [OR], 5.655; 95% confidence interval [CI], 2.114-15.131; P = 0.001), nuclear grooving (OR, 3.697; 95% CI, 1.409-9.701; P = 0.008), irregular nuclei (OR, 3.903; 95% CI, 1.442-10.560; P = 0.001) were shown to be significantly related to malignancy on univariate and multivariate analyses.
Conclusion
We recommend that surgical resection of thyroid nodules be considered in patients with AUS/FLUS showing the histologic findings such as nuclear grooving, irregular nuclei along with spiculated margin of ultrasonographic finding.
Keywords: Atypical, Malignant, Thyroid nodule, Fine needle aspiration
INTRODUCTION
Thyroid nodules are common and are mostly benign, the risk of malignancy is approximately 6.2% [1,2,3]. Ultrasound-guided fine needle aspiration (US-FNA) in thyroid nodules is a simple and useful access for the initial diagnosis of thyroid nodules and it is presently the most commonly used to identify whether these nodules are benign or malignant. Thereby, it can reduce unnecessary surgery [4,5,6]. However, 15% to 30% of FNAs are indeterminate [1,2]. According to the Bethesda system to help stratify risk for malignancy among FNA, these results of FNA are classified as atypia or follicular lesion of undetermined significance (AUS/FLUS) [7]. The category AUS/FLUS cannot be classified as benign or malignant because they present follicular cells with atypical nuclei or architecture, which is insufficient for characterization as malignant. Some recent studies reported that the risk of malignancy for the category AUS/FLUS is 5% to 19% [8,9,10]. So patients with AUS/FLUS require surgery (usually hemithyroidectomy) for definitive diagnosis. If malignancy is identified on the final pathology, a second operation (completion thyroidectomy) is usually required [1,2,3]. Hence, several clinical factor should be considered to assess the risk of malignancy in patients with AUS/FLUS. However, there are no clinical factors that are shown to be specific [11,12,13]. Therefore, the purpose of the present study was to determine which clinical factor increased the risk of malignancy in patients with AUS/FLUS.
METHODS
We retrospectively reviewed the medical records of patients who underwent FNA of thyroid nodules at Inha University Hospital from January 2011 through April 2015. A total of 129 patients who had their FNA interpreted as AUS/FLUS were included in this study. Patients were divided into 2 groups based on presence or absence of malignant findings on pathologic results confirmed by surgery or repeat FNA. Patients were excluded from the study if their cytologic findings presented AUS/FLUS or 'suspicious for malignancy' on repeat FNA. All patients underwent thyroid function test and ultrasonography for the initial work-up. AUS result is given when cytologic findings cannot be characterized as benign due to the presence of follicular cells with atypical nuclear or architecture, which is not sufficient for categorization as malignant [8,9,10].
The following parameters were analyzed: sex, age, size of nodule, ultrasonography criteria for malignancy, nuclear atypical descriptors, the presence of two or more of atypical descriptors, and final pathologic results. The following ultrasonography criteria for malignancy were analyzed: taller-thanwide shape, hypoechogenecity, spiculated margin, and calcification. Descriptors of atypical nuclei included any of following cytologic findings: nuclear clearing, nuclear grooving, nuclear inclusion, nuclei enlarged, irregular nuclear membrane, oval nuclei, pinpoint nuclei, peripheral nuclei.
We compared the previously mentioned variables between benign and malignant groups. The relationship between categorical variables (i.e., each atypical descriptor, ultrasonography criteria for malignancy, sex, the presence of two or more atypical descriptors) and final pathologic results were analyzed as appropriate by the chi-square test or Fisher exact test. The relationship between continuous variables (i.e., age, size of nodule) and final pathologic results were analyzed by t-test. Multivariate logistic regression with a backward stepwise variable selection procedure was performed to identify variables associated with an increased risk of malignancy. Variables associated with malignancy with a P-value of less than 0.10 in the univariate analysis were entered into the multivariate model, and nonsignificant variables were removed by means of a backward-selection procedure. P-value < 0.05 was considered to indicate statistical significance and 95% confidence intervals (CIs; for adjusted odds ratios [ORs]) were calculated to assess the precision of the obtained estimates. All analyses were performed by IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
This retrospective study group consisted of 21 males and 108 females. The mean age of the benign group and malignant group was 52.54 ± 11.84 and 49.32 ± 11.66 years, respectively. Of these 129 patients, 98 (76.5%) had benign cytologies and 31 had malignant cytologies. Final pathology was confirmed by surgery and repeat FNA. The 31 malignant diagnoses included 30 papillary carcinomas, 1 follicular carcinoma. The 98 benign diagnoses included 90 nodular hyperplasia, 4 follicular adenoma, 3 lymphocytic thyroiditis, and 1 Hurthle cell adenoma.
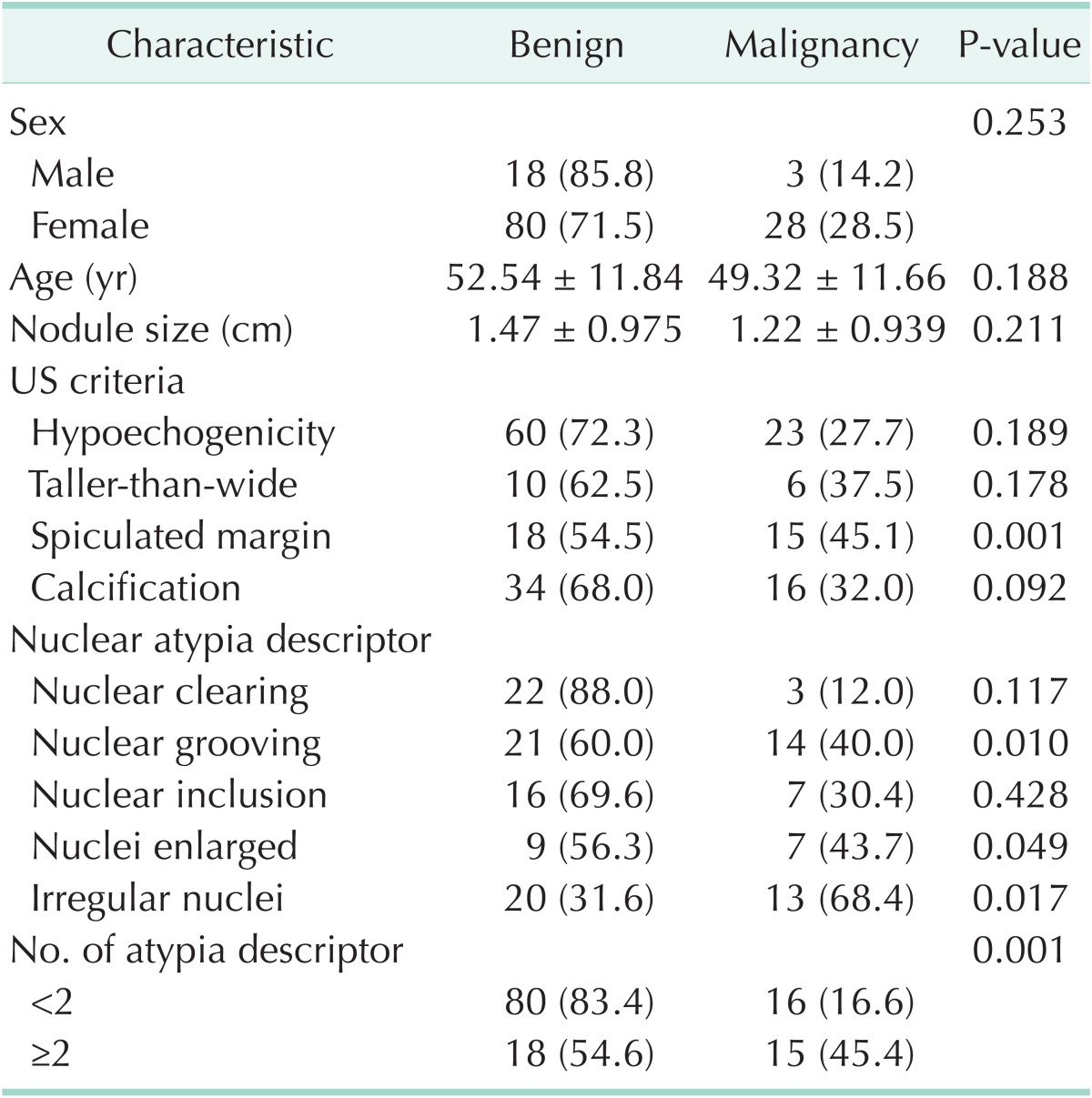
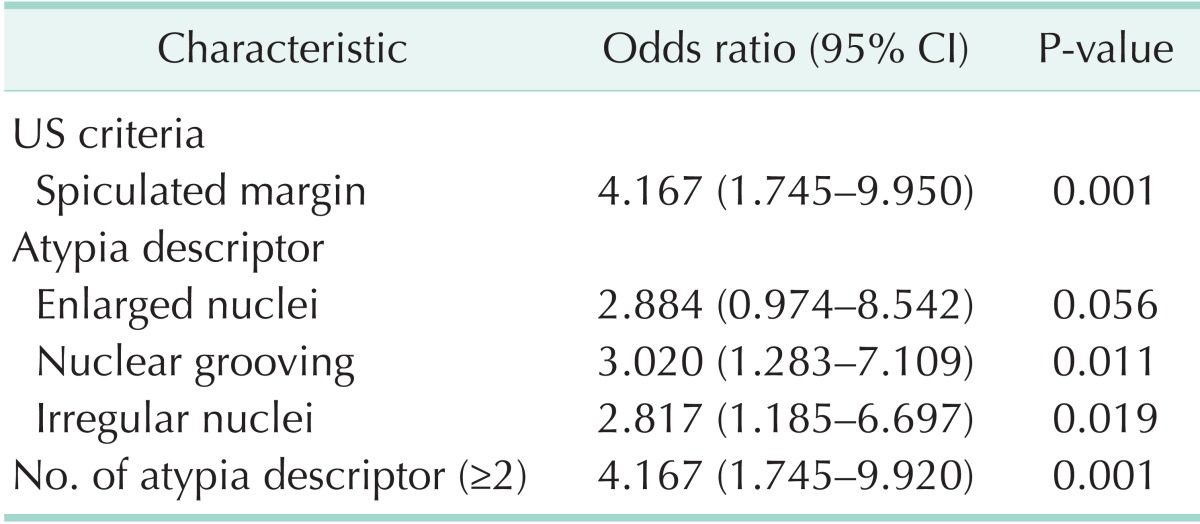
There was no significant difference in sex, age, size of nodule, nuclear clearing and inclusion, and the rest of the ultrasonography criteria for malignancy except speculated margin between the benign and malignant. The presence of spiculated margin (P = 0.001), nuclear grooving (P = 0.01), enlarged nuclei (P = 0.049), irregular nuclei (P = 0.017), and the presence of two or more of atypical descriptors (P = 0.001) were shown to be significantly related to malignancy compared to benign (Table 1). Because oval nuclei and peripheral nuclei were shown in just one patient, we did not show these results in the Table. Variables associated with malignancy with a P-value of less than 0.10 in the univariate analysis were as follows: spiculated margin (OR, 4.167; 95% CI, 1.745–9.950; P = 0.001), enlarged nuclei (OR, 2.884; 95% CI, 0.974–8.542; P = 0.056), nuclear grooving (OR, 3.020; 95% CI, 1.283–7.109; P = 0.011), irregular nuclei (OR, 2.817; 95% CI, 1.185–6.697; P = 0.019), and the presence of two or more of atypical descriptors (OR, 4.167; 95% CI, 1.745–9.920; P = 0.001) (Table 2). The previously mentioned variables were entered into the multivariate logistic model, and nonsignificant variables were removed by means of a backward-selection procedure. We identified the presence of spiculated margin (OR, 5.655; 95% CI, 2.114–15.131; P = 0.001), nuclear grooving (OR, 3.697; 95% CI, 1.409–9.701; P = 0.008), irregular nuclei (OR, 3.903; 95% CI, 1.442–10.560; P = 0.001) were shown to be significantly related to malignancy on multivariate logistic regression (Table 3).
Table 1. Demographics and risk factors of malignancy in patients with AUS/FLUS.
Values are presented as number (%) or mean ± standard deviation.
AUS/FLUS, atypical or follicular lesions of undetermined significance; US, ultrasonography.
Table 2. Univariate analysis of characteristics predicting the malignancy.
CI, confidence interval; US, ultrasonography.
Table 3. Multivariate analysis of characteristics predicting the malignancy.
CI, confidence interval; US, ultrasonography.
DISCUSSION
The risk for malignancy in AUS/FLUS, which may potentially represent disseminated, nonfollicular disease, is approximately 5%–15% [8,9,10]. A more correct initial judgment of the risk of malignancy is very important. Thus, we conducted the retrospective study to determine which clinical factor increased the risk of malignancy in patients with AUS/FLUS. Numerous studies have suggested that the age, sex, and thyroid nodule size of patient was a significant predictor of malignancy [5,12,13,14,15,16,17,18,19]. We analyzed these factors to find significant difference between benign and malignant thyroid nodules. In our study, age was not associated with an increased risk of malignancy. This finding corresponds with the earlier studies [11,12,15,17]. Contrary to our results, there are studies that report statistical difference in age between benign and malignant thyroid nodules [13,16,18,19]. Some reports have suggested that male gender was associated with malignancy [12,13]. But other studies have found no significant difference in the rate of malignancy between the sexes [11,14,15,16]. We also found no significant difference in the rate of malignancy between males and females. According to a certain study, tumor size was associated with an increases risk of malignancy [17]. In some studies, when nodule size was greater than 3 to 4 cm, this factor showed to be a predictor of malignancy [12,13,14,15,16]. In contrast to these studies, we found no significant difference in nodule size between benign and malignant. This result corresponds with the current study [20]. The reason for this result is postulated that thyroid nodules, of which mean size was smaller than 2 cm in both groups, were detected early with the recently frequent use of ultrasonography due to the rapid increase of the incidence of thyroid cancer in Korea [21].
Recent studies reported that the correlation of cytology and ultrasonographic findings could be helpful to manage thyroid nodules with AUS/FLUS [21,22]. Most previous studies have reported that ultrasonographic findings of malignancy such as hypoechogenicity, taller-than-wide shape, clacification, and spiculated margin are associated with malignancy [5,21,23]. We analyzed these findings to determine which were statistically significant in the risk of malignancy. Contrary to previous reports, spiculated margin alone was shown to be significantly related to malignancy on univariate analysis and multivariate logistic regression. The reason for this result is presumed that ultrasonography was performed by surgeon, endocrinologist and radiologist in our study. There might be an individual variation in the reading of ultrasonography, depending on the skill, experience, and preferences of clinicians. Also, our retrospective analysis was made up of all patients with AUS/FLUS, who were not randomly subjected, and this study had a small data set. Therefore, when cytologic findings associated with malignancy are considered, sonographic finding of spiculated margin may be a valuable clinical factor to determine whether or not to operate. Numerous studies reported that atypical nuclei or cytology on FNA are associated with increased the risk of malignancy [13,21,24]. However, uniform criteria for the definition of AUS/FLUS at cytology has yet to be used in the literature [20]. And patients' medical records in this study, which were retrospectively reviewed, briefly described descriptors of atypical cytology or architecture without providing details. In previous studies, specific, nuclear change or atypical lesion has both been recognized as an important prognostic factor for malignancy [13,25,26]. Therefore, we noted and analyzed only nuclear atypical descriptors to identify the independent effect of risk factors. Spiculated margin (OR, 4.167; 95% CI, 1.745–9.950; P = 0.001), enlarged nuclei (OR, 2.884; 95% CI, 0.974–8.542; P = 0.056), nuclear grooving (OR, 3.020; 95% CI, 1.283–7.109; P = 0.011), irregular nuclei (OR, 2.817; 95% CI, 1.185–6.697; P = 0.019), and the presence of two or more of atypical descriptors (OR, 4.167; 95% CI, 1.745–9.920; P = 0.001) were shown to be significantly related to malignancy on univariate analysis. Results of multivariate logistic regression showed that the presence of spiculated margin (OR, 5.655; 95% CI, 2.114–15.131; P = 0.001), nuclear grooving (OR, 3.697; 95% CI, 1.409–9.701; P = 0.008), and irregular nuclei (OR, 3.903; 95% CI, 1.442–10.560; P = 0.001) increased the risk of malignancy. After adjusting the association between variables, the presence of two or more of atypical descriptors was removed by means of a backward-selection procedure. This result is probably because variables shown to be significantly related to malignancy may be included in groups with the presence of two or more of atypical descriptors. Thus, this variable appeared to be considered statistically significant on the univariate analysis. There are few reports regarding the correlation between irregular nuclei and malignancy. However, according to our result, this descriptor could be considered as an important prognostic factor for malignancy. The presence of nuclear grooving has been not known as a specific feature of thyroid cancer. It might also present in benign thyroid nodules such as Hashimoto's thyroiditis, follicular adenoma, and Hurthle cell neoplasms. However, our finding is in contrast to the results of previous studies [27,28]. Several studies also have reported that nuclear grooving shows to be a helpful cytologic feature in the diagnosis of papillary carcinoma of the thyroid [13,25,26]. Although the presence of a nuclear groove is nonspecific, it may be the only finding for follicular variant of papillary carcinoma [28]. The reason for this result is presumed that, of the 31 patients with malignant in this study, 30 had papillary carcinoma (96.5%). Several studies suggested that the presence of nuclear inclusion increased the risk of malignancy [29]. However, in our study, nuclear inclusion was not found to be associated with an increased risk of malignancy. This result corresponds with the several earlier studies [30]. The reason for this result is conceivable in that the aspirated specimens might not include amount of cells, which is enough to show various changes or atypical features of the nucleus. It is inferred that another reason is small sample size.
This study has considerable limitations. This was a retrospective study at single institution and had a small sample size. Patients with AUS/FLUS were solely included in this study. They were not random subjects. These limitations might create a sampling bias and we were not able to find statistical significance of variables, which have shown significance in previous studies.
And, in categorization based on repeated FNA results, there is a point which is yet to be clarified. There is a potential for development of malignancy in patients with AUS/FLUS on first FNA, despite being categorized as benign group because repeated FNA demonstrated benign findings. The following limitation is a pathologic bias. Because the same pathologist did not review all cases, there might be individual variations in the description of nuclear features. The final limitation is that we noted only nuclear atypical descriptors because patients' medical records briefly described descriptors of atypical cytology or architecture without providing details. Although there have been some studies suggesting that atypical cytology on FNA is associated with high malignancy rate [24], it was difficult to categorize these descriptors nor we could analyze these factors. Therefore, larger prospective studies are necessary to confirm clinical factors known to show significance, and further certify other important prognostic factors. And consensual ultrasonographic criteria for malignancy should be established. Finally, if uniform cytologic atypical descriptors for definition of AUS/FLUS are used, these may help further guide management of patients with AUS/FLUS.
In summary, the presence of spiculated margin, nuclear grooving, and irregular nuclei were shown to be significantly related to malignancy. Therefore, we recommend that surgical resection of thyroid nodules may be considered in patients with AUS/FLUS showing the histologic findings such as nuclear grooving and irregular nuclei along with spiculated margin in ultrasonographic findings.
ACKNOWLEDGEMENTS
This study was supported by Inha University Hospital Research Grant.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Yang GC, Liebeskind D, Messina AV. Ultrasound-guided fine-needle aspiration of the thyroid assessed by Ultrafast Papanicolaou stain: data from 1135 biopsies with a two- to six-year follow-up. Thyroid. 2001;11:581–589. doi: 10.1089/105072501750302895. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 3.Greaves TS, Olvera M, Florentine BD, Raza AS, Cobb CJ, Tsao-Wei DD, et al. Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer. 2000;90:335–341. [PubMed] [Google Scholar]
- 4.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 5.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 6.Baloch ZW, Sack MJ, Yu GH, Livolsi VA, Gupta PK. Fine-needle aspiration of thyroid: an institutional experience. Thyroid. 1998;8:565–569. doi: 10.1089/thy.1998.8.565. [DOI] [PubMed] [Google Scholar]
- 7.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 8.Layfield LJ, Morton MJ, Cramer HM, Hirschowitz S. Implications of the proposed thyroid fine-needle aspiration category of "follicular lesion of undetermined significance": a five-year multi-institutional analysis. Diagn Cytopathol. 2009;37:710–714. doi: 10.1002/dc.21093. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, DeMay RM, Papas P, Yan B, Reeves W. Follicular lesions of the thyroid: aretrospective study of 1,348 fine needle aspiration biopsies. Diagn Cytopathol. 2012;40(Suppl 1):E8–E12. doi: 10.1002/dc.21477. [DOI] [PubMed] [Google Scholar]
- 10.Faquin WC, Baloch ZW. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol. 2010;38:731–739. doi: 10.1002/dc.21292. [DOI] [PubMed] [Google Scholar]
- 11.Wiseman SM, Baliski C, Irvine R, Anderson D, Wilkins G, Filipenko D, et al. Hemithyroidectomy: the optimal initial surgical approach for individuals undergoing surgery for a cytological diagnosis of follicular neoplasm. Ann Surg Oncol. 2006;13:425–432. doi: 10.1245/ASO.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 12.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998;8:377–383. doi: 10.1089/thy.1998.8.377. [DOI] [PubMed] [Google Scholar]
- 13.Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of "follicular neoplasm":a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26:41–44. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- 14.Davis NL, Gordon M, Germann E, Robins RE, McGregor GI. Clinical parameters predictive of malignancy of thyroid follicular neoplasms. Am J Surg. 1991;161:567–569. doi: 10.1016/0002-9610(91)90901-o. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Nicol TL, Zeiger MA, Dooley WC, Ladenson PW, Cooper DS, et al. Hürthle cell neoplasms of the thyroid: are there factors predictive of malignancy? Ann Surg. 1998;227:542–546. doi: 10.1097/00000658-199804000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlinkert RT, van Heerden JA, Goellner JR, Gharib H, Smith SL, Rosales RF, et al. Factors that predict malignant thyroid lesions when fine-needle aspiration is "suspicious for follicular neoplasm". Mayo Clin Proc. 1997;72:913–916. doi: 10.1016/S0025-6196(11)63360-0. [DOI] [PubMed] [Google Scholar]
- 17.Sippel RS, Elaraj DM, Khanafshar E, Kebebew E, Duh QY, Clark OH. Does the presence of additional thyroid nodules on ultrasound alter the risk of malignancy in patients with a follicular neoplasm of the thyroid? Surgery. 2007;142:851–857. doi: 10.1016/j.surg.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Kim ES, Nam-Goong IS, Gong GY, Hong SJ, Kim WB, Shong YK. Postoperative findings and risk for malignancy in thyroid nodules with cytological diagnosis of the so-called "follicular neoplasm". Korean J Intern Med. 2003;18:94–97. doi: 10.3904/kjim.2003.18.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyler DS, Winchester DJ, Caraway NP, Hickey RC, Evans DB. Indeterminate fine-needle aspiration biopsy of the thyroid: identification of subgroups at high risk for invasive carcinoma. Surgery. 1994;116:1054–1060. [PubMed] [Google Scholar]
- 20.Rago T, Di Coscio G, Basolo F, Scutari M, Elisei R, Berti P, et al. Combined clinical, thyroid ultrasound and cytological features help to predict thyroid malignancy in follicular and Hupsilonrthlecell thyroid lesions: results from a series of 505 consecutive patients. Clin Endocrinol (Oxf) 2007;66:13–20. doi: 10.1111/j.1365-2265.2006.02677.x. [DOI] [PubMed] [Google Scholar]
- 21.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Alexander EK, Marqusee E, Orcutt J, Benson CB, Frates MC, Doubilet PM, et al. Thyroid nodule shape and prediction of malignancy. Thyroid. 2004;14:953–958. doi: 10.1089/thy.2004.14.953. [DOI] [PubMed] [Google Scholar]
- 24.Kelman AS, Rathan A, Leibowitz J, Burstein DE, Haber RS. Thyroid cytology and the risk of malignancy in thyroid nodules: importance of nuclear atypia in indeterminate specimens. Thyroid. 2001;11:271–277. doi: 10.1089/105072501750159714. [DOI] [PubMed] [Google Scholar]
- 25.Rupp M, Ehya H. Nuclear grooves in the aspiration cytology of papillary carcinoma of the thyroid. Acta Cytol. 1989;33:21–26. [PubMed] [Google Scholar]
- 26.Mesonero CE, Jugle JE, Wilbur DC, Nayar R. Fine-needle aspiration of the macrofollicular and microfollicular subtypes of the follicular variant of papillary carcinoma of the thyroid. Cancer. 1998;84:235–244. [PubMed] [Google Scholar]
- 27.Jogai S, Al-Jassar A, Temmim L, Dey P, Adesina AO, Amanguno HG. Fine needle aspiration cytology of the thyroid: a cytohistologic study with evaluation of discordant cases. Acta Cytol. 2005;49:483–488. doi: 10.1159/000326192. [DOI] [PubMed] [Google Scholar]
- 28.Ylagan LR, Farkas T, Dehner LP. Fine needle aspiration of the thyroid: a cytohistologic correlation and study of discrepant cases. Thyroid. 2004;14:35–41. doi: 10.1089/105072504322783821. [DOI] [PubMed] [Google Scholar]
- 29.Kato MA, Buitrago D, Moo TA, Keutgen XM, Hoda RS, Ricci JA, et al. Predictive value of cytologic atypia in indeterminate thyroid fine-needle aspirate biopsies. Ann Surg Oncol. 2011;18:2893–2898. doi: 10.1245/s10434-011-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubitz CC, Faquin WC, Yang J, Mekel M, Gaz RD, Parangi S, et al. Clinical and cytological features predictive of malignancy in thyroid follicular neoplasms. Thyroid. 2010;20:25–31. doi: 10.1089/thy.2009.0208. [DOI] [PubMed] [Google Scholar]