Abstract
Purpose
This study aimed to evaluate the association between low body mass index (BMI) and morbidity after gastric cancer surgery.
Methods
A total of 1,805 patients were included in the study. These subjects had undergone gastric cancer surgery at a single institution between January 1997 and December 2013. Clinicopathologic and morbidity data were analyzed by dividing the patients into 2 groups: underweight patients (BMI < 18.5 kg/m2) and nonunderweight patients (BMI ≥ 18.5 kg/m2).
Results
The overall complication rate as determined by our study was 24.4%. Pulmonary complications occurred more frequently in the underweight group (UWG) than in the non-UWG (10.5% vs. 3.8%, respectively; P = 0.012). Multivariate analysis revealed two independent factors responsible for postoperative pulmonary complications—weight of the patients (UWG vs. non-UWG, 10.8% vs. 3.8%; P < 0.007) and stage of gastric cancer (early stage vs. advanced stage, 3.1% vs. 6.8%; P < 0.023). Multivariate analysis revealed that underweight (UWG vs. non-UWG, 10.8% vs. 3.8%, respectively, P < 0.007) and advanced cancer stage (early stage vs. advanced stage, 3.1% vs. 6.8%, respectively, P = 0.023) were significant risk factors for postoperative pulmonary complications.
Conclusion
We concluded that underweight patients had a higher pulmonary complication rate. Additionally, underweight and advanced cancer stage were determined to be independent risk factors for the development of postoperative pulmonary complications.
Keywords: Stomach neoplasm, Morbidity, Malnutrition
INTRODUCTION
Gastric cancer is the fourth most common form of cancer worldwide, and almost a million new cases are diagnosed annually [1]. Although the mortality rate due to gastric cancer decreased slightly from 774,000 in 1990 to 700,000 in 2012, it is the third-most common cause of cancer-related deaths, followed by lung cancer and hepatoma [2]. In Korea, gastric cancer has been the most commonly diagnosed cancer since 1999, when the Korea Central Cancer Registry first reported the incidence of gastric cancer [3].
Malnutrition is a condition that results from consuming a diet that has not enough or excess nutrients, resulting in health problems. Specifically, malnutrition is often used to refer to a state of under-nourishment, where there is insufficient intake of calories, proteins, carbohydrates, vitamins, and minerals [4]. Cancer patients tend to lose weight and suffer from malnutrition because of poor oral intake and alterations in metabolic pathway(s). The prevalence of malnutrition in cancer patients is reported to be around 10%–85%, which varies according to the type, location, grade, and stage of the tumor [5]. Underweight people typically find it difficult to sustain normal health. Being underweight is a condition generally defined as either having a body mass index (BMI) of < 18.5 kg/m2 [6] or body weight < 15%–20% of the normal value for a particular age and height [7].
Malnutrition has been shown to be an important risk factor for perioperative morbidity and mortality [8]. People with very low body weights may have insufficient stamina and weak immunity, along-with increased susceptibility to infections. Most immunological disorders are induced by the deficiency of specific nutrients, or malnutrition. Such disorders are frequently caused by low T-cell production, abnormal functioning of monocytes, macrophages, neutrophils, and leukocytes; thymus-mediated damage to the immune system (which results in diminished reaction to new antigens); and deterioration of lymphoid tissue.
Although many studies have examined the nutritional state after gastrectomy [9], few reports have in particular focused on the preoperative low BMI in relation to postoperative complications. This study aimed to evaluate the association between low BMI and postoperative morbidity following gastric cancer surgery.
METHODS
Between January 1997 and December 2013, data were collected for 1,805 patients who underwent gastric cancer surgery at a single institution. The following inclusion criteria were applied to select the patients: patients must have had primary gastric cancer, patients must have undergone gastrectomy, and he/she should not have been subject to preoperative chemotherapy. Patients who had other primary malignancies, and subjects with recurrent or remnant gastric cancer were excluded from this analysis. Demographic features including BMI, operative procedures, pathological results (based on the American Joint Committee on Cancer TNM 7th edition), and postoperative complications were reviewed [10].
The term complication was described according to our previous report on complications occurring after gastric ulcer operation. [11]. Among them is pulmonary complication, defined as the presence of atelectasis, pleural effusion, pneumonia, pulmonary edema, or pneumothorax on plain chest radiography or CT with clinical manifestations such as fever, leukocytosis, and dyspnea.
We divided the patients into 2 groups according to the BMI criteria 2013, namely the underweight group (UWG; BMI < 18.5 kg/m2) and the non-UWG (BMI ≥ 18.5 kg/m2) [12]. We investigated the types of complications and their rates between the 2 groups. Additional multivariate analysis was performed to analyze the independent risk factors for postoperative pulmonary complications.
Statistical analyses were performed with IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Chi-square test and t-test were conducted to compare nominal and continuous variables, respectively. Independent risk factors for morbidity were evaluated by binary logistic regression. Variables with a P-value of <0.05 in the univariate analysis were analyzed in the multivariate analysis. Values of P < 0.05 were considered statistically significant.
RESULTS
Patient characteristics
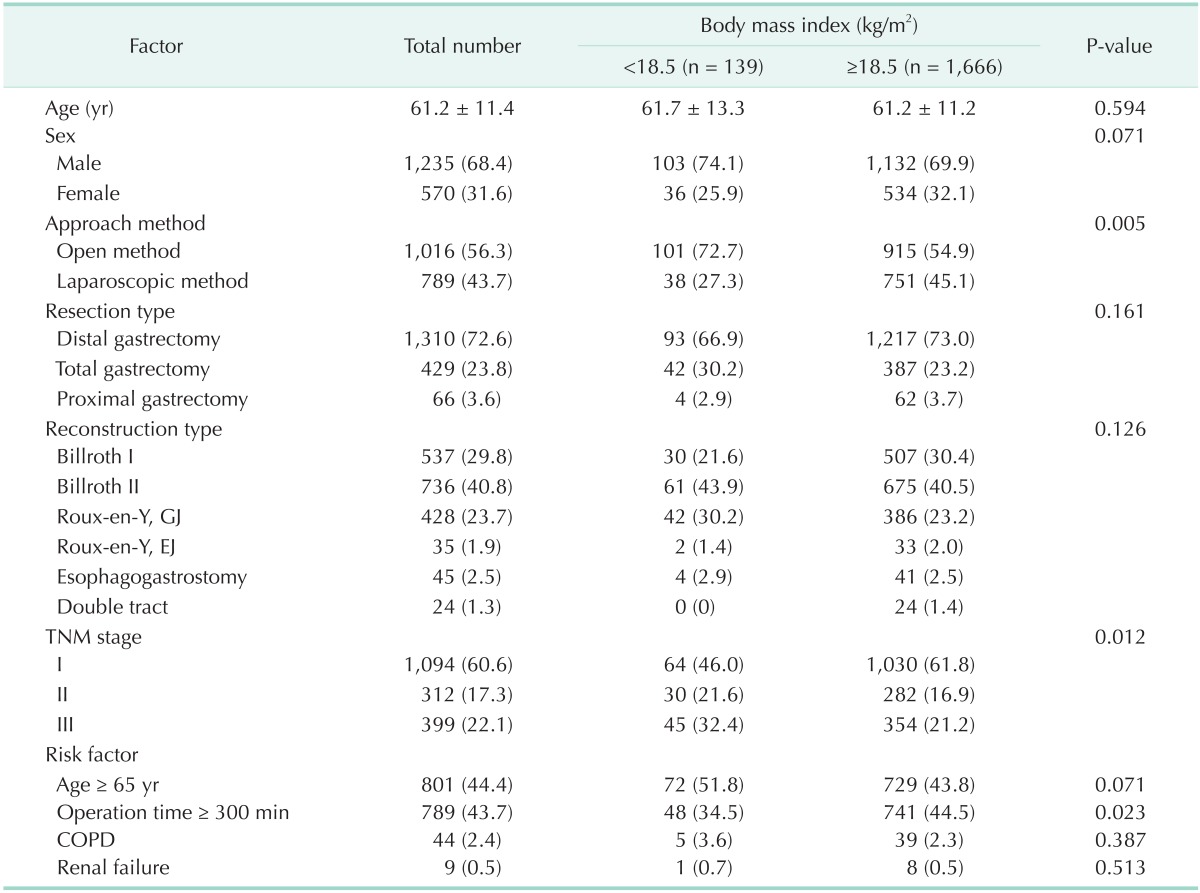
The average age of the patients was 61.2 ± 11.4 years. Of the subjects, 68.4% (n = 1,235) and 31.6% (n = 570) were male and female patients, respectively. Depending on the method of approach, 56.3% (n = 1,016) of the cases had undergone open surgery, while 43.7% (n = 789) had undergone laparoscopic surgery. In terms of the type of resection, 72.6% (n = 1,310) of the patients had undergone distal gastrectomy, 23.8% (n = 429) had undergone total gastrectomy, and 3.7% (n = 66) had undergone proximal gastrectomy. The most common reconstruction type was Billroth II (40.8%; n = 736), followed by Billroth I (29.8%; n = 537), Roux-en-Y gastrojejunostomy (24.4%; n = 450), esophagogastrostomy (2.4%; n = 45), Rouxen-Y esophagojejunostomy (2.0%; n = 36), and double tract anastomosis (1.3%, n = 24). Sixty point six percent (n = 1,094) had stage I cancer, 17.3% (n = 312) had stage II cancer, and 22.1% (n = 399) had stage III cancer (Table 1).
Table 1. Comparison of characteristics in patients between the UWG and non-UWG.
Values are presented as mean ± standard deviation or number (%).
UWG, underweight group; GJ, gastrojejunostomy; EJ, esophagojejunostomy; COPD, chronic pulmonary obstructive disease.
With respect to the patient characteristics, the surgical approach method and cancer stage were significantly different between groups. We assume that is because surgeons prefer open surgery more than laparoscopic surgery for patients with advanced stage, and that patients can lose weight more easily than early stage patients. Percentage of patients in the UWG who had undergone open surgery was higher than that of patients in the non-UWG (72.7% vs. 54.9%, respectively, P < 0.005). Also, the UWG included more stages II and III cancer patients (54.0% vs. 38.1%), while the non-UWG included more stage I cancer patients (61.8% vs. 46.0%), as shown in Table 1.
In old age, more patients tended to become underweight, but the difference was not statistically significant (P = 0.071). The incidence rate of underlying chronic pulmonary obstructive disease and renal failure were not significantly higher in the UWG than in the non-UWG. The UWG had shorter operation time than the non-UWG.
Complications in patients
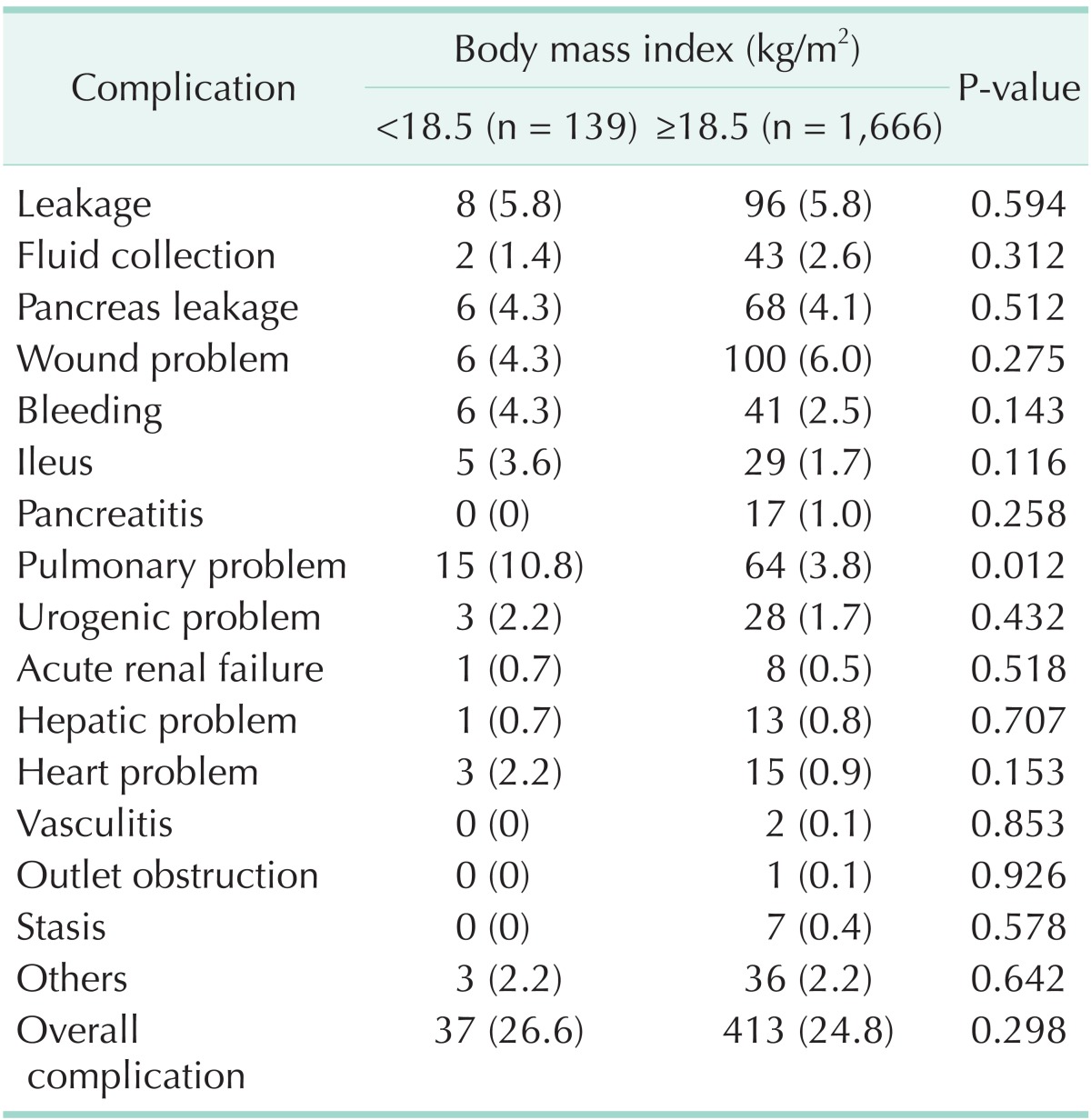
The overall complication rate amongst all patients was 24.4% (n = 450). With respect to the rates of each complication, wound problems were noted in 5.9% (n = 106) of the patients; anastomotic leakage, in 5.8% (n = 104); pulmonary problems, in 4.4% (n = 79); pancreatic leakage, in 4.1% (n = 74); bleeding, in 2.6% (n = 47); and ileus, in 1.9% (n = 34). In pulmonary problems, the most common complication was pleural effusion (50.6%), followed by atelectasis (25.3%), pneumonia (20.2%), pulmonary edema (10.1%), and pneumothorax (5.0%).
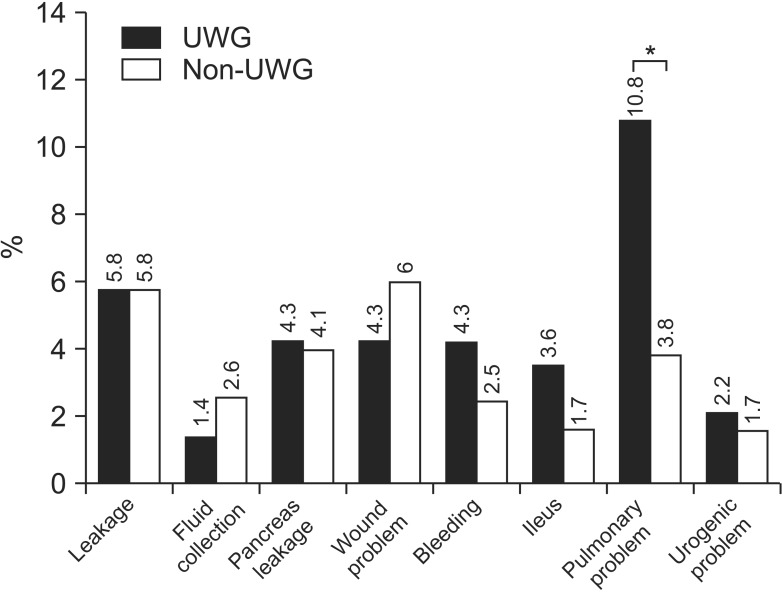
Total complication rates for both the UWG and non-UWG were similar (26.6% vs. 24.2%, respectively). The 2 groups differed significantly in terms of pulmonary problems (10.8% vs. 3.8%; P = 0.012). However, they did not exhibit any statistically significant difference when the following characteristics were compared: total complication rate, anastomotic leakage, fluid collection, pancreatic leakage, wound problem, bleeding, ileus, urologic problems, renal problems, hepatic problems, heart problems, vasculitis, outlet obstruction, and stasis (P > 0.05) (Table 2, Fig. 1).
Table 2. Characteristics of postoperative morbidity (classified according to body mass index).
Values are presented as number (%).
Fig. 1. Characteristics of postoperative morbidity between the underweight group (UWG) and non-UWG. *P = 0.005.
Multivariate analysis
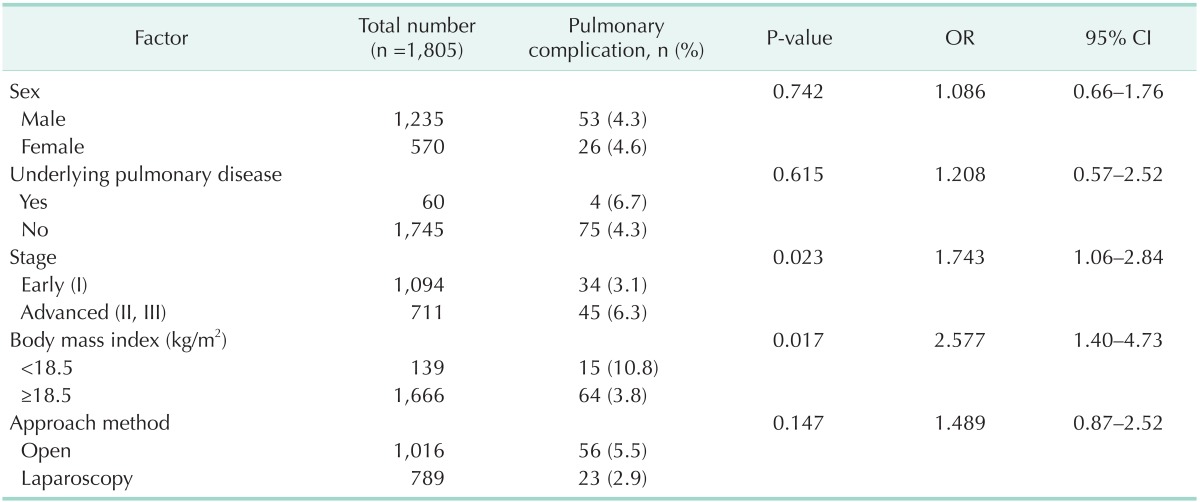
Comparison of UWG and non-UWG patients' characteristics revealed that pulmonary complication rates were significantly different between the 2 groups, as determined by univariate analysis. We performed multivariate analysis to investigate independent risk factors for postoperative pulmonary complications.
According to the multivariate analysis, underweight (odds ratio [OR], 2.58; 95% confidence interval [CI], 1.4–4.73) and advanced cancer stage (OR, 1.74; 95% CI, 1.06–2.84) were found to be significant independent risk factors for pulmonary complications (P < 0.05) (Table 3).
Table 3. Multivariate analysis of correlation between postoperative pulmonary complications and perioperative characteristics.
OR, odds ratio; CI, confidence interval.
DISCUSSION
This study was conducted to evaluate the association between low BMI and postoperative morbidity after gastric cancer surgery. The surgical approach method and cancer stage significantly differed between the UWG and non-UWG. Compared to those in the non-UWG, more patients in the UWG underwent open surgery and had advanced stage. This is because patients with advanced stage cancer tended to be underweight due to dyspepsia, cancer cachexia, anorexia, and so on and advanced stage cancer is more frequently treated with open surgery than with laparoscopic surgery. When the complication rates of UWG and non-UWG patients were compared, we observed that pulmonary problems were more apparent in the UWG. We speculated that underweight patients were unable to endure the aggressiveness of gastric surgery as compared to nonunderweight patients.
Multivariate analysis was performed to determine other factors responsible for pulmonary complications. In addition to our initial tentative conclusion, we observed a significantly higher rate of postoperative pulmonary complications in advanced stage patients.
Generally, advanced stage patients undergo more aggressive lymph node dissection and stomach resection than early stage patients. Therefore, advanced stage patients are subject to greater injury than early stage patients during surgery, and consequently may develop severe postoperative complications.
Additionally, with regards to postoperative lung problems, a study reported that protein deficiency can reduce respiratory muscle strength, vital capacity, and peak expiratory flow rate, resulting in the development of pneumonia and atelectasis [13].
There are some studies that explain the relation between specific factors and postoperative pulmonary complications. The nutritional state, for example, protein deficiency or low albumin levels, and recent weight loss can induce pulmonary complications [13,14,15,16,17]. Older patients can develop more serious pulmonary complications than younger patients [14,15,18,19]. Additional risk factors for postoperative pulmonary complications include smoking history, COPD, long operation time, upper abdominal surgery, chronic respiratory disease, emergency operation, renal failure, and significant intraoperative blood loss [18,19]. In our institution, there was no difference in the rate of underlying pulmonary disease between the UWG and non-UWG patients.
Contrary to previous studies, investigating with our patients, we have found out that risk factors such as old age, underlying COPD, and renal failure are not more frequent for the UWG. The operation time was even shorter for the UWG. A comprehensive view of all these findings suggests that low BMI is an independent risk factor of pulmonary complication regardless of the above-mentioned risk factors (Table 1).
Many studies have described the relationship between malnutrition and other postoperative complications. Numerous clinical studies have indicated that protein-calorie malnutrition is an important risk factor for the development of complications after major abdominal surgery [20,21,22,23]. Some studies suggest that malnutrition is an important factor leading to anastomotic leakage and wound infections [24,25]. According to a large prospective randomized trial of preoperative total parenteral nutrition (TPN) conducted by the Veterans Administration, patients receiving TPN showed significantly lower rates of anastomotic leakage and bronchopleural fistulas than those who did not receive TPN (5% vs. 43%, respectively; P = 0.03). It was concluded that TPN may be needed for preoperative nutritional support and massive postoperative nutritional support to prevent further complications. Bozzetti et al. [26] documented that pancreatic surgery, old age, weight loss, and low plasma albumin concentrations were independent risk factors for the development of postoperative complications in patients with gastrointestinal cancer, and that nutritional support significantly reduced the postoperative complications.
Contrary to the above studies, some studies suggest that no correlation exists between BMI and postoperative complications. A small-scale study reported that malnutrition was not associated with complications after colorectal surgery, and that perioperative TPN support was unable to reduce postoperative complications [27]. Hendry et al. [28] reported that BMI was not related to postoperative complications, unlike the American Society of Anesthesiologists score, sex, or rectal surgery. In contrast to our results, one study asserted that a BMI > 27 kg/m2 is a risk factor for developing complications after abdominal surgery [29]. Another study reported that the occurrence rate of ileus, after radical cystectomy for bladder cancer, had a positive correlation with BMI [30]. There was some controversy regarding the relationship of BMI with survival. One study reported the BMI is one of the prognostic factors for stages II and IIIA gastric cancer. On the other hand, another study reported that BMI does not adversely affect overall survival or disease-free survival.
However, there are certain limitations of our study. First, ours is a retrospective study, and second, the number of underweight patients included in the study is relatively small. Third, we failed to use objective scores for analyses of complications and nutrition research. Finally, we have too insufficient data about albumin level, recent weight loss, smoking history, and intraoperative blood loss to investigate risk factors of postoperative pulmonary complication thoroughly.
To conclude, this study demonstrates that underweight patients have a higher morbidity rate, especially in relation to pulmonary complications. Additionally, underweight and advanced cancer stage could be independent risk factors for postoperative pulmonary complications.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young EM. Food and development. London: Routledge; 2013. [Google Scholar]
- 5.Stratton RJ, Green CJ, Elia M. Disease-related malnutrition: an evidence-based approach to treatment. Cambridge (MA): CABI Pub.; 2003. [Google Scholar]
- 6.Aim for a Healthy Weight, Assessing your weight and health risk [Internet] Bethesda (MD): National Heart, Lung, and Blood Institute; [cited 2015 Mar 1]. Available from: https://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm. [Google Scholar]
- 7.Mahan LK, Escott-Stump S. Krause's food, nutrition, & diet therapy. Philadelphia: Saunders; 2004. [Google Scholar]
- 8.Jabaiti SK. Risk factors for wound complications following abdominoplasty. Am J Appl Sci. 2009;6:897–901. [Google Scholar]
- 9.Ryu SW, Kim IH. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J Gastroenterol. 2010;16:3310–3317. doi: 10.3748/wjg.v16.i26.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, et al. Analysis of risk factors for postoperative morbidity in perforated peptic ulcer. J Gastric Cancer. 2012;12:26–35. doi: 10.5230/jgc.2012.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. BMI classification 2013 [Internet] Geneva: World Health Organization; c2016. [cited 2015 Mar 1]. Available from: http://apps.who.int/bmi/index.jsp. [Google Scholar]
- 13.Windsor JA, Hill GL. Risk factors for postoperative pneumonia. The importance of protein depletion. Ann Surg. 1988;208:209–214. doi: 10.1097/00000658-198808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232:242–253. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arozullah AM, Khuri SF, Henderson WG, Daley J Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–857. doi: 10.7326/0003-4819-135-10-200111200-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ondrula DP, Nelson RL, Prasad ML, Coyle BW, Abcarian H. Multifactorial index of preoperative risk factors in colon resections. Dis Colon Rectum. 1992;35:117–122. doi: 10.1007/BF02050665. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 18.Rock P, Rich PB. Postoperative pulmonary complications. Curr Opin Anaesthesiol. 2003;16:123–131. doi: 10.1097/00001503-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Sin DD. Postoperative pulmonary complications: what every general practitioner ought to know. BC Med J. 2008;50:152–154. [Google Scholar]
- 20.Sungurtekin H, Sungurtekin U, Balci C, Zencir M, Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004;23:227–232. doi: 10.1080/07315724.2004.10719365. [DOI] [PubMed] [Google Scholar]
- 21.Putwatana P, Reodecha P, Sirapo-ngam Y, Lertsithichai P, Sumboonnanonda K. Nutrition screening tools and the prediction of postoperative infectious and wound complications: comparison of methods in presence of risk adjustment. Nutrition. 2005;21:691–697. doi: 10.1016/j.nut.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Vastine VL, Morgan RF, Williams GS, Gampper TJ, Drake DB, Knox LK, et al. Wound complications of abdominoplasty in obese patients. Ann Plast Surg. 1999;42:34–39. doi: 10.1097/00000637-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 23.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 24.Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148:65–71. doi: 10.1001/2013.jamasurg.2. [DOI] [PubMed] [Google Scholar]
- 25.Zhu QL, Feng B, Lu AG, Wang ML, Hu WG, Li JW, et al. Laparoscopic low anterior resection for rectal carcinoma: complications and management in 132 consecutive patients. World J Gastroenterol. 2010;16:4605–4610. doi: 10.3748/wjg.v16.i36.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007;26:698–709. doi: 10.1016/j.clnu.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Fasth S, Hulten L, Magnusson O, Nordgren S, Warnold I. Postoperative complications in colorectal surgery in relation to preoperative clinical and nutritional state and postoperative nutritional treatment. Int J Colorectal Dis. 1987;2:87–92. doi: 10.1007/BF01647698. [DOI] [PubMed] [Google Scholar]
- 28.Hendry PO, Hausel J, Nygren J, Lassen K, Dejong CH, Ljungqvist O, et al. Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg. 2009;96:197–205. doi: 10.1002/bjs.6445. [DOI] [PubMed] [Google Scholar]
- 29.Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564–571. doi: 10.1378/chest.111.3.564. [DOI] [PubMed] [Google Scholar]
- 30.Svatek RS, Fisher MB, Williams MB, Matin SF, Kamat AM, Grossman HB, et al. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology. 2010;76:1419–1424. doi: 10.1016/j.urology.2010.02.053. [DOI] [PubMed] [Google Scholar]