Abstract
Background/Objectives:
Iron deficiency anemia is a widespread public health problem, particularly in low- and middle-income countries. Maternal iron status around and during pregnancy may influence infant iron status. We examined multiple biomarkers to determine the prevalence of iron deficiency and anemia among breastfed infants and explored its relationship with maternal and infant characteristics in Bhaktapur, Nepal.
Subjects/Methods:
In a cross-sectional survey, we randomly selected 500 mother–infant pairs from Bhaktapur municipality. Blood was analyzed for hemoglobin, ferritin, total iron-binding capacity, transferrin receptors and C-reactive protein.
Results:
The altitude-adjusted prevalence of anemia was 49% among infants 2–6-month-old (hemaglobin (Hb) <10.8 g/dl) and 72% among infants 7–12-month-old (Hb <11.3 g/dl). Iron deficiency anemia, defined as anemia and serum ferritin <20 or <12 μg/l, affected 9 and 26% of infants of these same age groups. Twenty percent of mothers had anemia (Hb <12.3 g/dl), but only one-fifth was explained by depletion of iron stores. Significant predictors of infant iron status and anemia were infant age, sex and duration of exclusive breastfeeding and maternal ferritin concentrations.
Conclusions:
Our findings suggest that iron supplementation in pregnancy is likely to have resulted in a low prevalence of postpartum anemia. The higher prevalence of anemia and iron deficiency among breastfed infants compared with their mothers suggests calls for intervention targeting newborns and infants.
Introduction
The World Health Organization (WHO) estimates that globally ~293 million young children and 468 million non-pregnant women suffer from anemia, among which ~50% are estimated to be attributable to iron deficiency (ID).1 This makes iron one of the most common micronutrient deficiencies in the world. In addition to inadequate intake of iron, infection and blood loss are common causes of ID, and there is growing evidence suggesting that impairment of mucosal absorption of iron may contribute to the high rates of ID in developing countries.2 ID impairs physical activity and cognitive performance and may also cause irreversible intergenerational effects when it occurs in women of reproductive age.3, 4 ID anemia (IDA) during pregnancy increases the risk of adverse pregnancy and perinatal outcomes including maternal and neonatal deaths, particularly in severe anemia.5
Because of limited resources, ID is usually diagnosed based on the estimation of hemoglobin levels despite the gross lack of sensitivity and specificity of this method.6, 7 However, there is no feasible and universally accepted single indicator that can diagnose ID precisely. Low plasma ferritin indicates depletion of iron stores, but values between 20 and 100 μg/l are difficult to interpret because of its nature as a positive acute-phase protein.8 Transferrin receptor (TfR) is a relatively recent addition to the iron markers and is less affected by inflammation. However, it is not as widely used as ferritin.
Few studies have assessed determinants for poor iron status by using multiple iron parameters and at the same time measured the association between maternal iron status and that of their breastfed infants. The limited knowledge base is drawn primarily from studies conducted before the universal recommendation of iron and folic acid supplementation to pregnant women.9 Widespread use of iron and folic acid supplementation during pregnancy and lactation, secular changes in dietary patterns and rapid urbanization have likely changed the contributions of ID to anemia in low- and middle-income countries such as Nepal.10 In this paper, we aimed to describe prevalence and predictors of anemia and ID in breastfed infants and explore associations between maternal and infant iron status in a periurban Nepali population.
Materials and methods
Study area and population
Bhaktapur municipality is located in the Bhaktapur district in the Kathmandu valley at an altitude of 1400 m above sea level. The population according to census report of 2011 is 81 748 people, and the majority have agriculture as their main occupation. The study site includes a periurban area with a large proportion of low-income inhabitants. Bhaktapur is the one of the most densely populated districts of Nepal, with a population density of 11 058 people per square km. Newars constitute ~90% of the Bhaktapur municipality population.11
Enrollment procedure
The selection criteria and details on field procedures have been described in detail elsewhere.12 From January 2008 to February 2009, we enrolled 500 lactating women between 15 and 44 years of age and 474 of their infants below 1 year of age from Bhaktapur municipality in Nepal. We used a two-stage cluster sampling procedure whereby 66 neighborhood streets called 'Toles' were randomly selected as the primary sampling unit from a list of 160. We listed all women living in these Toles, and women and infant pairs were again randomly selected from this list.13 The inclusion criteria for the study were that both mothers and children had no ongoing infection (clinically assessed), resided in selected clusters, were willing to provide the household information and consented to participate. Because of acute blood loss and many physiological and hormonal changes during delivery that usually last up to 2 months,14 we enrolled lactating mothers 2 months after delivery. A total of 1101 eligible mother–infant pairs were recorded during the 1 year of longitudinal follow-up, and 582 were approached for enrollment. Of these, 500 mother–child pairs were enrolled in the study and provided a blood sample. All women gave written informed consent before the start of the study. The study had ethical clearance from the institutional review boards at the Institute of Medicine, Kathmandu, Nepal.
Sample size calculations
We assumed a prevalence of anemia of 25% in this population. Four hundred and fifty mothers were required to detect this prevalence with an absolute precision of 4%, that is, with a 95% confidence interval from 21 to 29%. Assuming 10% loss-to-follow-up, we enrolled 500 women and infant pairs.
Laboratory procedures'
Blood samples were collected from one of the cubital veins using micronutrient-free heparinized polypropylene tubes (Eppendorf, Sarstedt, Germany). Hemoglobin was tested on HemoCue immediately after blood collection (HemoCue, Vedbæk, Denmark) with regular calibration of the instrument as instructed by the manufacturer. The samples were then centrifuged (760 g, for 10 min, room temperature), and plasma was allocated onto micronutrient-free polypropylene vials (Eppendorf, Hinz, Germany) and stored at −20 °C in a freezer in the field site laboratory. Every day these samples were transported to the central laboratory in Kathmandu with an ice pack where they were then stored at −70 °C until they were transported on dry ice to Norway where they were temporarily stored at −80°C before analysis at the Laboratory of Clinical Biochemistry, Haukeland University Hospital (Bergen, Norway). The biochemical iron parameters were analyzed on a Modular Analytics System by Roche Diagnostics (Roche Diagnostics GmbH, Mannheim, Germany) with a 5% coefficient of analytical variation for each test. The plasma ferritin was analyzed by an electrochemiluminescence immunoassay, whereas the soluble TfR and transferrin were analyzed by immunoturbidimetry. Total iron-binding capacity (TIBC) was calculated from transferrin concentrations.
Definitions
According to the WHO, anemia among infants between 6 and 12 months of age is defined as hemoglobin <11 g/dl and depleted iron stores if ferritin <12 μg/l.15 The corresponding cutoffs for non-pregnant women are 12 g/dl and 15 μg/l, respectively. We also present the prevalence of depleted stores by plasma ferritin <30 μg/l, which is often used in a population where infection and inflammation are common.1 There is no standard cutoff to define anemia among infants <6 months of age; therefore, we used a cutoff of 10.5 g/dl for Hb and 20 μg/l for ferritin derived from a study among Swedish and Honduran infants.16 Because of lower partial pressure of oxygen at higher altitude, Centers for Disease Control recommends the adjustment of hemoglobin values when interpreting anemia prevalence among people residing altitude above 1000 m of sea level; hence, we added 0.3 g/dl to the standard cutoff values of Hb to adjust for a local altitude of 1400 m.17
To assess iron-deficient erythropoiesis (IDE), which is a transient stage of cellular iron insufficiency that can occur before the development of anemia, we measured the concentration of soluble TfR.18 TfR concentrations are less affected by inflammation than ferritin and can thus reflect early stages of ID at the cellular level provided there are no other causes of abnormal erythropoiesis.19 There is currently no consensus on a single cutoff value of TfR used to define ID, and hence we used thresholds from similar studies.20, 21, 22 We used a concentration of TIBC >83 μmol/l, which is the upper limit value of our laboratory, to indicate an alteration in iron status. We defined IDA as the concurrent presence of anemia and low plasma ferritin or a high TfR concentration. Anemia was considered to have other causes when it was accompanied with high ferritin or low TfR concentration.15 The TfR-F ratio was calculated by dividing TfR with the log transformation of plasma ferritin, and the TfR-F index was calculated by dividing TfR and ferritin concentrations after converting milligrams of TfR to microgram units. Maternal body mass index (BMI) was calculated as kg/m2, and BMI <18.5 kg/m2 was considered as undernourished. For children, weight for age, length for age and weight for length z-scores were calculated (WAZ, LAZ and WLZ) based on the WHO growth standards.23
Infants were considered exclusively breastfed when they reportedly had only consumed breast milk from mother (or wet nurse or expressed breast milk) since birth and had not consumed any liquids or semi/solid foods, except medicines.24
Statistical analysis
Differences between subjects with anemia or ID were tested by independent t-tests for continuous variables and a χ2 test for the categorical variables. A threshold of P<0.05 was used to indicate statistical significance for all tests. From a list of candidate covariates including age, gender of infant, C-reactive protein (CRP), breastfeeding status, anthropometry and socioeconomic factors, as well as mother's age, BMI and Hb and iron status, we manually selected and run unadjusted regression analysis.25 A forward purposeful stepwise selection process was carried out to identify important predictors for each of the iron-related variables. Only variables that were showing association at P<0.20 were retained in the final regression model. Final predictors for the multiple linear regression for iron status included infant age and sex, the prevalence of exclusive breastfeeding at 3 months of age, whether mother received iron supplements for at least 6 months and maternal ferrritin concentrations. Statistical analysis was performed by using Stata version 10 (StataCorp. 2013. Stata Statistical Software: Release 10.; StataCorp LP, College Station, TX, USA), and analyses were adjusted for the design effect of the cluster approach. The graph describing the relationship between plasma ferritin with age and gender of infant was constructed using generalized additive models in R statistical software (r-project.org).
Results
Baseline characteristics
The mean age of the 474 infants enrolled in this survey was 6.8 months (ranged 2–12 months), and 45% were below 6 months of age (Table 1). Most of the infants had been delivered at a hospital, were fed colostrum and initiated breastfeeding within 1 h of delivery. However, only 18% of infants practiced exclusive breastfeeding for the first 6 months of life, and average introduction of semisolid foods mostly in the form of home-made or local mixed grain porridge (lito) or readymade cerelac was 3 months.
Table 1. Characteristics of infant and mother enrolled in the micronutrient survey in Bhaktapur, Nepal.
| Characteristics | Valuesa |
|---|---|
| Infants (n=474) | |
| Mean age infant (s.d.) (months) | 6.8 (2.8) |
| Mean birth weight (s.d) (g)b | 2891 (491) |
| Newborn with <2500 g of birth weight) (%) | 13 |
| Home delivery | 10 |
| Initiation of breastfeeding within 1 h (n=487) | 80 |
| Colostrum given | 92 |
| Exclusive breastfeeding for >3 months of age | 42 |
| Exclusive breastfeeding for 6 months of age | 18 |
| Underweight infant (<−2 z-score weight for age) | 5 |
| Stunted infant (<−2 z-score length for age) | 10 |
| Mothers | |
| Mean age mother (s.d., years) | 25.8 (4.2) |
| Mother with <18.5 BMI (kg/m2) | 5 |
| Mother with >25 BMI (kg/m2) | 17 |
| Mother with two or more parity | 59 |
| Mother received large dose of vitamin A in the past 4 months | 2 |
| Household information | |
| Living in joint families | 51 |
| Family living in only one room | 38 |
| Family using tap drinking water | 99 |
| Family ownership of land | 55 |
| % of illiterate | |
| Mother | 19 |
| Father | 6 |
| No work or only seasonal agricultural work | |
| Mother | 73 |
| Father | 7 |
| Family planning | |
| Mother using Depo-Provera (currently) | 46 |
| Number of years of previous Depo use, mean (s.d.)c | 3.3 (1.8) |
| Supplementation during the last pregnancy | |
| Mothers with iron supplementation | 90 |
| Months of iron supplementation (n=449), mean (s.d.) | 4.8 (1.8) |
| Months of folic acid supplementation (n=115), mean (s.d.) | 2.9 (2.2) |
| Months of calcium supplementation (n=402), mean (s.d.) | 4.8 (1.8) |
Abbreviation: BMI, body mass index.
Values are %, all such cases, unless otherwise specified.
Among 438 newborns where birth weight was recorded.
Among 259 mothers who used Depo-Provera injection before.
Iron supplementation was reported by 90% of women during their last pregnancy, and one-third of them began using it in the first trimester. Although only about a quarter reported taking folic acid supplementation during their pregnancy, most were likely not aware that it was included in their iron supplements, as most preparations in the market combine the two.
Prevalence of anemia and ID
The mean Hb among infants was 10.7 g/dl, and more than two-thirds of infants >6 months of age (72%) were anemic (Hb <11.3 g/dl altitude-adjusted). Among infants <6 months of age, the prevalence of anemia (<10.8 g/dl age- and altitude-adjusted) was 49%. Only two infants had severe anemia (<7.3 g/dl). The mean hemoglobin concentration was 13.1 g/dl, and 20% of the women were found to be anemic using the altitude-specific cutoff value of Hb <12.3 g/dl (Tables 2a and b).
Table 2a. Hb, ferritin, TIBC, TfR and CRP concentrations among healthy infant and lactating mother in Bhaktapur, Nepal.
| Indicators | Infant (2–12 months) | Mother (15–44 years)a | Spearman's correlation (P-value)b |
| Hb, n | 474 | 500 | |
| Mean, s.d. (g/dl) | 10.7 (1.3) | 13.1 (1.3) | 0.16 (0.01) |
| Anemia adjusted for altitudec | 69 | 20 | |
| Mild anemia (%)c | 34 | 17 | |
| Moderate anemia | 35 | 3 | |
| Severe anemia | 0.4 | 0 | |
| Ferritin, n | 448 | 500 | |
| Mean (s.d.) (μg/l) | 54.8 (69.8) | 68.8 (45.9) | 0.12 (0.01) |
| Depleted iron storesd | 20 | 5 | |
| Iron deficiency anemiad | 17 | 4 | |
| TIBC, n | 448 | 500 | |
| Mean (s.d.) (μmol/l) | 72.6 (12.1) | 72.7 (10.7) | 0.09 (0.04) |
| High TIBC >83 μmol/l | 18 | 15 | |
| TfR, n | 449 | 500 | |
| Mean (s.d.) (mg/l) | 6.2 (3.1) | 3.4 (1.5) | 0.21 (<0.001) |
| Iron-deficient erythropoeisise | 76 | 16 | |
| Iron-deficient erythropoeisis and anemia | 20 | 6 | |
| CRP, n | 448 | 500 | |
| Mean (s.d.) (mg/l) | 4.3 (10.8) | 1.8 (6.5) | |
| > 5 mg/l | 21 | 5 | |
| Ratio TfR/log ferritin, mean (s.d.)f | 2.3 (3.1) | 0.95 (0.88) | |
| >2 | 38 | 5 | |
| TfR/ferritin index, mean (s.d.)f | 499 (1142) | 110 (289) | |
| >500 | 22 | 3 |
Abbreviations: CRP, C-reactive protein; Hb, hemoglobin; TfR, plasma transferrin receptor; TIBC, total iron-binding capacity.
All values are in %, unless specified otherwise.
Spearman's correlation coefficient between mother and their infant iron parameters.
The cutoffs for altitude-adjusted anemia, mild, moderate and severe were <12.3 , 10.3–12.2, 7.3–10.2 and <7.3 g/dl among mothers and <11.3, 9.3–11.2, 7.3–9.2 and <7.3 g/dl among infants.
Depleted iron store was defined as ferritin <15 μg/l among mothers and <12 μg/l for infants. Iron deficiency anemia was considered when depleted iron stores were combined with Hb <12.3 g/dl among mothers and <11.3 g/dl among infants.
Iron-deficient erythropoesis was defined TfR values of >4.4 mg/l.
The TfR-log ferritin ratio was calculated by dividing TfR with the log transformation of plasma ferritin, and the TfR-ferritin index was calculated by dividing TfR and ferritin concentrations in microgram unit.
Table 2b. Prevalence of anemia and iron deficiency according to age-specific cutoff values among healthy infants in Bhaktapur, Nepal.
| State | ⩽6 months of age | 7–12 months of age |
|---|---|---|
| 1. Anemia (Hb <10.8 g/dl among ⩽6 months and <11.3 g/dl among 7–12 months infants) | 49% | 72% |
| 2. Iron deficiency (plasma ferritin <20 μg/l among ⩽6 months and <12 μg/l among 7–12 months infants) | 17% | 30% |
| 3. Iron deficiency (plasma ferritin <30 μg/l) | 30% | 71% |
| 4. Iron deficiency anemia (anemia and plasma ferritin <30 μg/l)a | 16% | 54% |
| 5. Iron deficiency anemia (anemia and plasma ferritin <20 μg/l among ⩽6 months and <12 μg/l among 7–12 months infants)b | 9% | 26% |
Abbreviation: Hb, hemoglobin.
Among infants with normal CRP concentrations, IDA was 18% among ⩽ 6 months and 55% among 7–12-month-old infants.
Among infants with normal CRP concentrations, IDA was 18% among ⩽ 6 months and 55% among 7–12-month-old infants.
Interestingly, only 5% of the women and 17% of the infants ⩽6 months in our study were considered to be iron deficient when we assessed plasma ferritin with a cutoff of <15 and <20 μg/l, respectively. About one-third of infants >6 months of age (30%) were iron deficient (plasma ferritin <12 μg/l). When we used a cutoff value of plasma ferritin <30 μg/l, which was often used in the population where infection and inflammation are common, the prevalence of ID was 18%, 30% and 71% among mothers, infants ⩽6 months and infants >6 months of age, respectively. Using combined indicators of hemoglobin and plasma ferritin, the prevalence of IDA was 4% in mothers and 26% among infants >6 months of age. However, enrolling apparently healthy subjects and screened clinically, 5% women and 21% infants had a CRP concentration >5 mg/l, suggesting a possible subclinical acute infection. None of the iron-deficient women were having high CRP concentration (>5 mg/l), but 12% of iron-deficient infants of >6 months of age were having high CRP concentration. After restricting analyses to infants with normal CRP concentration (CRP <5 mg/l), the prevalence of ID and IDA was 19% and 10% among ⩽6 months and 32% and 29% among >6 months, respectively. Slightly more than one-third (36%) of infants ⩽6 months with normal CRP concentrations had plasma ferritin concentrations <30 μg/l, but the prevalence was 76% among >6 months of age. The prevalence of IDA in infants ⩽6 months (ferritin <30 μg/l and Hb <10.8 g/dl) was 16% in the whole group versus 18% in those with normal CRP. Corresponding figures in >6 months (ferritin <30 μg/l and a slightly higher threshold of Hb <11.3 g/dl) were 54% and 55%, respectively.
The mean TIBC concentration was 72.7 and 72.6 μmol/l in mothers and infants, respectively, with increased concentration >83 μmol/l in 15% (n=77) of the women and 18% (n=79) of the infants. Younger women (<25 years of age) were more vulnerable to ID than older women according to analysis of mean TfR concentrations (3.7 versus 3.2 mg/l, P=0.001). Seventy-six percent of infants (n=341) and 16% (n=79) of mothers had IDE based on the cutoff of TfR 4.4 mg/l. The corresponding figures were 14% (n=61) and 2% (n=9), respectively, when we used a cutoff of TfR 8.3 mg/l. Both the ratio of TfR to log ferritin and TfR to ferritin index showed that fewer than 5% of women were iron deficient, which is comparable to the estimates derived using plasma ferritin. The prevalence of ID among infants was 20–76%, depending mainly on which cutoff value was used for ferritin (Tables 2a and b).
Six percent of women had combination of high plasma TfR concentration and anemia, indicating ID at the cellular level. However, 82% of anemic women did not have a low ferritin concentration. Similarly, 63% of anemic women did not have elevated TfR concentrations. Most of the anemic infants had high TfR concentrations (81%) but not low plasma ferritin concentrations (24%).
Regression analysis
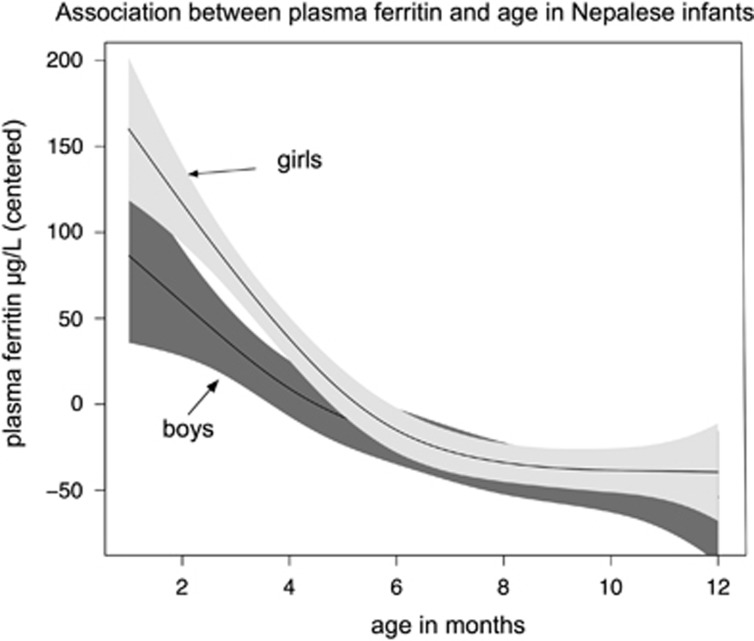
Results from the multiple regression analyses (Table 3) showed that each 1-month increase in infant age was associated with a significant decrease in plasma ferritin concentration of 8.2 μg/l (95% confidence interval: −10.1, −6.4). Mean plasma ferritin concentrations were more than threefold higher among young infants (6 or <6 months) compared with the older infants (88.6 μg/l and 26.8 μg/l, P⩽0.0001). Figure 1 depicts the association between plasma ferritin (restricted among infants with CRP value of <5 mg/l) and child age stratified according to gender. Female infants also had significantly higher Hb, ferritin and lower TIBC and TfR concentrations. Age of infants was also positively associated with TIBC and TfR concentrations. Exclusive breastfeeding for 3 months or more was negatively associated with the hemoglobin concentration. Maternal ferritin concentrations were also associated with infant ferritin concentration but not with other iron parameters.
Table 3. Association of age, gender, breastfeeding, infection, antenatal iron supplementation and maternal anemia with iron parameter of healthy infants in Bhaktapur, Nepala.
| Variables/iron parameters | Hemoglobin (g/dl), R2=0·09 | Ferritin (μg/l), R2=0.26 | TIBC (μmol/l), R2=0.09 | TfR (mg/l), R2=0.08 |
|---|---|---|---|---|
| Age (in months) | −0.01 (−0.06, 0.05), P=0.9 | −8.2 (−10.1,−6.4), P⩽0.0001 | 0.88 (0.37, 1.5), P=0.001 | 0.20 (0.05, 0.34), P=0.008 |
| Female | 0.43 (0.15, 0.72), P=0.003 | 14.0 (4.4, 23.6), P=0.004 | −4.0 (−6.6, −1.3), P=0.003 | −0.82 (−1.6, −0.08), P=0.03 |
| Exclusively breastfeeding for ⩾3 monthsb | −0.32 (−0.61, −0.02), P=0.03 | −8.5 (−18.6, 1.6), P=0.09 | 2.3 (−0.49, 5.0), P=0.1 | 0.40 (−0.37, 1.2), P=0.3 |
| Prenatal iron supplementation for at least 6 months to mother | 0.18 (−0.11, 0.37), P=0.2 | 4.3 (−5.4, 14.1), P=0.2 | −0.20 (2.9, 2.4), P=0.8 | −0.38, (−1.1, 0.38), P=0.3 |
| Maternal ferritin concentration (μg/l) | 0.12 (0.01, 0.23), P=0.04 | −0.01 (−0.01, 0.01), P=0.5 | ||
| Low birth weight (<2.5 kg of birth weight) | −0.41 (−0.83, −0.02), P=0.06 |
Abbreviations: CI, confidence interval; TfR, plasma transferrin receptor; TIBC, total iron-binding capacity.
Values are β-coefficient (95% CI) obtained from multiple linear regression analyses adjusted for variables listed in the table (total numbers ranged from 315 to 349).
Exclusively breastfeeding was defined when child had only breast milk since birth (except medicines).
Figure 1.
Association of plasma ferritin concentration and age of infant according to gender in Bhaktapur, Nepal. Only infants with C-reactive protein value <5 mg/l was included (n=448). The graph was made by using generalized additive models in R. The solid curves depict the estimated mean plasma ferritin concentration; the shaded areas represent the 95% CIs.
Discussion
Our findings from a representative sample of healthy infant and mother pairs from a periurban municipality in Nepal showed that anemia is highly prevalent among infants. Maternal iron stores reflected in plasma ferritin concentration offer protection against infant ID. Yet, even in this setting of greater availability of iron-rich foods than in many rural areas, the prevalence of anemia among infants aged 7–12 months is comparable to the most recent DHS survey in which anemia was observed in 73% and 78% among 9–11- and 6–8-month-old infants, respectively.26
Although we did not collect data on dietary intake of infants (except breast milk intake and introduction of solid/semisolid foods), evidence from other studies from Nepal suggests that dietary diversity is low27 and might also be responsible for IDA in this population. In Bhaktapur, animal-source foods are often not fed to young infants because of a general perception that they will be difficult to digest, which is reflected in their high prevalence of cobalamin deficiency.12 In the last national survey, iron-rich foods (meat, fish, poultry or eggs) were reported only by 13% of infants in the previous 24 h.26 Besides iron and cobalamin, deficiencies of other nutrients such as vitamin A, C, B9, vitamin D and zinc, as well as toxicity of lead, may also cause anemia.28, 29 Effective mucosal absorption of iron also may have been impaired by the presence of inhibiting and/or lack of enhancing factors, as well as intestinal pararasites and an unfavorable microbiome.2, 30
In contrast, the prevalence of maternal anemia in our population (20%) was lower compared with the national figure of 35% for non-pregnant women and is consistent with other estimates of the prevalence of anemia in the Kathmandu valley.26 Interestingly, ID explained only 20–25% of the total anemia burden in the women in this study irrespective of the iron parameters used. The Global Burden of Disease project estimated that the major causes of anemia among women in South Asia are ID, sickle cell anemia, thalassemias and malaria.31 However, in Bhaktapur, malaria, sickle cell disease and thalassemias are uncommon. More work is needed to identify other possible causes of anemia in this population, including chronic enteropathy, as well as other nutritional deficiencies such as vitamin B12 and folate. High coverage of iron supplementation during the last pregnancy,12 lactation amenorrhea, use of Depo-Provera family planning injection32 and the local practice of giving and withholding certain foods during the postpartum period may also contribute to the low prevalence of anemia and ID among women in this population.
Concentrations of Hb and CRP were comparable between male and female and younger and older (>6 months) infants. However, the average concentrations of plasma ferritin were more than threefold higher among the young infants (Figure 1) probably due to temporary redistribution of iron from the red blood cells to the iron stores during the first 6 months of life when fetal hemoglobin is replaced by adult hemoglobin.16, 33 In addition to this physiological process, the impact of inflammation and infectious diseases on plasma ferritin complicates selection of an adequate cutoff level to estimate the prevalence of ID and IDA. This is evident from the finding that IDA (17%), which did not explain more than 25% of the total prevalence of anemia (69%) in infants (Table 2a), was significantly increased when we used a ferritin cutoff of 30 μg/l (Table 2b). Most likely, an important reason for the discrepancy between prevalence of anemia and IDA is the use of erroneous ferritin cutoffs, which do not relate adequately to true ID. This is particularly relevant for infants in the first months of life where 30 μg/l may be is too low with respect to the enlarged iron stores with median ferritin levels above 80–100 μg/l.33 On the other hand, despite enrolling clinically healthy children, 21% had increased CRP. Therefore, we cannot rule out that subclinical, mild infection may have biased the true ferritin cutoff limit with the greatest impact in the older infants where the frequency of higher CRP concentration was higher compared with that in the younger. Plasma ferritin is very sensitive to infection and may even in mild virus infection raise 20–60 μg/l above the preclinical baseline.34
The plasma concentration of TfR is increased by ID, but in contrast to ferritin, it is not seriously affected by infection.19 Among the infants, there is good consistency between IDA diagnosed by combining anemia with either increased TfR (i.e., IDE) or with ferritin <12 μg/l, being 20% and 17%, respectively. As discussed above, a ferritin cutoff of 12 μg/l is obviously too low; thus, the prevalence of IDA is likely to be >17%. This point is strongly supported by the large difference between IDE (76%) and depleted iron stores (20%). Increased TIBC, which is a measure of long-standing reduced iron absorption, was increased in 18% of infants, which corresponded well with our IDA results. Because of lower iron status and faster growth rate, low birth weight infants are susceptible for developing ID,35, 36 which is also supported by our data as they had substantially lower Hb concentration compared with normal weight babies. Our finding of better iron status (reflecting higher Hb and ferritin and lower TfR and TIBC) among female infants is also consistent with previous findings.37, 38, 39 This is perhaps due to differences in iron requirements and metabolism40 or different sized iron stores in early infancy.41 Our findings of lower Hb and ferritin concentrations among infants who practiced more than 3 months of exclusive breastfeeding are also in accordance with findings from an earlier study42 and are a reflection of the low iron content of breast milk,43 but in discordance with others.44, 45 We used a cutoff of 3 months for exclusive breastfeeding instead of the standard WHO recommendation of 6 months. This is because about 50% of our enrolled infants were <6 months of age, and early introduction of complementary foods or drinks is common in this population. Caution is required while interpreting these finding as we used a strict definition of exclusive breastfeeding based on any introduction of food/drink since birth and because feeding patterns are constantly changing, particularly among young infants.
ID is considered the main underlying cause of anemia in developing countries. Our study clearly illustrates the well-known problem of using a common, single cutoff limit to get a true estimate of the prevalence of IDA and distinguish it from other causes that include deficiencies in other nutrients, inflammation, hematological abnormalities and parasitic infestation.15, 46, 47 Since the finding of an alarmingly high anemia prevalence, particularly among pregnant women and infants in a micronutrient survey conducted in 1998,48 the Government of Nepal, in collaboration with different agencies including the WHO, has endorsed several strategies to reduce anemia. The strategy includes the following: daily supplementation with 60 mg elemental iron sulfate for 180 days during pregnancy and the postpartum period irrespective of baseline iron status; single deworming drugs administered during the second trimester of pregnancy; insecticide-treated bed nets in areas where malaria is a common problem;49 and delayed cord clamping (after 2–3 min of birth).50, 51 Although we lack data on trends, our findings of a relatively low contribution of iron to overall anemia burden among women, high adherence to iron supplementation during pregnancy and the positive association between maternal ferritin with infant plasma ferritin suggest that these strategies have been effective, at least in the periurban area of Bhaktapur. Nevertheless, as shown in our study, combining tests associated with different stages of negative iron balance makes it possible to discriminate between ID, IDE and IDA. This may be a valuable approach to target more precisely subgroups with different need of iron in a population at risk.
Conclusion
The prevalence of ID and anemia among infants was high, particularly among male infants born to mothers with low iron stores. The contribution of ID to the total anemia prevalence, particularly among mothers, was low, suggesting that anemia may have other causes in this community, which require further exploration. The coverage of iron supplementation among pregnant and lactating women in this population was high, and the observation that high maternal plasma ferritin was associated with lower risk of anemia among infants suggests that increased coverage of this program in rural Nepal and other parts of South Asia could help reduce anemia burden.
Acknowledgments
We thank participants children and family of Bhaktapur and all the staff at Child Health Research Project, Department of Child Health, Tribhuvan University, Kathmandu, Nepal. We thank Shyam Dhaubhadel, founder of Siddhi Memorial Hospital in Bhaktapur, and the staff for their cooperation. The study was funded by grants from the GC Rieber foundation, the Research Council of Norway (Project No. 172226), South-Eastern Norway Regional Health Authority (Grant No. 2012090), a postdoctoral grant through University of Bergen and the Feed the Future Food Security Innovation Lab: Collaborative Research on Nutrition, which is funded by the United States Agency for International Development.
Author contributions
TAS, RKC, SH and PSS designed the study; RKC, MU, SH, ALT, WF, TAS and LL conducted the research and developed the first draft; RJU analyzed the blood samples, interpreted the results and contributed to manuscript writing; RKC had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
References
- WHO. Results and discussion. In: B de Benoist, E Mclean, I Egli, M Cogswell (eds). Worldwide Prevalence of Anemia 1993–2005: WHO Global Database on Anemia. WHO: Geneva, Switzerland, 2008.
- Thankachan P, Muthayya S, Walczyk T, Kurpad AV, Hurrell RF. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr Bull 2007; 28: 328–336. [DOI] [PubMed] [Google Scholar]
- Tran TD, Tran T, Simpson JA, Tran HT, Nguyen TT, Hanieh S et al. Infant motor development in rural Vietnam and intrauterine exposures to anaemia, iron deficiency and common mental disorders: a prospective community-based study. BMC Pregnancy Childb 2014; 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Hoorn SV, Lopez AD, Danaei G, Rodgers A, Mathers CD et al Comparative quantification of mortality and burden of disease attributable to selected risk factors. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL (eds). Global Burden of Disease and Risk Factors. World Bank: Washington, DC, USA, 2006. [PubMed] [Google Scholar]
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000; 71: 1280S–1284SS. [DOI] [PubMed] [Google Scholar]
- Cohen JH, Haas JD. The comparison of mixed distribution analysis with a three-criteria model as a method for estimating the prevalence of iron deficiency anaemia in Costa Rican children aged 12–23 months. Int J Epidemiol 1999; 28: 82–89. [DOI] [PubMed] [Google Scholar]
- Schneider JM, Fujii ML, Lamp CL, Lonnerdal B, Dewey KG, Zidenberg-Cherr S. Anemia, iron deficiency, and iron deficiency anemia in 12–36-mo-old children from low-income families. Am J Clin Nutr 2005; 82: 1269–1275. [DOI] [PubMed] [Google Scholar]
- WHO.. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System. World Health organization: Geneva, Switzerland. (WHO/NHD/MNM/11.2) 2011, available at http://www.who.int/vmnis/indicators/serum_ferritin.pdf. (last accessed date 19 May 2012. [Google Scholar]
- Dreyfuss ML, Stoltzfus RJ, Shrestha JB, Pradhan EK, LeClerq SC, Khatry SK et al. Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr 2000; 130: 2527–2536. [DOI] [PubMed] [Google Scholar]
- Pokhrel RK, Maharjan MR, Mathema P, Harvey PWJ. A Case Study of the Intensification of Maternal and Neonatal Micronutrient Program. The USAID Micronutrient and Child Blindness Project: Washington, DC, USA, 2011. [Google Scholar]
- CBS. National Population and Housing Census. Central Bureau of Statistics (CBS), Government of Nepal, National Planning Commission Secretariat: Kathmandu, Nepal, 2011. [Google Scholar]
- Henjum S, Manger M, Skeie E, Ulak M, Thorne-Lyman AL, Chandyo R et al. Iron deficiency is uncommon among lactating women in urban Nepal, despite a high risk of inadequate dietary iron intake. Br J Nutr 2014; 112: 1–10. [DOI] [PubMed] [Google Scholar]
- Valentiner-Branth P, Shrestha PS, Chandyo RK, Mathisen M, Basnet S, Bhandari N et al. A randomized controlled trial of the effect of zinc as adjuvant therapy in children 2–35 mo of age with severe or nonsevere pneumonia in Bhaktapur, Nepal. Am J Clin Nutr 2010; 91: 1667–1674. [DOI] [PubMed] [Google Scholar]
- Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol 2011; 90: 1247–1253. [DOI] [PubMed] [Google Scholar]
- WHO. Iron Deficiency Anemia. Assessment, Prevention and Control. A Guide for Programme Manager. World Health Organization: Geneva, Switzerland, 2001; contract no.: WHO/NHD/01.3.
- Domellof M, Dewey KG, Lonnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr 2002; 132: 3680–3686. [DOI] [PubMed] [Google Scholar]
- CDC. Centers for Disease Control (CDC). CDC Criteria for anemia in children and child bearing-aged women. Morb Mortality Wkly Rep 1989; 38: 400–404. [PubMed] [Google Scholar]
- Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Soluble transferrin receptor: longitudinal assessment from pregnancy to postlactation. Obstet Gynecol 2002; 99: 260–266. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Skikne BS, Simpson KM, Baynes RD, Cook JD. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med 1992; 119: 385–390. [PubMed] [Google Scholar]
- Kolbe-Busch S, Lotz J, Hafner G, Blanckaert NJ, Claeys G, Togni G et al. Multicenter evaluation of a fully mechanized soluble transferrin receptor assay on the Hitachi and cobas integra analyzers. the determination of reference ranges. Clin Chem Lab Med 2002; 40: 529–536. [DOI] [PubMed] [Google Scholar]
- Angeles Vazquez Lopez M, Molinos FL, Carmona ML, Morales AC, Munoz Vico FJ, Munoz JL et al. Serum transferrin receptor in children: usefulness for determinating the nature of anemia in infection. J Pediatr Hematol/Oncol 2006; 28: 809–815. [DOI] [PubMed] [Google Scholar]
- Widen EM, Bentley ME, Kayira D, Chasela CS, Daza EJ, Kacheche ZK et al. Changes in soluble transferrin receptor and hemoglobin concentrations in Malawian mothers are associated with those values in their exclusively breastfed, HIV-exposed infants. J Nutr 2014; 144: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO Anthro 2005, Beta Version, Feb 17th, 2006: Software for Assessing Growth and Development of the World's Children. World Health Organization: Geneva, Switzerland, 2006, available at http://www.who.int/childgrowth/software/en/. (last accessed date 9 July 2012. [Google Scholar]
- Labbok MH, Coffin CJ. A call for consistency in definition of breastfeeding behaviors. Soc Sci Med 1997; 44: 1931–1932. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley: New York, NY, USA, 2000. [Google Scholar]
- NDHS.. Ministry of Health and Population (Nepal), New ERA and ICF International Inc. 2012 Macro. Calverton, Maryland, USA. In Ministry of Health and Population (eds) Nepal Demographic Health Survey—2011. NDHS: Kathmandu, Nepal, 2011. [Google Scholar]
- Joshi N, Agho KE, Dibley MJ, Senarath U, Tiwari K. Determinants of inappropriate complementary feeding practices in young children in Nepal: secondary data analysis of Demographic and Health Survey 2006. Maternal & child nutrition. 2012 8: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah F, Kazi TG, Afridi HI, Baig JA, Khan S, Kolachi NF et al. Environmental exposure of lead and iron deficit anemia in children age ranged 1–5 years: a cross sectional study. Sci Total Environ 2010; 408: 5325–5330. [DOI] [PubMed] [Google Scholar]
- Yoon JW, Kim SW, Yoo EG, Kim MK. Prevalence and risk factors for vitamin D deficiency in children with iron deficiency anemia. Korean J Pediatr 2012; 55: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015; 64: 731–742. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandyo RK, Strand TA, Ulvik RJ, Adhikari RK, Ulak M, Dixit H et al. Prevalence of iron deficiency and anemia among healthy women of reproductive age in Bhaktapur, Nepal. Eur J Clin Nutr 2007; 61: 262–269. [DOI] [PubMed] [Google Scholar]
- Siimes MA, Addiego JE Jr, Dallman PR. Ferritin in serum: diagnosis of iron deficiency and iron overload in infants and children. Blood 1974; 43: 581–590. [PubMed] [Google Scholar]
- Eskeland B, Baerheim A, Ulvik R, Hunskaar S. Influence of mild infections on iron status parameters in women of reproductive age. Scand J Prim Health Care 2002; 20: 50–56. [DOI] [PubMed] [Google Scholar]
- Dallman PR, Siimes MA, Stekel A. Iron deficiency in infancy and childhood. Am J Clin Nutr 1980; 33: 86–118. [DOI] [PubMed] [Google Scholar]
- Eneroth H, Persson LA, El Arifeen S, Ekstrom EC. Infant anaemia is associated with infection, low birthweight and iron deficiency in rural Bangladesh. Acta Paediatr 2011; 100: 220–225. [DOI] [PubMed] [Google Scholar]
- Hay G, Refsum H, Whitelaw A, Melbye EL, Haug E, Borch-Iohnsen B. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am J Clin Nutr 2007; 86: 64–73. [DOI] [PubMed] [Google Scholar]
- Tamura T, Hou J, Goldenberg RL, Johnston KE, Cliver SP. Gender difference in cord serum ferritin concentrations. Biol Neonate 1999; 75: 343–349. [DOI] [PubMed] [Google Scholar]
- Agho KE, Dibley MJ, D'Este C, Gibberd R. Factors associated with haemoglobin concentration among Timor-Leste children aged 6–59 months. J Health Popul Nutr 2008; 26: 200–209. [PMC free article] [PubMed] [Google Scholar]
- Domellof M, Lonnerdal B, Dewey KG, Cohen RJ, Rivera LL, Hernell O. Sex differences in iron status during infancy. Pediatrics 2002; 110: 545–552. [DOI] [PubMed] [Google Scholar]
- Ziegler EE, Nelson SE, Jeter JM. Iron stores of breastfed infants during the first year of life. Nutrients 2014; 6: 2023–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and risk for iron deficiency in U.S. infants. Breastfeeding Med 2007; 2: 63–73. [DOI] [PubMed] [Google Scholar]
- Dewey KG. The challenge of meeting nutrient needs of infants and young children during the period of complementary feeding: an evolutionary perspective. J Nutr 2013; 143: 2050–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AS, Silva Rde C, Fiaccone RL, Pinto Ede J, Assis AM. Effect of length of exclusive breastfeeding and mixed feeding on hemoglobin levels in the first six months of life: a follow-up study. Cad Saude Publica 2010; 26: 409–417. [DOI] [PubMed] [Google Scholar]
- Sawasdivorn S, Taeviriyakul S. Are infants exclusively breastfed up to 6 months of age at risk of anemia? J Med Assoc Thailand 2011; 94: S178–S182. [PubMed] [Google Scholar]
- Jain S, Narayan S, Chandra J, Sharma S, Jain S, Malhan P. Evaluation of serum transferrin receptor and sTfR ferritin indices in diagnosing and differentiating iron deficiency anemia from anemia of chronic disease. Indian J Pediatr 2010; 77: 179–183. [DOI] [PubMed] [Google Scholar]
- Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet 2011; 378: 2123–2135. [DOI] [PubMed] [Google Scholar]
- NMSS. Nepal Micronutrient Status Survey (NMSS),1998. Ministry of Health, Child Health Division, HMG/N, New Era, Micronutrient Initiative, UNICEF Nepal and WHO: Kathmandu, Nepal. , 2001. [Google Scholar]
- MOHP. National Nutrition Policy and Strategy. Nutrition Section, Child Health Division, Ministry of Health and Population (MOHP), Government of Nepal: Kathmandu, Nepal, 2004. [Google Scholar]
- Mathew JL. Timing of umbilical cord clamping in term and preterm deliveries and infant and maternal outcomes: a systematic review of randomized controlled trials. Indian Pediatr 2011; 48: 123–129. [DOI] [PubMed] [Google Scholar]
- Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet 2006; 367: 1997–2004. [DOI] [PubMed] [Google Scholar]