Abstract
Background/Objectives:
Children aged 6 months to 5 years completing treatment for severe acute malnutrition (SAM) in a Médecins Sans Frontières Community Management of Acute Malnutrition (CMAM) program in Bihar, India, showed high cure rates; however, the program suffered default rates of 38%. This report describes the nutritional status of 1956 children followed up between 3 and 18 months after exiting the program.
Subjects/Methods:
All children aged 6–59 months discharged as cured with mid-upper arm circumference (MUAC) ⩾120 mm or who defaulted from the program with MUAC <115 mm were traced at 3, 6, 9, 12 and 18 months (±10 days) before three exit reference dates: first at the end of the food insecure period, second after the 2-month food security and third after the 4-month food security.
Results:
Overall, 68.7% (n=692) of defaulters and 76.2% (n=1264) of children discharged as cured were traced. Combined rates of non-recovery in children who defaulted with MUAC <115 mm were 41%, 30.1%, 9.9%, 6.1% and 3.6% at 3, 6, 9, 12 and 18 months following exit, respectively. Combined rates of relapse among cured cases (MUAC ⩾120 mm) were 9.1%, 2.9%, 2.1%, 2.8% and 0% at 3, 6, 9, 12 and 18 months following discharge, respectively. Prevalence of undernutrition increased substantially for both groups traced during low food security periods. Odds of death were much higher for children defaulting with MUAC <110 mm when compared with children discharged as cured, who shared the same mortality risk as those defaulting with MUAC 110–<115 mm.
Conclusions:
Seasonal food security predicted short-term nutritional status after exit, with relapse rates and non-recovery from SAM much higher during food insecurity. Mortality outcomes suggest that a MUAC of 110 mm may be considered an appropriate admission point for SAM treatment programs in this context.
Introduction
According to the third Indian National Family Health Survey-3 conducted in 2005–2006, 19.8% of Indian children aged 0–35 months were wasted, whereas 6.4% suffered from severe acute malnutrition (SAM) in India.1 In response to a household-based survey conducted in Darbhanga district after widespread flooding in Bihar, the prevalence of wasting and SAM in children between 6 months and 5 years was estimated to be 19.4 and 4.8%, respectively.2 In February 2009, Médecins Sans Frontières (MSF) initiated a Community-based Management of Acute Malnutrition (CMAM) program in Darbhanga district, with the objective to identify and treat SAM in children aged between 6 months and 5 years in Biraul block.
A typical CMAM program consists of fixed or ambulatory sites close to the community where children with ‘uncomplicated' SAM, who form the majority of patients, can be treated on a once-weekly basis, in addition to an inpatient facility where the minority of children suffering from SAM with associated serious medical complications are admitted until stable, when they are transferred into the community component to complete treatment.
Between February 2009 and September 2011, the MSF supported program treated 8274 children aged 6–59 months with SAM, with low mortality (0.9%), acceptable weight gain (4.9 g/kg/day) and a high cure rate in those children who completed the treatment (88.4%).3 However, consistent with outcomes from other SAM programs in India,4, 5 the program suffered from a high default rate (38%) limiting interpretation of the overall program success. This concern was compounded following the results of an in-program Semi-Quantitative Evaluation Of Access And Coverage (SQUEAC) survey, suggesting that substantial numbers of children relapsed after being cured.6
To monitor the overall impact and effectiveness of the program, MSF conducted a series of long-term follow-up surveys of children exiting the CMAM program. This observational cohort study describes the long-term outcomes of 1956 children followed up between 3 and 18 months following exit from the MSF supported CMAM program.
Materials and methods
Program description summary
The CMAM program reflected the worldwide format, using mid-upper arm circumference (MUAC) <115 mm and/or bilateral edema as admission criteria.7 One dedicated inpatient facility (the stabilization center) was established for children with severe illness, whereas five ambulatory therapeutic feeding centers were established in the community where children were provided treatment with World Health Organization (WHO)-standard8 pre-packaged F100-equivalent (per kcal) lipid-based ready-to-use therapeutic paste, produced in India (Eezeepaste, Compact, Gurgaon, India) on an outpatient basis.
Classification of exits
In order to be discharged as cured, children were required to meet the discharge criteria (MUAC ⩾120 mm with no edema for 1 week with good clinical condition and good appetite) on two consecutive visits. For the purposes of the study, defaulters were defined as children with MUAC <115 mm failing to attend the ambulatory therapeutic feeding center for 2 consecutive weeks or children who left the stabilization center and did not return for 2 consecutive days. Caregivers refusing for their children to be admitted into the stabilization center or leaving the stabilization center prematurely but agreeing to be seen in the ambulatory therapeutic feeding center were not considered defaulters.
Follow-up survey methodology
Considering the well-recognized effect of seasonality on food security and incidence of SAM,9 three periods of differing food securities were identified to survey the outcomes of children exiting from the program to ensure that a year-round picture emerged. Therefore, three reference dates were selected that would reflect any potentiating effect of the existing food security situation on children discharged 3, 6, 9, 12 and 18 months before that date. The food secure period lasts for 5 months in Darbhanga district (Supplementary Figure 1).
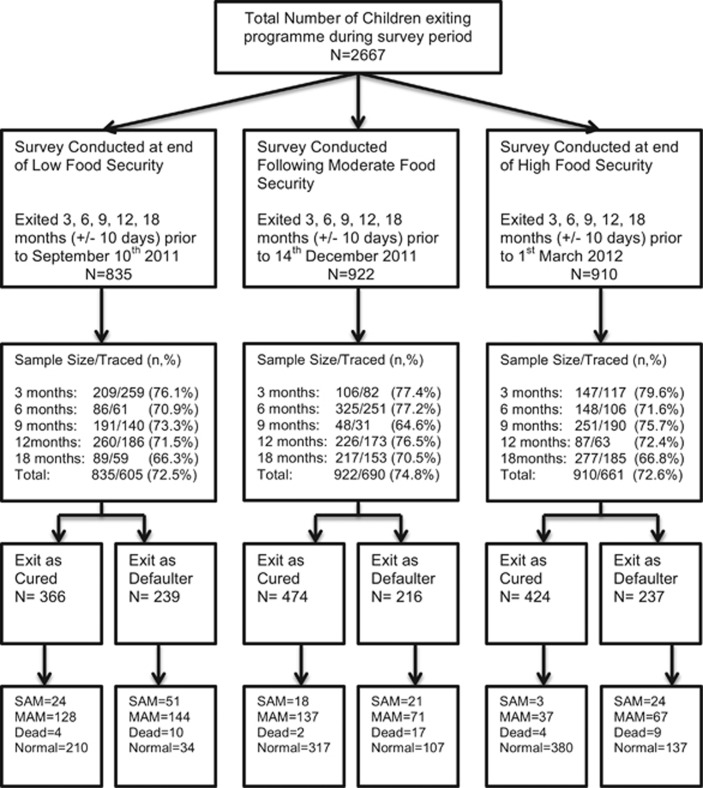
Therefore, the first reference date was at the end of the food insecure period (hereafter known as low food security), the second after 2 months of food security (hereafter known as moderate food security) and the third at the end of the food secure period (hereafter known as high food security). All children exiting the program within a range of ±10 days of each of these time points were then traced (Figure 1).
Figure 1.
Flowchart of analysis.
For example, the first reference date selected was the 10 September 2011, which at the end of the food insecure period represented the greatest point of ‘low food security'. As such, children exiting the program on the 10 June ±10 days (therefore children exiting the program between 1 and 20 June 2011) comprised the cohort to be traced at 3 months before this date. With the annual food insecure period lasting from April to September, this cohort would therefore reflect the nutritional status of children traced after experiencing 3 months of ‘low food security' following exit from the program. This framework would then be repeated for children exiting 6, 9, 12 and 18 months before this reference date.
Similarly, the third reference date selected was the 1 March 2012, and children exiting the program on the 1 December 2011 ±10 days (therefore children exiting the program between 21 November and 11 December) comprised the cohort to be traced at 3 months before this date. With the annual highest food security period lasting from November to March, this cohort would therefore reflect the nutritional status of children traced after have experienced 3 months of ‘high food security' following exit from the program. This framework would then be repeated for children exiting 6, 9, 12 and 18 months before this reference date.
Survey teams comprised a mixture of staff with extensive experience in the CMAM program and new staff. All surveyors underwent 3 days of training in anthropometric measurements by an MSF nutritionist and were pre- and post-tested on their ability to consistently and accurately use the measurement equipment. Each team comprised a newly trained surveyor and an experienced member of staff to ensure that counseling of caregivers in cases where children were found to be SAM was appropriate and effective. Each team carried a calibrated salter scale (precision to 100 g), locally produced height boards (precision 0.1cm) and MSF produced standard MUAC tapes. The Salter scales were calibrated before the surveys as per manufacturer guidelines.
Data handling and statistical analysis
All data were entered into an Excel (Microsoft, 2010) database; double data-entry was not done; however, audit of data transfer quality and database checks for inconsistencies were performed following each survey by an epidemiologist referring to source documents. World Health Organization Anthro software (Geneva, Switzerland) was used to calculate weight-for-height Z-scores. Analysis of data was conducted using SPSS version 19 statistical software (IBM, Chicago, IL, USA). A multivariate logistic regression model was also developed to determine risk factors independently associated with relapse, non-recovery and mortality among cured and defaulter children found to be significant (P<0.05) on bivariate analysis. Variables that could be justified a priori were also included and added step wise to the model in the multivariate analysis.
Ethics statement
This analysis met the Médecins Sans Frontières Institutional Ethics Review Committee criteria for a study involving the analysis of routinely collected program evaluation data. The program and survey were conducted under a memorandum of understanding with the district authorities and thereafter with consent of the Bihar State Health Society, the usual procedure for NGOs operating in this context. The purpose of the surveys was for monitoring and evaluation of program effectiveness and is a requirement under the agreement with the State Health Society. All electronic data were analyzed anonymously.
Results
A total of 1956 children were traced during the three surveys, which constituted 73.3% of the total sample. The proportions of children lost to follow-up remained similar across the three surveys and across stratification by age boundary and sex (Supplementary Table 1).
Overall, 76.2% (n=1264/1659) of children discharged as cured and 68.7% (n=692/1008) of children who exited the program as defaulter were traced. There were no significant differences in admission characteristics (age distribution, sex, severity of undernutrition and distance from facility) of children successfully traced compared with those who were not found during the surveys.
Long-term outcomes of children exiting as cured (MUAC ⩾120mm)
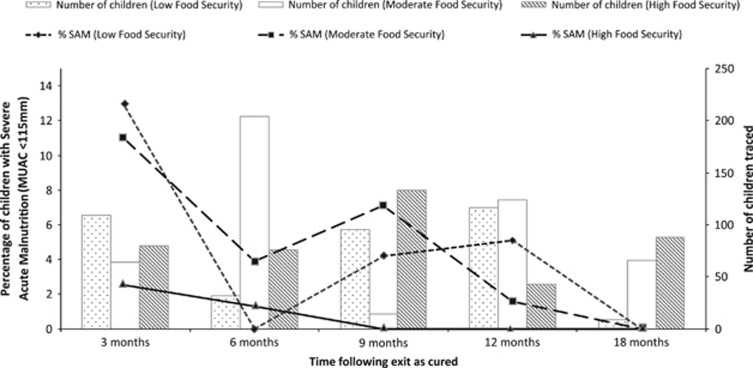
The combined rates of relapse into SAM across the surveys among children exiting as cured were 9.1% at 3 months, 2.9% at 6 months, 2.1% at 9 months, 2.8% at 12 months and 0% at 18 months following discharge. When stratified by food security at the time of follow-up survey, there was a marked difference between relapse rates at 3 months following discharge, where children exiting into 3 months of low food security exhibited relapse rates of 12.8%, compared with 10.9% and 2.5% of children exiting into moderate food security and 3 months of high food security, respectively (Figure 2). A similar pattern was seen when looking at overall wasting (Supplementary Figure 2).
Figure 2.
Prevalence of SAM (MUAC <115 mm) among children exiting as cured stratified by food security at the time of follow-up.
Of children exiting as cured, not using Anganwadi services, lower standard of living index, less time outside the program and being surveyed during low food security were all significant risk factors for relapse into SAM (MUAC <115 mm) at the time of follow-up (Table 1). There was no significant difference between the long-term outcomes of children discharged as cured with MUAC 120–⩽125 mm (n=1043) or with MUAC >125 mm (n=211), odds ratio 1.3; 95% confidence interval (CI) (0.6, 3.2), P=0.524.
Table 1. Risk factors for children exiting as cured relapsing into SAM (MUAC <115 mm) at the time of follow-up.
| Risk factor | Found to be SAM at time of survey N (%) | Not found to be SAM at time of survey, N (%) | OR (95% CI) | P-value | aOR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age at discharge | ||||||
| 6–<12 | 15 (5.3) | 269 (94.7) | 1.7 (0.7–4.0) | 0.241 | ||
| 12–<24 | 22 (3.1) | 699 (96.9) | 1.0 (0.4, 2.2) | 0.899 | ||
| ⩾24 | 8 (3.2) | 241 (96.8) | 1 | |||
| Sex | ||||||
| Female | 26 (3.3) | 752 (96.7) | 0.8 (0.5,1.5) | 0.548 | ||
| Male | 19 (4.0) | 457 (96.0) | ||||
| MUAC at time of discharge | ||||||
| 120–<125 | 39 (3.7) | 1004 (96.3) | 1.3 (0.6, 3.2) | 0.524 | ||
| ⩾125 | 6 (2.8) | 205 (97.2) | ||||
| WHZ | ||||||
| <–3 | 3 (10.7) | 25 (89.3) | 3.4 (0.6, 11.8) | 0.08 | 2.1 (0.5, 9.4) | 0.313 |
| ⩾–3 | 42 (3.4) | 1184 (96.6) | — | — | ||
| HAZ | ||||||
| <–3 | 35 (4.0) | 850 (96) | 1.5 (0.7, 3.0) | 0.280 | ||
| ⩾–3 | 10 (2.7) | 359 (97.3) | — | — | ||
| Use of Anganwadi services | ||||||
| No | 26 (6.0) | 404 (94.0) | 2.8 (1.5, 5.2) | 0.0006 | 2.0 (1.04, 3.9) | 0.037 |
| Yes | 18 (2.2) | 783 (97.8) | — | — | ||
| Episode of hospitalization after exit | ||||||
| Yes | 4(9.1) | 40 (90.9) | 2.8 (0.7, 8.3) | 0.07 | 2.5 (0.8, 8.4) | 0.128 |
| No | 41(3.4) | 1153 (96.6) | — | — | ||
| Standard of living index score | ||||||
| <8 | 20 (5.3) | 358 (94.7) | 4.4 (1.5,13.0) | 0.003 | 6.0 (1.7, 20.8) | 0.005 |
| 8–13 | 21 (3.8) | 533 (96.2) | 3.1 (1.03, 12.5) | 0.034 | 5.4 (1.6, 18.5) | 0.008 |
| >13 | 4 (1.3) | 315 (98.7) | — | — | ||
| Below poverty line card holder | ||||||
| Yes | 34 (4.0) | 821 (96.0) | 1.4 (0.7, 2.9) | 0.297 | ||
| No | 11 (2.8) | 383 (97.2) | ||||
| Time between exit from program and survey date | ||||||
| 3 Months | 23 (9.1) | 230 (90.9) | 3.5 (1.5, 7.9) | 0.002 | 4.2 (1.8, 9.9) | 0.001 |
| 6 Months | 9 (2.9) | 303 (97.1) | 1.02 (0.4, 2.7) | 0.960 | 1.4 (0.5, 3.8) | 0.554 |
| 9 Months | 5 (2.1) | 237 (97.9) | 0.7 (0.2, 2.2) | 0.580 | 1.2 (0.4, 3.9) | 0.767 |
| 12 Months | 8 (2.8) | 276 (97.2) | — | — | ||
| 18 Months | 0 (0) | 163 (100) | 0 (0, 1.008) | 0.03 | 0 (–) | 0.995 |
| Length of stay in program | ||||||
| ⩽7 Weeks | 21 (4.9) | 410 (95.1) | 1.7 (0.9, 3.2) | 0.08 | 1.6 (0.8, 3.1) | 0.157 |
| >7 Weeks | 24 (2.9) | 799 (97.1) | — | — | ||
| Admission in stabilization center | ||||||
| Yes | 4 (4.1) | 94 (95.9) | 1.1 (0.4, 3.3) | 0.784 | ||
| No | 41 (3.5) | 1115 (96.5) | — | — | ||
| Number of children <5 years in the household | ||||||
| ⩽2 | 12 (3.2) | 361 (96.8) | 0.9 (0.4, 1.7) | 0.628 | ||
| >2 | 33 (3.8) | 841 (96.2) | ||||
| FS at time of survey | ||||||
| Low FS | 24 (6.6) | 338 (93.4) | 9.9 (2.9, 51.5) | <0.001 | 5.4 (1.5, 19.3) | 0.009 |
| Moderate FS | 18 (3.8) | 454 (96.2) | 5.5 (1.6, 29.4) | 0.003 | 4.6 (1.2, 16.8) | 0.022 |
| High FS | 3 (0.7) | 417 (99.3) | — | — | ||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; FS, food security; HAZ, height for age Z-score; MUAC, mid-upper arm circumference; OR, odds ratio; SAM, severe acute malnutrition; WHZ, weight for height Z-score. Bolding refers to values that are statistically significant (P value<0.05).
A total of 10 children (0.8%) of those exiting as cured were found to have died at follow-up. Of the 10, 3 were reported to have died >3 months after discharge. No risk factors for mortality at follow-up were found in bivariate analysis.
Long-term outcomes of children exiting as default (MUAC <115mm)
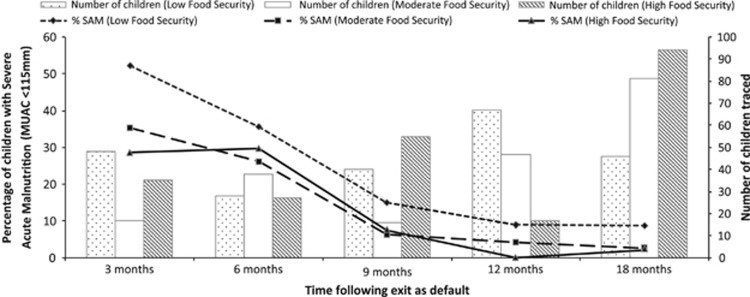
The combined prevalence of SAM in children exiting with MUAC <115 mm at the time of default across the surveys was 41.0% at 3 months, 30.1% at 6 months, 9.9% at 9 months, 6.1% at 12 months and 3.6% at 18 months following exit. When stratified by food security at the time of follow-up survey, there was a much more substantial difference between prevalence of SAM at 3 months following exit, where children experiencing 3 months of low food security following default showed a 52.1% prevalence of SAM, compared with 35.3% and 28.6% of children defaulting into moderate food security and 4 months of high food security, respectively (Figure 3). A similar pattern was seen when looking at overall wasting (Supplementary Figure 3).
Figure 3.
Prevalence of SAM (MUAC <115 mm) among children exiting as default (with MUAC <115 mm) stratified by food security at the time of follow-up.
Of children classified as having defaulted from the program, lower MUAC at the time of default, not using Anganwadi services, having experienced an episode of hospitalization after discharge, less time outside the program and having >2 children under the age of 5 years in the household were all significant risk factors for being found to have a MUAC <115 mm at the time of follow-up. Although showing a strong association on bivariate analysis, the food security at the time of survey did not retain significance on multivariate analysis. The adjusted odds ratio of having MUAC <115 mm at the time of follow-up for a child defaulting from the program with a MUAC of <100, 100–<105 and 105–<110 mm at the time of default was 8.0 (95% CI 3.1–20.5; P<0.001), 2.3 (95% CI 1.03, 5.0; P=0.042) and 2.0 (95% CI 1.01, 4.0; P=0.046) times higher, respectively, compared with that of a child exiting with MUAC of between 110 and <115 mm (Table 2).
Table 2. Risk factors for children exiting as default having MUAC <115 mm at the time of follow-up.
| Risk factor | Found to be SAM at time of survey N (%) | Not found to be SAM at time of survey N (%) | OR (95% CI) | P-value | aOR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age at exit as default | ||||||
| 6–<12 | 50 (22.8) | 169 (77.2) | 1.9 (0.9, 3.9) | 0.095 | ||
| 12–<24 | 36 (9.9) | 328 (90.1) | 0.7 (0.3, 1.5) | 0.333 | ||
| ⩾24 | 10 (13.7) | 63 (86.3) | — | — | ||
| Sex | ||||||
| Female | 70 (15.1) | 393 (84.9) | 1.1 (0.7, 1.9) | 0.587 | ||
| Male | 26 (13.5) | 167 (86.5) | — | — | ||
| MUAC at time of default | ||||||
| <100 | 18 (38.3) | 29 (61.7) | 5.1 (2.6, 10.0) | <0.001 | 8.0 (3.1, 20.5) | <0.001 |
| 100–<105 | 15 (16.3) | 77 (83.7) | 1.6 (0.9, 3.1) | 0.145 | 2.3 (1.03, 5.0) | 0.042 |
| 105–<110 | 22 (15.9) | 116 (84.1) | 1.6 (0.9, 2.7) | 0.115 | 2.0 (1.01, 4.0) | 0.046 |
| 110–<115 | 41 (10.8) | 338 (89.2) | — | — | ||
| WHZ at time of default | ||||||
| <–3 | 56 (19.0) | 239 (81.0) | 1.9 (1.2, 2.9) | 0.004 | 1.0 (0.6,1.8) | 0.954 |
| ⩾–3 | 40 (11.1) | 321 (88.9) | — | — | ||
| HAZ at time of default | ||||||
| <–3 | 70 (13.9) | 435 (86.1) | 0.8 (0.5, 1.3) | 0.306 | ||
| ⩾–3 | 26 (17.2) | 125 (82.8) | — | — | ||
| Use of Anganwadi services | ||||||
| No | 51 (23.2) | 169 (76.8) | 2.6 (1.7, 4.0) | <0.001 | 1.9 (1.1, 3.5) | 0.030 |
| Yes | 44 (10.5) | 376 (89.5) | — | — | ||
| Episode of hospitalization after exit as default | ||||||
| Yes | 13 (37.1) | 22 (62.9) | 3.9 (1.9, 8.0) | <0.001 | 3.3 (1.3, 8.1) | 0.01 |
| No | 81 (13.2) | 532 (86.8) | — | — | ||
| Standard of living index score | ||||||
| <8 | 39 (18.1) | 176 (81.9) | 1.6 (0.9, 2.8) | 0.138 | ||
| 8–13 | 37 (13.3) | 242 (86.7) | 1.1 (0.6, 1.9) | 0.819 | ||
| >13 | 20 (12.5) | 140 (87.5) | — | — | ||
| Below poverty line card holder | ||||||
| Yes | 77 (16.4) | 392 (83.6) | 1.7 (1.01, 2.9) | 0.045 | ||
| No | 19 (10.3) | 166 (89.7) | — | — | ||
| Time between exit from program and survey date | ||||||
| 3 Months | 41 (41.0) | 59 (59.0) | 18.5 (8.2, 41.6) | <0.001 | 10.3 (4.1, 25.7) | <0.001 |
| 6 Months | 28 (30.1) | 65 (69.9) | 11.5 (5.0, 26.4) | <0.001 | 7.9 (3.1, 20.4) | <0.001 |
| 9 Months | 11 (9.9) | 100 (90.1) | 2.9 (1.1, 7.5) | 0.02 | 1.3 (0.5, 3.8) | 0.627 |
| 12 Months | 8 (6.1) | 123 (93.9) | 1.7 (0.6, 4.7) | 0.279 | 0.6 (0.2, 1.8) | 0.374 |
| 18 Months | 8 (3.6) | 213 (96.4) | — | — | ||
| Length of stay in program | ||||||
| ⩽7 Weeks | 79 (16.3) | 406 (83.7) | 1.8 (1.01, 3.1) | 0.044 | 1.3 (0.7, 2.5) | 0.449 |
| >7 Weeks | 17 (9.9) | 154 (90.1) | — | — | ||
| Admission in stabilization center | ||||||
| Yes | 13 (21.0) | 49 (79) | 1.6 (0.9, 3.1) | 0.138 | ||
| No | 83 (14.0) | 511 (86.0) | ||||
| Number of children <5 years in the household | ||||||
| ⩽2 | 35 (19.3) | 146 (80.7) | 1.6 (1.02, 2.6) | 0.038 | 1.8 (1.003, 3.1) | 0.049 |
| >2 | 61 (12.9) | 411 (87.1) | — | — | ||
| FS at time of survey | ||||||
| Low FS | 51 (22.3) | 178 (77.7) | 2.4 (1.4, 4.1) | 0.0007 | 1.7 (0.8, 3.6) | 0.136 |
| Moderate FS | 21 (10.6) | 178 (89.4) | 1.0 (0.5, 1.9) | 0.993 | 0.9 (0.4, 2.0) | 0.875 |
Abbreviations: aOR, adjusted Odds Ratio; CI, confidence interval; FS, food security; HAZ, height for age Z-score; MUAC, mid-upper arm circumference; OR, odds ratio; SAM, severe acute malnutrition; WHZ, weight for height Z-score. Bolding refers to values that are statistically significant (P value<0.05).
A total of 36 children (5.2%) of those exiting the program as defaulter were found to have died at follow-up. Of the 36, 20 (55.6%) were reported to have died <1 month following the date of default.
Analysis of overall mortality following exit from the CMAM program
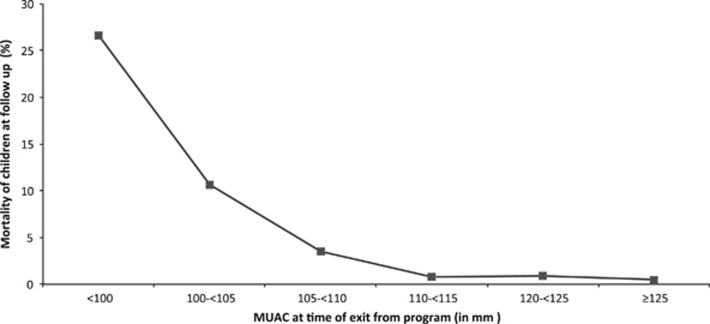
After combining all children traced over all food security periods, the long-term mortality rates for children who exited the program with a MUAC of <100, 100–<105, 105–<110, 110–<115, 120–<125 and ⩾125 mm were 26.6%, 10.7%, 3.5%, 0.8%, 0.9% and 0.5%, respectively (Figure 4). Overall, the adjusted relative risk of mortality following exit for a child defaulting from the program was 6.6 (95% CI 3.3–13.2; P<0.001) times higher compared with that of a child being discharged as cured. Note that it was not possible to relate overall mortality to food security since deaths identified during each survey may have occurred at any stage following time of exit from the program.
Figure 4.
Mortality rate at follow-up by MUAC at the time of discharge/exit from the CMAM program.
The adjusted odds of mortality for a child exiting the program with a MUAC of <100, 100–<105 and 105–<110 mm were 44.4 (95% CI 10.7, 184.3; P<0.001), 14.7 (95% CI 3.8, 57.5; P<0.001) and 4.6 (95% CI 1.1, 19.6; P=0.042) times higher, respectively, compared with that of a child exiting with MUAC of 110–<115 mm (Table 3).
Table 3. Risk factors for mortality in children exiting the program with MUAC <115 mm.
| Risk factor | Dead N (%) | Alive N (%) | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age at time of exit | ||||||
| 6–<12 | 16 (6.8) | 219 (93.2) | 0.8 (0.3, 1.9) | 0.564 | 0.5 (0.2, 1.7) | 0.279 |
| 12–<24 | 13 (3.4) | 364 (96.6) | 0.4 (0.1, 0.97) | 0.035 | 0.3 (0.1, 0.9) | 0.027 |
| ⩾24 | 7 (8.8) | 73 (91.3) | ||||
| Gender | ||||||
| Female | 31 (6.3) | 463 (93.7) | 2.6 (0.99, 6.8) | 0.045 | 2.6 (0.9, 7.2) | 0.069 |
| Male | 5 (2.5) | 193 (97.5) | ||||
| MUAC at time of exit | ||||||
| <100 | 17 (26.6) | 47 (73.4) | 45.7 (12.4, 248.2) | <0.001 | 44.4 (10.7, 184.3) | <0.001 |
| 100–<105 | 11 (10.7) | 92 (89.3) | 15.1 (3.9, 85.4) | <0.001 | 14.7 (3.8, 57.5) | <0.001 |
| 105–<110 | 5 (3.5) | 138 (96.5) | 4.6 (0.9, 29.8) | 0.038 | 4.6 (1.1, 19.6) | 0.042 |
| 110–<115 | 3 (0.8) | 379 (99.2) | 1 | |||
| WHZ | ||||||
| <–3 | 26 (8.1) | 295 (91.9) | 3.2 (1.5, 6.7) | 0.0014 | 1.01 (0.4, 2.6) | 0.982 |
| ⩾–3 | 10 (2.7) | 361 (97.3) | ||||
| HAZ | ||||||
| <–3 | 27 (5.1) | 505 (94.9) | 0.9 (0.4, 2.0) | 0.784 | ||
| ⩾–3 | 9 (5.6) | 151 (94.4) | ||||
| Length of stay in program | ||||||
| ⩽7 Weeks | 34 (6.6) | 485 (93.4) | 6.0 (1.5, 52.0) | 0.005 | 1.5 (0.1, 23.4) | 0.790 |
| >7 Weeks | 2 (1.2) | 171 (98.8) | ||||
| Admission in stabilization center | ||||||
| Yes | 6 (8.8) | 62 (91.2) | 1.9 (0.8,4.8) | 0.157 | ||
| No | 30 (4.8) | 594 (95.2) | ||||
Abbreviations: CI, confidence interval; HAZ, height for age Z-score; MUAC, mid-upper arm circumference; OR, odds ratio; WHZ, weight for height Z-score. Bolding refers to values that are statistically significant (P value<0.05).
Impact of being cured on prevalence of stunting at 12 months following exit from the program
No significant difference was seen in the mean degree of stunting at the time of exit from the program and 12-month follow-up for children defaulting from treatment (mean difference in height for age Z-score, 95% CI −0.2 (−0.1, 0.4), P=0.21). However, an improvement was seen in those children who completed the treatment and were discharged as cured (mean difference −0.7 (−0.5, −0.9) P<0.001).
Discussion
The ‘SPHERE' humanitarian charter suggests minimal standards that all therapeutic programs should achieve regardless of program structure—in-program mortality under 10%, <15% defaulting and >75% of children exiting the program should recover.10 However, evaluation of program performance becomes challenging when there is a high default rate, as it cannot be assumed that a high cure rate in those completing the treatment can be used as a proxy measure of program impact. In the Indian setting, the vast majority of therapeutic feeding initiatives suffer from exactly this conundrum—default rates up to 45%, but high cure rates in those children who complete the treatment.3, 4, 5, 11 Without an understanding of what ultimately happens to defaulting children or those leaving the program as cured, further interpretation of high in-program cure rates is challenging considering that the children are returning to the same socioeconomic dynamic as before admission.
There is surprisingly little evidence describing long-term outcomes following exit from SAM treatment programs. This may be explained in some part by the traditional focus on the African setting, where therapeutic feeding programs tend to be implemented in emergency settings and populations are mobile and difficult to track.12, 13, 14, 15, 16 One recent prospective cohort study based in a high HIV endemic area in Malawi described the outcomes of 1024 children admitted into an inpatient-based SAM treatment center. Long-term outcomes were available in 88% of the children; of 471 children discharged as nutritionally cured, 23% had died 12 months after their first admission.17 In this Malawian study, routine reporting would have given falsely optimistic impressions of post-SAM mortality—although much more evident than in our study, where the overall in-program mortality rate (0.9%) was similar to the long-term mortality rate of children discharged as cured (0.8%).
With an in-program default rate of 38%, tracing 68.7% (n=692) of all children who had defaulted during the reference period allowed us to ascribe a more accurate survival status to this important cohort—and the results were substantially different to what has been observed in the African setting where MUAC <115 mm has been associated with a much higher mortality.18 Overall, 5.6% of children traced who defaulted were found to have died when traced upto 18 months following exit from the program; however, once stratified by progressive MUAC boundaries, a clear association between lower MUAC and mortality emerged. The mortality rates for children who exited the program with a MUAC of <100, 100–<105, 105–<110, 110–<115, 120–<125 and ⩾125 mm were 26.6%, 10.7%, 3.5%, 0.8%, 0.9% and 0.5%, respectively, in this study; considering that the main purpose of interventions targeting children with SAM is to reduce mortality, it suggests that greater focus needs to be placed on ensuring that those children presenting with MUAC of <110 mm receive quality and sustained care.
This has an even greater implications when considering the scale of severe undernutrition in the Indian context—with a reported 8 million children with severe wasting in India, adequate coverage by any therapeutic program (inpatient or CMAM) becomes logistically, economically and practically challenging. For example, one recent study described the stark reality in the state of Madhya Pradesh where even if all 175 dedicated inpatient nutritional facilities were running at full capacity, it would take 15.5 years to provide this treatment method to the estimated 1.3 million children in the state suffering from SAM.19 In contrast, using MSF program admission data in Bihar, narrowing the admission criteria to MUAC <110 mm ±bilateral edema would reduce the burden of admissions by 57%.3 This would have the dual benefit of making implementation of widespread CMAM activities substantially more feasible and cost-effective, while allowing resources to be even better focussed on those children at highest risk of death.20 This is not to say that children with MUAC 110–115 mm should not be considered SAM, rather that they could be managed within existing routine supplementary feeding programs within the ICDS.21 The results of this study also suggest that, in this context, the incremental increase in admission time required to reach a discharge MUAC of >125 mm compared with >120 mm may not be necessary when considering long-term outcomes of children discharged as cured, rather that a focus on ensuring better food security for families whose children are discharged during periods of low food security may be a more cost-effective option.
This study has a number of limitations that should be considered when interpreting the results. Primarily, although there were no significant differences in the admission or demographic characteristics of children successfully traced compared with those lost to follow-up, 26.7% of the sample were not traced. Second, caution must be taken when extrapolating the long-term outcomes of the children who defaulted from the program to children with SAM in general who have never had any exposure to the CMAM program (and therefore did not receive systematic treatment on admission as per standard practice nor any therapeutic feeds). Third, although post-exit hospitalization was used as a proxy for illness during the follow-up period, more detailed information on the incidence of seasonal infections would have been useful in interpreting the full seasonal effect.
In conclusion, this study suggests that long-term outcomes following successful completion of treatment for SAM in a CMAM program in Bihar are good, albeit dependent on seasonal food security in the short term. It also suggests that a more narrow admission criteria for focused treatment of SAM of MUAC <110 mm ±bilateral edema may be more suitable for the Bihar context in order to ensure that limited resources are most appropriately used on targeting those children highest at risk of death.
Acknowledgments
We acknowledge the support of the State Health Society of Bihar and the Médecins Sans Frontières teams in Biraul who have made this work possible. We thank the pediatricians at the Darbangha Medical College Hospital (DMCH) and the Medical in Charge of Biraul Block PHC who have been pivotal in assisting the work of MSF. We also thank Patricia Kahn for editing assistance. Names for Pubmed indexing: Burza, Mahajan, Marino, Sunyoto, Shandilya, Tabrez, Kumari, Mathew, Jha, Salse, Casademont, Mishra. All funding for the implementation of the program was received as part of normal MSF fundraising activities. No donor had any part in the study design or implementation.
Author contributions
SB and RM analyzed the data and wrote the manuscript. SB, RM, TS, EM, AJ, AK, CS, NS and NKM were directly involved in program design and implementation and reviewed the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Supplementary Material
References
- International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005-2006. IIPS: Mumbai, India, 2007. [Google Scholar]
- Espie E, Pujol CR, Masferrer M, Saint-Sauveur JF, Urrutia PP, Grais RF. Acute malnutrition and under-5 mortality, northeastern part of India. J Trop Pediatr 2011; 57: 389–391. [DOI] [PubMed] [Google Scholar]
- Burza S, Mahajan R, Marino E, Sunyoto T, Shandilya C, Tabrez M et al. Community-based management of severe acute malnutrition in India: new evidence from Bihar. Am J Clin Nutr 2015; 101: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo VM, Jacob S, Badgaiyan N, Chandra P, Kumar A, Singh K. Providing care for children with severe acute malnutrition in India: new evidence from Jharkhand. Public Health Nutr 2012; 17: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Badgaiyan N, Ranjan A, Dixit HO, Kaushik A, Aguayo VM et al. Management of children with severe acute malnutrition in India: experience of nutrition rehabilitation centres in Uttar Pradesh, India. Indian Pediatr 2013; 51: 21–25. [DOI] [PubMed] [Google Scholar]
- Marino E, Salse N, Jha A, Burza S. Using SQUEAC methodology to assess CMAM for SAM programme coverage in India. Available at http://issuu.com/msfuk/docs/squeac (accessed 20 September 2014).
- WHO. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children; A Joint Statement by the World Health Organization and the United Nations Children's Fund. WHO: Geneva, Switzerland, 2009. [PubMed] [Google Scholar]
- Golden MH, Briend A. Treatment of malnutrition in refugee camps. Lancet 1993; 342: 360. [DOI] [PubMed] [Google Scholar]
- Brown KH, Black RE, Becker S. Seasonal changes in nutritional status and the prevalence of malnutrition in a longitudinal study of young children in rural Bangladesh. Am J Clin Nutr 1982; 36: 303–313. [PubMed] [Google Scholar]
- The Sphere Project. Humanitarian Charter and Minimum Standards in Humanitarian Response, 2011 edition. The Sphere Project in India: Bangalore, India, 2011. [Google Scholar]
- Aguayo VM, Agarwal V, Agnani M, Das Agrawal D, Bhambhal S, Rawat AK et al. Integrated program achieves good survival but moderate recovery rates among children with severe acute malnutrition in India. Am J Clin Nutr 2013; 98: 1335–1342. [DOI] [PubMed] [Google Scholar]
- Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 2009; 103: 541–548. [DOI] [PubMed] [Google Scholar]
- Pecoul B, Soutif C, Hounkpevi M, Ducos M. Efficacy of a therapeutic feeding centre evaluated during hospitalization and a follow-up period, Tahoua, Niger, 1987-1988. Ann Trop Paediatr 1992; 12: 47–54. [DOI] [PubMed] [Google Scholar]
- Hennart P, Beghin D, Bossuyt M. Long-term follow-up of severe protein-energy malnutrition in Eastern Zaire. J Trop Pediatr 1987; 33: 10–12. [DOI] [PubMed] [Google Scholar]
- van Roosmalen-Wiebenga MW, Kusin JA, de With C. Nutrition rehabilitation in hospital—a waste of time and money? Evaluation of nutrition rehabilitation in a rural district hospital in South-West Tanzania. II. Long-term results. J Trop Pediatr 1987; 33: 24–28. [DOI] [PubMed] [Google Scholar]
- Perra A, Costello AM. Efficacy of outreach nutrition rehabilitation centres in reducing mortality and improving nutritional outcome of severely malnourished children in Guinea Bissau. Eur J Clin Nutr 1995; 49: 353–359. [PubMed] [Google Scholar]
- Kerac M, Bunn J, Chagaluka G, Bahwere P, Tomkins A, Collins S et al. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PLoS One 2014; 9: e96030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangome MK, Fegan G, Fulford T, Prentice AM, Berkley JA. Mid-upper arm circumference at age of routine infant vaccination to identify infants at elevated risk of death: a retrospective cohort study in the Gambia. Bull World Health Organ 2012; 90: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja G, Dixit S, Khatri A, Yesikar V, Raghunath D, Chourasiya S. A study to evaluate the effect of nutritional intervention measures on admitted children in selected nutrition rehabilitation centers of Indore and Ujjain divisions of the state of Madhya Pradesh (India). Indian J Community Med 2012; 37: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens S, Bekele Y, Yun O, Harczi G, Ouannes M, Shepherd S. Mid-upper arm circumference based nutrition programming: evidence for a new approach in regions with high burden of acute malnutrition. PLoS One 2012; 7: e49320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker A, Shah N, Abraham. R Quantitative assessment: beneficiary nutritional status and performance of ICDS Supplementary Nutrition Programme in Bihar (Working Paper). International Growth Center 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.