Abstract
Persistent organohalogens (POHs) and metals have been linked to alterations in menstrual cycle function and fertility in humans. The Cree First Nations people living near James Bay in Ontario and Quebec, Canada, have elevated levels of POHs, mercury and lead compared to other Canadians. The present study further examines the interrelationships between selected POHs and elements on menstrual cycle function.
Forty-two women living in Cree communities in the James Bay region who were 19–42 years old at the start of the study were studied. Menstrual cycle characteristics were derived from structured daily diaries and endocrine measurements from daily urine samples collected during one cycle. POHs (n=31) were measured in blood plasma while elements (n=18) were measured in whole blood, yielding complete contaminant exposure measures for 31 of the participants. POHs and elements detected in ≥70% of the participants were submitted to principal component (PC) analysis to reduce the contaminant exposure data by generating a smaller number of summary PCA variables.
Multiple regression analysis revealed that, after adjusting for confounders, higher values of PC-1 were associated with significantly increased menstrual cycle length (p=0.02). PC-1 accounted for 44.7% of the total variance in the original matrix and summarized variation in the concentration of POHs. PC-3 values showed significant negative association with cycle length, after adjusting for confounders (P=0.002). PC-3 accounted for 9.2% of the variance and shows positive loadings for cadmium, selenium, and PBDE congeners 47 and 153, and a negative loading for copper. Sensitivity analysis of the model to quantify likely effect sizes showed a range of menstrual cycle length from 25.28— 28.34 d using the lower and upper 95% confidence limits of mean measured contaminant concentrations to predict cycle length. Other relationships are discussed. Our observations support the hypothesis that the menstrual cycle function of Cree women living in the James Bay area may be altered by exposure to POHs and elements.
Keywords: lead, mercury, cadmium, persistent organic pollutants, menstrual cycle, Cree, hormones
Introduction
The human menstrual cycle is a result of a series of interrelated hormonal changes within the hypothalamic–pituitary–ovarian axis. Epidemiologic studies have shown that subtle changes in the hormonal levels during the cycle are associated with disrupted, subfertile or infertile menstrual cycles (e.g., Baird et al., 1999; Brodin et al., 2008). This complex and delicate endocrine interplay of the menstrual cycle is vulnerable to toxicants and contaminants that can act as endocrine disruptors. This nexus of hormonally-active contaminants leading to menstrual cycle changes associated with infertility is compelling (Ouyang et al., 2005; Harley et al., 2010), yet little is known about the association between many specific environmental contaminants and menstrual cycle function.
Persistent organohalogens (POHs) are a group of chemicals linked to a number of disorders of reproductive function (Harley et al., 2010; Foster et al., 2008; Mendola et al., 2008). While the pesticide p,p'-dichlorodiphenyltrichloroethane (DDT) and its metabolite p,p'-dichlorodiphenyldichloroethylene (DDE) are associated with spontaneous abortion and neurodevelopmental delay (Eskenazi et al., 2009), their effects on reproductive health are not clear. Higher levels of DDT are associated with reduced levels of urinary estradiol and progesterone metabolites (Perry et al. 2006). Reports variously describe DDT and DDE exposures to be associated with decreasing (Windham et al., 2005; Ouyang et al., 2005; Toft et al., 2008) or not affecting (Chen et al., 2005) the length of the menstrual cycle. Exposure to polychlorinated biphenyls (PCBs) is associated with shorter menstrual cycles in Swedish fishermen’s wives (Mendola et al., 1997; Axmon et al., 2004; Toft et al., 2008) and lowered success rates of in vitro fertilization (Meeker et al., 2011). The women most heavily exposed to PCBs and dibenzofurans in the Yucheng incident have been found to have menstrual cycles that were 1.2 days shorter and a menstrual flow 0.5 days greater than controls (Yang et al., 2011).
Attribution of effects to an individual POH is seldom possible; exposure typically occurs simultaneously to a suite of POHs. Exposure to DDT and PCBs has been associated with decreased menstrual cycle length and luteal phase length (Axmon et al., 2004), as well as increased rate of abnormalities in menstrual flow (Yu et al., 2005). By contrast, Windham et al. (2005) stated that DDE and DDT, but not PCBs, were associated with decreased menstrual cycle length and that the change in cycle length was a result of a shorter luteal phase and associated with lower progesterone levels.
Metal exposures may also alter human reproductive health. The relationship between the essential metals copper and zinc and the menstrual cycle of eumenorrheic women suggests a relationship between these metals and estradiol (Michos et al., 2010). The effects of toxic metals on reproductive hormones and the menstrual cycle is not clear. Elevated blood levels of lead in women have been associated with higher circulating levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH) (Krieg, 2007), irregular menstruation, and infertility (Chang et al., 2006). Pollack et al. 2011 looked at the toxic metals cadmium, lead, and mercury and observed decreased FSH with increasing cadmium and increased progesterone with increased lead levels. Jackson et al. (2011) detected a positive relationship between cadmium and early follicular phase estradiol, but that relationship was not significant when the data was adjusted for lead, mercury, race/ethnicity and age. In contrast to Pollack et al. (2011), Jackson et al. (2011) found no relationship between lead and progesterone. Both Pollack et al. (2011) and Jackson et al. (2011) found that mercury was not related to reproductive hormone levels, but the levels of mercury, as well as lead and cadmium, were low and typical of a non-exposed population.
Cree communities on the Ontario west coast and Quebec east coast of James Bay have relatively high levels of POHs, lead, mercury and cadmium, compared to more southern urban populations in Canada (Tsuji et al., 2006, 2008; Dewailly and Nieboer, 2005; Bonnier-Viger, 2007; Nieboer et al., 2011; Charania et al. 2014). While the source of POHs is unknown for these populations, abandoned Mid-Canada Radar Line bases and accumulation within traditional foods are possible sources (Tsuji et al. 2005). Mercury exposure in this population is usually associated with fish consumption (Van Oostdam et al., 1999; Bonnier-Viger, 2007), whereas blood lead in this population has previously been correlated with hunting activities involving leaded ammunition (Tsuji et al., 2008). Cadmium exposure in this group is related to cigarette smoking rather than food (Charania et al. 2014).
In the present study, we further investigated the interrelationships of effects by selected POHs and elements on menstrual cycle characteristics in Cree women from eastern and western James Bay communities. In the current study we did not attempt to determine the source of contaminant exposure. Menstrual cycle function was assessed using diary entries and daily measurements of urinary reproductive hormones or their metabolites.
Materials and Methods
Study Group
Participants were women from two remote Cree First Nation communities in subarctic Canada. Population sizes (all individuals) in these communities are very limited (Fort Albany 850; Oujé-Bougoumou 622), and all residents between ages 18 and 40 that met study inclusion criteria (Fort Albany 169; Oujé-Bougoumou 137) were approached to participate in the study. Thus, the sample size represents a census of all available participants.
Participants were first recruited primarily through community presentations, and subsequently by personal interviews with community field coordinators. Written consent was obtained from all participants in person by community-based health care coordinators communicating in Cree language or English. This study was approved by the McMaster University Research Ethics Board (Study Number 98–47). Eligible participants were Cree women between 18–42 years old at the time of recruitment, who had not breast fed or used an intrauterine device, oral hormonal contraception or other hormonal replacement/medication for at least 3 months, or injectable hormonal contraception for 12 months. Participants had not been pregnant for at least 6 months, had not had surgery on their reproductive tract (ovaries, tubes, uterus) and did not have an endocrine disorder (diabetes, thyroid disease, adrenal disease, pituitary disease), reproductive disease (including chronic pelvic inflammatory disease and endometriosis), or cancer of the vagina, cervix, uterus, ovary or bladder. Subjects were not excluded for irregular or absent menstrual periods.
The study was conducted in two phases: a pilot and a larger expanded study. For the pilot study, participants were recruited from Fort Albany, Ontario, on the west shore of James Bay (n=9). For the larger study, 37 participants were recruited from Fort Albany, Ontario, and Oujé-Bougoumou, Quebec, a Cree community located 600 km southeast of Fort Albany and 360 km southeast of James Bay. Participation was from July 2000 through February 2001 for the pilot study, and from February through November 2004 for the larger study. Since four women from Fort Albany participated in both studies, only data from their participation in the larger study were included in the analyses to avoid bias due to multiple observations that are correlated or not independent.
Of the 42 eligible women, complete contaminant exposure measures were obtained for 31 women. Indices of menstrual cycle function were derived from endocrine hormones measured in daily urine collections and from menstrual bleeding recorded in daily diaries. Five participants failed to comply fully with the study design thus certain outcome variables could not be determined and sample size varied from 37 to 40 of the 42 participants.
At study entry, trained community-based study coordinators administered a questionnaire to participants to collect information about general health, reproductive history, diet, and potential confounders of the relationships between study exposures and outcomes.
Exposure Assessment- Blood Sample Collection and Analyses
Prior to the collection of the first urine sample, a blood sample was collected in three types of tube from each participant. The lag between blood and urine sampling was 22 ± 32 days (mean ± SD) with a median lag of 8 days. The blood was collected in 6-ml and 10-ml glass Vacutainer® tubes containing ethylenediaminetetraacetic acid (EDTA; Becton-Dickinson #367863 & #366457, respectively) for whole blood and plasma measurements of elemental and POH exposures, respectively, and in a 8.5-ml SST Vacutainer® tube (Becton-Dickinson #367953) for serum lipids. After sample collection and inversion of the 6 and 10 ml tubes to mix with EDTA, only the 10-ml samples were centrifuged, and the resulting plasma was transferred via hexane-washed polyethylene pipettes to shatter-resistant, hexane-cleaned glass vials with lids coated with polytetrafluoroethylene (PTFE; Supelco #2-3178) and stored at 20°C until shipped. Whole blood samples for elemental analyses were allowed to cool to room temperature and then stored in their Vacutainer tubes at 20°C until shipped. The 8.5-ml samples in the SST tubes were centrifuged and the serum was transferred to a hexane-cleaned glass vial (Supelco 2-3178) and frozen at −20°C until shipped. The plasma, whole blood and serum samples were shipped frozen to the Centre de toxicologie du Québec, Sainte-Foy, Quebec, for analyses of POHs, elements and lipids, respectively (Tsuji et al., 2005).
The following POHs were measured by gas chromatography-mass spectrometry (GC-MS; Tsuji et al., 2006): aldrin, alpha-chlordane, gamma-chlordane, oxychlordane, cis-nonachlor, trans-nonachlor, hexachlorobenzene, β-hexachlorohexane, mirex, polybrominated biphenyl (PBB) congener 153, polybrominated diphenyl ether (PBDE) congeners 47, 99, 100 and 153, PCB congeners 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 163, 170, 180, 183 and 187, DDE and DDT. Aroclor 1260 was imputed from PCB congener abundance by adding the levels of PCBs 138 and 153 then multiplying the sum by a factor of 5.2 (NIOSH 1997).
The following elements and their detection limits (nmol/L) were analyzed by inductively coupled plasma mass spectrometry (ICP-MS; Perkin Elmer Sciex Elan 6000): total arsenic (1.0), beryllium (50), bismuth (0.07), cadmium (0.4), cobalt (0.85), copper (7.9), lead (1.0), lithium (20), molybdenum (1.0), nickel (6.0), selenium (1.3), silver (10), tellurium (4.0) thallium (0.07), tin (2.0), total mercury (0.50), uranium (0.0080) and zinc (3.1). Antimony was measured but the collection tubes were contaminated with antimony during manufacture and thus the data were not used.
Total and free cholesterol, triglycerides, and phospholipids were individually measured using standard enzymatic methods (Technicon Automatic Analyser, RA-500) using the appropriate test packs (Randox for total cholesterol and triglycerides, BMC for free cholesterol, and Wako for phospholipids). Plasma total lipids were calculated using the summation method [total lipids=1.677(total cholesterol free cholesterol) + free cholesterol + triglycerides + phospholipids] following Patterson et al. (1991), and as recommended by Sandanger et al. (2003). The coefficients of variability for the plasma lipid measurements were 1.5–2.6% (Bonnier-Viger et al., 2007; Nieboer et al., 2011).
Outcome Assessment- Urine Sample Collection and Analysis
Women were instructed to collect daily samples of their first morning urine void for one complete menstrual cycle, beginning on the first day of spotting or bleeding and continuing through the third day after the end of the subsequent bleeding period. If bleeding was irregular or absent, participants were instructed to collect urine samples for at least 42 consecutive days.
Each participant maintained a daily diary to document sample collection, vaginal bleeding, sickness, medications and supplements. Onset of menses was determined from the vaginal bleeding records guided by an algorithm (Hornsby, 1991; Reutman et al., 2002a). In brief, the menstrual cycle and the menstrual period began on the first of two consecutive days of bleeding, only one of which could be spotting. Menses was preceded and followed by three consecutive days of non-bleeding or spotting. After day 2 of the period, 1–2 day intervals of non-bleeding or spotting were counted as part of the menses. Because urine collection and diary maintenance in this study typically began on about the first day of bleeding, the algorithm could not be strictly applied to counting non-bleeding days before onset of the first menses.
Each morning of the study, participants poured 5 mL of urine into a polypropylene vial containing glycerol (7% final dilution) to prevent freeze-induced activity loss of LH and FSH (Kesner et al., 1995). Samples were stored in the participant’s home freezer until they were picked up by a community-based study coordinator and stored at −20°C in a freezer at the local health clinic. Samples were shipped in dry ice by express courier to the NIOSH Reproductive Endocrinology Laboratory in Cincinnati, Ohio, USA, where they were stored at −80°C until endocrine analyses was conducted.
Urinary LH and FSH were assayed using immunofluorometric assays (Perkin-Elmer Cat. Nos. A031-101 and A017-201, respectively), modified and validated for analyzing urine samples (Kesner et al., 1994a; Kesner et al., 1998). Urinary estrone 3-glucuronide (E13G) and pregnanediol 3-glucuronide (Pd3G) were measured using competitive double-antibody time-resolved fluoroimmunoassays (Kesner et al., 1994b). Urinary creatinine (Cr) was measured using a Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics) that employs a slide composed of a dry, multilayered analytical element coated on a polyester support (Findlay et al., 1985; Mauck et al., 1986).
Assay analytical sensitivities were 0.05 mIU/ml for LH and FSH, 0.98 ng/ml for E13G and 0.035 μg/ml for Pd3G. The intra- and inter-assay coefficients of variation, respectively, were 7.67% and 9.71% for LH, 6.84% and 8.70% for FSH, 8.48% and 4.18% for E13G, 9.73% and 5.29% for Pd3G, and 2.67% and 1.64% for Cr. Urinary endocrine concentrations were divided by Cr concentrations to normalize for sample dilution.
Menstrual cycle function outcomes were derived from daily urinary endocrine measurements and daily menstrual bleeding records (Reutman et al., 2002a, 2002b). Five reliable endpoint variables indicative of various aspects of menstrual cycle function and fertility were selected for analyses a priori (Baird et al., 1999; Perry et al., 2006): cycle length, follicular phase length, luteal phase length, early-follicular phase FSH concentration and mid-luteal phase Pd3G concentration as previously defined (Luderer et al., 2013). The last two endpoints are indices of ovarian reserve and luteal function, respectively. Day of ovulation (the last day of the follicular phase) was defined as the day of luteal transition based on the E13G:Pd3G ratio (Baird et al., 1995) or, if that was indeterminate, the day of the LH surge onset (Kesner et al., 1998). Out of 41 participants recruited to the study, 10 individuals did not comply with study protocol and were excluded from analyses of contaminant and menstrual cycle variables.
Statistical Analyses
Contaminant variables were screened to use only those present at detectable concentrations in 70% or more of the study participants. A detection frequency of ≥70% was used because statistical analyses of data with more than 30% imputed values may lead to a skewed distribution (Tsuji et al, 2008). The POHs retained for the statistical analyses were trans-nonachlor, oxychlordane, DDE, PBDEs 47 and 153, and PCBs 118, 138, 153, 163, 170, 180 and 187; the retained elements were total arsenic, cadmium, cobalt, copper, lead, molybdenum, nickel, selenium, thallium, total mercury, uranium and zinc. For these selected contaminants, values below the detection limit were imputed at a concentration equal to one-half the detection limits, and all plasma organic contaminant concentrations were then adjusted for plasma total lipid concentration to give values of μg/kg lipid. Lipid-adjusted concentrations of organic contaminants were then transformed as log10 (1 + [μg/kg lipid]) to improve the normality of the data distribution and remove negative values. Elemental contaminants do not vary with lipid content of the sample and thus were not lipid adjusted, but were similarly transformed to better approximate normality.
Values for the suite of selected contaminant concentrations were subjected to principal components analysis (PCA) to generate a smaller number of summary PCA predictor variables (Green 1979, Gauch 1982), and used to explain variation in reproductive cycle measures in regression models. By definition, these PCA scores are uncorrelated with each other and, therefore, can be used optimally as predictor variables in multiple regression analysis. Using the original concentration data as variables in regression models (or generalized linear models) is not defensible: correlations between related contaminants such as PCB congeners was observed to reach r-values of 0.90 and greater, and this multicollinearity biases the estimation of individual regression coefficients to cause misleading interpretation of the effects of individual predictor variables. Using PCA essentially as a data transformation is a common tool to deal with multicollinearity in multiple regression models (Freund and Wilson 1997). Only PC variables with eigenvalues >1 were retained in the analysis, since eigenvalues of ≤1 indicate that the PC axis is essentially a random skewer through an almost spherical cloud of points in the multi-dimensional contaminant space.
The confounders investigated for inclusion in the statistical models included age, gravidity, total months of breastfeeding for all pregnancies, smoking, alcohol consumption, and caffeinated drink consumption. The last three variables where each assessed using the following yes/no dichotomous questions “Do you drink tea, coffee or soft drinks”; “Do you drink beer, liquor or wine” and “Do you smoke an average of at least one cigarette per day”. These factors have been previously associated with changes in menstrual cycle function and/or fertility (Curtis et al., 1997; Hakim et al., 1998; Dunson et al., 2002; Rowland et al., 2002). Confounders showing significant correlation (p<0.05) with menstrual cycle function endpoints were included with contaminant variables in regression analyses against endocrine endpoints. Age is a primary correlate of bio-accumulated POHs (Quinn and Wania, 2012), so using age as one of the confounders removes the age effect from the exposure variables incorporating these organic contaminants. Body mass index (BMI) was not included as a potential confounder because it was not significantly correlated with the selected reproductive endpoints.
The effects of contaminants on menstrual cycle function variability were examined for each of the five chosen menstrual cycle function endpoints. For each outcome variable, the effects of confounder variables were examined in one block of a regression model. The influence of contaminant variables was judged by the additive predictive power of a second block of the regression model that included both confounder and contaminant variables. The difference (change in F-ratio) between the two models determined the effect of the contaminants, and the slope coefficients of the PCA contaminant variables were examined for significance and for direction of influence (positive or negative slope). Slope coefficients were calculated by the SPSS software and presented in both raw form (in units of the predictor variable) and standardized, which allows comparison of the relative influence of various predictor variables. This approach is conceptually similar to regression of the PCA contaminant variables on the residuals (unexplained variance) after regression of the confounder variables on the menstrual cycle function endpoint response variable, but is a more statistically valid model. Owing to the low numbers of available participants, we used a bootstrapping technique (1000 iterations of analysis, randomized with Mersenne Twister seed; SPSS version 22, SPSS Chicago, IL). Bootstrapping allows the internal generation of mean values of regression coefficients (and associated measures of bias and uncertainty) to produce conservative assessment of results, less biased by unique observations or outliers.
To examine reproductive cycle variation ascribable to the realistic range of variation in contaminant concentrations, we also conducted effect-size (sensitivity) analysis for models where significant influences of contaminant PC variables were found after consideration of confounders (age, alcohol, caffeine) by generating two hypothetical participant cases, one with high contaminant burdens, and one with low contaminant burdens. To generate these two “case” values, 95% confidence limits of the mean values for each contaminant were calculated, representing the low (lower 95% C.I. value for all contaminants) and high burden (upper 95% C.I value for all contaminants) as hypothetical cases. These contaminant values were projected a posteriori, into the existing PCA space to obtain scores on PC axes 1–6. The resultant PC scores were then used in the existing regression models to compute expected values of menstrual cycle measures for the hypothetical cases, giving a realistic measure of effect size for variation in contaminant concentrations.
Data analyses were performed using SPSS software version 22 (SPSS, 2014).
Results
Characteristics of the study participants are presented in Table 1. Participants ranged in age from 19.9 to 42.6 years at the time of participation, with a mean ± SD age of 31.2 ± 7.2. Compared to an age-matched reference population of adult Canadian women, the study participants were similar height (165.9 ± 7.2 cm versus 164.0 ± 11.5 cm), but heavier (77.5 ± 16.9 kg versus 66.1 ± 28.4 kg) (Personal Communication, Statistics Canada, Canadian Community Health Survey, 2000/1). Approximately 60% of the study women were smokers which is lower than the rate of 70.2% of females 15–39 in this First Nation population (Charania et al. 2014).
Table 1.
Characteristics of study participants.
| Characteristic | n | Mean | Median | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age (years) | 31 | 31.2 | 32.4 | 6.6 | 19.9 | 42.6 |
| Height (cm) | 28 | 65.1 | 65.0 | 2.8 | 60.0 | 70.0 |
| Body mass (kg) | 29 | 173.3 | 170.0 | 37.0 | 115.0 | 270.0 |
| BMI (kg/m2) | 26 | 28.4 | 28.7 | 5.4 | 18.8 | 40.5 |
| Gravidity | 31 | 2.75 | 3 | 2.32 | 0 | 10 |
| Breastfeeding (months)* | 30 | 3.9 | 0.0 | 7.8 | 0.0 | 30.0 |
| Smoking** | 31 | 41.9% no; 58.1% yes | ||||
| Alcohol consumption** | 31 | 64.5% no; 35.5% yes | ||||
| Caffeine** | 31 | 80.6% no; 19.4% yes | ||||
Total duration of breastfeeding summed across all pregnancies
Smoking, alcohol and caffeine consumption were dichotomized to 1 (no) or 2 (yes); see Materials & Methods for details.
The menstrual cycle characteristics used as study outcomes are summarized in Table 2. The mean ± SD (median) menstrual cycle length of 28.5 ± 3.6 (28) days is similar to the mean of 27.2 days and 29.2 ± 3.4 days, respectively, for healthy women 30–34 years old (Treloar et al., 1967) and 25–35 years old (Fehring et al., 2006). The follicular phase and luteal phase lengths were 16.0 ± 5.5 (14) and 13.4 ± 2.1 (13) days, respectively, which are also similar to those reported for normal women (Lenton et al., 1984a & b). The follicular and luteal phase lengths are also close to the respective normal ranges of 13–14 days and 13–15 days reported in the review by Harlow (2000) for women with cycles less than 40 days. The mean urinary FSH level on follicular phase days 1–3 was 11.2 ± 17.5 mIU/mg Cr, which is comparable to those previously described (Reutman et al., 2002b; Steiner et al., 2008). Mean urinary Pd3G level measured on luteal phase days 5–6 was 13.6 ± 6.5 μg/mg Cr, similar to those previously reported for Caucasian women of the same age, but higher than that for African American women of similar age (Reutman et al., 2002a).
Table 2.
Menstrual cycle characteristics for all study participants. Urinary concentrations of early-follicular phase FSH and mid-luteal phase Pd3G are adjusted for creatinine (Cr).
| n | Mean | Median | SD | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Cycle Length (days) | 38 | 28.53 | 28.00 | 3.62 | 23 | 40 |
| Follicular Phase Length (days) | 40 | 16.03 | 14.00 | 5.49 | 10 | 35 |
| Luteal Phase Length (days) | 38 | 13.39 | 13.00 | 2.07 | 9 | 19 |
| Early Foll. Phase FSH (mIU/mg Cr) | 40 | 11.17 | 8.71 | 17.47 | 1.97 | 115.90 |
| Mid-Luteal Phase Pd3G (μg/mg Cr) | 39 | 13.55 | 14.03 | 6.51 | 0.69 | 25.92 |
Summary statistics for the contaminants with a detection frequency ≥70% (31 women) used in the PCA are found in Table 3.These circulating POH levels are similar to those measured one to three years earlier in the same communities (Tsuji et al. 2005; Dewailly and Nieboer, 2005). The organohalogen contaminant detected at highest concentration was DDE with a geometric mean concentration of 144.1 μg/kg lipid, which is greater than that for Canadian women aged 20–39 (102.2 μg/kg lipid; Health Canada, 2010). Oxychlordane was also higher in our population in a similar comparison (3.2 μg/kg lipid versus 2.3 μg/kg lipid; Health Canada 2010).The pesticide contaminant trans-nonachlor was present at about the same level as in age-matched Canadian women (4.5 μg/kg lipid versus 4.2 μg/kg lipid). The PBDEs were represented by congeners 47 and 153, which are typically the most abundant congeners detected in blood of Canadian women. The observed concentrations are comparable to those reported by Health Canada (2010) for women 20–39 years old, but lower than those typically encountered in the Great Lakes Basin (Turyk et al., 2010) or for aboriginal peoples of James Bay coastal communities (Liberda et al., 2011). Of the 7 PCB congeners listed in Table 3, the PCB congeners 138, 153 and 180 constituted 71% of the total PCBs; these three congeners were present at levels 2–3 times higher than observed in other Canadian women of similar age (Tsuji et al., 2006; Health Canada, 2010).
Table 3.
Descriptive statistics for the contaminants with detection frequencies ≥70% and used in the principal component analysis (n=31). POHs were measured in plasma, elements were measured in blood, and lipids were measured in serum. The number of decimal places is determined by the analytical resolution for each analyte.
| Descriptive Statistics | |||||||
|---|---|---|---|---|---|---|---|
| Geometric | Arithmetic | ||||||
| Contaminant | Min | Max | Mean | 95% Confidence Limits | Mean | SD | |
| Lower | Upper | ||||||
| trans-Nonachlor (μg/kg lipid) | 0.9 | 44.0 | 4.5 | 3.2 | 6.1 | 6.5 | 8.6 |
| Oxychlordane (μg/kg lipid) | 0.7 | 26.0 | 3.2 | 2.4 | 4.3 | 4.3 | 5.1 |
| p,p'-DDE (μg/kg lipid) | 42.1 | 920.0 | 144.1 | 108.0 | 192.2 | 198.8 | 189.4 |
| PBDE 47 (μg/kg lipid) | 2.2 | 217.2 | 14.1 | 9.5 | 20.9 | 26.1 | 41.9 |
| PBDE 153 (μg/kg lipid) | 0.7 | 23.1 | 4.5 | 3.3 | 6.3 | 6.1 | 5.3 |
| PCB 118 (μg/kg lipid) | 2.5 | 164.0 | 9.9 | 6.4 | 15.0 | 20.7 | 34.4 |
| PCB 138 (μg/kg lipid) | 2.6 | 300.0 | 19.9 | 12.6 | 31.3 | 44.6 | 73.3 |
| PCB 153 (μg/kg lipid) | 4.3 | 860.0 | 38.3 | 23.0 | 63.3 | 105.4 | 199.4 |
| PCB 163 (μg/kg lipid) | 0.9 | 180.0 | 6.9 | 4.0 | 11.5 | 20.1 | 42.7 |
| PCB 170 (μg/kg lipid) | 0.9 | 198.0 | 8.3 | 4.9 | 13.7 | 23.0 | 47.2 |
| PCB 180 (μg/kg lipid) | 2.6 | 700.0 | 26.6 | 15.7 | 44.7 | 79.9 | 164.2 |
| PCB 187 (μg/kg lipid) | 0.9 | 240.0 | 9.5 | 5.4 | 16.1 | 28.0 | 56.0 |
| Cadmium (nmol/L) | 2.50 | 66.00 | 12.01 | 8.39 | 17.02 | 17.66 | 15.69 |
| Cobalt (nmol/L) | 1.30 | 8.50 | 3.51 | 2.93 | 4.17 | 3.84 | 1.92 |
| Copper (μmol/L) | 12.00 | 23.00 | 15.60 | 14.75 | 16.50 | 15.77 | 2.47 |
| Lead (μmol/L) | 0.02 | 0.31 | 0.08 | 0.06 | 0.11 | 0.08 | 0.07 |
| Molybdenum (nmol/L) | 8.60 | 16.00 | 11.56 | 10.90 | 12.25 | 11.69 | 1.85 |
| Nickel (nmol/L) | 3.00 | 69.00 | 19.12 | 15.50 | 23.53 | 22.19 | 13.79 |
| Selenium (μmol/L) | 1.90 | 2.70 | 2.19 | 2.12 | 2.26 | 2.19 | 0.19 |
| Thallium (nmol/L) | 0.04 | 0.30 | 0.15 | 0.12 | 0.17 | 0.15 | 0.06 |
| Total Arsenic (nmol/L) | 7.40 | 21.00 | 10.76 | 9.91 | 11.68 | 11.02 | 2.76 |
| Total Mercury (nmol/L) | 0.03 | 100.00 | 5.23 | 3.21 | 8.21 | 11.57 | 20.95 |
| Uranium (nmol/L) | 0.00 | 0.03 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 |
| Zinc (μmol/L) | 81.00 | 110.00 | 95.91 | 93.07 | 98.84 | 96.23 | 7.94 |
All trace elements except beryllium and tellurium were detected in at least some participants. The observed mean plasma concentrations for the essential elements copper, selenium and zinc provided in Table 3 are similar to those reported in the recent Canadian population survey for women of reproductive age (Health Canada, 2010), while that for the essential element molybdenum (11.6 nmol/L) is higher by nearly a factor of 1.7. However, even higher molybdenum concentrations have been reported in “unexposed” healthy individuals (Goullé et al., 2005). Uranium and total arsenic levels in our study are similar to those previously described (Health Canada, 2010). Most of blood arsenic is in non-toxic forms, and usually reflects fish consumption (Brantsaeter et al., 2010).
Blood lead levels in these communities correlate with the amount of wild game shot with lead ammunition that is consumed (Tsuji et al., 2008), while mercury concentrations are associated with fish consumption (Liberda et al. 2014). Not surprisingly, therefore, mean blood lead (0.08 μmol/L) and mercury levels (5.23 nmol/L) in this population were 200% and 56% higher, respectively, than those reported in the recent Health Canada survey (2010). Cadmium, a marker of smoking, also was considerably higher in our study population than the general Canadian population (12.01 versus 3.2 nmol/L) (Health Canada 2010). The elevated cadmium levels in the current study are similar to the geometric mean for cadmium (10 nmol/L) measured in nine First Nations of Eeyou Istchee in Northern Quebec (Charania et al. 2014). Nickel levels in the current study were also elevated relative to the general Canadian population (19.12 nmol/L versus 6.98 nmol/L) (Health Canada 2010). Stainless steel household items (e.g., water taps, appliances, etc.) can be a source of nickel. On the other hand, the mean concentrations of the nickel and cadmium measured in the current study are near or within reference ranges measured for occupationally unexposed individuals (≤8.9 nmol cadmium/L and ≤34 nmol nickel/L) (London Laboratory Services Group, 2011).
Compared to trace elements measured in healthy populations, blood levels in our population are normal for thallium and cobalt (Bárány et al., 2005; Heitland and Koster, 2006). Bismuth levels are slightly higher in our study population than in a reference population, but well below the accepted toxicity level (Lambert, 1991; Slikkerveer et al., 1992). Silver, which is a component of silverware, jewellery, and dental amalgam (Armitage et al., 1996), was not detected in any specimen, whereas lithium was detected in only five specimens all of which were within the normal range (Donahoo et al., 2004). Tin was observed in only nine samples.
Table 4 shows the loadings on the first six PC variables which account for 81% of the variation in the original contaminant data. Figure 1 is graphical representation of the loadings on the first three PC variables which account for 63.5% of the total variation. The graph emphasizes the groupings of the elements and organohalogens that constitute the individual PC variables. PC variables 7–24 had eigenvalues less than 1, and the sum of the variances explained by the final 18 variables accounts for the remaining 19% of the variation. As a consequence of the small amount of variance explained by each additional PC variable, they can be considered to add little reliable explanatory power.
Table 4.
Principal Components Analyses. Organohalogens and trace elements that are in each of the six principal components (PCs) with eigenvalues (in parentheses) >1. Variation of total contaminant burden attributed to each PC variable accounts is presented [in brackets]. Analytes included in the PCA were detectable in ≥70% of blood samples and were expressed in units of μg/kg lipid (POHs), nmol/L (*) or μmol/L (**). All analytes were transformed by log10(x+1). These six PCs account for 81% of the variance in the original matrix. Loadings ≥ 0.45 are underlined.
| Contaminant | PC-1 (10..6) [44.7%] | PC-2 (2.3) [9.6%] | PC-3 (2.2) [9.2%] | PC-4 (1.78) [7.4%] | PC-5 (1.31) [5.7%] | PC-6 (1.15) [4.8%] |
|---|---|---|---|---|---|---|
| trans-Nonachlor | 0.918 | −0.154 | −0.130 | 0.069 | −0.028 | −0.081 |
| Oxychlordane | 0.884 | −0.116 | −0.118 | 0.134 | −0.002 | −0.029 |
| p,p'-DDE | 0.923 | −0.208 | −0.161 | 0.131 | 0.018 | −0.025 |
| PBDE 47 | 0.282 | −0.298 | 0.679 | −0.039 | 0.113 | −0.030 |
| PBDE 153 | 0.606 | −0.310 | 0.536 | 0.264 | 0.082 | −0.064 |
| PCB 118 | 0.956 | −0.021 | −0.107 | −0.095 | −0.028 | −0.019 |
| PCB 138 | 0.986 | −0.018 | −0.011 | −0.043 | −0.021 | −0.025 |
| PCB 153 | 0.985 | −0.014 | 0.002 | −0.044 | −0.030 | 0.006 |
| PCB 163 | 0.985 | −0.010 | −0.014 | −0.026 | −0.079 | 0.049 |
| PCB 170 | 0.985 | 0.001 | −0.010 | 0.002 | −0.069 | −0.011 |
| PCB 180 | 0.982 | −0.020 | −0.004 | 0.026 | −0.055 | −0.029 |
| PCB 187 | 0.982 | 0.009 | −0.005 | −0.039 | −0.054 | 0.010 |
|
| ||||||
| Cadmium* | −0.116 | −0.058 | 0.635 | 0.271 | 0.451 | 0.363 |
| Cobalt* | −0.392 | −0.107 | 0.048 | 0.674 | −0.256 | 0.204 |
| Copper** | −0.239 | −0.298 | −0.502 | 0.344 | −0.217 | 0.383 |
| Lead** | 0.412 | 0.637 | −0.122 | 0.413 | 0.091 | 0.172 |
| Molybdenum* | −0.434 | −0.110 | −0.135 | −0.594 | 0.167 | −0.152 |
| Nickel* | −0.363 | 0.412 | −0.018 | 0.388 | −0.258 | −0.223 |
| Selenium** | −0.106 | 0.128 | 0.513 | −0.075 | −0.530 | 0.039 |
| Thallium* | 0.362 | 0.528 | 0.093 | −0.320 | 0.006 | 0.418 |
| Total Arsenic* | 0.131 | 0.658 | 0.399 | 0.068 | 0.277 | −0.110 |
| Total Mercury* | 0.294 | 0.577 | −0.393 | −0.058 | 0.300 | 0.219 |
| Uranium* | −0.132 | −0.519 | −0.251 | 0.109 | 0.523 | 0.275 |
| Zinc** | 0.037 | −0.102 | 0.196 | −0.455 | −0.363 | 0.632 |
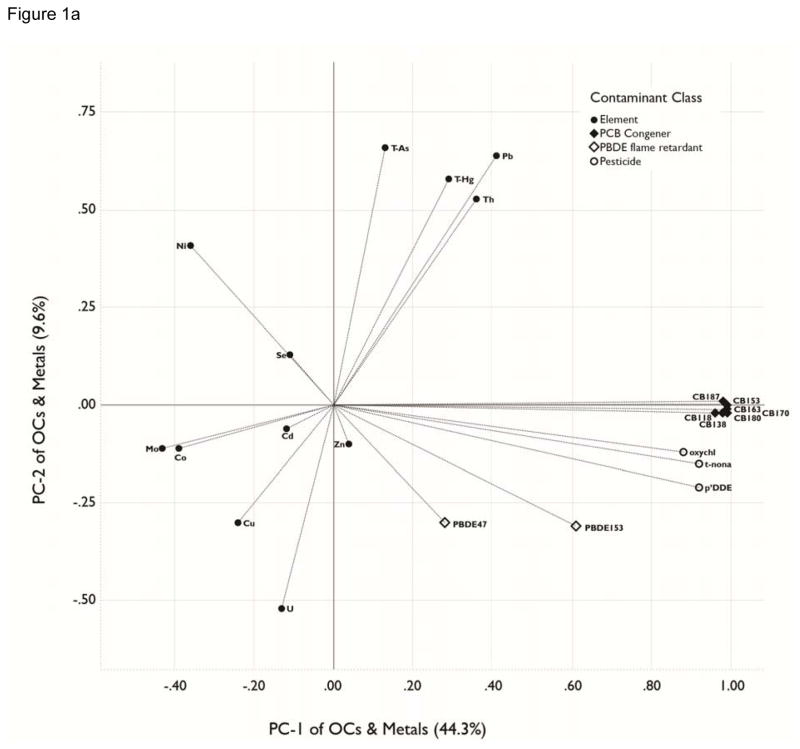
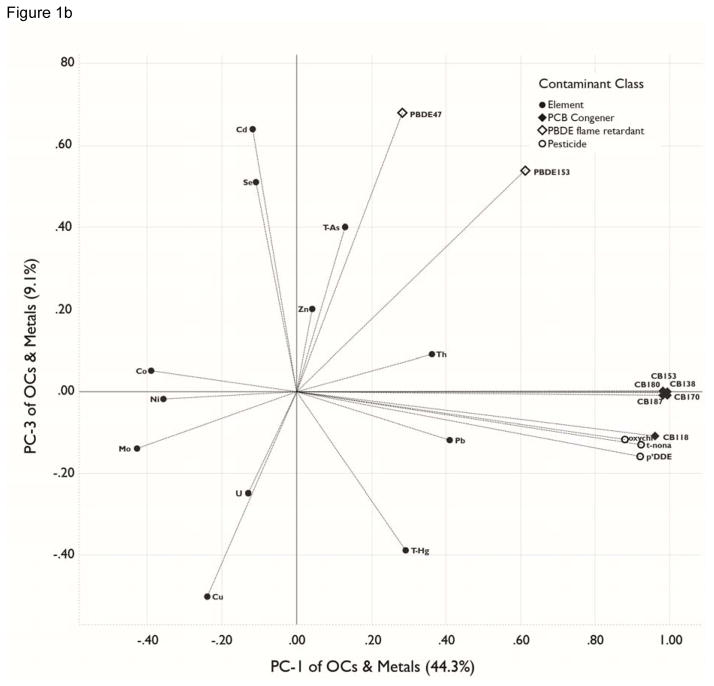
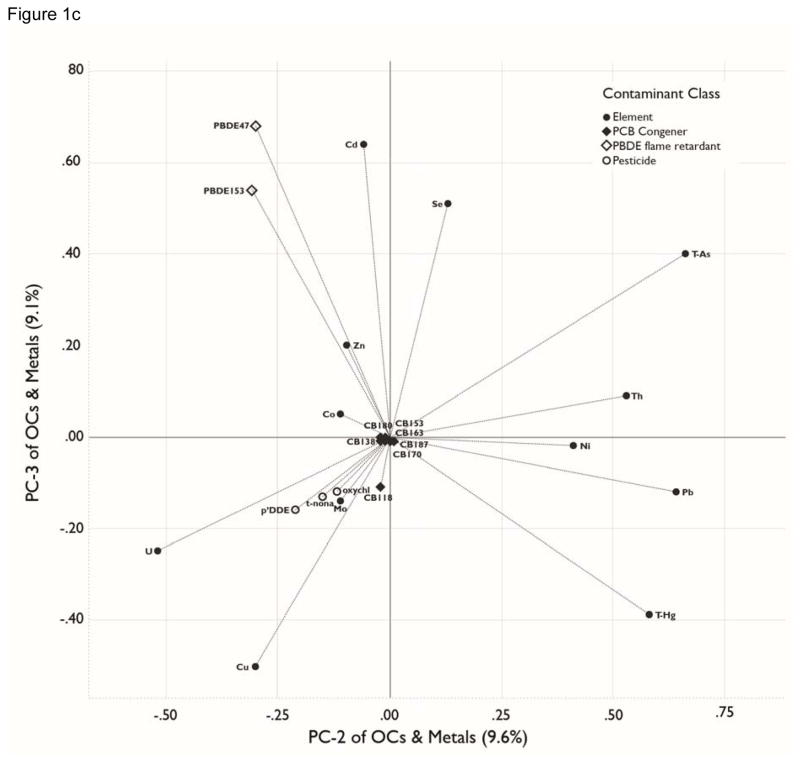
Figure 1.
a, b, c. Principal Components Analyses. Loadings for organohalogens and elements for the three largest principal components (PCs), which collectively account for 63.5% of the variance in the original matrix. The percentage of variation explained by each PC variable is presented in parenthesis in the axis label. Analytes included in the PCA were detectable in ≥70% of blood samples. See Table 4 for contaminant units. . All analytes were transformed by log10(x+1). . Figure 1a plots PC-1 loadings against PC-2 loadings. Figure 1b plots PC-1 against PC-3. Figure 1c plots PC-2 against PC-3.
PC-1 variable accounts for 44.7% of the variation in total contaminant burden, owing primarily to the PCB congeners and pesticides which generally have strongly positive loadings (Figure 1a, 1b). PBDE 47 and the elements do not load as heavily on PC-1. Thus, PC-1 can be considered to be a variable summarizing exposure to most POHs, and that this exposure measure is mostly independent of elemental exposure.
PC-2 and PC-3, respectively, account for 9.6% and 9.2% of the variance in the total contaminant body burden (Figure 1c). PC-2 contrasts relatively high positive loadings for lead, thallium, arsenic and mercury with a relatively large negative loading for uranium. These loadings infer that the former elements have a common or correlated pattern of exposure for study participants and that, in contrast, uranium has a contrasting exposure pattern. In a similar fashion, PC-3 exhibits relatively high positive loadings for cadmium, selenium and PBDE 47 and 153, and a relatively large negative loading for copper.
The remaining 3 axes account for 7.4 (PC-4), 5.7% (PC-5) and 4.8% (PC-6) of the model variance; they are dominated by the elements measured, with no significant loadings for the organic contaminants. PC-4 has a high positive loading for cobalt and large negative loading for molybdenum and zinc; PC-5 has high positive loadings for cadmium and uranium and a large negative loading for selenium; and PC-6 has a high positive loading for zinc.
The ANOVA of the menstrual cycle function outcomes and potential confounders (measured as binary (yes/no) variables) indicated that only alcohol and caffeinated drink consumption, and the continuous variable of age (by correlation analysis) were related to any of the five menstrual cycle variables (results not presented). These confounders were retained for the regressions.
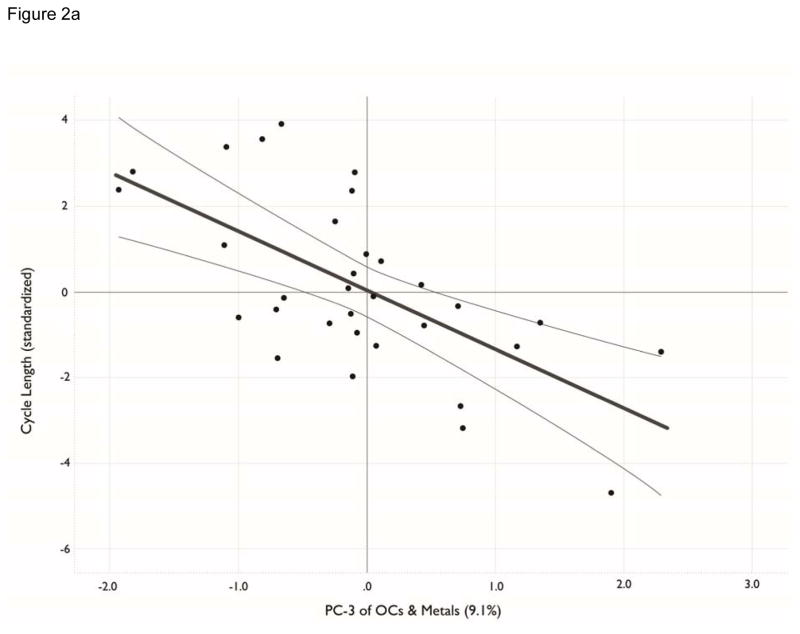
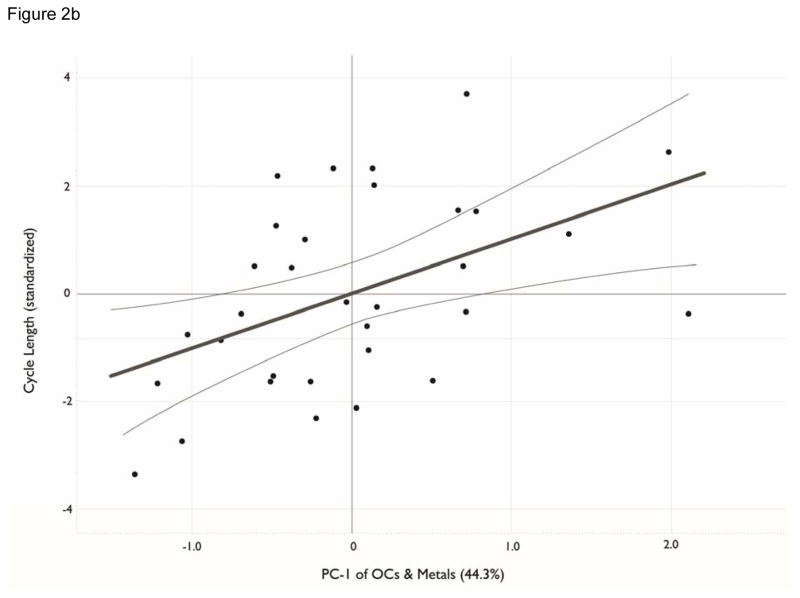
The regression of menstrual cycle length against the confounders (Model 1, Table 5) was significant (p=0.046) owing to the age of the women. Regression of the 3 confounders and the 6 PC variables against menstrual cycle length (Model 2, Table 5) showed a significant improvement (p=0.003) in the amount of variation explained compared to Model 1. In Model 2, the constant and the age of the participants remained significant, as did caffeinated drink consumption. Of the PC variables added to Model 2, only PC-3 had a significant regression coefficient in our bootstrapped regression model. This analysis indicates that menstrual cycle length is negatively related to age and PC-3 score. A suggestive positive but non-significant association with caffeine, PC-1, and PC-6 score also was noted. The standardized regression coefficients for PC-3 of -0.496 indicate that changes in PC-3 have a strong effect on menstrual cycle, though not as strong as the effect of age (−0.594). A partial regression plot, holding other variables constant, illustrates the significant effect of PC-3 on menstrual cycle length (Figure 2a). A similar plot for PC-1 illustrates the non-significant effect of PC-1 on menstrual cycle length (Figure 2b). The first stage of the regression model for menstrual cycle length explained approximately 25% of the variation in this dependent variable (r2 = 0.253), but with the addition of the contaminant variables, 68% of variation in menstrual cycle length was explained in the second stage regression (r2 = 0.683, Table 5).
Table 5.
Multiple regressions for menstrual cycle length computed against potential confounders (Model 1) and also against the contaminant principal component (PC) analysis scores (Model 2). The regression coefficient (B) is the coefficient of the slope for predictor variables. The PC scores used in Model 2 are generated from organohalogens and elements detectable in ≥70% of the sample population. *Regression coefficient slope B and model are significant (p<0.05).
| Menstrual Cycle Length | ||||
|---|---|---|---|---|
| Regression Coefficient (B) | t-statistic | p-value1 | ||
| Model 1 | Mean Raw1 (95% C.I.) | Standardized | ||
| Constant | 33.46 (27.40—41.58) | 9.988 | 0.001* | |
| Age (y) | −0.21 (−0.37—0.08) | −0.493 | −2.678 | 0.011* |
| Alcohol (Y/N) | −0.38 (−3.37—2.10) | −0.066 | −0.341 | 0.766 |
| Caffeine (Y/N) | 1.28 (−1.82—5.38) | 0.186 | 1.052 | 0.424 |
| Model 1 significance: F = 3.045, p = 0.046*; r2 = 0.253 | ||||
| Model 2 | ||||
| Constant | −32.56 (−219.93—247.94) | 10.861 | 0.773 | |
| Age (y) | −0.25 (−0.40—0.04) | −0.594 | −3.317 | 0.023* |
| Alcohol (Y/N) | −0.28 (−2.15—2.01) | −0.049 | −0.326 | 0.788 |
| Caffeine (Y/N) | 2.98 (−0.08—5.69) | 0.443 | 2.751 | 0.073 |
| PC-1 | 1.01 (−0.22—2.09) | 0.364 | 2.508 | 0.073 |
| PC-2 | 0.17 (−0.75—1.26) | 0.062 | 0.500 | 0.728 |
| PC-3 | −1.37 (−2.39—0.58) | −0.496 | −3.602 | 0.011* |
| PC-4 | −0.36 (−1.40—0.61) | −0.129 | −0.878 | 0.421 |
| PC-5 | 0.37 (−0.89—1.27) | 0.134 | 1.038 | 0.512 |
| PC-6 | 0.83 (−0.10—1.69) | 0.300 | 2.073 | 0.086 |
| Model 2 improvement over Model 1: F ratio change = 4.749; p = 0.003*; r2 = 0.683. | ||||
results from Bootstrap procedure, n=1000 samples.
Figure 2.
a, b. Principle component (PC) scores for organohalogen and elements versus menstrual cycle length. Analytes included in the PCA were detectable in ≥70% of blood samples. See Table 4 for contaminant units. All analytes were transformed by log10(x+1). Figure 2a plots PC-3 loadings against menstrual cycle length. Figure 2b plots PC-1 loadings versus menstrual cycle length.
The only other outcome variable that had a significant regression against our predictive suite of confounder and exposure variables was follicular phase length (Table 6). In Model 1, again, only the regression coefficient for the age of participants was significant (p = 0.025). In the regression with the three confounders and the 6 PC variables against follicular phase length (Model 2), the age coefficient remained significant. However, there was not a significant increase in the amount of variation explained by the second model, although there is a weak but non-significant effect of PC-1 (POH exposure) on follicular phase length after accounting for the effect of age. The standardized regression coefficient for PC-1 in Model 2 is of lower magnitude than that of age suggesting that the effect of PC-1 on follicular phase length is less than that of age. The variance in follicular phase length explained by age and the other confounder variables (Model 1) was approximately 25% (r2 = 0.249, Table 6), and with the addition of contaminant variables (Model 2) the explained variance was increased to 49% (r2 = 0.494).
Table 6.
Multiple regressions for follicular phase length were computed against potential confounders (Model 1) and also against the contaminant principal component (PC) analysis scores (Model 2). The regression coefficient (B) is the coefficient of the slope for predictor variables. The PC scores used in Model 2 are generated from organohalogens and elements detectable in ≥70 % of the sample population. * Regression coefficient slope B and model are significant (p< 0.05).
| Follicular Phase Length | ||||
|---|---|---|---|---|
| Regression Coefficient (B) | t-statistic | p-value1 | ||
| Model 1 | Mean Raw1 (95% C.I.) | Standardized | ||
| Constant | 22.06 (14.36—30.08) | 5.957 | 0.001* | |
| Age (y) | −0.21 (−0.37—−0.04) | −0.459 | −2.487 | 0.025* |
| Alcohol (Y/N) | 0.37 (−2.66—3.06) | 0.058 | 0.299 | 0.819 |
| Caffeine (Y/N) | −1.14 (−3.26—2.23) | −0.149 | −0.842 | 0.375 |
| Model 1 significance: F = 2.984, p = 0.049*; r2 = 0.249. | ||||
| Model 2 | ||||
| Constant | −79.33 (−321.95—373.20) | 5.369 | 0.677 | |
| Age (y) | −0.28 (−0.46—−0.01) | −0.611 | −2.700 | 0.022* |
| Alcohol (Y/N) | 0.35 (−2.38—3.51) | 0.055 | 0.291 | 0.797 |
| Caffeine (Y/N) | 0.39 (−2.96—3.11) | 0.051 | 0.256 | 0.791 |
| PC-1 | 1.32 (−0.62—2.77) | 0.432 | 2.355 | 0.113 |
| PC-2 | −0.04 (−1.02—1.09) | −0.012 | −0.075 | 0.941 |
| PC-3 | −0.78 (−2.30—0.24) | −0.255 | −1.466 | 0.229 |
| PC-4 | 0.07 (−1.37—1.05) | 0.023 | 0.126 | 0.901 |
| PC-5 | −0.24 (−1.69—0.84) | −0.079 | −0.488 | 0.642 |
| PC-6 | 0.68 (−0.61—1.56) | 0.224 | 1.222 | 0.205 |
| Model 2 improvement over Model 1: F ratio change = 1.692; p = 0.172; r2 = 0.494. | ||||
results from Bootstrap procedure, n=1000 samples.
Early follicular phase FSH (Table 7), luteal phase length and mid-luteal phase Pd3G levels were not significantly related to the PC variables when they were considered with the confounders. There was, however, a significant association (p=0.001) between age of the participants and early follicular phase FSH. Model 1 (confounders only) explained 35% of the variation in early follicular phase FSH levels (r2 = 0.346), and addition of the contaminant variables in Model 2 increased the explanatory power to 51% of the variation in the dependent variable (r2 = 0.509, Table 7).
Table 7.
Multiple regressions for early follicular phase FSH were computed against potential confounders (Model 1) and also against the contaminant principal component (PC) analysis scores (Model 2). The regression coefficient (B) is the coefficient of the slope for predictor variables. The PC scores used in Model 2 are generated from organohalogens and elements detectable in ≥70% of the sample population. * Regression coefficient slope B and model are significant (p<0.05).
| Early Follicular Phase FSH | ||||
|---|---|---|---|---|
| Regression Coefficient (B) | t-statistic | p-value1 | ||
| Model 1 | Mean Raw1 (95% C.I.) | Standardized | ||
| Constant | −6.38 (−15.30—1.99) | −1.127 | 0.138 | |
| Age (y) | 0.42 (0.19—0.64) | 0.628 | 3.622 | 0.005* |
| Alcohol (Y/N) | 1.53 (−0.78—4.06) | 0.151 | 0.827 | 0.194 |
| Caffeine (Y/N) | 0.10 (−3.02—3.49) | −0.028 | −0.170 | 0.958 |
| Model 1 significance: F = 4.582, p = 0.011*; r2 = 0.346. | ||||
| Model 2 | ||||
| Constant | −88.72 (−457.50—280.06) | −0.410 | 0.621 | |
| Age (y) | 0.34 (0.03—0.65) | 0.528 | 2.290 | 0.034* |
| Alcohol (Y/N) | 1.81 (−1.81—5.42) | 0.155 | 0.802 | 0.310 |
| Caffeine (Y/N) | −0.02 (−4.94—4.91) | −0.111 | −0.548 | 0.995 |
| PC-1 | −0.53 (−3.13—1.92) | −0.168 | 0.901 | 0.658 |
| PC-2 | 0.22 (−1.99—2.51) | 0.127 | 0.796 | 0.871 |
| PC-3 | −0.66 (−2.54—1.34) | −0.114 | −0.643 | 0.472 |
| PC-4 | 1.00 (−1.74—2.86) | 0.209 | 1.104 | 0.269 |
| PC-5 | 1.29 (−0.38—3.20) | 0.216 | 1.301 | 0.124 |
| PC-6 | −0.47 (−2.06—1.15) | −0.084 | −0.451 | 0.507 |
| Model 2 improvement over Model 1: F ratio change = 1.104; p = 0.395; r2 = 0.509 | ||||
results from Bootstrap procedure, n=1000 samples.
Since contaminant concentrations summarized by PC-3 scores were found to comprise a significant source of variation in menstrual cycle length, effect size was evaluated at the upper and lower 95 percent confidence limits for the mean of observed contaminant concentrations. At the lower confidence limit of mean contaminant concentrations, an expected menstrual cycle length of 25.28 day was calculated while at the higher confidence limit a length of 28.34 day was calculated.
Discussion
The population in this study has circulating levels of DDE, oxychlordane, PCBs, molybdenum, lead, mercury, cadmium, nickel and bismuth that are higher than in a similar aged Health Canada reference population (Health Canada, 2010). Our study reveals that, after adjusting for confounding variables, some of these contaminants may be altering menstrual cycles. Specifically, the overall menstrual cycle length may be shortened by exposure to greater concentrations of PBDE 47, PBDE 153, cadmium, and selenium, while higher concentrations of copper may offset this effect. The decrease in cycle length associated with PC-3 is similar in magnitude and direction to the changes associated with age of participants.
PC-3, with positive loading of PBDE 47 and 153, cadmium and selenium, and a negative loading of copper, is negatively associated with cycle length. But even though the majority of the women in our study smoked, and smoking was a dichotomous variable in our population, smoking was not a significant predictor of cycle length. Blood cadmium is linearly related to frequency of cigarette smoking (White and Sabbioni, 1998), so blood cadmium may be a better indicator of smoking intensity than the dichotomous variable that we used. Cadmium levels may also be related to consumption of the liver or kidneys of moose (Alces alces; Crête et al. 1987; Crichton and Paquet 2000) or bear (Ursus americanus; Crichton and Paquet 2000). Yet there is no evidence that cadmium from the consumption of organ meat is a major source of this toxic metal in the Cree from James Bay. Rather, cadmium levels are associated with cigarette smoking in this population (Charania et al., 2014).
Although the loadings for copper and selenium in PC-3 are somewhat difficult to interpret, blood levels of copper are strongly homeostatically regulated by the body, and those for selenium vary directly with dietary intake (Shenkin et al., 2006). The decrease in cycle length associated with PC-3 is of similar magnitude, but opposite direction to changes associated with caffeine consumption and less than the menstrual cycle length changes associated with age. Like menstrual cycle length in our study, follicular phase length is positively related to the POH levels in PC-1, and inversely related to age, though the effect of the POH exposure on follicular phase length appears to be relatively weak in the additive regression model we employed, perhaps in part due to the relatively small size of the study. Yet, the changes detected for cycle length would appear to most likely reflect changes in follicular phase length, the more variable phase of the menstrual cycle (Fehring et al., 2006). The endocrine indices we examined in this study were not linked to these effects on menstrual cycle and follicular phase lengths.
Regression analyses in this study recognized the well-established relationships that women’s age is associated with shorter menstrual cycles (Rowland et al., 2002; Brodin et al., 2008) and follicular phases (Lenton et al. 1984b) and with elevated follicular phase levels of FSH (Steiner et al., 2008). The shortening of the menstrual cycle appears to be an age-independent marker of infertility (Brodin et al., 2008).
We also found that the consumption of caffeinated beverages had a suggestive, but non-significant association with longer menstrual cycles, but only when the regression analysis included the PC variables. Fenster et al. (1999) found that heavy caffeine consumption was associated with shorter cycles, while Liu et al. (2004) found no significant relationship between caffeine and any menstrual cycle characteristic.
The effect-size of variation in concentrations of contaminants upon menstrual cycle indicates statistically significant variation in menstrual cycle may result from alterations in contaminant loads. At the lower 95% confidence limit for contaminant loads, our data suggest a menstrual cycle 2.5 days shorter than a normal 28 day cycle. The effect-size for contaminants at their upper 95% confidence limit are very small and suggest normal menstrual cycle and follicular phase length with the highest contaminant loads.
There are significant data limitations when carrying out detailed studies with large numbers of variables on small populations such as the Cree women of reproductive age in the James Bay region. Large number of participants are simply not available thus a census of willing participants, rather than large-scale epidemiological studies, is inevitable. We attempted to ameliorate the effects of low sample size by reducing variables with PC analyses and bootstrapping the data.
Conclusions
As pointed out by Singh (2014) it is difficult to make conclusive statements about the role of environmental contaminants in indigenous peoples because of “mixed-results, small number of studies, and studies restricted to a small number of regions.” We present data describing the association of POHs and elemental exposure on menstrual cycle function in Cree First Nation women living in the region of James Bay, Canada. Levels for most of the POHs, except PBDE, were substantially greater than for those of other Canadian women of similar age. The concentrations of trace elements were not different from other Canadian women of similar age except for lead, mercury and cadmium, and the essential trace elements molybdenum and nickel. These elements were found at elevated levels in the study participants. The menstrual cycle characteristics measured in this study were not grossly different from those in other populations of women of similar age.
Yet, our findings suggest menstrual cycle length was significantly and directly related to concentrations of some POHs and elements, principally the PBDEs, cadmium, selenium, and copper summarized in PC-3 scores. Menstrual cycle length was affected by scores on PC-3 in confounder controlled, multi-stage regression models, where our results indicated a negative trend in cycle length with increasing concentrations of PBDEs, cadmium and selenium, and a positive association with higher concentrations of copper. The magnitudes of the changes were less than those attributable to increasing age but greater than to changes attributable to caffeine consumption. The possible offsetting effects of PC-1 and PC-3 on menstrual cycle length appear to limit the overall change attributable to contaminant exposure in our effect size sensitivity analysis. It is difficult to know if these changes in the menstrual cycle are attributable to synergistic or additive effects of the variables in PC-1 or PC-3, or simply the result of one of the variables, or if these contaminants are correlated but not causally linked to the effect. Variation in contaminant exposure did not show statistically significant effects on luteal phase length or the two hormonal indices evaluated.
Acknowledgments
Partial funding was provided by Canadian Institutes of Health Research, Institute of Aboriginal Peoples Health.
We are very grateful to the women of Fort Albany, Ontario, and Oujé-Bougoumou, Quebec, for participating in the study. We would also like to thank Anna Bosum and Weena Bosum of Oujé-Bougoumou plus Celine Sutherland and Ruby Edwards-Wheesk of Fort Albany who provided essential support with recruitment and sampling in their communities.
Footnotes
Competing Financial Interests: The authors declare they have no competing financial interests.
Disclaimers:
Mention of company names and/or products does not constitute endorsement by the Centers for Disease Control and Prevention (CDC).
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Armitage SA, White MA, Wilson HK. The determination of silver in whole blood and its application to biological monitoring of occupationally exposed groups. Ann Occup Hyg. 1996 Jun;40(3):331–8. doi: 10.1016/0003-4878(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Axmon A, Rylander L, Strömberg U, Hagmar L. Altered menstrual cycles in women with a high dietary intake of persistent organochlorine compounds. Chemosphere. 2004;56:813–9. doi: 10.1016/j.chemosphere.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Baird DD, McConnaughey DR, Weinberg CR, Musey PI, Collins DC, Kesner JS, Knecht EA, Wilcox AJ. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995;6:547–50. doi: 10.1097/00001648-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, Zhou H, Kamel F, McConnaughey DR, Kesner JS, Wilcox AJ. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71:40–9. doi: 10.1016/s0015-0282(98)00419-1. [DOI] [PubMed] [Google Scholar]
- Bárány E, Bergdahl IA, Bratteby LE, Lundh T, Samuelson G, Skerfving S, Oskarsson A. Iron status influences trace element levels in human blood and serum. Environ Res. 2005;98(2):215–3. doi: 10.1016/j.envres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Bonnier-Viger Y, Dewailly E, Egeland GM, Nieboer E, Pereg D. Nituuchischaayihtitauu Aschii. Multi-community environment-and-health longitudinal study in Iiyiyiu Achii: Mistissini. In: Pereg D, Nieboer E, editors. Technical report: Summary of activities, results and recommendations. Montreal, QC: Cree Board of Health and Social Services of James Bay; 2007. p. 389. [Google Scholar]
- Brantsaeter AL, Haugen M, Thomassen Y, Ellingsen DG, Ydersbond TA, Hagve TA, Alexander JH, Meltzer M. Exploration of biomarkers for total fish intake in pregnant Norwegian women. Public Health Nutr. 2010;13(1):54–62. doi: 10.1017/S1368980009005904. [DOI] [PubMed] [Google Scholar]
- Brodin T, Bergh T, Berglund L, Hadziosmanovic N, Holte J. Menstrual cycle length is an age-independent marker of female fertility: results from 6271 treatment cycles of in vitro fertilization. Fertil Steril. 2008;90(5):1656–6. doi: 10.1016/j.fertnstert.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Chang SH, Cheng BH, Lee SL, Chuang HY, Yang CY, Sung FC, Wu TN. Low blood lead concentration in association with infertility in women. Environ Res. 2006;101(3):380–6. doi: 10.1016/j.envres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Charania NA, Tsuji LJ, Martin ID, Liberda EN, Coté S, Ayotte P, Dewailly E, Nieboer E. An examination of traditional foods and cigarette smoking as cadmium sources among the nine First Nations of Eeyou Istchee, Northern Quebec, Canada. Environ Sci Process Impacts. 2014;16(6):1422–33. doi: 10.1039/c4em00064a. [DOI] [PubMed] [Google Scholar]
- Chen A, Zhang J, Zhou L, Gao ES, Chen L, Rogan WJ. DDT serum concentration and menstruation among young Chinese women. Environ Res. 2005;99(3):397–402. doi: 10.1016/j.envres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Crête M, Potvin F, Walsh P, Benedetti J-L, Lefebvre MA, Weber J-P, Paillard G, Gagnon J. Pattern of cadmium contamination in the liver and kidneys of moose and white-tailed deer in Québec. Science of the Total Environment. 1987;66:45–53. doi: 10.1016/0048-9697(87)90076-3. [DOI] [PubMed] [Google Scholar]
- Crichton V, Paquet PC. Cadmium in Manitoba's wildlife. Alces. 2000;36:205–216. [Google Scholar]
- Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. Am Epidemiol. 1997;146:32–41. doi: 10.1093/oxfordjournals.aje.a009189. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Nieboer E. Exposure and preliminary health assessments of the Oujé-Bougoumou Cree population to mine tailings residues: Report of the survey. Montréal, QC: Institut National deSanté Publique du Québec; Jan, 2005. p. 278. [cited 2011 Jan16]. Available from: http://www.inspq.qc.ca. [Google Scholar]
- Donahoo WT, Bessesen DH, Higbee DR, Lei S, Grunwald GK, Higgins JA. Serum lithium concentration can be used to assess dietary compliance in adults. J Nutr. 2004;134(11):3133–36. doi: 10.1093/jn/134.11.3133. [DOI] [PubMed] [Google Scholar]
- Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17(5):1399–403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, de Jager C, Henshel DS, Leipzig F, Leipzig JS, Lorenz EC, Snedeker SM, Stapleton D. The Pine River statement: human health consequences of DDT use. Environ Health Perspect. 2009;117(9):1359–67. doi: 10.1289/ehp.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35:376–84. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Fenster L, Quale C, Waller K, Windham GC, Elkin EP, Benowitz N, Swan SH. Caffeine consumption and menstrual function. Am J Epidemiol. 1999;149:550–7. doi: 10.1093/oxfordjournals.aje.a009851. [DOI] [PubMed] [Google Scholar]
- Findlay JB, Wu AL, Knott V, Mauck L, Frickey PH, Norton GE. Development of a Kodak Ektachem® clinical chemistry slide for CK-B activity. Clin Chem. 1985;31(Abstract 509):1000. [Google Scholar]
- Foster WG, Neal MS, Han MS, Dominguez MM. Environmental contaminants and human infertility: hypothesis or cause for concern? J Toxicol Environ Health B Crit Rev. 2008;11:162–76. doi: 10.1080/10937400701873274. [DOI] [PubMed] [Google Scholar]
- Freund RJ, Wilson WJ. Regression Analysis: statistical modeling of a response variable. Elsevier; 1997. p. 496. [Google Scholar]
- Gauch HG., Jr . Multivariate analysis in community ecology. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Bouige D, Lacroix C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values Forensic Sci Int. 2005;153(1):39–44. doi: 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Green RH. Sampling design and statistical methods for environmental biologists. New York: Wiley; 1979. [Google Scholar]
- Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70(4):632–7. doi: 10.1016/s0015-0282(98)00257-x. [DOI] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjödin A, Eskenazi B. PBDE concentrations in women's serum and fecundability. Environ Health Perspect. 2010;118(5):699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD. Menstruation and menstrual disorders: the epidemiology of menstruation and menstrual dysfunction. In: Goldman M, Hatch M, editors. Women and Health. Academic Press; San Diego, CA: 2000. pp. 99–113. [Google Scholar]
- Health Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009) Ottawa, Canada: Health Canada; 2010. Report on human biomonitoring of environmental chemicals in Canada. [cited 2012 Jan16]. Available from: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/chms-ecms/report-rapport-eng.pdf. [Google Scholar]
- Heitland P, Köster H. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP–MS. Journal of Trace Elements in Medicine and Biology. 2006;20(4):253–62. doi: 10.1016/j.jtemb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Hornsby PP. Dissertation. Chapel Hill, NC: University of North Carolina; 1991. The effects of in utero exposure to diethylstilbestrol (DES) on the menstrual cycle. [Google Scholar]
- Jackson LW, Howards PP, Wactawski-Wende J, Schisterman EF. The association between cadmium, lead and mercury blood levels and reproductive hormones among healthy, premenopausal women. Hum Reprod. 2011;26(10):2887–95. doi: 10.1093/humrep/der250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner JS, Knecht EA, Krieg EF., Jr Time-resolved immunofluorometric assays for urinary luteinizing hormone and follicle stimulating hormone. Analyt Chim Acta. 1994a;285:13–22. [Google Scholar]
- Kesner JS, Knecht EA, Krieg EF, Jr, Barnard G, Mikola H, Kohen F, Gani MM, Coley J. Validation of time-resolved fluoroimmunoassays for urinary estrone 3-glucuronide and pregnanediol 3-glucuronide. Steroids. 1994b;59:205–11. doi: 10.1016/0039-128x(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Kesner JS, Knecht EA, Krieg EF. Stability of urinary female reproductive hormones stored under various conditions. Reprod Toxicol. 1995;9:239–44. doi: 10.1016/0890-6238(95)00005-u. [DOI] [PubMed] [Google Scholar]
- Kesner JS, Knecht EA, Krieg EF, Jr, Wilcox AJ, O'Connor JF. Detecting preovulatory luteinizing hormone surges in urine. Human Reprod. 1998;13:15–21. doi: 10.1093/humrep/13.1.15. [DOI] [PubMed] [Google Scholar]
- Krieg EF., Jr The relationships between blood lead levels and serum follicle stimulating hormone and luteinizing hormone in the third National Health and Nutrition Examination Survey. Environ Res. 2007;104(3):374–82. doi: 10.1016/j.envres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Lambert JR. Pharmacology of bismuth-containing compounds. Symposium on Helicobacter pylori: A cause of gastroduodenal disease. Rev Infect Dis. 1991;13:S691–5. doi: 10.1093/clinids/13.supplement_8.s691. [DOI] [PubMed] [Google Scholar]
- Lenton EA, Landgren B-M, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol. 1984a;91(7):685–89. doi: 10.1111/j.1471-0528.1984.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Lenton EA, Landgren BM, Sexton L, Harper R. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984b;91(7):681–4. doi: 10.1111/j.1471-0528.1984.tb04830.x. [DOI] [PubMed] [Google Scholar]
- Liberda EN, Tsuji LJS, Martin ID, Ayotte P, Dewailly E, Nieboer E. The complexity of hair/blood mercury concentration ratios and its implications. Environ Res. 2014;134:286–294. doi: 10.1016/j.envres.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Liberda EN, Wainman BC, LeBlanc A, Dumas P, Martin ID, Tsuji LJS. Dietary exposure of PBDEs resulting from a subsistence diet in three First Nation communities in the James Bay Region of Canada. Environ Int. 2011;37(3):631–6. doi: 10.1016/j.envint.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160(2):131–40. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- London Laboratory Services Group. Panels with reference ranges. London, ON Canada: Trace Elements Laboratory; 2008. Mar, [cited Feb 18, 2011]. Available from: http://www.lhsc.on.ca/lab/metals/icpms.htm. [Google Scholar]
- Luderer U, Kesner JS, Fuller JM, Krieg EF, Jr, Meadows JW, Tramma SL, Yang H, Baker DB. Effects of gestational and lactational exposure to heptachlor epoxide on age at puberty and reproductive function in men and women. Environmental Research. 2013;121:84–94. doi: 10.1016/j.envres.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Mauck JC, Mauck L, Novros J, Norton GE, Toffaletti J. Development of a single slide Kodak Ektachem® thin-film assay for serum and urine creatinine. Clin Chem. 1986;32(Abstract 735):1197–8. [Google Scholar]
- Meeker JD, Maity A, Missmer SA, Williams PL, Mahalingaiah S, Ehrlich S, Berry KF, Altshul L, Perry MJ, Cramer DW, Hauser R. Serum Concentrations of polychlorinated biphenyls (PCBs) in relation to in vitro fertilization (IVF) outcomes. Environ Health Perspect. 2011;119(7):1010–16. doi: 10.1289/ehp.1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola P, Buck GM, Sever LE, Zielezny M, Vena JE. Consumption of PCB-contaminated freshwater fish and shortened menstrual cycle length. Am J Epidemiol. 1997;146(11):955–60. doi: 10.1093/oxfordjournals.aje.a009222. [DOI] [PubMed] [Google Scholar]
- Mendola P, Messer LC, Rappazzo K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril. 2008;89(2 suppl):e81–e94. doi: 10.1016/j.fertnstert.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Michos C, Kalfakakou V, Karkabounas S, Kiortsis D, Evangelou A. Changes in copper and zinc plasma concentrations during the normal menstrual cycle in women. Gynecological Endocrinology. 2010;26(4):250–255. doi: 10.3109/09513590903247857. [DOI] [PubMed] [Google Scholar]
- Nieboer E, Dewailly E, Egeland GM, Chateau-Degat M-L, Bonnier-Viger Y. Nituuchischaayihtitaau Aschii. Multi-Community environment-and-health longitudinal study in Iiyiyiu Aschii: Eastmain and Wemindji. In: Nieboer E, Robinson E, Petrov K, editors. Technical report: summary of activities, results and recommendations. Montreal, QC: Cree Board of Health and Social Services of James Bay; 2011. Available from: http://creehealth.org/clinical-protocols/nituuchischaayihtitaau-aschii-multi-community-environment-and-health-longitudinal. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health) Occupational exposure to poly-chlorinated biphenyls (PCBs) Rockville, Md: US DHEW, PHS, CDC; 1997. Criteria for a recommended standard. No. 77–225. [Google Scholar]
- Ouyang F, Perry MJ, Venners SA, Chen C, Wang B, Yang F, Fang Z, Zang T, Wang L, Xu X, Wang X. Serum DDT, age at menarche, and abnormal menstrual cycle length. Occup Environ Med. 2005;62:878–84. doi: 10.1136/oem.2005.020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson DG, Jr, Isaacs SG, Alexander LR, Turner WE, Hampton L, Bernert JT, Needham LL. Determination of specific polychlorinated dibenzo-p-dioxins and dibenzofurans in blood and adipose tissue by isotope dilution-high-resolution mass spectrometry. IARC Sci Publ. 1991;108:299–342. [PubMed] [Google Scholar]
- Perry M, Ouyang F, Korrick S, Venners S, Chen C, Xu X, Lasley B, Wang X. A prospective study of serum DDT and progesterone and estrogen levels across the menstrual cycle in nulliparous women of reproductive age. Am J Epidemiol. 2006;164:1056–64. doi: 10.1093/aje/kwj329. [DOI] [PubMed] [Google Scholar]
- Pollack AZ, Schisterman EF, Goldman LR, Mumford SL, Albert PS, Jones RL, Wactawski-Wende J. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ Health Perspect. 2011;119(8):1156–61. doi: 10.1289/ehp.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CL, Wania F. Understanding differences in the body burden–age relationships of bioaccumulating contaminants based on ppulation cross sections versus individuals. Environ Health Perspect. 2012;120(4):554–559. doi: 10.1289/ehp.1104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutman SR, LeMasters GK, Knecht EA, Shukla R, Lockey JE, Burroughs GE, Kesner JS. Evidence of reproductive endocrine effects in women with occupational fuel and solvent exposures. Environ Health Perspect. 2002a;110(8):805–11. doi: 10.1289/ehp.02110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutman SR, Lemasters GK, Kesner JS, Shukla R, Krieg EF, Jr, Knecht EA, Lockey JE. Urinary reproductive hormone level differences between African American and Caucasian women of reproductive age. Fertil Steril. 2002b;78:383–91. doi: 10.1016/s0015-0282(02)03204-1. [DOI] [PubMed] [Google Scholar]
- Rowland AS, Baird DD, Long SL, Wegienka G, Harlow SD, Alavanja M, Sandler DP. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–74. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Sandanger TM, Brustad M, Lund E, Burkow IC. Changes in levels of persistent organic pollutants in human plasma after consumption of a traditional northern Norwegian fish dish – Molje (cod, cod liver, cod liver oil and hard roe) J Environ Monit. 2003;5:160–5. doi: 10.1039/b210517a. [DOI] [PubMed] [Google Scholar]
- Shenkin A, Baines M, Fell GS, Lyon TDG. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. In: Burtis CA, Ashwood ER, Bruns DE, editors. Vitamins and Trace Elements. 4. USA: Elsevier Saunders; 2006. pp. 1075–164. [Google Scholar]
- Singh K, Bjerregaard P, Chan HM. Association between environmental contaminants and health outcomes in indigenous populations of the Circumpolar North. Int J Circumpolar Health. 2014;73:25808. doi: 10.3402/ijch.v73.25808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikkerveer A, Jong HB, Helmich RB, de Wolff FA. Development of a therapeutic procedure for bismuth intoxication with chelating agents. J Lab Clin Med. 1992;119:529–37. [PubMed] [Google Scholar]
- SPSS Inc. SPSS Statistics for Mac OS X. Chicago: SPSS Inc; [Accessed Dec 19, 2013]. Released 2014. Statistics Canada. Statistics Canada, Canadian Community Health Survey, 2000/1. Table 105–0027. No date. from Statistics Canada: http://www5.statcan.gc.ca/cansim/a47. [Google Scholar]
- Steiner AZ, Baird DD, Kesner JS. Mother's menopausal age is associated with her daughter's early follicular phase urinary follicle stimulating hormone level. Menopause. 2008;15:940–4. doi: 10.1097/gme.0b013e31816429e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Axmon A, Lindh CH, Giwercman A, Bonde JP. Menstrual cycle characteristics in European and Inuit women exposed to persistent organochlorine pollutants. Hum Reprod. 2008;23(1):193–200. doi: 10.1093/humrep/dem349. [DOI] [PubMed] [Google Scholar]
- Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- Tsuji LJS, Wainman BC, Martin ID, Weber J-P, Sutherland C, Elliott JR, Nieboer E. The mid-Canada radar line and First Nations' people of the James Bay region, Canada: an evaluation using log-linear contingency modeling to analyze organochlorine frequency data. J Environ Monit. 2005;7:888–98. doi: 10.1039/b500524h. [DOI] [PubMed] [Google Scholar]
- Tsuji LJS, Wainman BC, Martin ID, Weber JP, Sutherland C, Nieboer E. Abandoned mid-Canada radar line sites in the western James region of northern Ontario, Canada: a source of organochlorines for First Nations people? Sci. Total Environ. 2006;370:452–66. doi: 10.1016/j.scitotenv.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Tsuji LJS, Wainman BC, Martin ID, Nieboer E. Lead shot contribution to blood lead of First Nations People: The use of lead isotopes to identify the source of exposure. Sci Total Environ. 2008;393:291–8. doi: 10.1016/j.scitotenv.2008.06.048. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Steenport D, Buelow C, Imm P, Knobeloch L. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere. 2010;81(4):517–22. doi: 10.1016/j.chemosphere.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oostdam J, Gilman A, Dewailly E, Usher P, Wheatley B, Kuhnlein H, Neve S, Walker J, Tracy B, Feely M, Jerome V, Kwavnick B. Human Health implications of environmental contaminants in Arctic Canada: a Review. Sci Total Environ. 1999;230:1–82. doi: 10.1016/s0048-9697(99)00036-4. [DOI] [PubMed] [Google Scholar]
- White MA, Sabbioni E. Trace element reference values in tissues from inhabitants of the European Union. X A study of 13 elements in blood and urine of a United Kingdom population. Science of the Total Environment. 1998;216(3):253–270. doi: 10.1016/s0048-9697(98)00156-9. [DOI] [PubMed] [Google Scholar]
- Windham GC, Lee D, Mitchell P, Anderson M, Petreas M, Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005;16:182–90. doi: 10.1097/01.ede.0000152527.24339.17. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Huang T-S, Lin K-C, Kuo P, Tsai P-C, Guo YL. Menstrual effects among women exposed to polychlorinated biphenyls and dibenzofurans. Environmental Research. 2011;111(2):288–94. doi: 10.1016/j.envres.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Yu M, Guo Y, Hsu C, Rogan W. Menstruation and reproduction in women with polychlorinated biphenyl (PCB) poisoning: long-term follow-up interviews of the women from the Taiwan Yucheng cohort. Int J Epidemiol. 2005;29:672–677. doi: 10.1093/ije/29.4.672. [DOI] [PubMed] [Google Scholar]